Abstract
We evaluated phage therapy in experimental infections due to S242, a fatal neonatal meningitis Escherichia coli strain belonging to the worldwide-distributed O25b:H4-ST131 clone that produces extended-spectrum beta-lactamase CTX-M-15. A lytic phage, EC200PP, active against S242, was isolated from environmental water. After determining in vitro and ex vivo stabilities and pharmacokinetic properties of EC200PP in rat pups, we assessed the therapeutic efficacy of a single dose of 108 PFU using models of sepsis and meningitis in which fatality was 100%. EC200PP was partially neutralized by human serum. In contrast to the high concentration of phage in the spleen and the kidney, low titers in urine and the central nervous system were observed. Nevertheless, in the sepsis model, EC200PP administered 7 h or 24 h postinfection resulted in 100% and 50% pup survival, respectively. In the meningitis model, EC200PP administered 1 h or 7 h postinfection rescued 100% of the animals. The most delayed treatments were associated with the selection of phage-resistant S242 mutants. However, a representative mutant was highly sensitive to killing serum activity and avirulent in an animal model. EC200PP is a potential therapeutic agent for sepsis and meningitis caused by the widespread E. coli O25:H4-ST131 multidrug-resistant clone.
INTRODUCTION
Escherichia coli is the leading bacterial pathogen responsible for extraintestinal infections, including urinary tract infections, bacteremia, and meningitis. Neonatal meningitis is one of the most severe E. coli infections, killing up to one-quarter of those infected (18). The future prognosis of this disease may be worsened by the increasing incidence of multidrug-resistant strains of E. coli, especially those producing extended-spectrum beta-lactamases (ESBL) such as CTX-M-type enzymes. Until 2005, no E. coli meningitis strain resistant to expanded-spectrum cephalosporins had been reported in France (20). In 2008, we described the first fatal neonatal meningitis due to a CTX-M-15-producing E. coli strain resistant to fluoroquinolones (5). We along with others have since reported several cases of ESBL-producing E. coli meningitis (16, 34), with carbapenem being the sole therapeutic alternative. The recent finding of a worldwide dissemination in Enterobacteriaceae of the NDM-1 enzyme which hydrolyzes carbapenem may soon lead to cases with no therapeutic issue (3).
An alternative approach, phage therapy, has the potential to circumvent antibiotic resistance by lysing pathogenic bacteria. Though used for decades in Eastern Europe, successful phage therapy has been demonstrated only recently in experimental models (8, 11, 15, 27, 36, 40, 43, 44). Because bacteriophage replicate at infection sites, efficacy may require just a single dose (23, 40). Although phage-resistant mutants may be selected during treatment, they are often less virulent (8, 23) and thus do not usually compromise treatment. In general, the specificity of phage infection ensures that the bulk of the body's microflora is unaffected (6, 12).
Although experimental phage therapy against E. coli infections has been previously reported, there are, to our knowledge, no data on phage therapy for E. coli meningitis. The aim of this study was to isolate a lytic phage of a CTX-M-15-producing E. coli strain belonging to the widespread, multidrug-resistant O25b:H4-ST131 clone to determine its pharmacokinetic properties and to assess its efficacy in neonatal sepsis and meningitis rat models.
MATERIALS AND METHODS
Bacterial strains.
E. coli S242, a ciprofloxacin resistant, CTX-M-15 ESBL-producing strain, was isolated from a neonate with fatal meningitis (5). This strain is capsular antigen K1 negative and belongs to the B2 phylogenetic group. We further characterized S242 by determining its serotype, sequence type (ST), and virulence genotype, as described previously (2, 14, 29), in order to determine whether S242 belongs to the clone O25b:H4-ST131 that produces CTX-M-15.
Sixty-three E. coli strains representative of the E. coli reference (ECOR) collection and E. coli meningitis strains of different serotypes and sequence types previously described (2), as well as reference strains and strain TN03, representative of the O25b:H4-CTX-M-15-ST131 clone (14, 35), were used for phage host range determination (Table 1).
Table 1.
Host range determination of phage EC200PPa
| Bacterial strain | Phylogenetic group | Serotype | EC200PP activityb |
|---|---|---|---|
| ECOR1 | A | ON:HN | |
| ECOR2 | A | ON:H32 | |
| ECOR5 | A | O79:NM | |
| ECOR10 | A | O6:H10 | |
| ECOR4 | A | ON:HN | |
| ECOR6 | A | ON:NM | |
| ECOR13 | A | ON:HN | |
| ECOR24 | A | O15:NM | |
| ECOR15 | A | O25:NM | |
| ECOR28 | B1 | O104:NM | |
| ECOR71 | B1 | O78:NM | |
| ECOR72 | B1 | O144:H8 | |
| ECOR54 | B2 | O25:H1 | |
| ECOR55 | B2 | O25:H1 | + |
| ECOR56 | B2 | O6:H1 | |
| ECOR59 | B2 | O4:H40 | |
| ECOR60 | B2 | O4:HN | |
| ECOR62 | B21 | O2:NM | |
| ECOR64 | B2 | O75:NM | |
| ECOR35 | D | O1:NM | |
| ECOR38 | D | O7:NM | |
| ECOR40 | D | O7:NM | |
| ECOR46 | D | O1:H6 | |
| ECOR48 | D | ON:HN | |
| ECOR50 | D | O2:HN | + |
| CFT073 | B2 | O6:K2:H1 | |
| J96 | B2 | O4:K6 | |
| 536 | B2 | O6:K15:H31 | |
| TN03 | B2 | O25b:H4 | + |
| S5 | B2 | O18:H7 | |
| S11 | B2 | O35:H4 | |
| S12 | B2 | O18:H7 | |
| S13 | D | O7:H4 | |
| S15 | B2 | O83:H− | |
| S22 | B2 | O6:H1 | + |
| S25 | B2 | O18:H7 | |
| S39 | D | O7:H− | |
| S41 | A | O2:H− | |
| S43 | B2 | O6:H1 | |
| S49 | B2 | O25:H− | + |
| S51 | B1 | O128:H2 | |
| S55 | A | O16:H− | |
| S63 | D | O1:H7 | |
| S69 | B2 | O18:H7 | |
| S88 | B2 | O45:H7 | |
| S97 | B2 | O8:H6 | |
| S102 | D | O166:H15 | |
| S104 | B2 | O1:H7 | |
| S105 | B2 | O14:H7 | |
| S106 | B2 | O16:H6 | |
| S113 | A | O12:H− | |
| S120 | B1 | O23:H− | |
| S123 | A | O78:H4 | |
| S124 | B2 | O1:H7 | |
| S130 | B2 | O83:H5 | + |
| S133 | B2 | O18:H7 | |
| S138 | A | O21:H4 | |
| S149 | B2 | O83:H1 | |
| S176 | D | O77:H18 | |
| S182 | D | O1:H− | |
| S191 | B2 | O16:H− | |
| S192 | B2 | O83:H7 | |
| S242 | B2 | O25:H4 | + |
Lytic spectra of isolated phage EC200PP against different E. coli strains representative of the ECOR collection and E. coli meningitis strains (S prefix) of different serotypes and phylogenetic groups as well as reference strains (CFT073, 536, and J96) and strain TN03 representative of clone O25b:H4-ST131 producing CTX-15.
