Abstract
An agar plate assay was developed for detecting the induction of drug-resistant mycobacterial mutants during exposure to inhibitors of DNA gyrase. When Mycobacterium smegmatis on drug-containing agar, resistant colonies arose over a period of 2 weeks. A recA deficiency reduced mutant recovery, consistent with involvement of the SOS response in mutant induction. The C-8-methoxy compounds gatifloxacin and moxifloxacin allowed the recovery of fewer resistant mutants than either ciprofloxacin or levofloxacin when present at the same multiple of the MIC; a quinolone-like 8-methoxy-quinazoline-2,4-dione was more effective at restricting the emergence of resistant mutants than its cognate fluoroquinolone. Thus, the structure of fluoroquinolone-like compounds affects mutant recovery. A spontaneous mutator mutant of M. smegmatis, obtained by growth in medium containing both isoniazid and rifampin, increased mutant induction during exposure to ciprofloxacin. Moreover, the mutator increased the size of spontaneous resistant mutant subpopulations, as detected by population analysis. Induction of ciprofloxacin resistance was also observed with Mycobacterium tuberculosis H37Rv. When measured with clinical isolates, no difference in mutant recovery was observed between multidrug-resistant (MDR) and pansusceptible isolates. This finding is consistent with at least some MDR isolates of M. tuberculosis lacking mutators detectable by the agar plate assay. Collectively, the data indicate that the use of fluoroquinolones against tuberculosis may induce resistance and that the choice of quinolone may be important for restricting the recovery of induced mutants.
INTRODUCTION
Fluoroquinolones are broad-spectrum antimicrobials that are important for the treatment of multidrug-resistant (MDR) tuberculosis (TB) (6, 37). Unfortunately, fluoroquinolone resistance is emerging, often in strains of Mycobacterium tuberculosis that are already MDR (62). When the resulting fluoroquinolone-resistant MDR mutants are also resistant to an injectable drug such as kanamycin, amikacin, or capreomycin, they are considered to be extensively drug resistant (XDR) (12). At that point, treatment is still possible but obtaining a successful outcome is quite difficult (3, 19, 38). Thus, having a new, highly effective fluoroquinolone is desirable to halt the progression to XDR status. During the last decade, several new quinolones were developed for other Gram-positive bacteria, and two of these agents, moxifloxacin and gatifloxacin, are now being considered as additions to the anti-TB armamentarium (14, 39, 58). However, a well-known problem of fluoroquinolone action with other bacteria is the induction of the mutagenic SOS response (21, 42, 52). If this phenomenon extends to mycobacteria, the quinolones are expected to induce resistance to themselves and to other agents commonly employed. Thus, understanding and restricting the emergence of resistance during quinolone exposure are likely to be important.
Work with Escherichia coli suggests that an agar plate assay can be used to detect the induction of resistant mutants during drug exposure (7, 8, 31). Induced mutants appear as colonies that gradually accumulate over a period of 10 to 14 days on fluoroquinolone-containing agar; mutant subpopulations present prior to drug exposure appear as colonies within 1 to 2 days after plating. Mutant induction requires RecA and inducible LexA (activation of RecA promotes self-cleavage of LexA, the repressor of the SOS regulon (28). It also requires a large parental population, making the readout sensitive to the lethal action of quinolones. Some quinolone class compounds also suppress mutant growth, which will reduce the recovery of induced mutants. Thus, the agar plate assay is a composite test for several important quinolone activities that are likely to depend on drug structure.
To determine whether the agar plate assay is suitable for mycobacteria, we plated Mycobacterium smegmatis on quinolone-containing agar and measured colony accumulation over a 2-week period. To test for mutant induction, we blocked the induction of the mutagenic SOS response with a recA mutation and measured the effect on the accumulation of ciprofloxacin-resistant colonies. The sensitivity of the assay to fluoroquinolone structure was then examined with four commercially available compounds, and assay sensitivity to mutator mutants was assessed with a spontaneous mutator. When we applied the agar plate assay to M. tuberculosis using ciprofloxacin, a fluoroquinolone known to enrich resistant mutants (65), mutant induction was readily observed. We expect the assay to be useful for comparing anti-TB agents and for assessing the mutator status of bacterial isolates.
MATERIALS AND METHODS
Bacterial strains and culture conditions.
The M. smegmatis and M. tuberculosis strains used in the study are listed in Tables 1 and 2, respectively. All mycobacteria were cultured in 7H9 liquid medium or on 7H10 agar plates, in both cases supplemented with 10% albumin-dextrose-catalase, 0.2% glycerol, and 0.05% Tween 80 (18). Incubation was at 37°C; all M. tuberculosis work was conducted in a biosafety level 3 containment facility.
Table 1.
M. smegmatis strains used in this study
| Strain | Relevant genotype or phenotype | Source or reference |
|---|---|---|
| KD1163 | Wild type (mc2155) | Laboratory stock; S. Cole |
| KD2859 | KD1163 Inhr Rifr mutator | This worka |
| KD2860 | KD1163 Inhr Rifr | This workb |
| mc2155 (KD2045) | recA+ | K. G. Papavinasasundaram |
| HS42 (KD2046) | mc2155 ΔrecA | K. G. Papavinasasundaram |
Obtained by selection of double resistance in a single step.
Obtained by stepwise selection.
Table 2.
M. tuberculosis strains used in this study
| Strain | sSNP clustera | RFLPb | Resistancec | Spoligotype octal coded | PGGe | Source |
|---|---|---|---|---|---|---|
| H37Rv | VII | H37Rv | None | 777777477760771 | 3 | Laboratory strain, PHRIf TB Center |
| 10536 | II | KY | R, I | 000000000000771 | 1 | Clinical isolate, PHRI TB Center |
| 10775 | II | W4 | None | 000000000003771 | 1 | Clinical isolate, PHRI TB Center |
| 18996 | IV | H | I, R, E, PZA | 777776777760601 | 2 | Clinical isolate, PHRI TB Center |
| 13571 | IV | H | None | 777776777760601 | 2 | Clinical isolate, PHRI TB Center |
| 16644 | V | AH | I, R, E, PZA, S | 777776777760771 | 2 | Clinical isolate, PHRI TB Center |
| 12850 | V | AH | None | 777776777760771 | 2 | Clinical isolate, PHRI TB Center |
sSNP, synonymous single nucleotide polymorphism. Isolates have been grouped based on closely related genotypes (1).
RFLP (restriction fragment length polymorphism) genotype method based on IS6110 (1).
Abbreviations: E, ethambutol; I, isoniazid; PZA, pyrazinamide; R, rifampin; S, streptomycin.
Spoligotyping is a PCR-based genotyping method focused on directly repeated nucleotide sequences (5).
PHRI, Public Health Research Institute.
Chemicals and reagents.
Ciprofloxacin and moxifloxacin were products of Bayer Healthcare (West Haven, CT), and gatifloxacin was obtained from Bristol-Myers Squibb (Princeton, NJ). PD160793 was a generous gift from John Domagala (Parke-Davis Division of Pfizer Chemical Co., Ann Arbor, MI). Dione UING5-207 and the cognate fluoroquinolone UING5-249 were prepared as previously outlined (13). Levofloxacin, isoniazid, and rifampin were obtained from Sigma-Aldrich (St. Louis, MO). Fluoroquinolones were dissolved in 1 N NaOH at 1/10 of the final volume, and then sterile water was added to obtain a final concentration of 10 mg/ml. Dione UING5-207 was dissolved in dimethyl sulfoxide at 10 mg/ml. Isoniazid was dissolved in water, and rifampin was dissolved in ethanol. Dilutions were prepared with sterile distilled water, and solutions were kept at −20°C for several weeks during testing.
