Abstract
ABCG2/BCRP is an ATP-binding cassette transporter that extrudes compounds from cells in the intestine, liver, kidney, and other organs, such as the mammary gland, affecting pharmacokinetics and milk secretion of antibiotics, anticancer drugs, and other compounds and mediating drug-drug interactions. In addition, ABCG2 expression in cancer cells may directly cause resistance by active efflux of anticancer drugs. The development of ABCG2 modulators is critical in order to improve drug pharmacokinetic properties, reduce milk secretion of xenotoxins, and/or increase the effective intracellular concentrations of substrates. Our purpose was to determine whether the anthelmintic triclabendazole (TCBZ) and its main plasma metabolites triclabendazole sulfoxide (TCBZSO) and triclabendazole sulfone (TCBZSO2) inhibit ABCG2 activity. ATPase assays using human ABCG2-enriched membranes demonstrated a clear ABCG2 inhibition exerted by these compounds. Mitoxantrone accumulation assays using murine Abcg2- and human ABCG2-transduced MDCK-II cells confirmed that TCBZSO and TCBZSO2 are ABCG2 inhibitors, reaching inhibitory potencies between 40 and 55% for a concentration range from 5 to 25 μM. Transepithelial transport assays of ABCG2 substrates in the presence of both TCBZ metabolites at 15 μM showed very efficient inhibition of the Abcg2/ABCG2-mediated transport of the antibacterial agents nitrofurantoin and danofloxacin. TCBZSO administration also inhibited nitrofurantoin Abcg2-mediated secretion into milk by more than 2-fold and increased plasma levels of the sulfonamide sulfasalazine by more than 1.5-fold in mice. These results support the potential role of TCBZSO and TCBZSO2 as ABCG2 inhibitors to participate in drug interactions and modulate ABCG2-mediated pharmacokinetic processes.
INTRODUCTION
ABCG2/BCRP is a described member of the ABC transporter family, a group of proteins that transport certain chemicals out of cells (29). These ABC drug efflux transporters extrude a wide range of xenotoxins from cells in intestine, liver, and other organs and thus affect the bioavailability of many compounds and participate in drug-drug interactions. In addition, ABCG2 mediates secretion into the milk of its substrates (both therapeutic and toxic), such as antibiotics, antitumoral agents, carcinogens, or vitamins (31, 32). Recently, the International Transporter Consortium has included ABCG2 in the group of transporters that are clinically relevant (8). Moreover, the overexpression of ABC transporters has been associated with multidrug resistance (MDR), a major impediment to successful cancer chemotherapy. Increasing interest has been given to the development of inhibitors to overcome MDR and to increase oral bioavailability and tissue penetration or to decrease milk secretion of its substrates (21, 28).
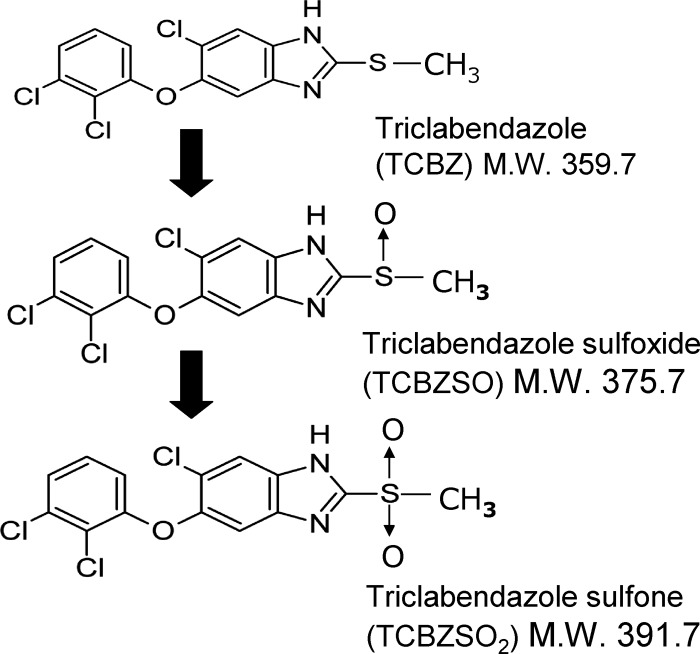
Some benzimidazole drugs, such as the anthelmintics albendazole sulfoxide and oxfendazole and the antacid pantoprazole, have been reported to interact with ABCG2 (3, 19). In the case of pantoprazole, its use as an ABCG2 inhibitor to improve plasma pharmacokinetics and brain penetration of ABCG2 substrates has been reported (2, 3). Triclabendazole (TCBZ) is a flukicidal halogenated benzimidazole thiol derivative used for treating liver fluke infections in livestock and is the drug of choice against human Fascioliasis (6). The TCBZ parent drug is not detected in plasma after its oral administration because it is rapidly metabolized into its metabolites triclabendazole sulfoxide (TCBZSO) and triclabendazole sulfone (TCBZSO2) (10) (Fig. 1). TCBZ and TCBZSO have been shown to interact with other ABC transporters in vitro (4); however, the interaction of TCBZ and its metabolites with ABCG2 has not yet been investigated.
Fig 1.
Chemical structures of triclabendazole (TCBZ) and its metabolites triclabendazole sulfoxide (TCBZSO) and triclabendazole sulfone (TCBZSO2). The molecular weight (M.W.) for each compound is shown.
In this paper, we studied whether TCBZ and its metabolites (TCBZSO and TCBZSO2) in vitro inhibit the ABCG2 transporter in ATPase assays using ABCG2-enriched membranes and in mitoxantrone (MXR) accumulation and transepithelial transport assays using ABCG2-transduced cell lines. In vivo inhibition of the transporter was assessed by studying the Abcg2-mediated effect of TCBZSO coadministration on the secretion into milk of the antibacterial agent nitrofurantoin and on plasma levels of the sulfonamide sulfasalazine using Abcg2−/− and wild-type mice. Experiments with murine Abcg2-transduced cells and mice are included in this study, as mice are extensively used as experimental models to study the transporter function in vivo.
MATERIALS AND METHODS
Reagents and drugs.
Mitoxantrone, sulfasalazine, and nitrofurantoin were purchased from Sigma-Aldrich (St. Louis, MO), danofloxacin was purchased from Fluka Chemie (Buchs, Switzerland), TCBZ was purchased from Sequoia Research Products (Pangbourne, United Kingdom), TCBZSO and TCBZSO2 were purchased from LGC Standards (Barcelona, Spain), isoflurane (Isovet) was purchased from Schering-Plough (Madrid, Spain), oxytocin (Oxiton) was purchased from Ovejero (León, Spain), and Ko143 was purchased from Tocris (Bristol, United Kingdom). All the other chemicals were analytical grade and available from commercial sources.
Animals.
