Abstract
A single inhaled dose of laninamivir octanoate (LO), a long-acting neuraminidase inhibitor, exhibits efficacy in treating both adult and pediatric patients with influenza virus infection. The intrapulmonary pharmacokinetics (PK) of LO and laninamivir, a pharmacologically active metabolite, were investigated by a single-center, open-label study of healthy adult volunteers. Subgroups of five subjects each underwent bronchoalveolar lavage (BAL) 4, 8, 24, 48, 72, 168, and 240 h following a single inhaled administration of LO (40 mg). Plasma, BAL fluid, and alveolar macrophages (AM) were analyzed to determine LO and laninamivir concentrations, using validated liquid chromatography-tandem mass spectrometry methods. The concentrations in epithelial lining fluid (ELF) and AM from the first and subsequent BAL fluid samples were determined separately to explore the drug distribution in airways. Mean laninamivir concentrations in ELF, calculated using the first BAL fluids and BAL fluids collected 4 h after inhaled administration, were 8.57 and 2.40 μg/ml, respectively. The laninamivir concentration in ELF decreased with a longer half-life than that in plasma, and it exceeded the 50% inhibitory concentrations for viral neuraminidases at all time points examined for 240 h after the inhalation. Laninamivir exposure in ELF from the first BAL samples was 3.2 times higher than that in ELF from the subsequent BAL fluid samples. ELF concentration profiles of laninamivir support its long-lasting effect for treatment of patients with influenza virus infection by a single inhaled administration.
INTRODUCTION
Laninamivir potently inhibits the neuraminidase activities of various influenza A and B viruses, including viruses of subtypes N1 to N9, A(H1N1)2009 virus, highly pathogenic avian influenza H5N1 viruses, and oseltamivir-resistant viruses (24). Neuraminidase inhibitors interrupt an established influenza virus infection at a late stage of virus replication by inhibiting the release of virions from infected cells, and consequently, they prevent viral spread across the mucous lining of the respiratory tract. The worldwide spread of seasonal H1N1 viruses resistant to oseltamivir, currently the most widely used neuraminidase inhibitor for treatment of influenza virus infections, and the emergence of highly pathogenic H5N1 avian influenza viruses have become considerable public health concerns (16).
Laninamivir octanoate (LO) is an octanoyl prodrug of laninamivir which has shown lower inhibitory activities toward neuraminidase than those of laninamivir in all laboratory strains, vaccine strains, and clinical isolates of influenza viruses tested in vitro (25). Though LO has low inhibitory activities toward neuraminidase, a single inhaled dose of LO exhibited efficacy for treatment of patients with influenza virus infection (23). The efficacy of a single inhaled 40-mg dose of LO was not inferior to that of 10 doses of oseltamivir administered orally over 5 days for treatment of seasonal influenza, including that caused by oseltamivir-resistant viruses in adult patients. In addition, a single dose of LO significantly reduced the duration of influenza illness compared with oseltamivir against an H1N1 virus with an H274Y mutation in pediatric patients (20). This long-acting characteristic of LO is partly supported by the pharmacokinetics (PK) of laninamivir. Laninamivir was retained in the trachea and lungs over long periods after a single intranasal/intratracheal administration of LO in mice and rats (14, 15). In humans, the PK of laninamivir after an inhaled dose of LO revealed a long plasma half-life in healthy young adults and in subjects with renal impairment (10, 11, 26). Moreover, laninamivir bound to viral neuraminidase in vitro more stably than did other neuraminidase inhibitors, including oseltamivir, zanamivir, and peramivir (13). Though these PK and binding characteristics support its potential as a long-acting neuraminidase inhibitor, leading to its efficacy against influenza virus by a single administration, laninamivir concentrations in human target tissues such as the lung and trachea have not yet been clarified.