Blank, absence of phage plaques; +: presence of phage plaques.
Phage isolation, preparation, and characterization.
S242 was used for isolating and enriching virulent bacteriophage from the environment (mainly sewage water from different areas of France). Environmental samples and cells from an overnight culture of E. coli S242 in Luria Bertani (LB) medium were mixed and incubated at 37°C for 24 h with shaking to enrich for E. coli-specific bacteriophage. After addition of chloroform, debris and unlysed cells were removed by centrifugation at 11,000 × g for 5 min, followed by passage of the supernatant through a 0.2-μm-pore-size filter. The enriched phage suspension was plated on LB agar medium with E. coli S242. Plaques formed on the plates after 24 h of incubation at 37°C. Single plaques were picked for phage purification and amplification. The phage were then stored at 4°C in a suspension in LB broth or physiological saline. Phage titers were determined by spreading diluted samples on a lawn of E. coli S242; after overnight incubation, plaques were counted, and their morphologies were assessed.
Determination of phage-resistant bacteria rate.
A total of 109 to 102 CFU of S242 was plated on a LB agar, and then 1 ml of concentrated phage suspension containing 109 phage particles was poured over the surface. After incubation overnight at 37°C, isolated colonies representing phage-resistant bacteria were counted, and the rate of phage-resistant bacteria was calculated (37).
Adsorption rate, latent period, and phage burst size.
One-step growth experiments were carried out according to previous descriptions (13) to determine first the adsorption rate and then the latent period and phage burst size. To determine the adsorption rate, samples were taken at different time intervals to analyze the free phage particles in the solutions. For latent period and phage burst size determinations, E. coli bacteria were mixed with phage solutions, and phage were allowed to adsorb for 15 min. The mixture was subjected to centrifugation immediately at 5,000 rpm for 10 min to remove free phage particles. The pellet was resuspended in fresh LB medium, and the culture was continuously incubated at 37°C. Samples were taken at 3-min intervals, and phage titers were determined. These results permitted us to calculate the number of phage produced per bacteria (burst size, [(phage titer at the plateau phase) − (phage titer at the latent phase)]/number of infective centers) and the latent period.
Determination of the MOI.
Serial dilutions of bacteriophage stock solution (107 to 1 PFU/ml) were mixed with the same amount of E. coli cells (108 CFU/ml). The multiplicity of infection (MOI) was calculated by determining the ratio of phage titer to the number of cells per ml. After 4 h of incubation at 37°C, bacteriophage titers were determined and compared.
pH and thermal stability tests.
pH stability and thermal stability tests were carried out as previously described (9, 13). Briefly, suspensions of 109 PFU phage/ml in LB medium were incubated at different pH and temperatures, and samples were taken at intervals for assay of surviving PFU.
Restriction fragment analysis of genomic DNA.
Phage were precipitated from culture supernatant with 10% polyethylene glycol (6000) and 1 M sodium chloride and concentrated by centrifugation at 9,000 × g for 2 h at 4°C and resuspension in phage buffer (10 mM MgSO, 10 mM Tris [pH 7.6], and 1 mM EDTA) (10, 41). Phage DNA was isolated by phenol extraction, followed by ethanol precipitation. Phage DNA (300 ng) was digested with the endonucleases HindIII and PvuII as described by the manufacturer (New England BioLabs), and the fragments were separated and visualized in a 0.7% agarose gel as described previously.
Host range determination.
Ability of phage to infect 68 E. coli clinical isolates was tested. A total of 109 cells were mixed with melted agar, and this mixture was poured on solid agar to make double-layer agar plates. After solidification, 2 × 106 PFU of bacteriophage stock suspensions was spotted on plates carrying each bacterial strain. After adsorption of the spots, the plates were inverted and incubated for 24 h at 37°C before the degree of lysis was scored (37, 46).
Endotoxin purification of phage before animal injection.
Phage were purified from endotoxins using a commercially available kit (EndoTrapH Blue; Cambrex BioScience, Verviers, Belgium), according to the instructions of the manufacturer and as previously used by Merabishvili et al. for human application (32). The titer of phage in sterile physiological water was adjusted to 2 × 109 PFU/ml for animal injections.
Phage stability in different media.
Stability assays were conducted by titrating phage at intervals during incubation in vitro at different temperatures and pH and ex vivo in rat serum, urine, and EDTA-treated blood and in human serum and urine. When necessary, serum was heated at 56°C for 30 min to inactivate complement. Human sera from 20 consenting healthy donors were obtained after approval from the institutional review board of Etablissement Français du Sang. Urine was from a healthy human volunteer (female, 30 years of age).
Pharmacokinetics.
A total of 107 PFU of phage was injected into 15 animals at day 5, intraperitoneally (i.p.) or subcutaneously (s.c.) in the lower back, using a Microfine syringe (0.3 ml, 30-gauge; Becton Dickinson). Groups of five animals were sacrificed at 2, 6, and 24 h after injection. EDTA-treated blood (100 μl), cerebrospinal fluid (CSF; 10 μl), urine (50 μl), spleen, kidney, and half-brain specimens were sampled. To evaluate the impact of contamination by red cells, all CSF samples were examined by microscopy for red cell contamination as previously described (22). Spleen, kidney, and half-brains were weighed and then crushed into 500 μl of physiological saline. At 2 and 6 h postinjection, samples were kept at 4°C for analysis in parallel with 24-h samples.