Animals were housed and handled according to procedures approved by the Research Committee of Animal Use of the University of León (Spain) and carried out according to the Principles of Laboratory Animal Care and the European guidelines described in the EC Directive 86/609. The animals used were male or lactating female Abcg2−/− and wild-type mice, all of >99% FVB genetic background and between 9 and 13 weeks of age. Animals were kindly provided by A. H. Schinkel (The Netherlands Cancer Institute, Amsterdam, The Netherlands), were kept in a temperature-controlled environment with a 12-h-light/12-h-dark cycle, and received a standard diet (Panlab; Barcelona, Spain) and water ad libitum.
Cell cultures.
MDCK-II cells and their human ABCG2- and murine Abcg2-transduced subclones were kindly provided by A. H. Schinkel (The Netherlands Cancer Institute, Amsterdam, The Netherlands). Culture conditions were as previously described (12, 23).
Transport studies.
Transepithelial transport assays using Transwell plates were carried out as previously described (19) with minor modifications. Transepithelial resistance was measured in each well using a Millicell ERS ohmmeter (Millipore, Bedford, MA); wells registering a resistance of 150 Ω or greater, after correcting for the resistance obtained in blank control wells, were used in the transport experiments. The measurement was repeated at the end of the experiment to check the tightness of the monolayer. Experiments were performed using Opti-MEM medium, a reduced serum medium that is a modification of Eagle's minimum essential medium, buffered with HEPES and sodium bicarbonate. Active transport across MDCK-II monolayers was expressed by the relative transport ratio, defined as the apically directed transport percentage divided by the basolaterally directed translocation percentage, after 4 h (30).
ATPase assay.
ABCG2-associated ATP hydrolysis was determined by quantifying the release of inorganic phosphate (Pi) with a colorimetric assay with small modifications (1). Experiments were carried out in 96-well microtiter plates (F96 MicroWell plates, nontreated; Nalge Nunc, Rochester, NY). Plasma membrane vesicle preparations from isolated mammalian cells containing human ABCG2 (BCRP-M-ATPase) were obtained from Solvo Biotechnology (Budapest, Hungary) (9). Vesicles were diluted in reaction volumes of 60 μl containing a protein concentration of 0.075 mg/ml in ice-cold phosphate release assay buffer (25 mM Tris-HCl including 50 mM KCl, 3 mM ATP, 2.5 mM MgSO4, 3 mM dithiothreitol [DTT], 0.5 mM EGTA, 2 mM ouabain, and 3 mM sodium azide) adjusted to pH 7 at 37°C (1). Incubation of compounds and membranes was started by transferring the plate from ice to a water bath kept at 37°C for 1 h and was terminated by rapidly cooling the plate on ice. The phosphate release assays were performed in parallel in the presence of vanadate to inhibit ABCG2 ATPase activity, and the vanadate values were subtracted from the measurements. At least two independent measurements in plasma membrane vesicles were performed. Each independent experiment consisted of one 96-well plate with two measurements.
Accumulation assays.
In vitro accumulation assays were carried out as previously described (23). Mitoxantrone (MXR; 10 μM) was used as a fluorescent substrate. Relative cellular accumulation of MXR of at least 5,000 cells was determined by flow cytometry using a CyAn cytometer (Beckman Coulter, Fullerton, CA). The fluorescence of the accumulated substrate in tested populations was quantified from histogram plots using the median of fluorescence (MF). Flow cytometry data were processed and analyzed using SUMMIT version 4.3 software (Innovation Drive, Fort Collins, CO). Inhibitory potencies of compounds were calculated as previously described (23) in MDCK-II-ABCG2 or MDCK-II-Abcg2 cells according to the following equation: inhibitory potency = (MF with tested compound − MF without inhibitor)/(MF with Ko143 − MF without inhibitor) × 100%.
Plasma levels of sulfasalazine.
Sulfasalazine (20 mg/kg of body weight) was intragastrically administered to wild-type and Abcg2−/− male mice by oral gavage feeding in 4-h-fasted mice as a solution of 6% ethanol, 42% PEG400, and 52% water. Oral administration consisted of 300 μl of solution per 30 g of body weight. TCBZSO (50 mg/kg) or the vehicle (6% ethanol, 42% PEG400, and 52% water) was orally administered 15 min before oral administration of sulfasalazine (20 mg/kg). Blood was collected after 30 min of administration of sulfasalazine by cardiac puncture after anesthesia with isoflurane. At the end of the experiment, the mice were killed by cervical dislocation. Heparinized blood samples were centrifuged immediately at 1,500 × g for 10 min, and collected plasma was stored at −20°C until high-performance liquid chromatography (HPLC) analysis. Between 4 and 7 animals were used for each experimental group.
Milk secretion experiments.
Pups of approximately 10 days old were separated from their mother approximately 4 h before the start of the experiment. Nitrofurantoin (5 mg/kg) was administered in the tail vein to wild-type and Abcg2−/− lactating female mice as a solution of 6% ethanol, 42% PEG400, and 52% water. The intravenous (i.v.) administration consisted of 150 μl of solution per 30 g of body weight. TCBZSO (50 mg/kg or 100 mg/kg) or the vehicle (6% ethanol, 42% PEG400, and 52% water) was administered intraperitoneally (i.p.) (500 μl of solution per 30 g of body weight) 5 min before intravenous administration of nitrofurantoin. Oxytocin (200 μl of a 1-IU/ml solution) was administered subcutaneously to lactating dams in order to stimulate milk secretion 20 min after the administration of nitrofurantoin. Blood and milk were collected 30 min after substrate administration under anesthesia with isoflurane. Blood was collected by orbital bleeding, and heparinized blood samples were centrifuged immediately at 1,500 × g for 10 min. Milk was collected from the mammary glands by gentle pinching. At the end of the experiment, mice were subsequently killed by cervical dislocation. Collected plasma and milk samples were stored at −20°C until HPLC analysis. Between 4 and 7 animals were used for each experimental group.
HPLC analysis.
The chromatographic system consisted of a Waters 2695 separation module and a Waters 2998 UV photodiode array detector.
The conditions for HPLC analysis of danofloxacin were modified according to previously published methods (17, 18). Samples from the transport assays were not processed, and 50 μl of the culture medium was injected directly into the HPLC system. Separation of the samples was performed on a reverse-phase column (Phenomenex Synergi 4-μm Hydro-RP 80A). The mobile phase consisted of 25 mM orthophosphoric acid (pH 3.0)-acetonitrile (75:25), the flow rate of the mobile phase was set to 1.5 ml/min, and UV absorbance was measured at 278 nm. The temperature of the samples was 4°C. Standard samples were prepared in the appropriate drug-free matrix, yielding a concentration range from 0.02 μg/ml to 5 μg/ml.