Measurement of antimicrobial susceptibility.
Inhibition of growth (MIC) was measured by agar or broth dilution. About 104 to 105 cells were applied to a series of agar plates or broth cultures containing antimicrobials at various concentrations. M. smegmatis colonies were counted after incubation for 3 days or turbid growth was detected after 2 days. The MIC was taken as the minimal concentration that blocked the growth of liquid cultures or inhibited the formation of colonies by 99%, as determined from interpolation of log-log plots. MICs for M. tuberculosis were determined by the broth dilution method after incubation for 10 days.
Lethal activity was measured with liquid cultures of exponentially growing M. smegmatis in which aliquots containing 5 × 108 CFU/ml were incubated for 18 h in the presence of various concentrations of ciprofloxacin. The number of surviving cells was determined by dilution and plating on drug-free agar, followed by incubation at 37°C for 3 days. Percent survival was determined relative to that of an untreated control sampled at the time of drug addition.
Detection of quinolone-induced, quinolone-resistant mutants.
The agar plate method previously described for E. coli (31) was used, with slight modification. Briefly, concentrated quinolone solutions were added to molten agar, and mycobacterial cells (104 to 108) were plated on solidified agar. The cumulative number of colonies forming during incubation at 37°C was determined at regular intervals.
Enrichment of mutator mutants.
The presence of a mutator mutation was defined by the concurrent recovery of resistance to two drugs (single-step double mutants) under conditions in which the number of bacteria in the culture was below that required for recovery of resistance to one drug alone times the number of bacteria required for the recovery of resistance to the other drug. To obtain single-step double mutants, cultures of wild-type M. smegmatis (KD1163) were grown to mid-log phase and diluted to generate sets of cultures (16 1-ml cultures) such that sets contained 3 × 105 CFU/ml, 1.3 × 106 CFU/ml, 5 × 106 CFU/ml, or 2 × 107 CFU/ml. All cultures received both rifampin and isoniazid at 3 times the MIC (rifampin MIC, 1.5 μg/ml; isoniazid MIC, 6.25 μg/ml). After the cultures were incubated at 37°C for 10 days with shaking, those exhibiting growth (turbidity) were presumed to contain a mutator mutation. Single colonies were isolated and grown into cultures for analysis.
Population analysis profiles.
Population analysis was performed as previously described (65). Briefly, a series of agar plates was prepared in which the concentration of the fluoroquinolone PD160793 varied over a broad range. Test strains of M. smegmatis, grown to stationary phase in liquid medium, were plated in amounts that allowed a small number of colonies to form during incubation for 3 days. To verify that the colonies represented mutants, they were transferred to drug-free agar for a second round of growth and then tested on agar containing the drug at the same concentration used initially for selection. All colonies grew when retested on fluoroquinolone-containing agar.
Ethidium bromide-resistant mutants were identified by the ability to form colonies on agar containing 6.25 μg/ml ethidium bromide, which was equivalent to the MIC measured with cultures grown in liquid medium.
DNA isolation and nucleotide sequence determination.
Cultures of stationary-phase M. smegmatis were harvested by centrifugation, and DNA was extracted as described previously (65). Briefly, cells were treated with lysozyme, proteinase K, and sodium dodecyl sulfate; lysates were extracted with mixtures of chloroform-isoamyl alcohol; and DNA was precipitated with alcohol. PCR was performed to amplify the quinolone resistance-determining regions (QRDR) of gyrA and gyrB, and the nucleotide sequences of those regions were determined. The oligonucleotides used for amplification were 5′-ACG AAC TGT TCT CCA TCC TCA TGG GCG AAG-3′ (forward primer), 5′-ACA TCT CCA TCG CCA ACG GAG TG-3′ (reverse primer), and 5′-CAG CGC AGC TAC ATC GAC TAC GC-3′ (sequencing primer) for gyrA and 5′-CCG ACT GCC GTT CGA CGG AT-3′ (forward and sequencing primer) and 5′-CGG CCA TCA ACA CGA TCT TG-3′ (reverse primer) for gyrB.
RESULTS
Induction of quinolone-resistant mutants of M. smegmatis during quinolone exposure.
To determine whether fluoroquinolones induce resistance mutations in mycobacteria, we plated an exponentially growing culture of M. smegmatis on ciprofloxacin-containing agar and recorded the appearance of colonies over a 14-day period (the structures of the compounds used are shown in Fig. 1). Increasing the ciprofloxacin concentration from 0.4 to 3.2 μg/ml (2 to 16 times the MIC) decreased the accumulation of colonies (Fig. 2A). Increasing the number of cells applied to agar increased the number of mutants recovered (Fig. 2B), but the fraction of cells recovered was roughly constant at a given concentration of ciprofloxacin (Fig. 2C; the slight decline in colony number seen with large inocula probably arose from crowding of colonies interfering with growth). Approximately 100 colonies were obtained from ciprofloxacin-containing agar, regrown on drug-free agar, and then retested on agar containing ciprofloxacin at the same concentration initially used to obtain the colonies. All colonies regrew rapidly (data not shown), which indicated that the colonies recovered from the initial plating contained resistant mutants. Nucleotide sequence analysis of DNA isolated from colonies recovered from ciprofloxacin-containing agar plates after incubation for 10 days revealed alterations in gyrA and gyrB (Table 3; mutants were from the experiment shown in Fig. 2A). Ten mutants whose colonies were visible only after a week of incubation regrew at the same rate as wild-type cells (data not shown), indicating that their late recovery from agar was not due to slow growth of mutants present prior to drug exposure. A recA mutation restricted the increase in mutant colony recovery (Fig. 2D), consistent with involvement of the SOS response in mutant induction (4). Collectively, these data indicate that fluoroquinolones induce resistance in M. smegmatis.
Fig 1.
Structures of the compounds used in this study.
Fig 2.