Sepsis models.
All experiments and animal housing procedures complied with University Paris Diderot guidelines and were approved by the institutional review board (number 2011-14/676-0032). Four-day-old Sprague Dawley rat pups (Charles River, France) were received with mothers. For sepsis induction, inocula of S242 in physiological saline were injected i.p. into rat pups at day 5. S242 inocula were prepared by overnight growth in brain heart infusion broth at 37°C. After centrifugation, cells were resuspended in physiological saline, and the optical density at 600 nm (OD600) was adjusted to 1.3, corresponding to 2 × 108 CFU/ml. Suspensions were serially diluted to obtain correct inocula and for enumeration. To evaluate bacteremia, 5 μl of blood was obtained by tail incision at 7, 24, and 48 h and diluted in 200 μl of physiological saline and after 5 days by intracardiac puncture using EDTA. Quantitative cultures were obtained by sample serial dilution. To assess S242 meningeal invasion, CSF was obtained by cisterna magna puncture from animals anesthetized with isoflurane (Abbott).
Meningitis model by intrathecal injection.
Meningitis by intrathecal inoculation via the cisterna magna was induced as previously described (38); 10 μl of different S242 inocula was injected into anesthetized pups using a Microfine syringe.
Experimental treatment by phage.
According to the infection model under study, phage were administered by i.p. or s.c. route at different times postinfection, taking care to separate the sites of phage administration and bacterial inoculation. The bacteremia and sterility of CSF were evaluated at intervals (24 h, 48 h, and 5 days) as described above. Phage counts in treated pups were performed as indicated above, at 24 h and 5 days in blood and CSF and at 5 days in crushed spleen.
Electron microscopy.
Electron micrographs were taken with a transmission electron microscope (Philips CM10).
Statistical analysis.
Data are given as means ± standard errors. Significant differences in survival rates were determined among treatment groups by a log rank test (GraphPad Prism, version 4.00). Phage survival differences in different media were evaluated through two-way analysis of variance (ANOVA).
RESULTS
S242 belongs to the widespread E. coli clone O25:H4-ST131 that produces CTX-M-15.
Phenotyping and molecular characterization indicated that S242 belongs to serotype O25:H4 with a positive PCR for O25b (14). It belongs to ST131 in the Achtmann multilocus sequence typing (MLST) scheme, and tests for all the genes sought were negative except iucA (aerobactin), fyuA (yersiniabactin), hra or hek (adhesion), and sat (secreted auto-transporter toxin). These results showed that S242 belongs to the E. coli clone O25b:H4-ST131 that produces CTX-M-15 (14, 35).
Characterization of a bacteriophage with therapeutic potential.
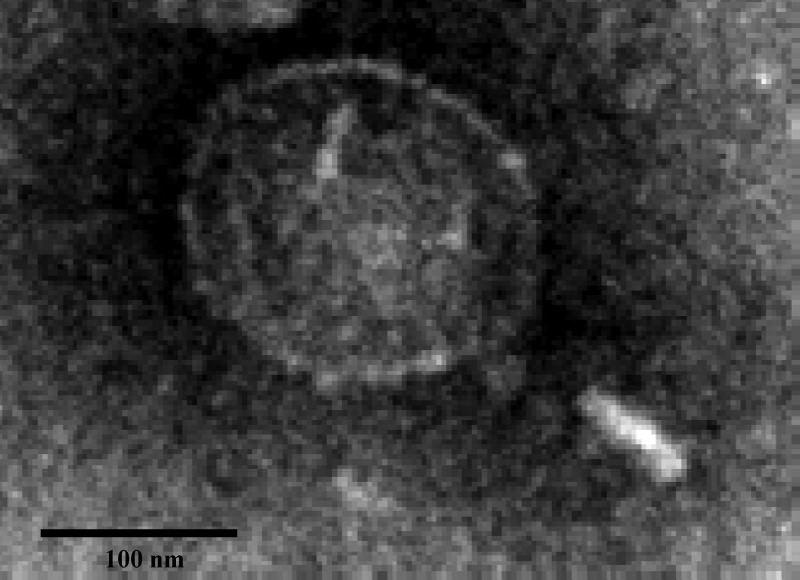
Three phages active against S242 were isolated from environmental samples and purified. One, EC200PP, was found in preliminary experiments to be more stable in vitro, and this was chosen for the study and further characterization. Electron microscopy revealed a virion of 200 nm with an icosahedral head and a short tail of 100 nm, indicating that this phage probably belongs to the Podoviridae family (Fig. 1). Genome size was estimated by restriction endonuclease digestion to be 36 to 40 kbp.
Fig 1.
Transmission electron micrograph of a particle (size, 200 nm) of bacteriophage EC200PP, a member of the Podoviridae family. Magnification, ×48,000.
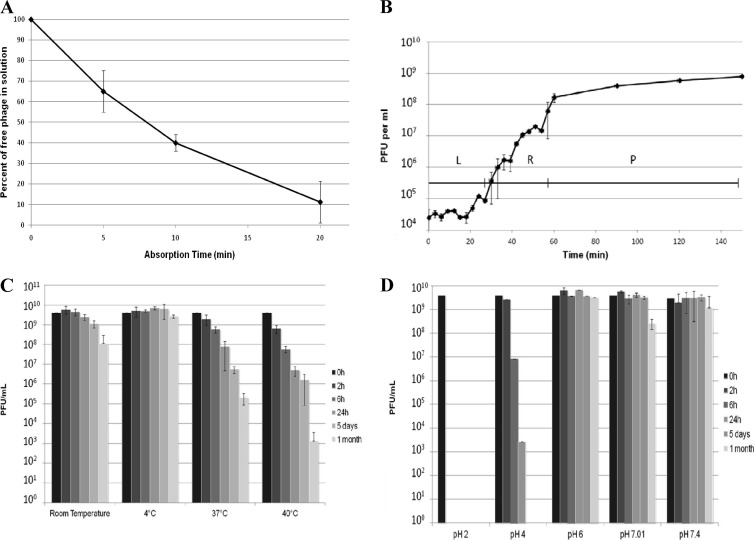
EC200PP formed plaques on 7 of 63 E. coli strains tested (Table 1), notably TN03. Therefore, EC200PP has a narrow spectrum and is potentially active against the CTX-M-15-producing O25b:H4-ST131 E. coli clonal group. The fraction of S242 cells resistant to EC200PP was 7 × 10−6. The main in vitro characteristics of EC200PP against S242 (Fig. 2A and B) were an adsorption time of 20 min, a 25-min latent period, a burst size estimated to 10, and an MOI of 10−3 giving the highest phage progeny yield.