The conditions for HPLC analysis of nitrofurantoin were modified according to a previously published method (20). Samples from the transport assays were not processed, and 50 μl of the culture medium was injected directly into the HPLC system. For the mouse samples of nitrofurantoin, to each 50-μl aliquot of plasma or milk, 5 μl of furazolidone (12.5 μg/ml) was incorporated as an internal standard and 50 μl of cold methanol was added. Samples were shaken and kept at −20°C for 15 min, the organic and water phases were separated by centrifugation at 16,000 × g for 5 min, and 50 μl of the supernatant was injected into the HPLC system. Separation of the samples was performed on a reverse-phase column (Phenomenex Synergi 4-μm Hydro-RP 80A). The mobile phase consisted of 25 mM potassium phosphate buffer (pH 3)-acetonitrile (75:25), the flow rate of the mobile phase was set to 1.2 ml/min, and UV absorbance was measured at 366 nm. The temperature of the samples was 4°C, and the temperature of the column was 30°C. Standard samples in the appropriate drug-free matrix were prepared, yielding concentration ranges from 0.039 μg/ml to 5 μg/ml for transport samples, from 0.125 μg/ml to 4 μg/ml for plasma mouse samples, and from 0.0312 μg/ml to 4 μg/ml for milk mouse samples.
The conditions for HPLC analysis of sulfasalazine were modified according to previously published methods (13). For the mouse samples of sulfasalazine, to each 100-μl aliquot of plasma, 10 μl of probenecid (37.5 μg/ml in methanol) was incorporated as an internal standard and 300 μl of methanol was added. Samples were shaken and kept at −20°C for 15 min, and the organic and water phases were separated by centrifugation at 1,500 × g for 2 min. The supernatant was collected in a new Eppendorf tube and evaporated to dryness under a nitrogen stream. The samples were resuspended in 100 μl of methanol and injected into the HPLC system. Separation of the samples was performed on a reverse-phase column (Chemcobond 5-ODS-H, 5-μm particle size, 4.6 by 250 mm). The mobile phase consisted of 12 mM phosphate buffer containing 0.06% tetrabutylammonium hydrogen sulfate (pH 7.4)-methanol (50:50), the flow rate of the mobile phase was set to 1 ml/min, and UV absorbance was measured at 260 nm. The temperature of the samples was 4°C, and the temperature of the column was 40°C. Standard samples in the appropriate drug-free matrix were prepared, yielding a concentration range from 0.04 μg/ml to 40 μg/ml. Integration was performed using Empower software (Waters).
Statistical analysis.
The two-sided unpaired Student t test was used throughout to assess the statistical significance of differences between the two sets of data. Results are presented as the means and standard deviations (SDs). Differences were considered to be statistically significant when P was <0.05.
RESULTS
Effect of TCBZ and its metabolites TCBZSO and TCBZSO2 on ABCG2 ATPase activity.
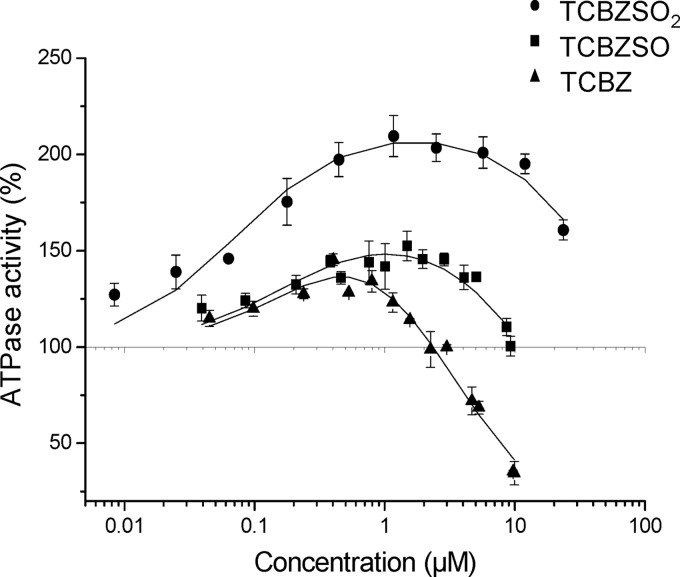
To characterize the interaction of TCBZ and its metabolites (TCBZSO and TCBZSO2) with ABCG2, drug-stimulated ATPase activity in inside-out plasma membrane vesicles from isolated mammalian cells containing human ABCG2 was measured by monitoring the phosphate release rate at pH 7 and 37°C. Figure 2 shows the rate of ABCG2 ATPase activity as a function of compound concentration (log scale). Drug-stimulated ABCG2 ATPase activity is expressed as a percentage of the basal activity (taken as 100%). ABCG2 titration curves of the three compounds showed typical bell-shaped curves previously observed for P-glycoprotein (1), with activation at lower drug concentrations and clear inhibition at higher drug concentrations, indicating an important interaction with the transporter. Maximum activity increases in the order of TCBZ to TCBZSO to TCBZSO2, and the concentration of half-maximum inhibition increases in the same order. The higher the half-maximum inhibition, the lower the inhibitory power of the compound. Note that, in all cases, the inhibition in ABCG2 ATPase activity is achieved at rather low concentrations. As has been seen for ATPase activity, all three compounds are probably effectively transported by ABCG2, with the best activation curve being for TCBZSO2.
Fig 2.
ATPase activity in inside-out plasma membrane vesicles as a function of the compound concentration for ABCG2. The titration curves shown represent the averages of two to four measurements; standard deviations are given. Curves are fits to the modified Michaelis-Menten equation proposed by Litman et al. (16).
Mitoxantrone accumulation assays.
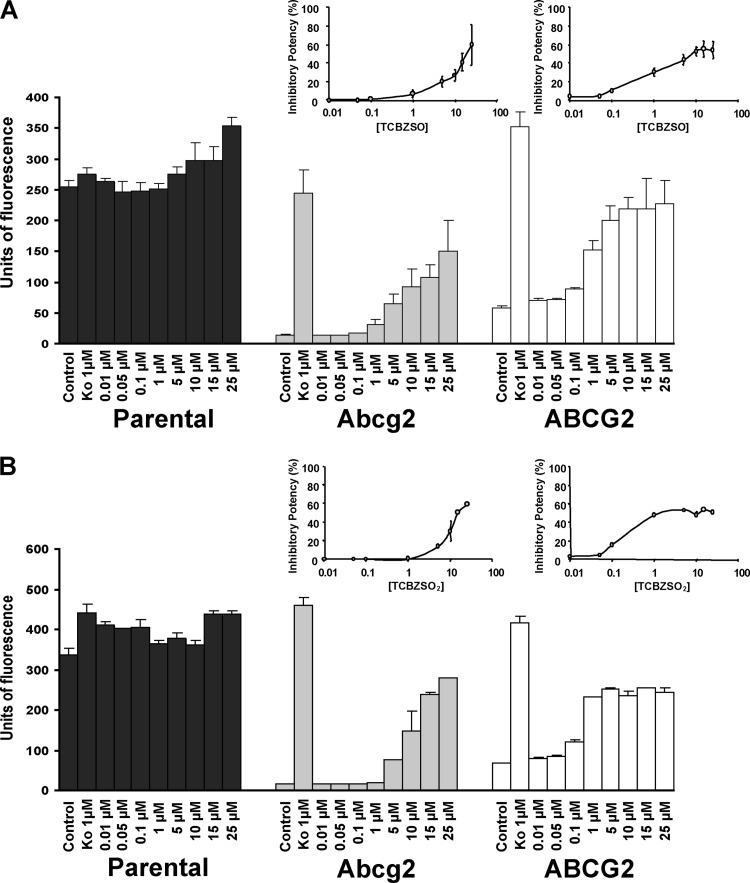
To further study the Abcg2/ABCG2 inhibitory effect of the major plasma metabolites TCBZSO and TCBZSO2, the ability of these compounds to reverse the reduced mitoxantrone accumulation in murine Abcg2- and human ABCG2-expressing cell lines was tested in flow cytometry experiments. Abcg2/ABCG2 inhibition with the model inhibitor Ko143 increased the accumulation of mitoxantrone in Abcg2- and ABCG2-transduced cells and thus increased the median of fluorescence (MF) to levels similar to those in the parental cells.