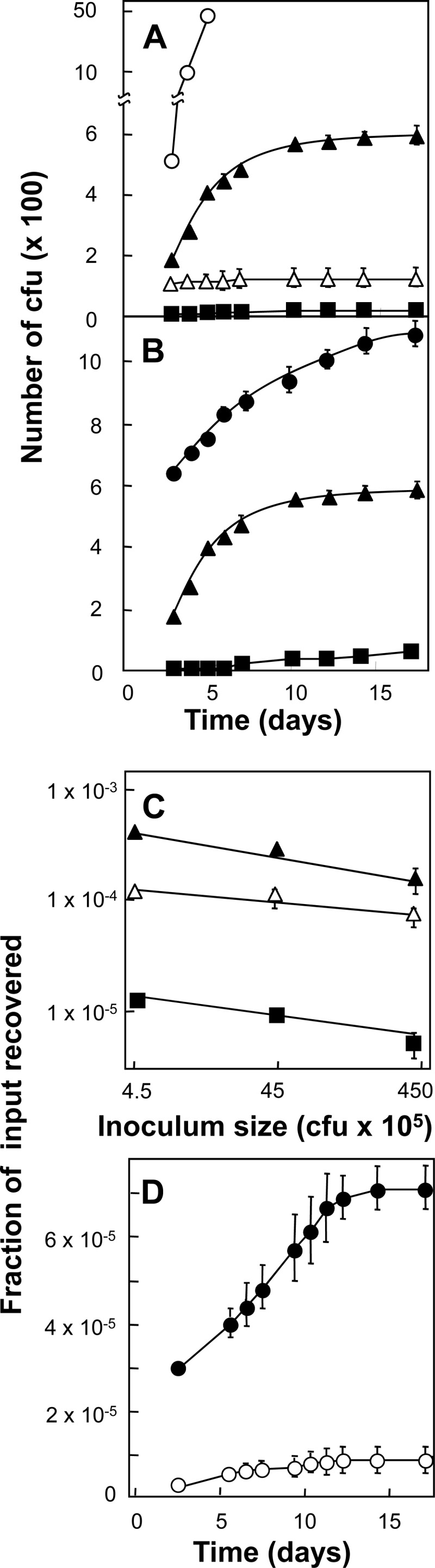
Ciprofloxacin-resistant mutants of M. smegmatis induced by ciprofloxacin. Exponentially growing cultures of M. smegmatis strain KD1163 were applied to ciprofloxacin-containing agar and incubated at 37°C, and at daily intervals, the cumulative number of colonies was determined. (A) Effect of ciprofloxacin concentration. An inoculum of 4.5 × 106 CFU was applied to agar containing various concentrations of ciprofloxacin (MIC = 0.2 μg/ml). Symbols: empty circles, 0.4 μg/ml; filled triangles, 0.8 μg/ml; empty triangles, 1.6 μg/ml; filled squares, 3.2 μg/ml. (B) Effect of inoculum size. Various numbers of cells were applied to agar containing 0.8 μg/ml ciprofloxacin (4 times the MIC). Symbols: filled circles, 4.5 × 107 CFU; filled triangles, 4.5 × 106 CFU; filled squares, 4.5 × 105 CFU. (C) Effect of inoculum size on fractional recovery of mutants. Data such as those shown in panel B were used to determine the number of colonies measured at several concentrations of ciprofloxacin on day 10, corrected for pre-existing mutants by subtraction of the number of colonies found on day 3. Symbols: filled triangles, 0.8 μg/ml (4 times the MIC); empty triangles, 1.6 μg/ml (8 times the MIC); filled squares, 3.2 μg/ml (16 times the MIC). (D) Effect of a recA deficiency on mutant induction. Wild-type strain mc2155 (KD2045; filled circles, MIC = 0.5 μg/ml) and recA-deficient strain HS42 (KD2046; empty circles, MIC = 0.2 μg/ml) were applied to agar containing ciprofloxacin at 2.5 times the MIC, and at the indicated incubation times, colony numbers were determined and plotted as a fraction of the number of CFU applied to the plates. Error bars for panels A, B, and D represent standard deviations; similar results were obtained with two replicate experiments.
Table 3.
Characterization of M. smegmatis mutants used in this study
| Parental strain and source of mutants | Drug used for selection | Mutation |
No. of mutants | |
|---|---|---|---|---|
| GyrA | GyrB | |||
| KD1163 (wild type) | ||||
| Fig. 2A, day 3 | Ciprofloxacin | None | Y524H | 1 |
| Fig. 2A, day 3 | Ciprofloxacin | V38G | None | 1 |
| Fig. 2A, day 10 | Ciprofloxacin | None | Y524H | 5 |
| Fig. 2A, day 10 | Ciprofloxacin | A91V | None | 1 |
| Fig. 2A, day 10 | Ciprofloxacin | D95G | None | 1 |
| Fig. 2A, day 10 | Ciprofloxacin | D95S | None | 1 |
| Fig. 2A, day 10 | Ciprofloxacin | D95Y | None | 1 |
| KD2859 (mutator) | ||||
| Fig. 4A, day 3 | Ciprofloxacin | None | Y524H | 1 |
| Fig. 4A, day 3 | Ciprofloxacin | E44K | None | 2 |
| Fig. 4A, day 3 | Ciprofloxacin | E44R | None | 1 |
| Fig. 4A, day 10 | Ciprofloxacin | None | Y524H | 5 |
| Fig. 4A, day 10 | Ciprofloxacin | A91V | None | 1 |
| Fig. 4A, day 10 | Ciprofloxacin | S92P | None | 3 |
| KD1163 (wild type) | ||||
| Fig. 4C, a | PD160793 | None | Y524H | 1 |
| Fig. 4C, b | PD160793 | None | Y524H | 1 |
| Fig. 4C, b | PD160793 | A91V | None | 1 |
| Fig. 4C, b | PD160793 | D95Y | None | 1 |
| Fig. 4C, c | PD160793 | None | Y524H | 3 |
| Fig. 4C, c | PD160793 | D95S | None | 1 |
| Fig. 4C, c | PD160793 | D95Y | None | 1 |
| KD2859 (mutator) | ||||
| Fig. 4C, b′ | PD160793 | None | Y524H | 2 |
| Fig. 4C, b′ | PD160793 | S92P | None | 2 |
| Fig. 4C, c′ | PD160793 | None | Y524H | 4 |
| Fig. 4C, c′ | PD160793 | G89A | None | 1 |
| Fig. 4C, c′ | PD160793 | A91V | Y524H | 1 |
| Fig. 4C, c′ | PD160793 | S92P | None | 1 |
Effect of drug structure on recovery of M. smegmatis mutants.
M. smegmatis was plated on agar containing a fluoroquinolone (moxifloxacin, gatifloxacin, levofloxacin, or ciprofloxacin) at 2 times the MIC, and then colonies were scored at daily intervals for 2 weeks. The relative order for restricting the recovery of induced mutants was moxifloxacin ∼ gatifloxacin > levofloxacin > ciprofloxacin (Fig. 3A). Similar results were observed when M. smegmatis was plated on agar containing drugs at several other concentrations (data not shown).
Fig 3.
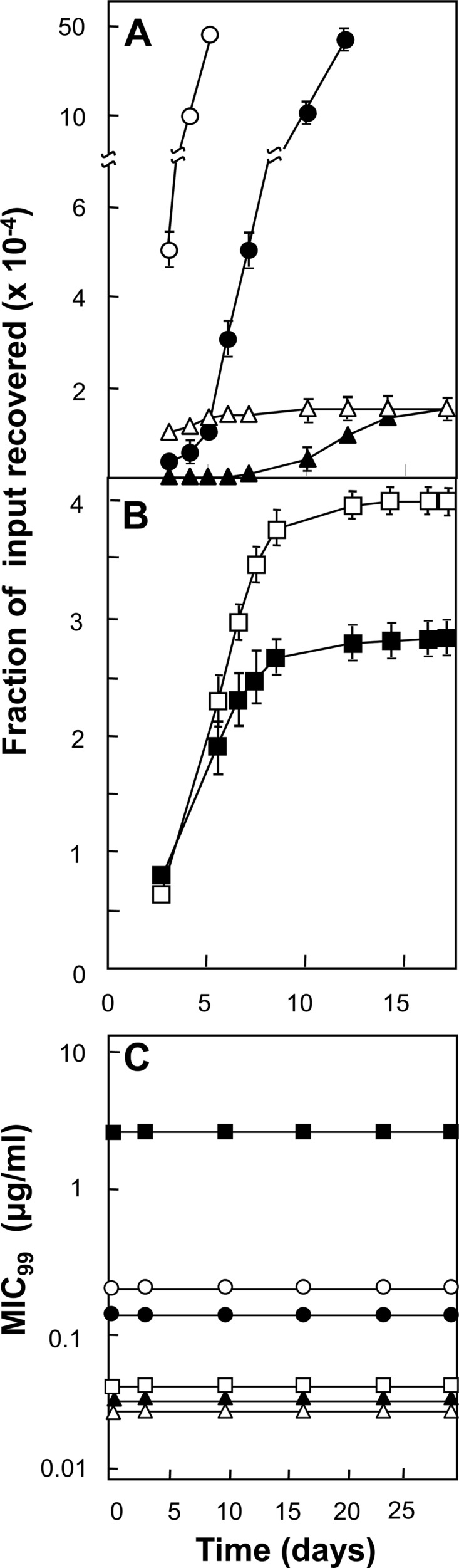
Effect of drug structure on induction of quinolone resistance. (A) Comparison of fluoroquinolones. An exponentially growing culture of M. smegmatis KD1163 (4.5 × 106 CFU) was applied to agar containing the following fluoroquinolones at 2 times the MIC: ciprofloxacin at 0.4 μg/ml, empty circles; levofloxacin at 0.25 μg/ml, filled circles; gatifloxacin at 0.06 μg/ml, empty triangles; moxifloxacin at 0.06 μg/ml, filled triangles. Plates were incubated at 37°C, and at daily intervals, the cumulative number of colonies was determined and expressed as a fraction of the input number of CFU. Replicate experiments produced similar results, as did similar experiments at inocula ranging from 4.5 ×105 to 4.5 ×107. (B) Comparison of quinazoline-2,4-dione and cognate fluoroquinolone. M. smegmatis KD1163 (5 × 106 CFU) was applied as described for panel A to agar containing 2.5 times the MIC of either dione UING5-207 (5 μg/ml, filled squares) or the fluoroquinolone UING5-249 (0.08 μg/ml, open squares). Replicate experiments gave similar results. (C) Stability of fluoroquinolones and 2,4-dione. Sets of agar plates were prepared with various concentrations of each compound and incubated at 37°C, and at the indicated times, drug-containing plates were used to determine the MICs for M. smegmatis KD1163. The symbols are the same as in panels A and B. Similar results were obtained with replicate experiments. Error bars represent standard deviations.