Fig 2.
In vitro characterization of phage EC200PP comprising an adsorption rate test (A), single-step growth experiment where latent time and burst size of phage EC200PP were inferred from the curve with a triphasic pattern (L, latent phase; R, rise phase; P, plateau phase) (B), thermal stability test (C), and pH stability (D).
Stability of EC200PP.
Storage and body temperature stability are two important parameters in the therapeutic application of phage (Fig. 2C). After 24 h and 5 days at 37°C in saline, the titer had fallen by 1 and 2 orders of magnitude, respectively. Furthermore, EC200PP was relatively sensitive to acidic pH (Fig. 2D) but was stable for 1 month at room temperature (20°C) and pH 6.0 to 7.4.
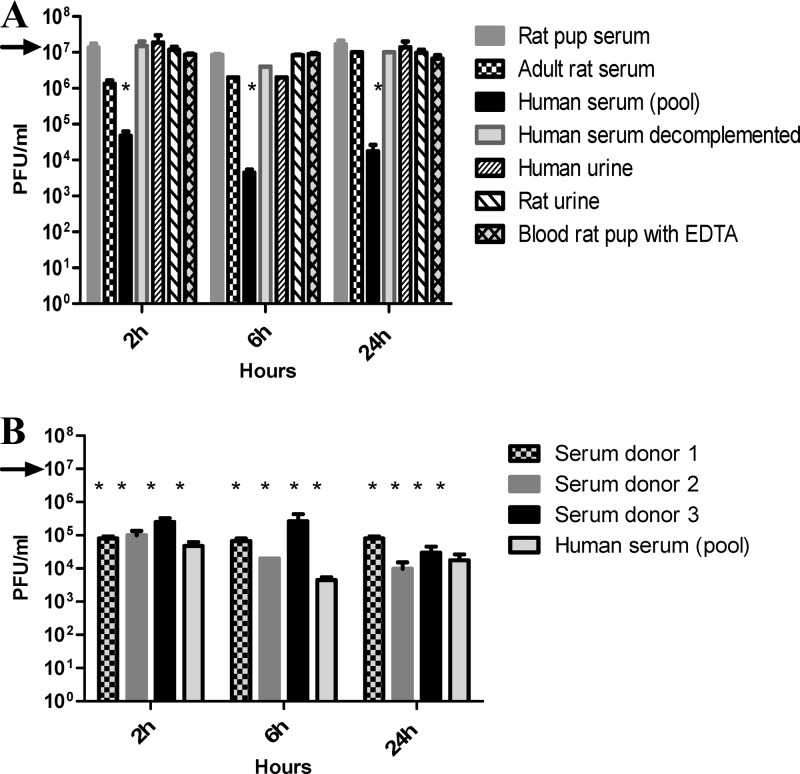
EC200PP was stable in rat pup blood containing EDTA (anticoagulant used to sample blood) and in serum for at least 24 h (Fig. 3A). In contrast, in human serum the free-phage titer fell by 2 to 3 logs in 2 h and then remained stable, indicating a partial inhibitory activity of human serum. The same result was obtained with three separate human sera, rendering unlikely the presence of a phage-specific antibody in the serum pool (Fig. 3B). Neutralization was not observed in heated human serum, suggesting that complement is involved in the serum inhibitory effect. To determine whether the lack of phage neutralizing effect of rat pup serum was related to animal age and depletion in complement activity (28), we repeated the stability test using adult rat serum; no difference was observed between adult and rat pups. Finally, the inhibitory effect of serum was not specific to EC200PP as the same partial neutralizing effect was observed with T4 phage (data not shown). In rat, as well as in human urine, EC200PP appeared stable during 24 h at 37°C (Fig. 3A).
Fig 3.
Stability of EC200PP inoculated at 107 PFU (black arrow) in different media (A) and in sera of different donors (B) during 24 h (*, P < 0.001 versus initial inoculum).
Innocuity and pharmacokinetics of EC200PP.
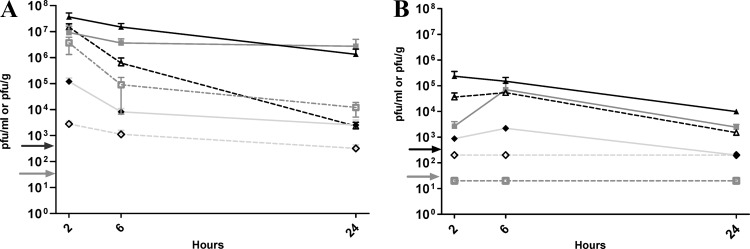
We examined innocuity and pharmacokinetics of EC200PP introduced by i.p. and s.c. routes in uninfected animals. For the innocuity test, 10 animals received purification buffer without phage, 10 received 108 PFU of EC200PP in saline, and 10 received saline. No sign of toxicity was observed, and weight gain averaged 11 g in 5 days for all groups. The i.p. pharmacokinetics assay showed a high concentration of EC200PP in blood at 2 h (107 PFU/ml); the phage were still detectable (103 PFU/ml) at 24 h. In spleen and kidney, EC200PP concentrations were higher than in blood and relatively stable, suggesting trapping by each organ (Fig. 4A). The blood/tissue ratio in the brain extract was estimated to be 0.1 to 0.15 (39). Therefore, most phage detected in brain samples at 2 and 6 h presumably originated from blood. However, at 24 h the same phage concentration was found in brain and blood, implying that phage cross the blood-brain barrier (BBB). Unfortunately, the very low phage concentration in CSF meant that even minimal contamination of CSF by blood prevented us from drawing conclusions about phage passage through the blood-CSF barrier (BCB).
Fig 4.