Our results showed that the addition of TCBZSO or TCBZSO2 at different concentrations (0.01 to 25 μM; higher concentrations were cytotoxic) (Fig. 3) increased, in a concentration-dependent manner, the accumulation of mitoxantrone (10 μM) in Abcg2/ABCG2-transduced cells. The strongest inhibitory potency for TCBZSO was reached at 25 μM for murine Abcg2-transduced cells (40%) and at 10 μM in the human ABCG2-transduced cells (55%). In the case of TCBZSO2, the strongest inhibitory potency was reached at 25 μM for Abcg2-transduced cells and at 5 μM for ABCG2-transduced cells, with values of 55% in both cases. All these data indicate that TCBZSO and TCBZSO2 are inhibitors of Abcg2/ABCG2.
Fig 3.
Effect of TCBZSO (A) and TCBZSO2 (B) on accumulation of mitoxantrone (10 μM) at different concentrations in parent MDCK-II cells and in their murine Abcg2- and human ABCG2-transduced derivatives. Cells were preincubated with or without Ko143 (1 μM). Results (units of fluorescence) are expressed as the means of at least three experiments; error bars indicate SDs. In addition, inhibitory potencies of the different concentrations of the tested compounds for Abcg2 and ABCG2 are represented at the top of each graph. Inhibitory potency was related to the effect of the reference inhibitor Ko143 (set at 100% inhibition of Abcg2/ABCG2).
In vitro transport of nitrofurantoin and danofloxacin in the presence of TCBZSO and TCBZSO2.
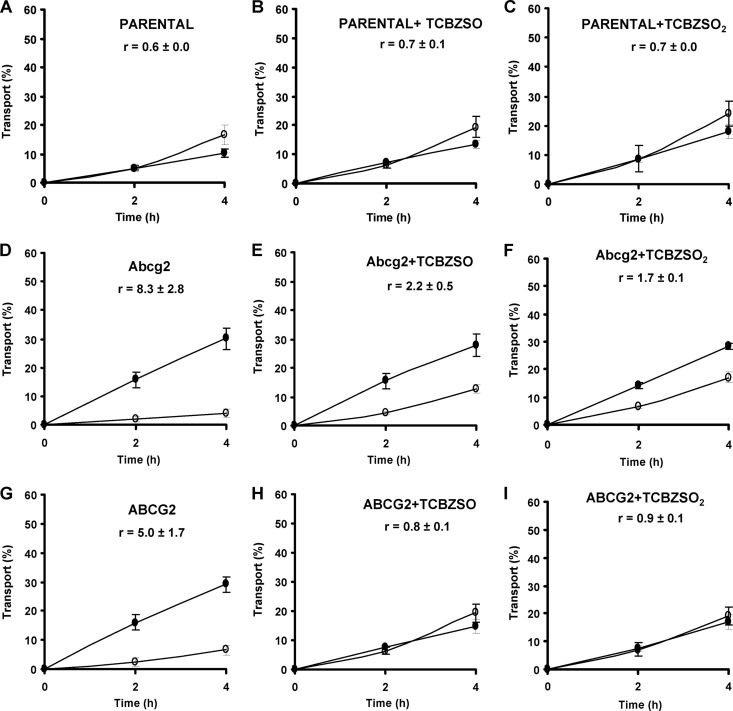
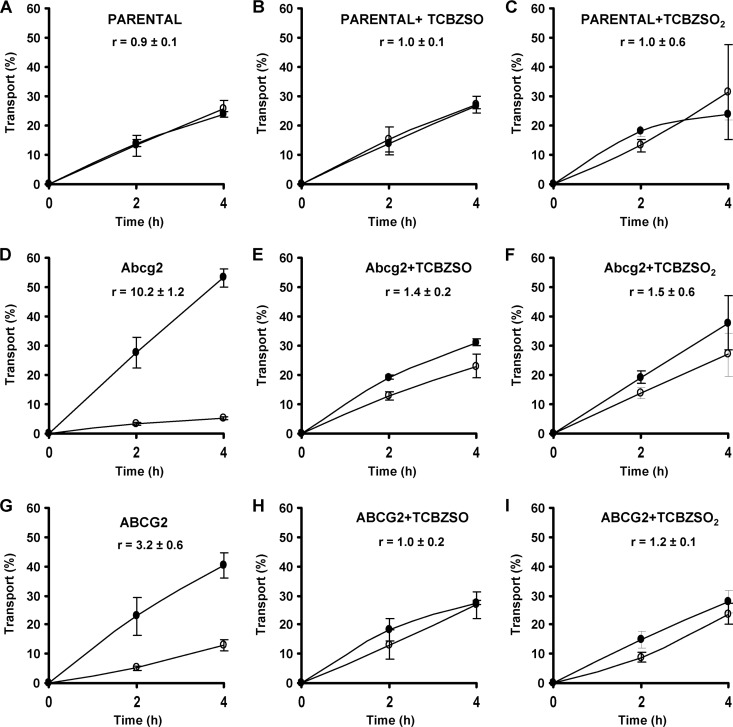
To complete the characterization of the inhibitory behavior of the TCBZ metabolites on Abcg2/ABCG2 using other assays and Abcg2/ABCG2 substrates, we tested the effect of these compounds (15 μM TCBZSO and 15 μM TCBZSO2) on the Abcg2/ABCG2-mediated in vitro transport of two known Abcg2/ABCG2 substrates, the antibacterial agents nitrofurantoin (10 μM) and danofloxacin (10 μM). As has already been reported (20, 26), we observed for nitrofurantoin (Fig. 4) and danofloxacin (Fig. 5) that in the MDCK-II parental cell line, apically and basolaterally directed translocations were similar (Fig. 4A and 5A), but that in the Abcg2/ABCG2-transduced MDCK-II cell lines, apically directed translocation was highly increased and basolaterally directed translocation dramatically decreased (Fig. 4D and G and 5D and G), since these drugs are excellent Abcg2/ABCG2 substrates. When we added TCBZSO (15 μM) and TCBZSO2 (15 μM) as inhibitors, apically directed translocation decreased and, subsequently, basolaterally directed translocation increased compared to the control situation without an inhibitor in Abcg2/ABCG2-transduced cells (Fig. 4E, F, H, and I and 5E, F, H, and I). Murine Abcg2-mediated transport was moderately inhibited, and in the case of the human ABCG2, transport was almost completely inhibited in both cases, with relative transport ratios similar to those of the parental cells.