To determine whether an 8-methoxy-quinazoline-2,4-dione is more effective than a comparable fluoroquinolone at restricting the recovery of induced mutants, we plated M. smegmatis on agar containing 2.5 times the MIC of the dione UING5-207 or a fluoroquinolone (UING5-249) that was identical to the dione except at the dione/carboxyl region (Fig. 1). The dione allowed the recovery of fewer resistant mutants (Fig. 3B). Similar results were observed when the inoculum size was varied at various drug concentrations (data not shown).
Drug stability is potentially important when agar plates are incubated for extended times. As a test of stability, we placed the fluoroquinolones and dione in the agar medium used for mycobacteria and, after various incubation times, measured the ability of the drug-containing agar to block the growth of M. smegmatis. Ciprofloxacin, levofloxacin, gatifloxacin, moxifloxacin, 2,4-dione UING5-207, and fluoroquinolone UING5-249 were stable for the duration of the experiment (Fig. 3C). These data indicate that the differences among the compounds described above are due to differences in activity, not stability.
Enrichment of M. smegmatis mutator mutant.
To determine whether mycobacterial mutators can be detected by the agar plate assay, we first prepared spontaneous mutator strains by requiring growth of M. smegmatis in the presence of two antimicrobials (rifampin and isoniazid). Conditions were used in which resistant growth was unlikely to occur in liquid cultures unless a mutator was present to increase the frequency of rifampin and isoniazid resistance (see Materials and Methods for details). When sets of cultures were inoculated with 3 × 105 or 1.3 × 106 CFU, no culture (0/16 at each inoculum size) became turbid following incubation at 37°C. However, at an inoculum of 5 × 106 CFU, 7 of 16 cultures exhibited growth; at 2 × 107 CFU, 16 of 16 cultures grew. The cell numbers at which growth occurred were below the number needed to obtain isoniazid and rifampin resistance concurrently in the absence of a mutator [(frif × finh) = (2 × 10−5) × (3 × 10−6) = 6 × 10−11, where f is the frequency of resistance] (20, 50; data not shown).
Three independent cultures obtained from the smallest inoculum showing growth (5 × 106 CFU) in rifampin plus isoniazid were studied as putative mutator strains. Each mutator strain exhibited growth on agar plates containing both isoniazid and rifampin at 3 times the MIC, and the MICs of the drugs measured individually in liquid culture were 2 times and 8 times the MICs of isoniazid and rifampin for the wild type, respectively. The mutator strains lacked mutations in the QRDR of gyrA and gyrB, and they exhibited no increase in the MIC of ciprofloxacin (data not shown). However, when mutator strains were plated on agar containing ciprofloxacin at 2.5 times the MIC, a larger increase in colony recovery during incubation was seen than with the parental wild-type strain (Fig. 4A shows an example). As a control, a rifampin-isoniazid double mutant was prepared in a stepwise manner using agar containing 5 μg/ml rifampin or 12.5 μg/ml isoniazid. When one of these two-step double mutant strains was assayed for induced resistance to ciprofloxacin, it behaved the same way as the wild-type strain (Fig. 4A). The MIC for the two-step double mutants, determined with liquid cultures, was 12.5 μg/ml (2 times the MIC with the wild type) for isoniazid and 100 μg/ml (80 times the MIC with the wild type) for rifampin.
Fig 4.
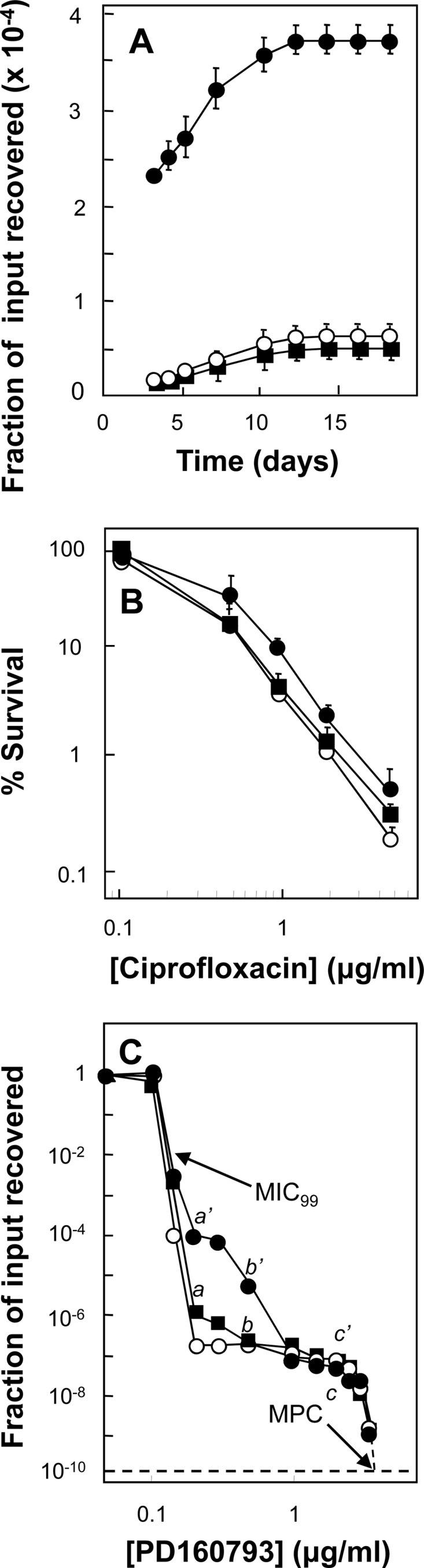
Characterization of a mutator mutation of M. smegmatis. (A) Induction of ciprofloxacin-resistant mutants. M. smegmatis strains were grown to exponential phase, and 3.5 × 106 CFU were applied to agar containing 0.5 μg/ml ciprofloxacin (2.5 times the MIC). At daily intervals, the cumulative number of mutants was recorded and expressed as a fraction of the number of input cells. Symbols: wild type (KD1163), open circles; InhR Rifr mutator (KD2859), filled circles; InhR Rifr mutant obtained by stepwise selection (KD2860), filled squares. Error bars represent standard deviations; replicate experiments gave similar results. (B) Lethal susceptibility to ciprofloxacin. M. smegmatis was incubated at the indicated concentrations of ciprofloxacin for 18 h, and then samples were diluted, applied to agar, and incubated and CFU were counted. Symbols represent the same strains as in panel A. Error bars represent standard deviations; replicate experiments gave similar results. (C) Population analysis with PD160793. Stationary-phase cultures were applied to agar plates containing the indicated concentrations of ciprofloxacin and incubated for 3 days. Colonies were counted and confirmed to be resistant mutants by regrowth on agar containing the same concentration of ciprofloxacin that was initially used for selection. Similar results were obtained in replicate experiments. The symbols are the same as in panel A. The MIC99 and MPC are indicated by arrows. The characteristics of the mutants recovered at points a, b, c, a′, b′, and c′ are listed in Table 3. The dashed line indicates the recovery of 1 mutant per 1010 cells applied to agar, a threshold often used to determine the MPC.