Pharmacokinetic study of EC200PP after intraperitoneal injection (A) or subcutaneous injection (B) of 108 PFU in rat pups. Representation of mean (n = 3 to 5) PFU/ml or PFU/g and standard error of the mean (SEM) in different organs or biologic fluids at 2, 6 and 24 h: ▲, spleen; ♦, brain; ■, kidney; △, blood; ♢, CSF; and □, urine. Detection thresholds: blood and urine (gray arrow) 20 PFU/ml; spleen, kidney, brain, and CSF (black arrow) (200 PFU/ml or/g).
After s.c. administration phage concentration in blood was low and delayed compared to i.p. injection, with a peak at 6 h postinjection (105 PFU/ml), although phage were still detectable at 24 h. High concentrations of phage were observed in spleen and kidney. We could not determine whether phage crossed the BBB or BCB after s.c. injection (Fig. 4B) because of the low level of phage detected and possible blood contamination. Finally, the passage of phage into urine was highly variable after i.p. administration as large differences were observed between individuals, and no phage were detected in urine after s.c. administration (Fig. 4A and B).
Sepsis model and treatment.
The virulence of S242 in rat pups was evaluated by i.p. injection of 103 to 106 CFU. An inoculum of 104 CFU was chosen as it produced bacteremia of 3.08 ± 0.4 log CFU/ml at 7 h, 5.7 ± 0.03 log CFU/ml associated with peritonitis at 24 h, and finally death of all animals between 36 and 48 h. At 24 h postinfection meningitis was observed in only 15% (2/13) of the pups.
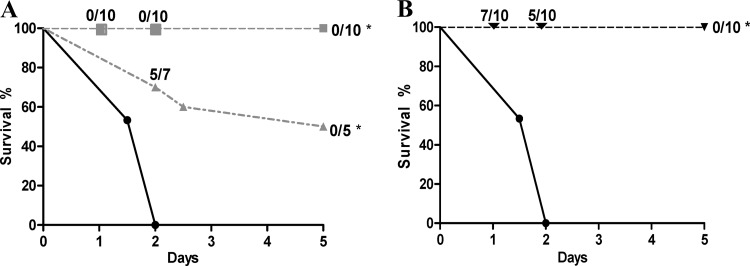
Two groups of 10 rat pups were infected with 104 CFU of S242 and treated at 7 h or 24 h postinfection by s.c. injection of 108 PFU of EC200PP (Fig. 5A). All rats survived when injection was at 7 h, and blood cultures were sterile as soon as 24 h postinfection. When injection was delayed to 24 h, half the rats survived until day 5, with negative blood cultures. In the group treated at 24 h postinfection, atypical colonies with rough morphology were observed upon plating blood samples taken 48 h after infection (5 ± 0.3 log CFU/ml). These colonies were resistant to EC200PP. One rough strain, named O4-EC200PPR, was further characterized.
Fig 5.
(A) Survival curves after intraperitoneal injection of 2 × 104 CFU of E. coli S242 in rat pups without treatment (●; n = 30) or treated at 7 h (■; n = 10) or 24 h (▲; n = 10) after infection by 108 PFU of EC200PP subcutaneously administered (*, P < 0.001 versus no treatment). Ratios on the figure indicate at different time points the rate of positive blood culture. (B) Survival curves after i.p. injection of 2 × 104 CFU of E. coli S242 (●; n = 30) or 2 × 104 CFU of O4-EC200PPR (▼; n = 10) in rat pups (*, P < 0.001 versus S242). Ratios on the figure indicate at different time points the rate of positive blood culture.
Characterization of mutant O4-EC200PPR.
Growth rates of S242 and O4-EC200PPR in LB medium were identical (see Fig. S1 in the supplemental material), and biochemical properties, antibiotic susceptibility, and virulence factor expression were identical for mutant and parent strains (data not shown). However, whereas S242 survived and grew in human serum, O4-EC200PPR was very serum sensitive; a 2-h exposure to serum reduced viability of the phage-resistant strain by at least 5 logs (see Fig. S2). To explore in vivo properties of O4-EC200PPR, we examined its behavior in a sepsis model. After i.p. injection of 104 CFU, we observed sepsis in 7/10 animals at day 1 (4.3 ± 0.9 log CFU/ml) (Fig. 5B). Animal survival at day 5 was 100%, with all blood cultures being negative. These results indicated that the O4-EC200PPR strain selected after EC200PP treatment had a dramatically attenuated virulence associated with a pronounced susceptibility to serum. Additionally, a pool of rough colonies was tested as O4-EC200PPR had been, and similar results were obtained, indicating that attenuated virulence was not unique to O4-EC200PPR (data not shown).
Meningitis model and treatment.
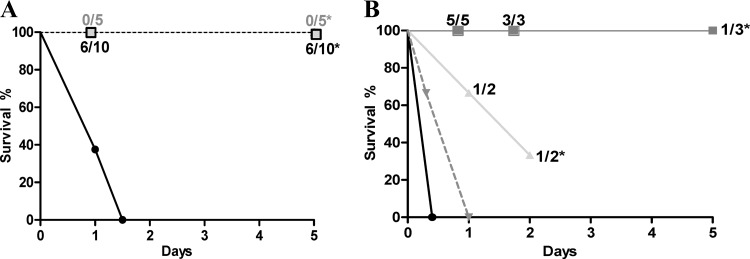
Since the sepsis model with 104 CFU injected by the i.p. route induced meningitis in only 15% of rat pups, an intrathecal model was chosen for assessment of meningitis treatment to use a minimum number of animals. Two hundred CFU of S242 was injected in the cisterna magna. Death ensued for 70% animals at 24 h and for all animals at 36 h. At 24 h, CSF samples of living animals yielded at least 106 CFU/ml. A group of 5 pups was treated with 108 PFU of EC200PP by the i.p. route 1 h after infection. After 24 h all animals had sterile CSF and an average concentration of phage in CSF of 4.5 ± 0.2 log PFU/ml. All animals survived to day 5 with no phage detected in CSF, blood, or spleen (Fig. 6A).
Fig 6.