Fig 4.
Transepithelial transport of nitrofurantoin (10 μM) in parent MDCK-II (A) and in their murine Abcg2- and human ABCG2-transduced derivatives (D and G) in the absence or presence of TCBZSO (15 μM) or TCBZSO2 (15 μM). The experiment was started with the addition of nitrofurantoin to one compartment (basolateral or apical). After 2 and 4 h, the percentage of drug appearing in the opposite compartment was measured by HPLC and plotted. TCBZSO (B, E, and H) and TCBZSO2 (C, F, and I) were present as indicated. Results are means, and error bars (sometimes smaller than the symbols) indicate SDs (n = 3). ●, translocation from the basolateral to the apical compartment; ○, translocation from the apical to the basolateral compartment. r represents the relative transport ratio (i.e., the apically directed translocation divided by the basolaterally directed translocation) at 4 h.
Fig 5.
Transepithelial transport of danofloxacin (10 μM) in parent MDCK-II (A) and in their murine Abcg2- and human ABCG2-transduced derivatives (D and G) in the absence or presence of TCBZSO (15 μM) or TCBZSO2 (15 μM). The experiment was started with the addition of danofloxacin to one compartment (basolateral or apical). After 2 and 4 h, the percentage of drug appearing in the opposite compartment was measured by HPLC and plotted. TCBZSO (B, E, and H) and TCBZSO2 (C, F, and I) were present as indicated. Results are means, and error bars (sometimes smaller than the symbols) indicate SDs (n = 3). ●, translocation from the basolateral to the apical compartment; ○, translocation from the apical to the basolateral compartment. r represents the relative transport ratio (i.e., the apically directed translocation divided by the basolaterally directed translocation) at 4 h.
These results therefore showed that TCBZSO (15 μM) and TCBZSO2 (15 μM) very efficiently inhibit the Abcg2/ABCG2-mediated transport of antibacterial substrates such as nitrofurantoin and danofloxacin.
Effect of coadministration of TCBZSO on plasma levels of sulfasalazine.
To assess whether the in vitro Abcg2/ABCG2 inhibitory role of the major plasma metabolites TCBZSO and TCBZSO2 was also relevant in vivo, we studied the effect of the coadministration of TCBZSO on plasma levels of the sulfonamide sulfasalazine, a model ABCG2 substrate (35). Danofloxacin was not used for these pharmacokinetic experiments because Abcg2 does not affect plasma levels of danofloxacin in mice (26), and therefore, this antibacterial cannot be considered as an in vivo model substrate to study Abcg2-mediated effects on plasma levels.
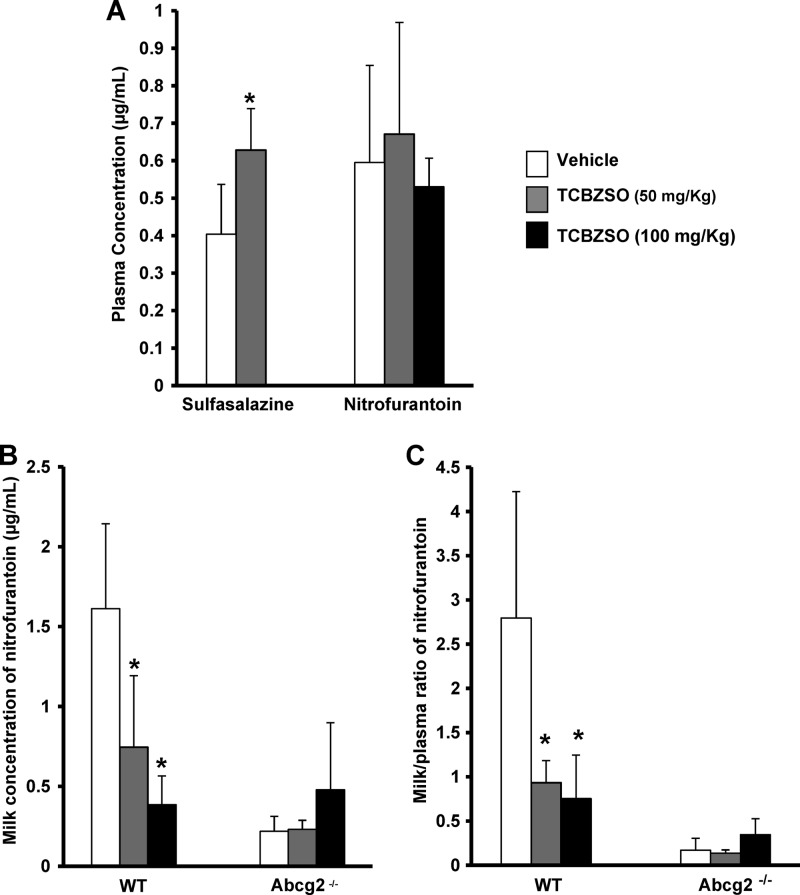
TCBZSO (50 mg/kg) or the vehicle was orally administered to wild-type and Abcg2−/− male mice 15 min prior to oral administration of sulfasalazine (20 mg/kg), and plasma samples were collected 30 min after sulfasalazine administration. The plasma concentration of sulfasalazine was more than 1.5-fold higher in wild-type mice coadministered with TCBZSO than in control wild-type mice (0.63 ± 0.11 μg/ml versus 0.40 ± 0.13 μg/ml, respectively; P < 0.05) (Fig. 6A). No significant differences in plasma concentrations of sulfasalazine were observed with TCBZSO treatment in the Abcg2−/− mice (4.91 ± 1.67 and 5.83 ± 1.70 μg/ml for control and TCBZSO-treated animals, respectively), indicating that the TCBZSO effect is Abcg2 specific. Plasma concentrations of sulfasalazine in Abcg2−/− mice were approximately 10-fold higher than those in the wild-type animals (4.91 ± 1.67 μg/ml versus 0.40 ± 0.13 μg/ml, respectively) according to the results obtained by Zaher et al. (35), confirming that this compound is a very good in vivo substrate of Abcg2. We thus demonstrated that the coadministration of TCBZSO affects oral plasma levels of sulfasalazine through inhibition of Abcg2 at the dosage used.
Fig 6.