Two other features of mutator strain KD2859 were also examined. One was susceptibility to the lethal action of ciprofloxacin. When we measured bacterial survival after incubation at various concentrations of ciprofloxacin, little difference was observed among the wild-type, mutator, and two-step mutant strains (Fig. 4B). The second feature was the nature of mutant subpopulations. We performed population analysis (54) using the fluoroquinolone PD160793. Since this quinolone has a broad population profile (65), its use was expected to facilitate the detection of changes in mutant subpopulation size. The population profile of mutator KD2859 exhibited a shoulder not seen with the double mutant obtained by two-step selection or with the wild-type strain (Fig. 4C). Extending the incubation period from 3 to 14 days increased the recovery of mutants by about 15-fold for the mutator in the shoulder region of the population profile (data not shown). A similar but smaller increase was seen with wild-type cells in the same region of the profile (data not shown). The MIC and the mutant prevention concentration (MPC) were the same for the wild-type and mutator strains (Fig. 4C), even after the incubation time was increased from 3 to 14 days (data not shown).
Colonies obtained at intermediate concentrations of PD160793 contained two general mutant subpopulation types. One was represented by variants of the GyrA and GyrB gyrase subunits. Both gyrase types were present in the wild-type and mutator populations (Table 3, mutants from Fig. 4C). The second general type increased drug efflux, as defined by resistance to ethidium bromide (51). In both wild-type and mutator populations, the fraction that was ethidium resistant declined as the fluoroquinolone concentration increased (with wild-type cells, ethidium-resistant mutants constituted 89, 45, and 11% of the cells recovered at positions a, b, and c, respectively, of Fig. 4C; with the mutator strain, ethidium-resistant mutants constituted 86, 41, and 5% of the cells recovered at positions a′, b′, and c′, respectively, of Fig. 4C). Thus, we observed no indication of preferential amplification of efflux mutants.
Quinolone-induced quinolone resistance in M. tuberculosis.
We next applied the agar plate mutant induction assay to M. tuberculosis strain H37Rv. Cells were plated on agar containing ciprofloxacin at various concentrations, and after various incubation times (between 30 and 60 days), colonies were counted. Mutant accumulation was similar to that observed with M. smegmatis, but it occurred over a longer time (Fig. 5A), presumably due to the lower growth rate of M. tuberculosis. When cells from 20 colonies obtained after incubation for 50 days were examined in liquid culture, each showed a 16-fold increase in ciprofloxacin MIC (data not shown). By this criterion, the colonies represented resistant mutants. Since ciprofloxacin was stable in agar for at least 60 days during incubation at 37°C (data not shown), we attribute the accumulation of colonies to the induction of mutants. Together, these data support the use of agar plate induced-mutant assays with M. tuberculosis.
Fig 5.
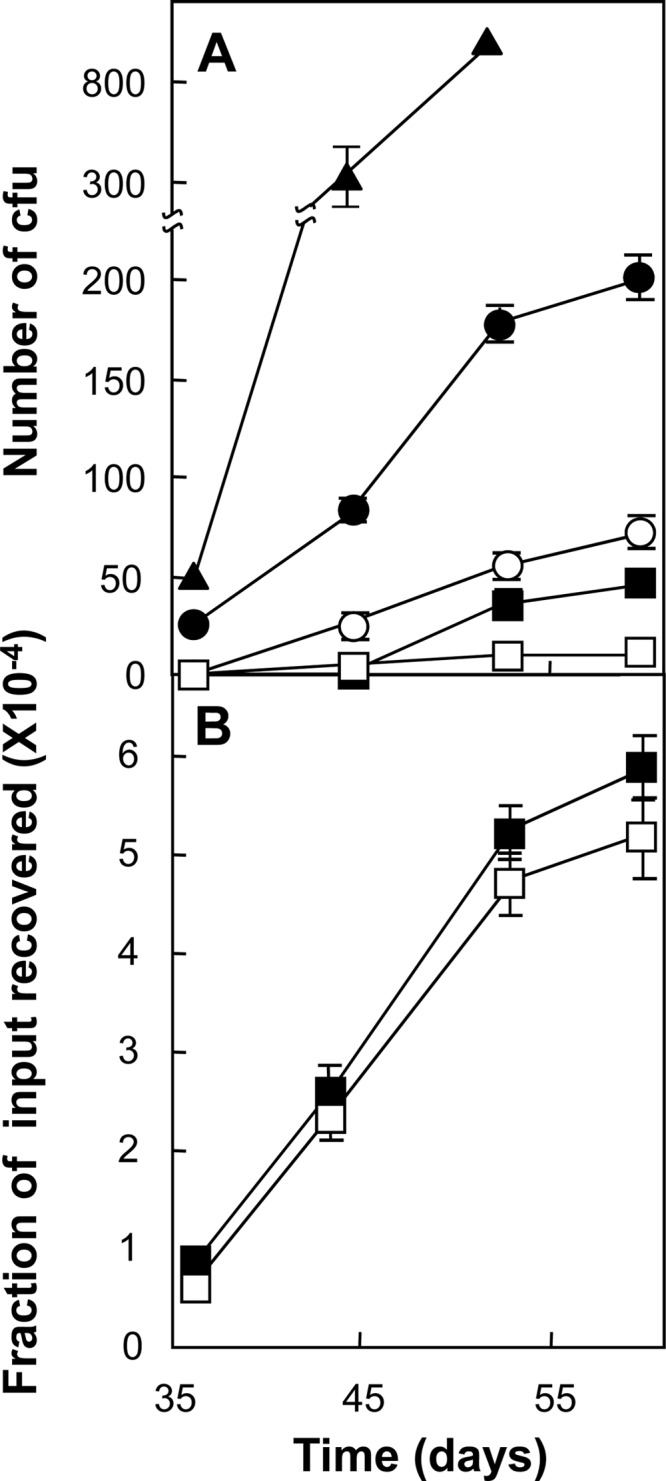
Ciprofloxacin-resistant mutants of M. tuberculosis induced by ciprofloxacin. (A) Exponentially growing cultures of M. tuberculosis H37Rv (1.5 × 107 CFU) were applied to ciprofloxacin-containing agar and incubated at 37°C. At the indicated times, the number of colonies was determined. Symbols: filled triangles, 0.3 μg/ml (1.5 times the MIC); filled circles, 0.4 μg/ml (2 times the MIC); open circles, 0.5 μg/ml (2.5 times the MIC); filled squares, 0.6 μg/ml (3 times the MIC); open squares, 0.8 μg/ml (4 times the MIC). (B) Induction of ciprofloxacin-resistant mutants by pansusceptible and MDR clinical isolates. Cultures (2.5 × 106 CFU) of a pansusceptible isolate (strain 12850, filled squares) and an MDR isolate (strain 16644, empty squares) were applied to agar containing ciprofloxacin at 2 times the MIC and incubated at 37°C. At the indicated times, the cumulative number of colonies was determined and expressed as a fraction of the number of input cells. Similar results were obtained with two other pairs of pansusceptible and MDR isolates (strains 10775 and 10536 and strains 13571 and 18996). Error bars represent standard deviations; replicate experiments gave similar results.