(A) Survival curves after intrathecal injection of 200 CFU of S242 in rat pups without treatment (●; n = 10) or treated at 1 h (■; n = 5) or 7 h (□; n = 10) after infection by 108 PFU of EC200PP by i.p. injection. Ratios on the figure indicate at different time points the rate of positive CSF cultures (*, P < 0.001 versus no treatment). (B) Survival curves after intrathecal injection of 2 × 106 CFU of S242 in rat pups without treatment (●; n = 10) or treated at 1 h (■; n = 5), 2 h (▲; n = 3), or 4 h (▼; n = 3) postinfection by 108 PFU of EC200PP by i.p. injection. Ratios on the figure indicate at different time points the rate of positive CSF cultures (*, P < 0.005 versus no treatment).
Treatment initiated 7 h postinfection allowed a survival of 10/10 animals at 5 days. However, 6/10 cultures from corresponding CSF samples were positive at days 1 and 5 (4.8 ± 0.9 and 3.8 ± 0.5 log CFU/ml, respectively). At day 5, both rough and typical S242 colonies were observed. At day 1, CSF contained on average 5.3 ± 0.8 log PFU/ml, whereas phage quantity in blood was equal or inferior to the detection threshold. No phage were found at day 5 in CSF, blood, or spleen.
In light of these encouraging results, we wondered whether phage therapy could be effective with a higher inoculum such as that observed during human meningitis (26). A total of 2 × 106 CFU was injected in pup cisterna magna. Without treatment, 100% of animals died in less than 12 h (Fig. 6B). At 1 h after infection 108 PFU of EC200PP was injected i.p. All animals were alive at day 5. For all animals at 24 h, CSF cultures were positive but without bacteremia. Phage concentration in CSF (5.8 ± 0.3 log PFU/ml) was 10-fold higher than that in blood (4.6 ± 0.3 log PFU/ml), demonstrating the capacity of phage to cross the BCB and grow within the bacteria. At 48 h, all CSF samples were positive, but at day 5, two of three animals displayed sterile CSF; the third was positive. No CSF sample could be obtained from the two other animals. When treatments were delayed 2 h (n = 3) and 4 h postinfection (n = 4), efficiency and survival rate declined dramatically (Fig. 6B).
DISCUSSION
In the last 5 years, significant increases in E. coli strains resistant to extended-spectrum cephalosporins have been observed in 14 of 33 European countries (www.ecdc.europa.eu). At present, carbapenems appear to be the last available treatment for severe infections caused by ESBL-producing E. coli. The recent emergence of carbapenemase in E. coli and the lack of new antibiotic discovery emphasize the need to develop alternatives. We have shown here, for the first time, that Podoviridae bacteriophage can cure sepsis and meningitis induced by an E. coli strain belonging to O25b:H4-ST131 clone, which produces CTX-M-15 and is spread throughout the world.
Our phage EC200PP displayed a potent lytic activity as well as stability within physiological ranges of temperature and pH. To assess EC200PP lytic activity in human tissues, we conducted ex vivo assays of phage in serum and urine. Our phage were stable in urine and moderately inactivated in human serum though not by serum of rat (or mouse) (data not shown). The neutralizing effect appeared to be related to complement activity. Specific antibodies, which phage are known to induce, are a potential alternative source of inhibitory activity (8, 43). However, the similar neutralizing effects of the sera of three donors and the pool strongly suggest that human innate immunity is mainly responsible for the partial inactivation. Very few studies have noted that mammalian serum is able to neutralize bacteriophage (42). Antibodies have been assumed to be responsible, but the exact source and mechanisms of neutralization have not been studied during the last 50 years. Our results thus suggest that human innate immunity might limit the efficacy of phage therapy in systemic experimental infection models. Similar results for phage T4 imply that this might be true of phage more generally. On the other hand, serum neutralization was rapid but incomplete, leaving 104 to 105 PFU/ml after 24 h of incubation, and phage could replicate in the presence of their pathogenic bacterial host and so compensate for phage losses (45). Whether different phages have various abilities to resist human serum inactivation remains to be evaluated; we suggest that future assessments of experimental phage therapy should include this evaluation.
Pharmacokinetic properties of phage in experimental models using adult mice have been described previously (19, 31, 36, 40, 44) but, to our knowledge, they have never been studied in a neonatal murine model. Our data indicated that EC200PP was partially retained by spleen and kidney, where phage concentrations were at any time as high or higher than in blood, especially after intraperitoneal injection, as in mouse models (19, 31, 36). The basis of this trapping phenomenon remains to be determined (19). Of relevance to reduction of phage elimination by the host defense system, Merril et al. showed that serial passages in mice selected for phage that remain in the circulatory system (33).
Although our infection models did not involve the urinary tract, phage diffusion into urine was studied since 20% of neonatal meningitis cases are secondary to a pyelonephritis (21). Despite the high concentrations of phage observed in kidney, phage were present in urine after i.p. injection at a 1,000-fold lower concentration; no phage could be detected in urine after s.c. administration. Since we excluded the degradation of EC200PP in pup urine, low diffusion into urine is the likely explanation. Nishikawa et al. (36) examined secretion of phage T4 into urine after i.p. injection in mice and observed a much lower count than in kidney. Such low concentrations were sufficient to treat animals with urinary tract infection, suggesting that a limited diffusion may be counterbalanced by phage growth in the inoculum bacteria. Finally, low brain concentrations of EC200PP and nondetection in CSF suggest its low capacity to cross the BBB or BCF. Such pharmacokinetics have been described in mice (36, 40), but diffusion in CSF has not been reported before.
Using a severe model of sepsis, which leads to 100% lethality in less than 48 h, we showed that phage EC200PP administered in a single dose up to 7 h postinfection resulted in 100% survival. Even when the treatment was delayed by 24 h postinfection, 50% animal rescue was obtained. Smith et al., using a model of E. coli K1 septicemia, found only one natural phage, among 15 phages active in vitro, that cured 100% of animals at 8 h postinfection. However, when the treatment was delayed to 16 h postinfection, all animals died (7, 40). This underlines the remarkable in vivo activity of EC200PP. Moreover, in vivo activity of EC200PP was not hampered by the selection of phage-resistant S242 since the mutants were avirulent. Reduced in vivo virulence without in vitro fitness cost has been reported previously for phage-resistant enterobacterial strains (8, 23), as in the present study (see Fig. S1 in the supplemental material). In our case, the high sensitivity to serum killing may explain the lack of virulence of the phage-resistant isolate.