In vivo effect of TCBZSO coadministration. (A) Plasma concentrations of sulfasalazine and nitrofurantoin in wild-type mice. TCBZSO (50 mg/kg) or the vehicle was administered orally to males 15 min prior to oral administration of sulfasalazine (20 mg/kg). TCBZSO (50 or 100 mg/kg) or the vehicle was administered i.p. to lactating females 5 min prior to i.v. administration of nitrofurantoin (5 mg/kg). (B and C) Milk concentrations (B) and milk/plasma ratios (C) of nitrofurantoin in wild-type and Abcg2−/− lactating females. TCBZSO (50 or 100 mg/kg) or the vehicle was administered i.p. to mice 5 min prior to i.v. administration of nitrofurantoin (5 mg/kg). Plasma and milk were collected after 30 min of drug administration and analyzed by HPLC. Results are means, and error bars indicate SDs (n = 4 to 7). *, P < 0.05 (significant differences between control and TCBZSO treatments in wild-type mice).
Effect of TCBZSO coadministration on plasma and milk levels of nitrofurantoin.
To further demonstrate an in vivo Abcg2/ABCG2 inhibitory role of TCBZ metabolites in other relevant drug-drug interactions and biological processes, the effect of the coadministration of TCBZSO on the secretion into milk of the antibacterial nitrofurantoin, an in vivo Abcg2/ABCG2 model substrate, was studied. Nitrofurantoin transfer into milk has been previously used as an experimental setting to test the in vivo effect of ABCG2 inhibitors (21, 33).
TCBZSO (50 and 100 mg/kg) was administered i.p. to lactating Abcg2−/− and wild-type females 5 min prior to an intravenous administration of nitrofurantoin (5 mg/kg). Thirty minutes after nitrofurantoin administration, milk and blood were collected. No significant differences were observed in plasma concentrations in wild-type mice after coadministration of TCBZSO at both doses (Fig. 6A). Plasma concentrations of nitrofurantoin in Abcg2−/− mice were approximately 3-fold higher than those in wild-type animals (1.70 ± 0.71 versus 0.59 ± 0.25 μg/ml, respectively; P < 0.05), confirming that this compound is a very good in vivo substrate of Abcg2. The milk concentration of nitrofurantoin (Fig. 6B) was more than 2-fold lower in wild-type mice treated with TCBZSO (50 mg/kg) (0.74 ± 0.44 μg/ml) and more than 4-fold lower in wild-type mice treated with TCBZSO (100 mg/kg) (0.38 ± 0.18 μg/ml) than in control wild-type mice (1.61 ± 0.53 μg/ml) (P < 0.05). No differences were observed after TCBZSO treatment in Abcg2−/− mice, indicating that the TCBZSO effect is Abcg2 specific. Consequently, TCBZSO inhibits Abcg2-mediated secretion of nitrofurantoin into milk since the milk-to-plasma ratio of this compound (Fig. 6C) was 3-fold lower in wild-type mice treated with TCBZSO (50 mg/kg) (0.93 ± 0.25) and almost 4-fold lower in wild-type mice treated with TCBZSO (100 mg/kg) (0.75 ± 0.49) than in control wild-type mice (2.79 ± 1.42) (P < 0.05).
Our results show that coadministration of TCBZSO inhibits Abcg2/ABCG2-mediated secretion of nitrofurantoin into milk at the dosage used.
DISCUSSION
The concomitant administration of multiple drugs is often used in pharmacotherapy and may affect their kinetics and pharmacological activity. There is increasing evidence to suggest that interference between drugs and ATP-binding cassette (ABC) proteins is a key mechanism underpinning clinically important drug interactions (17). It is therefore of interest to study the potential effect of the major active plasma metabolites of the widely used fasciolicide TCBZ (TCBZSO and TCBZSO2) in drug interactions with Abcg2/ABCG2 substrates affecting pharmacokinetics and milk secretion. In this study, we have shown that TCBZSO and TCBZSO2 efficiently inhibit in vitro and in vivo ABCG2 transporter activity by using different in vitro and in vivo assays with different substrates.
In ATPase assays (Fig. 2), ABCG2 inhibition was observed for all three compounds studied, TCBZ, TCBZSO, and TCBZSO2, at concentrations higher than 1 μM, with the strongest inhibition observed in the case of TCBZ, the most hydrophobic compound. Subsequent inhibition studies were performed with the major plasma metabolites TCBZSO and TCBZSO2, since due to its high metabolism, the TCBZ parent drug is not detected in plasma. In mitoxantrone accumulation assays with a concentration range from 5 to 25 μM, both compounds showed inhibitory potencies between 40 and 55% for murine Abcg2/human ABCG2. Some drugs considered to be good ABCG2 inhibitors showed 50% inhibitory concentrations (IC50s) in the same range for the same cell line (34): for lopinavir, 7.66 μM; for nelfinavir, 13.50 μM; for saquinavir, 27.40 μM; and for delavirdine, 18.60 μM. For other benzimidazole drugs considered to interact with ABCG2, such as pantoprazole and omeprazole, the IC50s were 13 μM and 36 μM, respectively (3). Our concentration values with an inhibitory potency close to 50% are in the same range as the plasma concentrations of the active metabolite TCBZSO that were reported in humans (25 μM ≈ 9.4 μg/ml) (5) and in veterinary species (30 μM ≈ 11.3 μg/ml) (7) after treatment at the therapeutic dose.
The Abcg2/ABCG2 inhibitory potential of the TCBZ metabolites was also confirmed for other known Abcg2/ABCG2 substrates, such as the antibacterial agents nitrofurantoin and danofloxacin, in transepithelial transport experiments at a concentration of 15 μM, showing a moderate inhibition for murine Abcg2 and a complete inhibition for human ABCG2 (Fig. 4 and 5). The 15 μM concentration was chosen based on the stronger inhibition observed in the mitoxantrone accumulation assays for human ABCG2. Inhibition of the in vitro transepithelial transport of both compounds at concentrations of TCBZ metabolites below 15 μM could not be excluded. Inhibition in transepithelial transport experiments can be expected as long as the concentration of the drug is higher than the concentration at maximum activity in an ATPase assay (for all three compounds, in the ABCG2 ATPase activity profiles, the maximum activity was reported at around 1 μM) (27). The similar inhibitory power of TCBZSO and TCBZSO2 that was observed in transport assays is due to the similar concentration of half-maximum inhibition in ATPase assays (Fig. 2). Although the interaction of these compounds with other ABC transporters, such as P-glycoprotein, has been previously reported (4), a lack of effect of these compounds on vectorial transport in parental cells (Fig. 4A, B, and C and 5A, B, and C) indicates that this interaction is probably ABCG2 specific in our experimental setting. All these data indicate that both TCBZ metabolites are good in vitro inhibitors of Abcg2/ABCG2.