Since a mutator phenotype was readily detected in M. smegmatis using the agar plate assay (Fig. 4A), we carried out a pilot experiment to determine whether clinical isolates of MDR M. tuberculosis have a mutator phenotype that could be detected by the agar plate assay. For comparison, we used pansusceptible isolates that belong to the same genetic group and have the same IS6110 genotype and spoligotype as a given MDR isolate (Table 2). Nucleotide sequence analysis of DNA showed that at the beginning of the experiment all of the strains were wild type for gyrA in the QRDR. MDR and pansusceptible isolates exhibited similar increases in colony formation during incubation on ciprofloxacin-containing agar (Fig. 5B shows an example). The same result was obtained with two other pairs of M. tuberculosis isolates (data not shown). These results suggest that ciprofloxacin is not more mutagenic with MDR than with pansusceptible isolates.
DISCUSSION
Fluoroquinolones rapidly inhibit bacterial DNA replication (56), and some derivatives induce the SOS response (27, 30, 47). Indeed, induction of recA, a key gene in the response, was discovered in part by treating cells with nalidixic acid (16, 17), the prototype of this antimicrobial class. Since error-prone repair occurs during the SOS response (55), the quinolones are generally considered to be mutagenic (15, 31, 34, 36, 46, 49). Differences in the induction of mutants among structural variants of the fluoroquinolones were easily seen with M. smegmatis. When the compounds were present at comparable concentrations (2 to 3 times the MIC), gatifloxacin and moxifloxacin allowed the recovery of fewer induced mutants than levofloxacin, which in turn was more restrictive than ciprofloxacin (Fig. 3A). A quinazoline-2,4-dione that lacks critical structural features of the quinolone pharmacophore was more restrictive than the cognate fluoroquinolone (Fig. 3B). Thus, the agar plate mutant accumulation assay can be used to evaluate quinolones and related compounds for the ability to generate resistance during drug exposure.
The agar plate assay tests the combined effects of at least three factors: survival of the parental population, induction of the SOS response, and growth of resistant mutants. Since the size of the parental population affects whether a new mutation will occur, bactericidal activity is important. Quinolones are “concentration-dependent killers”; consequently, higher concentrations are more restrictive for mutant induction due to lethal activity (Fig. 2A). Likewise, aspects of drug structure that increase lethal activity will restrict the induction and recovery of resistant mutants. With mycobacteria, appending a C-8-methoxy group to the quinolone structure improves lethal activity against both wild-type and resistant cells (9, 32), which explains why gatifloxacin and moxifloxacin treatment resulted in the recovery of fewer mutants (Fig. 3A).
Quinolone-mediated DNA damage induces the SOS response through the activation of RecA (28), and increased expression of RecA has been taken as a marker of induction (48). Induction occurs over a broad range of quinolone concentrations (16, 48), with maximal induction correlating with the MIC (48). Two sources of DNA damage are likely to be involved. One probably arises directly from the formation of drug-gyrase/topoisomerase IV-DNA complexes blocking DNA replication (43, 48). The other derives from the cascade of reactive oxygen species that is associated with lethal antimicrobials (22, 59). The final step, accumulation of hydroxyl radical, damages DNA and would explain how ampicillin can induce the SOS response (35). We note that some very bactericidal fluoroquinolones kill cells without a contribution from hydroxyl radical (60). Additional work is needed to fully define the role of drug structure in the induction of the SOS regulon.
Drug structure also affects the propensity of a given compound to selectively restrict the growth of resistant mutants. We have termed this propensity antimutant activity, which is defined as the ratio of the MIC for mutant cells to the MIC for wild-type cells (13, 33). If mutant growth is restricted, resistance is less likely to emerge. The presence of an 8-methoxy group on fluoroquinolones contributes to increased antimutant activity, as does replacement of the 3-carboxylate group of quinolones with the dione motif in quinazoline-2,4-diones (Fig. 3) (13, 33). X-ray structures of drug-topoisomerase-DNA complexes (2, 24, 25, 61) suggest that the preferential activity of quinazoline-2,4-diones with gyrA mutants may be due to (i) the dione binding at the DNA nick site being farther from the common resistance residues and (ii) bound dione not requiring magnesium coordination with aspartic acid 95 (M. smegmatis numbering system [24, 33]). The latter reduces the effect of substitutions at position 95 on the MIC. Thus, finding that the 2,4-dione restricts the recovery of induced mutants on agar plates is consistent with structural analyses. Since the lethal activity of 2,4-diones is similar to that of cognate fluoroquinolones with E. coli when concentrations are normalized to the MIC (13, 33), lethal activity may contribute little to dione UING5-207 allowing the recovery of fewer induced mutants than the cognate fluoroquinolone.
Mutator strains exhibit an elevated mutation frequency and are a significant factor in the emergence of resistance during infection with some Gram-negative bacteria (29, 44, 45, 63). Here we obtained spontaneous mutators in cultures of M. smegmatis by forcing growth in the presence of both rifampin and isoniazid. The resulting cells exhibited elevated induction of ciprofloxacin resistance (Fig. 4A). The population profiles of mutator strains of Pseudomonas aeruginosa show an overall shift to a higher quinolone concentration and an increase in the mutant subpopulation size (39). With M. smegmatis, only part of the population profile was shifted (Fig. 4C). Thus, the effects of mutators can vary. Nucleotide sequence analysis revealed alterations in M. smegmatis gyrA and gyrB at these intermediate fluoroquinolone concentrations (Table 3). A more extensive analysis is required to determine whether particular gyrase mutants are enriched. Ethidium bromide resistance, which was taken as a marker for mutants having elevated efflux, showed no difference in frequency between mutator and wild-type populations.
Effects of mutators can be interpreted within the framework of the mutant selection window hypothesis (10, 64), which postulates that resistant mutant subpopulations are enriched by drug treatment within a specific drug concentration range (roughly between the MIC and the MPC, where the MPC is the MIC of the least susceptible mutant subpopulation). With short incubation times, population profiles are expected to reflect mutant subpopulations present prior to drug exposure. In a study of P. aeruginosa, mutator mutants exhibited increased MICs, shoulders in the population profiles, and increased MPCs (39). Thus, the mutant selection window for a P. aeruginosa mutator is populated by a larger subpopulation of resistant mutants, and the MICs for at least some of the mutants are elevated. In the present study with M. smegmatis, the subpopulation size was increased (shoulder in the profile shown in Fig. 4C) without changes in the susceptibility of the least susceptible mutant subpopulation (without changes in the MPC). Increasing the incubation time to allow the scoring of induced mutants also increased only the subpopulation size and not the MPC (data not shown). These data are consistent with the M. smegmatis mutator generating more of the same mutant subpopulations that were present in the wild-type population (Table 3).
Fluoroquinolones also induce resistance in M. tuberculosis (Fig. 5). No mutator phenotype was observed when we applied the assay to three MDR clinical isolates. Previous work reported that clinical isolates of the M. tuberculosis W-Beijing lineage contain alterations in orthologues of mutator genes of other bacteria (11, 26). However, no evidence of mutator activity was detected. Other work showed that the DnaE2 polymerase is induced by UV irradiation and that the absence of DnaE2 lowers the UV-mediated mutation frequency (4). Moreover, DnaE2 is important for the acquisition of rifampin resistance in infected mice. If the DnaE2 polymerase is a mutator, a dnaE2 deficiency should reduce the accumulation of mutants in the agar plate assay used in Fig. 5, especially following UV treatment.