K1 capsular antigen is known to be a major virulence factor involved in crossing the BCB (25). Yet 10 to 20% of E. coli meningitis strains, like S242, do not harbor this antigen (4). Although our strain was able to induce bacteremia levels compatible with BCB crossing (25), low rates of meningitis were observed. Consequently, we used a previously published intracisternal infection method (24). Others studies had evaluated phage therapy in experimental central nervous system infections by intracranial inoculation with E. coli (1, 17, 40). This model does not match the usual site of E. coli infection since intracerebral, in contrast to CSF, infections are very rare in neonates (20). Although EC200PP has a low propensity for crossing the BCB, early administration after bacterial inoculation gave a major boost to survival, even when a high inoculum, such as that encountered in clinics, was used (30). Even if a low quantity of phage reaches the subarachnoidal space, it may be sufficient to infect bacteria, multiply, and finally sterilize CSF. The sterilization of CSF, as well as the higher concentration of phage in CSF than in animal blood treated at 1 h or 7 h after infection, strongly supports the notion that EC200PP crosses the BCB.
Our work demonstrates the genuine potential of bacteriophage for treating sepsis and meningitis infections induced by the multidrug-resistant O25b:H4-ST131 E. coli. Further studies will aim to optimize this strategy through reduction of phage elimination by the host defense system and by enhancing barrier crossing.
Supplementary Material
ACKNOWLEDGMENTS
This work was supported in part by the Société Française de Pédiatrie.
We thank P. Lebon who performed the electronic microscopy study and G. Arlet for providing strain TN03. We thank Pascale Neveu and José Alvez, from the Subdivision Contrôle des Eaux, SATESE 75, Section d'assainissement de Paris, STEA, Mairie de Paris, Direction de la Propreté et de l'Eau, and the SIAAP, Direction du Développement et de la Prospective, Service Etudes et Méthodes, for providing environmental water samples.
Footnotes
Published ahead of print 9 April 2012
Supplemental material for this article may be found at http://aac.asm.org/.
REFERENCES
- 1. Barrow P, Lovell M, Berchieri A., Jr 1998. Use of lytic bacteriophage for control of experimental Escherichia coli septicemia and meningitis in chickens and calves. Clin. Diagn. Lab. Immunol. 5:294–298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Bidet P, et al. 2007. Combined multilocus sequence typing and o serogrouping distinguishes Escherichia coli subtypes associated with infant urosepsis and/or meningitis. J. Infect. Dis. 196:297–303 [DOI] [PubMed] [Google Scholar]
- 3. Birgy A, et al. 2011. Early detection of colonization by VIM-1-producing Klebsiella pneumoniae and NDM-1-producing Escherichia coli in two children returning to France. J. Clin. Microbiol. 49:3085–3087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bonacorsi S, et al. 2003. Molecular analysis and experimental virulence of French and North American Escherichia coli neonatal meningitis isolates; identification of new virulent clone. J. Infect. Dis. 187:1895–1906 [DOI] [PubMed] [Google Scholar]
- 5. Boyer-Mariotte S, et al. 2008. CTX-M-15-producing Escherichia coli in fatal neonatal meningitis: failure of empirical chemotherapy. J. Antimicrob. Chemother. 62:1472–1474 [DOI] [PubMed] [Google Scholar]
- 6. Bruttin A, Brussow H. 2005. Human volunteers receiving Escherichia coli phage T4 orally: a safety test of phage therapy. Antimicrob. Agents Chemother. 49:2874–2878 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Bull JJ, Levin BR, DeRouin T, Walker N, Bloch CA. 2002. Dynamics of success and failure in phage and antibiotic therapy in experimental infections. BMC Microbiol. 2:35 doi:10.1186/1471-2180-2-35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Capparelli R, et al. 2010. Bacteriophage therapy of Salmonella enterica: a fresh appraisal of bacteriophage therapy. J. Infect. Dis. 201:52–61 [DOI] [PubMed] [Google Scholar]
- 9. Capra ML, Quiberoni A, Reinheimer J. 2006. Phages of Lactobacillus casei/paracasei: response to environmental factors and interaction with collection and commercial strains. J. Appl. Microbiol. 100:334–342 [DOI] [PubMed] [Google Scholar]
- 10. Carey-Smith GV, Billington C, Cornelius AJ, Hudson JA, Heinemann JA. 2006. Isolation and characterization of bacteriophages infecting Salmonella spp. FEMS Microbiol. Lett. 258:182–186 [DOI] [PubMed] [Google Scholar]
- 11. Carmody LA, et al. 2010. Efficacy of bacteriophage therapy in a model of Burkholderia cenocepacia pulmonary infection. J. Infect. Dis. 201:264–271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Chibani-Chennoufi S, et al. 2004. In vitro and in vivo bacteriolytic activities of Escherichia coli phages: implications for phage therapy. Antimicrob. Agents Chemother. 48:2558–2569 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Chow JJ, Batt CA, Sinskey AJ. 1988. Characterization of Lactobacillus bulgaricus bacteriophage ch2. Appl. Environ. Microbiol. 54:1138–1142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Clermont O, et al. 2008. The CTX-M-15-producing Escherichia coli diffusing clone belongs to a highly virulent B2 phylogenetic subgroup. J. Antimicrob. Chemother. 61:1024–1028 [DOI] [PubMed] [Google Scholar]
- 15. Debarbieux L, et al. 2010. Bacteriophages can treat and prevent Pseudomonas aeruginosa lung infections. J. Infect. Dis. 201:1096–1104 [DOI] [PubMed] [Google Scholar]
- 16. Dubois D, et al. 2009. CTX-M beta-lactamase production and virulence of Escherichia coli K1. Emerg. Infect. Dis. 15:1988–1990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Dubos RJ, Straus JH, Pierce C. 1943. The multiplication of bacteriophage in vivo and its protective effect against an experimental infection with Shigella Dysenteriae. J. Exp. Med. 78:161–168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Gaschignard J, et al. 2011. Neonatal bacterial meningitis: 444 cases in 7 years. Pediatr. Infect. Dis. J. 30:212–217 [DOI] [PubMed] [Google Scholar]