Furthermore, we demonstrated the relevance of the ABCG2 inhibition properties of these compounds in mice using two different ABCG2 substrates in two different pharmacokinetic processes. Plasma levels of sulfasalazine and milk levels of nitrofurantoin (Fig. 6) were significantly affected by the coadministration of TCBZSO only in wild-type animals, with no effect on Abcg2−/− mice, indicating the Abcg2-specific effect. This effect is most likely due not only to the inhibition exerted by TCBZSO itself but also to that by its metabolite TCBZSO2. TCBZSO coadministration did not affect nitrofurantoin plasma levels. Some authors have reported local effects mediated by Abcg2 (fetal distribution and milk secretion) but no differences in plasma systemic profiles between wild-type and knockout mice for some substrates (24, 30, 36). Unlike the nitrofurantoin experiment, there seems to have been an Abcg2-mediated effect of TCBZSO coadministration on plasma levels of sulfasalazine, since the difference in plasma concentrations of this compound after oral administration between untreated Abcg2−/− and wild-type mice was approximately 10-fold, whereas in the case of nitrofurantoin (i.v. administration), it was only 3-fold, thus indicating a higher effect of Abcg2 on the systemic disposition of sulfasalazine after oral administration. In addition, the different routes of TCBZSO administration (oral for the sulfasalazine experiment and intraperitoneal for the nitrofurantoin experiment) and/or the gender or physiological status of the animals may influence the TCBZSO inhibitory effect.
This in vivo interaction between drugs resulting in higher plasma levels or lower secretion of the substrate into milk can be applied not only to the substrates tested but also to other ABCG2 substrates. This finding is highly relevant considering that concurrent administration of different drugs is a usual clinical practice. In addition, TCBZ is marketed in combination with other anthelmintics to improve efficacy, to broaden the spectrum of activity, and to limit resistance emergence (4). Some of these drug combinations include drugs, such as ivermectin (15) or oxfendazole (19), that are known to interact with ABC transporters. It will therefore be of interest to further study the possible in vivo effect of these TCBZ metabolites in the potential drug interactions with other known Abcg2/ABCG2 substrates in therapeutic target species (humans and livestock).
ABCG2 inhibitors can be used in combination therapy with substrates of the transporter in order to modulate their pharmacokinetics, brain penetration, milk secretion, and, thus, their efficacy. Several studies have managed to increase the bioavailability and milk secretion of antibacterial agents, such as nitrofurantoin, or antitumorals, such as topotecan, or to improve brain penetration of the antitumoral imatinib with the use of ABCG2 and P-glycoprotein inhibitors, such as elacridar, the benzimidazole pantoprazole, or isoflavones (2, 11, 14, 21, 25). However, it has to be noted that the use of TCBZ for this purpose may be controversial in animals whose products are destined for human consumption or in areas of parasite endemicity due to the potential development of resistance.
In addition, inhibitors of ABCG2 may be useful in other application fields, e.g., for reversal resistance in chemotherapy (22). Further studies are needed to show the application of these compounds in this field.
In summary, in this study, we have shown clear in vitro and in vivo interactions between the major plasma metabolites of TCBZ and ABCG2. These compounds are excellent ABCG2 inhibitors, and their relevance may be important for ABCG2-mediated drug-drug interactions affecting drug bioavailability.
ACKNOWLEDGMENTS
This work was supported by the research project grant AGL2009-11730 and the Ramon y Cajal grant (to G.M.) from the Ministry of Science and Technology and the European Regional Development Fund (Spain) and by a predoctoral grant (FPU) (to B.B.) from the Ministry of Education (Spain).
We thank A. H. Schinkel (The Netherlands Cancer Institute, Amsterdam, The Netherlands) for providing MDCK-II cells and their transduced cell lines and Abcg2−/− mice. We are grateful to James McCue for assistance in language editing.
Footnotes
Published ahead of print 16 April 2012
REFERENCES
- 1. Aanismaa P, Seelig A. 2007. P-glycoprotein kinetics measured in plasma membrane vesicles and living cells. Biochemistry 46:3394–3404 [DOI] [PubMed] [Google Scholar]
- 2. Breedveld P, et al. 2005. The effect of Bcrp1 (Abcg2) on the in vivo pharmacokinetics and brain penetration of imatinib mesylate (Gleevec): implications for the use of breast cancer resistance protein and P-glycoprotein inhibitors to enable the brain penetration of imatinib in patients. Cancer Res. 65:2577–2582 [DOI] [PubMed] [Google Scholar]
- 3. Breedveld P, et al. 2004. Mechanism of the pharmacokinetic interaction between methotrexate and benzimidazoles: potential role for breast cancer resistance protein in clinical drug-drug interactions. Cancer Res. 64:5804–5811 [DOI] [PubMed] [Google Scholar]
- 4. Dupuy J, Alvinerie M, Menez C, Lespine A. 2010. Interaction of anthelmintic drugs with P-glycoprotein in recombinant LLC-PK1-mdr1a cells. Chem. Biol. Interact. 186:280–286 [DOI] [PubMed] [Google Scholar]
- 5. El-Tantawy WH, Salem HF, Mohammed Safwat NA. 2007. Effect of Fascioliasis on the pharmacokinetic parameters of triclabendazole in human subjects. Pharm. World Sci. 29:190–198 [DOI] [PubMed] [Google Scholar]
- 6. Fairweather I. 2009. Triclabendazole progress report, 2005-2009: an advancement of learning? J. Helminthol. 83:139–150 [DOI] [PubMed] [Google Scholar]
- 7. Fairweather I, Boray JC. 1999. Fasciolicides: efficacy, actions, resistance and its management. Vet. J. 158:81–112 [DOI] [PubMed] [Google Scholar]
- 8. Giacomini KM, et al. 2010. Membrane transporters in drug development. Nat. Rev. Drug Discov. 9:215–236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Glavinas H, et al. 2007. ABCG2 (breast cancer resistance protein/mitoxantrone resistance-associated protein) ATPase assay: a useful tool to detect drug-transporter interactions. Drug Metab. Dispos. 35:1533–1542 [DOI] [PubMed] [Google Scholar]