Bronchoalveolar lavage (BAL) of human subjects by use of a flexible bronchofiberscope offers an easy, low-risk means of sampling fluids and cells from the respiratory tract (7). Drug concentrations in epithelial lining fluid (ELF) and alveolar macrophages (AM) obtained from BAL fluid have been evaluated in healthy volunteers (1, 5, 8, 18) as well as lung transplant recipients (3, 22) and critically ill patients (9) to consider the efficacy of drugs against respiratory pathogens and the appropriate dosage regimens for anti-infective drugs. Among the neuraminidase inhibitors, the concentration of zanamivir in ELF after intravenous (i.v.) administration was evaluated to demonstrate drug distribution from the systemic circulation to the pulmonary compartment to support the i.v. dose regimens used in clinical studies (19). The distribution of zanamivir after multiple oral inhaled administrations was also evaluated by analyzing the concentration of zanamivir in both the first BAL fluid and subsequent BAL fluid aliquots, since the contents of BAL fluid may vary according to the segment of the washed lung. The first aliquot of BAL fluid is considered to be more related to bronchi, and subsequent aliquots are considered to be more related to the more distal parts of the lung, including bronchioles and alveoli (7, 12). Given that LO is administered as a dry powder formulation containing various particle sizes of LO, inhaled LO particles do not necessarily distribute evenly in the respiratory tract.
The purpose of this study was to evaluate the BAL fluid concentrations of laninamivir after a single inhaled administration in healthy subjects to consider its long-acting efficacy based on its intrapulmonary PK. In addition, the concentrations in ELF from the first BAL fluid and subsequent aliquots were determined separately to explore the distribution of laninamivir in the lungs.
MATERIALS AND METHODS
Study design and subjects.
This open-label, single-dose study was conducted at the Osaka Pharmacology Clinical Research Hospital (Osaka, Japan). The study protocol was approved by the Institutional Review Boards of the study site. The study was conducted in accordance with the guidelines on good clinical practice and ethical standards for human experimentation established by the Declaration of Helsinki. All subjects gave written informed consent. The registry's URL and registration number are as follows: http://www.clinicaltrials.jp/ and JapicCTI-111441.
Healthy male Japanese subjects between the ages of 20 and 45 years and with a normal body mass index (18.5 to 25.0) and no history of smoking were eligible for inclusion in the study. Subjects were excluded from this study if any of the following conditions existed: evidence of organ dysfunction or any clinically significant deviation from the normal range in the physical examination, vital signs, electrocardiogram (ECG), or clinical laboratory determinations; hypersensitivity to neuraminidase, lactose, lidocaine, atropine, or midazolam; forced expiratory volume in 1 second percentage (FEV1.0%) of <70% or other pulmonary function test abnormality; a history of alcohol abuse or drug abuse; and an inability to communicate satisfactorily with the investigator. Each subject underwent a physical examination, 12-lead electrocardiogram, and laboratory screening test.
A total of 36 subjects were enrolled and evaluated during March 2011 to May 2011. Of these, one participant dropped out at the time of BAL because of the pharyngeal reflex during the insertion of bronchoscopy. The subjects' mean age, weight, and body mass index (± standard deviation [SD]) were 24.7 ± 4.6 years, 65.0 ± 7.0 kg, and 21.5 ± 1.5 kg/m2, respectively. Subjects were allocated to 7 groups of 5 subjects each according to the time of BAL. All subjects were given a single inhaled dose of 40 mg of LO. The subjects were instructed to inhale each dose from a dry powder inhaler along with deep breathing. The total nominal dose of LO was administered as separate doses of 10 mg, with a minimal interval, in a sitting position. The subjects were prohibited from resting in a supine position for 2 h after inhalation. Subjects fasted for 4 h predosing, and no food was permitted for 2 h postdosing. BAL was performed 4, 8, 24, 48, 72, 168, and 240 h after dosing.
BAL.
All subjects underwent fiber-optic bronchoscopy with BAL once per person at their respective times after the dose of LO. The blood pressure, respiratory rate, and heart rate of each subject were recorded before, at the completion of, and approximately 1 h after bronchoscopy. Oxygenation was monitored by fingertip oximetry throughout the procedure. As a pretreatment, inhalation of 1% lidocaine through a nebulizer and injection of atropine sulfate were started 15 min before fiber-optic bronchoscopy. After that, regional anesthesia of the pharynx with lidocaine through a Jackson-type spray was performed. Systemic sedation was not used. The bronchoscope was inserted up to the right middle lobe and then wedged. BAL was carried out by infusion of four 50-ml volumes of sterile saline into the subsegmental bronchus of the right middle lobe, and each specimen was aspirated immediately. Instillation of 50 ml of saline and its aspiration were performed within 1 min. The volume of each aspirate from the instillations was measured immediately, and the aspirates were kept on ice. The aspirate of the first aliquot was kept separate, and the aspirates of the remaining three aliquots were pooled.