In conclusion, to minimize the induction of new resistant mutants and to restrict the amplification of pre-existing mutant subpopulations, we suggest that TB treatment regimens keep quinolone concentrations high and avoid agents that are not highly effective, such as ciprofloxacin, ofloxacin, and levofloxacin. Keeping activity high is important even when other active antimicrobials are present, since pharmacokinetic differences can create times at which the equivalent of monotherapy occurs. Whether mutators in M. tuberculosis can be enriched by the methods used with M. smegmatis is not known. In preliminary work with Mycobacterium bovis BCG, we failed to recover mutators at cell densities 1,000 times higher than those used for M. smegmatis. Thus, the genome of M. bovis, and presumably that of M. tuberculosis, may be exceptionally stable. Such a conclusion for M. tuberculosis is supported by (i) the paucity of synonymous (silent) mutations in structural genes (40, 41, 57) and (ii) the failure of sequential isolates obtained during the development of MDR TB to show mutations other than those in the resistance genes (53).
ACKNOWLEDGMENTS
We thank Marila Gennaro and Bo Shopsin for critical comments on the manuscript.
This work was supported by NIH grants AI73491 and AI087671.
Footnotes
Published ahead of print 7 May 2012
REFERENCES
- 1. Barnes P, Cave M. 2003. Molecular epidemiology of tuberculosis. N. Engl. J. Med. 349:1149–1156 [DOI] [PubMed] [Google Scholar]
- 2. Bax B, et al. 2010. Type IIA topoisomerase inhibition by a new class of antibacterial agents. Nature 466:935–940 [DOI] [PubMed] [Google Scholar]
- 3. Bonilla C, et al. 2008. Management of extensively drug-resistant tuberculosis in Peru: cure is possible. PLoS One 3:e2957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Boshoff H, Reed M, Barry C, Mizrahi V. 2003. DnaE2 polymerase contributes to in vivo survival and the emergence of drug resistance in Mycobacterium tuberculosis. Cell 113:183–193 [DOI] [PubMed] [Google Scholar]
- 5. Brudey K, et al. 2006. Mycobacterium tuberculosis complex genetic diversity: mining the fourth international spoligotyping database (SpolDB4) for classification, population genetics and epidemiology. BMC Microbiol. 6:23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Caminero J, Sotgiu G, Zumla A, Migliori G. 2010. Best drug treatment for multidrug-resistant and extensively drug-resistant tuberculosis. Lancet Infect. Dis. 10:621–629 [DOI] [PubMed] [Google Scholar]
- 7. Cirz R, et al. 2005. Inhibition of mutation and combating the evolution of antibiotic resistance. PLoS Biol. 3:e176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Cirz R, Romesberg F. 2006. Induction and inhibition of ciprofloxacin resistance-conferring mutations in hypermutator bacteria. Antimicrob. Agents Chemother. 50:220–225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Dong Y, Xu C, Zhao X, Domagala J, Drlica K. 1998. Fluoroquinolone action against mycobacteria: effects of C8 substituents on bacterial growth, survival, and resistance. Antimicrob. Agents Chemother. 42:2978–2984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Drlica K, Zhao X. 2007. Mutant selection window hypothesis updated. Clin. Infect. Dis. 44:681–688 [DOI] [PubMed] [Google Scholar]
- 11. Ebrahimi-Rad M, et al. 2003. Mutations in putative mutator genes of Mycobacterium tuberculosis strains of the W-Beijing family. Emerg. Infect. Dis. 9:838–845 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Gandhi NR, et al. 2006. Extensively drug-resistant tuberculosis as a cause of death in patients co-infected with tuberculosis and HIV in a rural area of South Africa. Lancet 368:1575–1580 [DOI] [PubMed] [Google Scholar]
- 13. German N, Malik M, Rosen J, Drlica K, Kerns R. 2008. Use of gyrase resistance mutants to guide selection of 8-methoxy-quinazoline-2,4-diones. Antimicrob. Agents Chemother. 52:3915–3921 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Ginsberg A. 2010. Drugs in development for tuberculosis. Drugs 70:2201–2214 [DOI] [PubMed] [Google Scholar]
- 15. Gocke E. 1991. Mechanism of quinolone mutagenicity in bacteria. Mutat. Res. 248:135–143 [DOI] [PubMed] [Google Scholar]
- 16. Gudas LJ, Pardee AB. 1976. DNA synthesis inhibition and the induction of protein X in Escherichia coli. J. Mol. Biol. 101:459–477 [DOI] [PubMed] [Google Scholar]
- 17. Inouye M, Pardee A. 1970. Changes of membrane proteins and their relation to deoxyribonucleic acid synthesis and cell division of Escherichia coli. J. Biol. Chem. 245:5813–5819 [PubMed] [Google Scholar]
- 18. Jacobs WR, et al. 1991. Genetic systems in mycobacteria. Methods Enzymol. 204:537–555 [DOI] [PubMed] [Google Scholar]
- 19. Jacobson K, Tierney D, Jeon C, Mitnick C, Murray M. 2010. Treatment outcomes among patients with extensively drug-resistant tuberculosis: systematic review and meta-analysis. Clin. Infect. Dis. 51:6–14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Karunakaran P, Davies J. 2000. Genetic antagonism and hypermutability in Mycobacterium smegmatis. J. Bacteriol. 182:3331–3335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kim S, et al. 1997. Multiple pathways for SOS-induced mutagenesis in Escherichia coli: an overexpression of dinB/dinP results in strongly enhancing mutagenesis in the absence of any exogenous treatment to damage DNA. Proc. Natl. Acad. Sci. U. S. A. 94:13792–13797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kohanski M, Dwyer D, Hayete B, Lawrence C, Collins J. 2007. A common mechanism of cellular death induced by bactericidal antibiotics. Cell 130:797–810 [DOI] [PubMed] [Google Scholar]
- 23. Kong Y, et al. 2007. Association between Mycobacterium tuberculosis Beijing/W lineage strain infection and extrathoracic tuberculosis: insights from epidemiologic and clinical characterization of the three principal genetic groups of M. tuberculosis clinical isolates. J. Clin. Microbiol. 45:409–414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Laponogov I, et al. 2010. Structural basis of gate-DNA breakage and resealing by type II topoisomerases. PLoS One 5:e11338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Laponogov I, et al. 2009. Structural insight into the quinolone-DNA cleavage complex of type IIA topoisomerases. Nat. Struct. Mol. Biol. 16:667–669 [DOI] [PubMed] [Google Scholar]
- 26. Lari N, Rindi L, Bonanni D, Tortolli E, Garzelli C. 2006. Mutations in mutT genes of Mycobacterium tuberculosis isolates of Beijing genotype. J. Med. Microbiol. 55:599–603 [DOI] [PubMed] [Google Scholar]
- 27. Lewin C, Howard B, Ratcliffe N, Smith J. 1989. 4-Quinolones and the SOS response. J. Med. Microbiol. 29:139–144 [DOI] [PubMed] [Google Scholar]