- 10. Hennessy DR, Lacey E, Steel JW, Prichard RK. 1987. The kinetics of triclabendazole disposition in sheep. J. Vet. Pharmacol. Ther. 10:64–72 [DOI] [PubMed] [Google Scholar]
- 11. Jonker JW, et al. 2005. The breast cancer resistance protein BCRP (ABCG2) concentrates drugs and carcinogenic xenotoxins into milk. Nat. Med. 11:127–129 [DOI] [PubMed] [Google Scholar]
- 12. Jonker JW, et al. 2000. Role of breast cancer resistance protein in the bioavailability and fetal penetration of topotecan. J. Natl. Cancer Inst. 92:1651–1656 [DOI] [PubMed] [Google Scholar]
- 13. Kita T, et al. 2001. N-Acetyltransferase 2 genotype correlates with sulfasalazine pharmacokinetics after multiple dosing in healthy Japanese subjects. Biol. Pharm. Bull. 24:1176–1180 [DOI] [PubMed] [Google Scholar]
- 14. Kruijtzer CM, et al. 2002. Increased oral bioavailability of topotecan in combination with the breast cancer resistance protein and P-glycoprotein inhibitor GF120918. J. Clin. Oncol. 20:2943–2950 [DOI] [PubMed] [Google Scholar]
- 15. Lifschitz A, Virkel G, Ballent M, Sallovitz J, Lanusse C. 2009. Combined use of ivermectin and triclabendazole in sheep: in vitro and in vivo characterisation of their pharmacological interaction. Vet. J. 182:261–268 [DOI] [PubMed] [Google Scholar]
- 16. Litman T, Zeuthen T, Skovsgaard T, Stein WD. 1997. Competitive, non-competitive and cooperative interactions between substrates of P-glycoprotein as measured by its ATPase activity. Biochim. Biophys. Acta 1361:169–176 [DOI] [PubMed] [Google Scholar]
- 17. Marchetti S, Mazzanti R, Beijnen JH, Schellens JH. 2007. Concise review: clinical relevance of drug-drug and herb-drug interactions mediated by the ABC transporter ABCB1 (MDR1, P-glycoprotein). Oncologist 12:927–941 [DOI] [PubMed] [Google Scholar]
- 18. Merino G, et al. 2006. Breast cancer resistance protein (BCRP/ABCG2) transports fluoroquinolone antibiotics and affects their oral availability, pharmacokinetics, and milk secretion. Drug Metab. Dispos. 34:690–695 [DOI] [PubMed] [Google Scholar]
- 19. Merino G, et al. 2005. Transport of anthelmintic benzimidazole drugs by breast cancer resistance protein (BCRP/ABCG2). Drug Metab. Dispos. 33:614–618 [DOI] [PubMed] [Google Scholar]
- 20. Merino G, Jonker JW, Wagenaar E, van Herwaarden AE, Schinkel AH. 2005. The breast cancer resistance protein (BCRP/ABCG2) affects pharmacokinetics, hepatobiliary excretion, and milk secretion of the antibiotic nitrofurantoin. Mol. Pharmacol. 67:1758–1764 [DOI] [PubMed] [Google Scholar]
- 21. Merino G, et al. 2010. In vivo inhibition of BCRP/ABCG2 mediated transport of nitrofurantoin by the isoflavones genistein and daidzein: a comparative study in Bcrp1 (−/−) mice. Pharm. Res. 27:2098–2105 [DOI] [PubMed] [Google Scholar]
- 22. Noguchi K, Katayama K, Mitsuhashi J, Sugimoto Y. 2009. Functions of the breast cancer resistance protein (BCRP/ABCG2) in chemotherapy. Adv. Drug Deliv. Rev. 61:26–33 [DOI] [PubMed] [Google Scholar]
- 23. Pavek P, et al. 2005. Human breast cancer resistance protein: interactions with steroid drugs, hormones, the dietary carcinogen 2-amino-1-methyl-6-phenylimidazo(4,5-b)pyridine, and transport of cimetidine. J. Pharmacol. Exp. Ther. 312:144–152 [DOI] [PubMed] [Google Scholar]
- 24. Perez M, et al. 2009. In vitro and in vivo interaction of moxidectin with BCRP/ABCG2. Chem. Biol. Interact. 180:106–112 [DOI] [PubMed] [Google Scholar]
- 25. Perez M, et al. 2009. Milk secretion of nitrofurantoin, as a specific BCRP/ABCG2 substrate, in assaf sheep: modulation by isoflavones. J. Vet. Pharmacol. Ther. 32:498–502 [DOI] [PubMed] [Google Scholar]
- 26. Real R, et al. 2011. Involvement of breast cancer resistance protein (BCRP/ABCG2) in the secretion of danofloxacin into milk: interaction with ivermectin. J. Vet. Pharmacol. Ther. 34:313–321 [DOI] [PubMed] [Google Scholar]
- 27. Seelig A. 2007. The role of size and charge for blood-brain barrier permeation of drugs and fatty acids. J. Mol. Neurosci. 33:32–41 [DOI] [PubMed] [Google Scholar]
- 28. Shukla S, Ohnuma S, Ambudkar SV. 2011. Improving cancer chemotherapy with modulators of ABC drug transporters. Curr. Drug Targets 12:621–630 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Szakacs G, Paterson JK, Ludwig JA, Booth-Genthe C, Gottesman MM. 2006. Targeting multidrug resistance in cancer. Nat. Rev. Drug Discov. 5:219–234 [DOI] [PubMed] [Google Scholar]
- 30. Tang SC, et al. 2012. Brain accumulation of sunitinib is restricted by P-glycoprotein (ABCB1) and breast cancer resistance protein (ABCG2) and can be enhanced by oral elacridar and sunitinib coadministration. Int. J. Cancer 130:223–233 [DOI] [PubMed] [Google Scholar]
- 31. van Herwaarden AE, Schinkel AH. 2006. The function of breast cancer resistance protein in epithelial barriers, stem cells and milk secretion of drugs and xenotoxins. Trends Pharmacol. Sci. 27:10–16 [DOI] [PubMed] [Google Scholar]
- 32. van Herwaarden AE, et al. 2006. Breast cancer resistance protein (Bcrp1/Abcg2) reduces systemic exposure of the dietary carcinogens aflatoxin B1, IQ and Trp-P-1 but also mediates their secretion into breast milk. Carcinogenesis 27:123–130 [DOI] [PubMed] [Google Scholar]
- 33. Wang L, Leggas M, Goswami M, Empey PE, McNamara PJ. 2008. N-(4-[2-(1,2,3,4-tetrahydro-6,7-dimethoxy-2-isoquinolinyl)ethyl]-phenyl)-9,10-dihydro-5-methoxy-9-oxo-4-acridine carboxamide (GF120918) as a chemical ATP-binding cassette transporter family G member 2 (Abcg2) knockout model to study nitrofurantoin transfer into milk. Drug Metab. Dispos. 36:2591–2596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Weiss J, et al. 2007. Modulation of human BCRP (ABCG2) activity by anti-HIV drugs. J. Antimicrob. Chemother. 59:238–245 [DOI] [PubMed] [Google Scholar]
- 35. Zaher H, et al. 2006. Breast cancer resistance protein (Bcrp/abcg2) is a major determinant of sulfasalazine absorption and elimination in the mouse. Mol. Pharm. 3:55–61 [DOI] [PubMed] [Google Scholar]
- 36. Zhou L, et al. 2008. The breast cancer resistance protein (Bcrp1/Abcg2) limits fetal distribution of glyburide in the pregnant mouse: an Obstetric-Fetal Pharmacology Research Unit Network and University of Washington Specialized Center of Research Study. Mol. Pharmacol. 73:949–959 [DOI] [PubMed] [Google Scholar]