The BAL fluid was filtered through sterile gauze and centrifuged at 400 × g for 5 min at 4°C. The supernatants were separated, and the volumes were measured. An aliquot of the supernatant was stored at −70°C for urea nitrogen measurement. Acidified 4-(2-aminoethyl)benzenesulfonyl fluoride hydrochloride (AEBSF) solution was added to the remaining supernatants (1:40 [vol/vol]) as an enzyme inhibitor to minimize LO degradation and was mixed to prepare BAL fluid samples. The sediment cells from the BAL fluid were washed twice by the addition of an aliquot of 1 ml of ice-cold phosphate-buffered saline (PBS) and were centrifuged at 400 × g for 5 min at 4°C. Part of the cells was suspended in an aliquot of 1 ml of ice-cold PBS for determination of the cell counts with a hemocytometer. A differential count of BAL fluid cells was performed with a stained cytocentrifuged sample of BAL fluid. The remaining BAL fluid cells and supernatants were both stored at −70°C until they were assayed for LO and laninamivir concentrations.
Blood samples for determination of LO, laninamivir, and urea concentrations.
Blood (5 ml) was collected into Vacutainers containing heparin as an anticoagulant predosing, 0.25, 2, and 3.5 h after dosing, and 30 min before each BAL procedure. After the addition of an aliquot of 0.125 ml of AEBSF to 5 ml of blood, followed by centrifugation at 1,700 × g for 10 min at 4°C, the plasma supernatants were stored at −20°C until the assay. Blood samples (2 ml) for determination of the urea concentration were collected into Vacutainers containing heparin after finishing BAL and then centrifuged, and plasma was stored at −70°C until assay.
Sample analysis.
LO and laninamivir concentrations in plasma were analyzed by solid-phase extraction and liquid chromatography-tandem mass spectrometry (LC-MS/MS) in the positive ionization mode (10) at Covance Laboratories Ltd. (North Yorkshire, United Kingdom). The lower limit of quantitation for the plasma assay was 1 ng/ml, and the linear calibration range was 1 to 1,000 ng/ml. The interassay accuracies of the plasma quality control (QC) samples for LO and laninamivir were within 100.0 to 102.1% and 98.7 to 100.0% of nominal values, respectively, and the interassay precisions were less than 10%. Coefficients of variation (CVs) of the intra-assay QC samples for LO and laninamivir in plasma were 2.1 to 4.7% and 2.8 to 11.5%, respectively.
LO and laninamivir in BAL fluid and AM were extracted by deproteinization using methanol, and their concentrations were determined by validated LC-MS/MS methods at Shin Nippon Biomedical Laboratories Ltd. (Wakayama, Japan). Intraday, interday, and dilution reproducibility, freeze-thaw stability, and storage stability were evaluated, and the results met the predefined acceptance criteria. The lower limit of quantitation was 0.1 ng/ml, and the linear calibration range was 0.1 to 10 ng/ml. CVs of the intra-assay QC samples (AEBSF-spiked pulmonary surfactant in physiological saline) for LO and laninamivir were 3.8 to 4.7% and 2.7 to 3.1%, respectively, and those of the interassay QC samples were 3.2 to 4.1% and 2.4 to 4.7%, respectively.
The concentrations of urea in plasmas and BAL supernatants were analyzed by use of a modified diagnostic kit (Urea N B; Wako Pure Chemical Industries, Ltd., Tokyo, Japan) which was validated and measured at Shin Nippon Biomedical Laboratories, Ltd. (Kagoshima, Japan). Plasma was diluted 200-fold with physiological saline before measurement. The absorbance of standards, samples, and QC samples at 570 nm was measured against the absorbance of the blank. QC samples with concentrations of 7.5, 15, and 35 mg/dl were run with every standard curve. Standard curves ranging from 5 to 50 mg/dl were linear (r > 0.99). CVs of the intra- and interassay QC samples for urea concentration were 0.5 to 4.9% and 0.5 to 7.1%, respectively.
Determination of ELF volume and calculations of drug concentrations in ELF and AM.
The ELF volume was determined by the urea dilution method (17). The volume of ELF in BAL fluid (VELF) was derived from the following relationship: VELF = VBAL × (ureaBAL/ureaplasma), where VBAL is the volume of aspirated BAL fluid, ureaBAL is the concentration of urea in supernatant BAL fluid, and ureaplasma is the concentration of urea in plasma. Since erythrocytes in BAL fluid were minimal (<1,000 cells/μl) in all BAL fluids examined, the urea concentration was not corrected for possible contamination with urea from blood (4).