- 19. Geier MR, Trigg ME, Merril CR. 1973. Fate of bacteriophage lambda in non-immune germ-free mice. Nature 246:221–223 [DOI] [PubMed] [Google Scholar]
- 20. Houdouin V, et al. 2008. Association between mortality of Escherichia coli meningitis in young infants and non-virulent clonal groups of strains. Clin. Microbiol. Infect. 14:685–690 [DOI] [PubMed] [Google Scholar]
- 21. Houdouin V, et al. 2008. Clinical outcome and bacterial characteristics of 99 Escherichia coli meningitis in young infants. Arch. Pediatr. 15(Suppl 3):S138–S147 [DOI] [PubMed] [Google Scholar]
- 22. Houdouin V, et al. 2002. A uropathogenicity island contributes to the pathogenicity of Escherichia coli strains that cause neonatal meningitis. Infect. Immun. 70:5865–5869 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Hung CH, Kuo CF, Wang CH, Wu CM, Tsao N. 2011. Experimental phage therapy in treating Klebsiella pneumoniae-mediated liver abscesses and bacteremia in mice. Antimicrob. Agents Chemother. 55:1358–1365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Kim KS. 1985. Comparison of cefotaxime, imipenem-cilastatin, ampicillin-gentamicin, and ampicillin-chloramphenicol in the treatment of experimental Escherichia coli bacteremia and meningitis. Antimicrob. Agents Chemother. 28:433–436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kim KS, et al. 1992. The K1 capsule is the critical determinant in the development of Escherichia coli meningitis in the rat. J. Clin. Invest. 90:897–905 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Kim KS, Manocchio M, Bayer AS. 1984. Efficacy of cefotaxime and latamoxef for Escherichia coli bacteremia and meningitis in newborn rats. Chemotherapy 30:262–269 [DOI] [PubMed] [Google Scholar]
- 27. Kutter E, et al. 2010. Phage therapy in clinical practice: treatment of human infections. Curr. Pharm. Biotechnol. 11:69–86 [DOI] [PubMed] [Google Scholar]
- 28. Lassiter HA, Watson SW, Seifring ML, Tanner JE. 1992. Complement factor 9 deficiency in serum of human neonates. J. Infect. Dis. 166:53–57 [DOI] [PubMed] [Google Scholar]
- 29. Mahjoub-Messai F, et al. 2011. Escherichia coli isolates causing bacteremia via gut translocation and urinary tract infection in young infants exhibit different virulence genotypes. J. Infect. Dis. 203:1844–1849 [DOI] [PubMed] [Google Scholar]
- 30. Mariani-Kurkdjian P, et al. 1999. Bacterial concentration in the cerebrospinal fluid in childhood meningitis. Presse Med. 28:1227–1230 [PubMed] [Google Scholar]
- 31. McVay CS, Velasquez M, Fralick JA. 2007. Phage therapy of Pseudomonas aeruginosa infection in a mouse burn wound model. Antimicrob. Agents Chemother. 51:1934–1938 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Merabishvili M, et al. 2009. Quality-controlled small-scale production of a well-defined bacteriophage cocktail for use in human clinical trials. PLoS One 4:e4944 doi:10.1371/journal.pone.0004944 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Merril CR, et al. 1996. Long-circulating bacteriophage as antibacterial agents. Proc. Natl. Acad. Sci. U. S. A. 93:3188–3192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Moissenet D, et al. 2010. Meningitis caused by Escherichia coli producing TEM-52 extended-spectrum beta-lactamase within an extensive outbreak in a neonatal ward: epidemiological investigation and characterization of the strain. J. Clin. Microbiol. 48:2459–2463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Nicolas-Chanoine MH, et al. 2008. Intercontinental emergence of Escherichia coli clone O25:H4-ST131 producing CTX-M-15. J. Antimicrob. Chemother. 61:273–281 [DOI] [PubMed] [Google Scholar]
- 36. Nishikawa H, et al. 2008. T-even-related bacteriophages as candidates for treatment of Escherichia coli urinary tract infections. Arch. Virol. 153:507–515 [DOI] [PubMed] [Google Scholar]
- 37. Postic B, Finland M. 1961. Observations on bacteriophage typing of Pseudomonas aeruginosa. J. Clin. Invest. 40:2064–2075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Scheld M. 1986. Experimental animal models of bacterial meningitis, p 139–186 In Zak O, Sands MA. (ed), Experimental models in antimicrobial chemotherapy, vol. 1 Academic Press, New York, NY [Google Scholar]
- 39. Sherwood L. 2006. Blood vessel and arterial tension, p 286 In Boeck D. (ed), Human physiology, 2nd ed Thomson Brooks/Cole, Belmont, CA [Google Scholar]
- 40. Smith HW, Huggins MB. 1982. Successful treatment of experimental Escherichia coli infections in mice using phage: its general superiority over antibiotics. J. Gen. Microbiol. 128:307–318 [DOI] [PubMed] [Google Scholar]
- 41. Stenholm AR, Dalsgaard I, Middelboe M. 2008. Isolation and characterization of bacteriophages infecting the fish pathogen Flavobacterium psychrophilum. Appl. Environ. Microbiol. 74:4070–4078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Toussaint AJ, Muschel LH. 1959. Neutralization of bacteriophage by normal serum. Nature 183:1825–1827 [DOI] [PubMed] [Google Scholar]
- 43. Wang J, et al. 2006. Therapeutic effectiveness of bacteriophages in the rescue of mice with extended spectrum beta-lactamase-producing Escherichia coli bacteremia. Int. J. Mol. Med. 17:347–355 [PubMed] [Google Scholar]
- 44. Watanabe R, et al. 2007. Efficacy of bacteriophage therapy against gut-derived sepsis caused by Pseudomonas aeruginosa in mice. Antimicrob. Agents Chemother. 51:446–452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Weber-Dabrowska B, Mulczyk M, Gorski A. 2003. Bacteriophages as an efficient therapy for antibiotic-resistant septicemia in man. Transplant. Proc. 35:1385–1386 [DOI] [PubMed] [Google Scholar]
- 46. Yang H, Liang L, Lin S, Jia S. 2010. Isolation and characterization of a virulent bacteriophage AB1 of Acinetobacter baumannii. BMC Microbiol. 10:131 doi:10.1186/1471-2180-10-131 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.