- 28. Little J. 1993. LexA cleavage and other self-processing reactions. J. Bacteriol. 175:4943–4950 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Maciá M, et al. 2005. Hypermutation is a key factor in development of multiple-antimicrobial resistance in Pseudomonas aeruginosa strains causing chronic lung infections. Antimicrob. Agents Chemother. 49:3382–3386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Maguin E, Lutkenhaus J, D'Ari R. 1986. Reversibility of SOS-associated division inhibition in Escherichia coli. J. Bacteriol. 166:733–738 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Malik M, Hoatam G, Chavda K, Kerns R, Drlica K. 2010. Novel approach for comparing abilities of quinolones to restrict the emergence of resistant mutants during quinolone exposure. Antimicrob. Agents Chemother. 54:149–156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Malik M, et al. 2005. Lethality of quinolones against Mycobacterium smegmatis in the presence or absence of chloramphenicol. Antimicrob. Agents Chemother. 49:2008–2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Malik M, et al. 2011. Fluoroquinolone and quinazolinedione activity with wild-type and gyrase mutants of Mycobacterium smegmatis. Antimicrob. Agents Chemother. 55:2335–2343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Mamber S, Kolek B, Brookshire K, Bonner D, Fung-Tomc J. 1993. Activity of quinolones in the Ames Salmonella TA102 mutagenicity test and other bacterial genotoxicity assays. Antimicrob. Agents Chemother. 37:213–217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Miller C, et al. 2004. SOS response induction by β-lactams and bacterial defense against antibiotic lethality. Science 305:1629–1631 [DOI] [PubMed] [Google Scholar]
- 36. Miller JH, Low KB. 1984. Specificity of mutagenesis resulting from the induction of the SOS system in the absence of mutagenic treatment. Cell 37:675–682 [DOI] [PubMed] [Google Scholar]
- 37. Mitnick C, Castro K, Harrington M, Sacks L, Burman W. 2007. Randomized trials to optimize treatment of multidrug-resistant tuberculosis. PLoS Med. 4:e292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Mitnick C, et al. 2008. Comprehensive treatment of extensively drug-resistant tuberculosis. N. Engl. J. Med. 359:563–574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Morero N, Monti M, Argaraña CE. 2011. Effect of ciprofloxacin concentration on the frequency and nature of resistant mutants selected from Pseudomonas aeruginosa mutS and mutT hypermutators. Antimicrob. Agents Chemother. 55:3668–3676 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Musser J. 2001. Single nucleotide polymorphisms in Mycobacterium tuberculosis structural genes. Emerg. Infect. Dis. 7:486–488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Musser J, Amin A, Ramaswamy S. 2000. Negligible genetic diversity of Mycobacterium tuberculosis host immune system protein targets: evidence of limited selective pressure. Genetics 155:7–16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Napolitano R, Janel-Bintz R, Wagner J, Fuchs R. 2000. All three SOS-inducible DNA polymerases (Pol II, Pol IV and Pol V) are involved in induced mutagenesis. EMBO J. 19:6259–6265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Oishi M, Smith CL, Friefeld B. 1979. Molecular events and molecules that lead to induction of prophage and SOS functions. Cold Spring Harbor Symp. Quant. Biol. 43:897–907 [DOI] [PubMed] [Google Scholar]
- 44. Oliver A, Canton R, Campo P, Baquero F, Blazquez J. 2000. High frequency of hypermutable Pseudomonas aeruginosa in cystic fibrosis lung infection. Science 288:1251–1253 [DOI] [PubMed] [Google Scholar]
- 45. Oliver A, Levin B, Juan C, Baquero F, Blazquez J. 2004. Hypermutation and the preexistence of antibiotic-resistant Pseudomonas aeruginosa mutants: implications for susceptibility testing and treatment of chronic infections. Antimicrob. Agents Chemother. 48:4226–4233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Phillips I. 1987. Bacterial mutagenicity and the 4-quinolones. J. Antimicrob. Chemother. 20:771–773 [DOI] [PubMed] [Google Scholar]
- 47. Piddock LJV, Wise R. 1987. Induction of the SOS response in Escherichia coli by 4-quinolone antimicrobial agents. FEMS Microbiol. Lett. 41(3):289–294 [Google Scholar]
- 48. Piddock LJ, Walters RN, Diver JM. 1990. Correlation of quinolone MIC and inhibition of DNA, RNA, and protein synthesis and induction of the SOS response in Escherichia coli. Antimicrob. Agents Chemother. 34:2331–2336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Power E, Phillips I. 1993. Correlation between umuC induction and Salmonella mutagenicity assay for quinolone antimicrobial agents. FEMS Microbiol. Lett. 112:251–254 [DOI] [PubMed] [Google Scholar]
- 50. Quan S, Venter H, Dabbs E. 1997. Ribosylative inactivation of rifampin by Mycobacterium smegmatis is a principle contributor to its low susceptibility to this antibiotic. Antimicrob. Agents Chemother. 41:2456–2460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Rodrigues L, Ramos J, Couto I, Amaral L, Viveiros M. 2011. Ethidium bromide transport across Mycobacterium smegmatis cell-wall: correlation with antibiotic resistance. BMC Microbiol. 11:35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Sanders L, Rockel A, Lu H, Wozniak D, Sutton M. 2006. Role of Pseudomonas aeruginosa dinB-encoded DNA polymerase IV in mutagenesis. J. Bacteriol. 188:8573–8585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Saunders NJ, et al. 2011. Deep resequencing of serial sputum isolates of Mycobacterium tuberculosis during therapeutic failure due to poor compliance reveals stepwise mutation of key resistance genes on an otherwise stable genetic background. J. Infect. 62:212–217 [DOI] [PubMed] [Google Scholar]
- 54. Sieradzki K, Roberts R, Haber S, Tomasz A. 1999. The development of vancomycin resistance in a patient with methicillin-resistant Staphylococcus aureus infection. N. Engl. J. Med. 340:517–523 [DOI] [PubMed] [Google Scholar]
- 55. Smith B, Walker G. 1998. Mutagenesis and more: umuDC and the Escherichia coli SOS response. Genetics 148:1599–1610 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Snyder M, Drlica K. 1979. DNA gyrase on the bacterial chromosome: DNA cleavage induced by oxolinic acid. J. Mol. Biol. 131:287–302 [DOI] [PubMed] [Google Scholar]
- 57. Sreevatsan S, et al. 1997. Restricted structural gene polymorphism in the Mycobacterium tuberculosis complex indicates evolutionary recent global dissemination. Proc. Natl. Acad. Sci. U. S. A. 94:9869–9874 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Wang J, et al. 2010. Adding moxifloxacin is associated with a shorter time to culture conversion in pulmonary tuberculosis. Int. J. Tuberc. Lung Dis. 14:65–71 [PubMed] [Google Scholar]
- 59. Wang X, Zhao X. 2009. Contribution of oxidative damage to antimicrobial lethality. Antimicrob. Agents Chemother. 53:1395–1402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Wang X, Zhao X, Malik M, Drlica K. 2010. Contribution of reactive oxygen species to pathways of quinolone-mediated bacterial cell death. J. Antimicrob. Chemother. 65:520–524 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Wohlkonig A, et al. 2010. Structural basis of quinolone inhibition of type IIA topoisomerases and target-mediated resistance. Nat. Struct. Mol. Biol. 17:1152–1153 [DOI] [PubMed] [Google Scholar]
- 62. World Health Organization 2010. Multidrug and extensively drug-resistant TB (M/XDR-TB) 2010 global report on surveillance and response. World Health Organization, Geneva, Switzerland: http://whqlibdoc.who.int/publications/2010/9789241599191_eng.pdf [Google Scholar]
- 63. Yang H, Wolff E, Kim M, Diep A, Miller J. 2004. Identification of mutator genes and mutational pathways in Escherichia coli using a multicopy cloning approach. Mol. Microbiol. 53:283–295 [DOI] [PubMed] [Google Scholar]
- 64. Zhao X, Drlica K. 2001. Restricting the selection of antibiotic-resistant mutants: a general strategy derived from fluoroquinolone studies. Clin. Infect. Dis. 33(Suppl 3):S147–S156 [DOI] [PubMed] [Google Scholar]
- 65. Zhou J, et al. 2000. Selection of antibiotic resistant bacterial mutants: allelic diversity among fluoroquinolone-resistant mutations. J. Infect. Dis. 182:517–525 [DOI] [PubMed] [Google Scholar]