The drug concentrations in ELF (CELF) were determined as follows: CELF = CBAL× (ureaplasma/ureaBAL), where CBAL is the measured concentration of LO or laninamivir in the supernatant of BAL fluid. The calculation of CELF was performed with BAL fluids recovered from the first aliquot and the remaining aliquots.
The drug concentrations in AM (CAM) were determined as follows: CAM = AAM/VAM, where AAM is the measured amount of LO or laninamivir in a cell suspension and VAM is the volume of AM in a cell suspension. Cell counts by type were performed to determine the number of AM. The reported AM volume of 2.42 μl/106 cells was used in calculations for the volume of AM in the suspension (17).
Pharmacokinetic analysis.
Pharmacokinetic parameters were calculated by a noncompartmental analysis using the computer software WinNonlin Professional (version 6.1; Pharsight Corp., CA). The area under the concentration-time curve from 0 to 3.5 h after the inhalation (AUC0–3.5) was obtained by the linear trapezoidal method. The AUC up to the last measurable time (AUClast), the maximum drug concentration (Cmax), the time to reach Cmax (Tmax), and standard errors for AUClast and Cmax were estimated using the sparse sampling option in WinNonlin. The elimination half-life (t1/2) was obtained by linear regression of 3 or more log-transformed data points in the terminal phase.
Safety.
Safety was assessed by clinical evaluation (including physical examinations, vital signs, and 12-lead electrocardiograms) and laboratory measurements (including hematology, serum chemistry, and urinalysis). Adverse events (AEs) were assessed through questioning and spontaneous reporting. A 12-lead ECG and chest X-rays were assessed at screening, 1 day before the administration of LO, 1 day after BAL, and at the end-of-study visit. Investigators evaluated all clinical AEs in terms of intensity (mild, moderate, or severe), duration, severity, outcome, and relationship to the study drug.
RESULTS
Recovery of ELF and cells from BAL fluid.
The volume of fluid recovered from the first BAL aliquot (BAL1) and the pooled volume obtained from the 2nd, 3rd, and 4th BAL aliquots (BAL2) were 14.8 ± 5.4 and 103.0 ± 15.2 ml (mean ± SD; n = 35), respectively. The calculated volumes of ELF recovered from BAL1 and BAL2 were 0.24 ± 0.17 and 1.62 ± 0.67 ml, respectively. The most predominant cell type was AM (88.1% ± 6.5% for BAL1 and 91.4% ± 5.4% for BAL2).
LO and laninamivir concentrations in ELF, AM, and plasma.
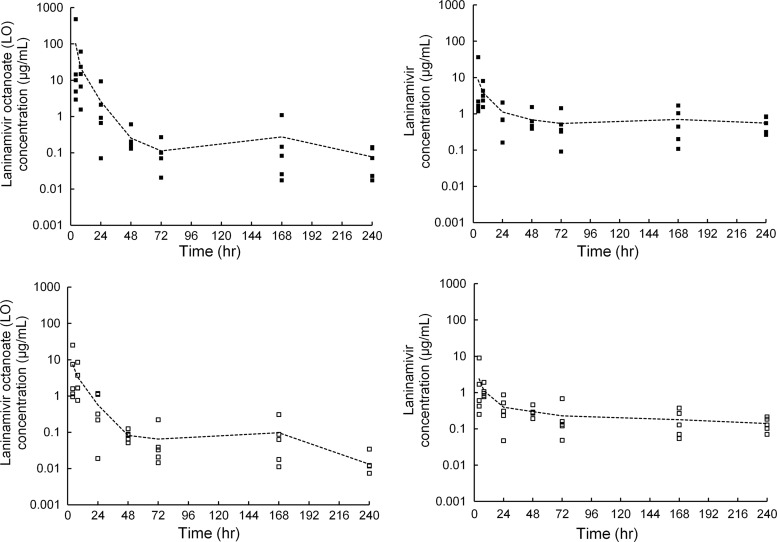
Mean and individual concentrations of LO and laninamivir in ELF from BAL1 and BAL2 after a single inhaled administration of 40 mg of LO are shown in Fig. 1, and drug concentrations in ELF and AM for each group are summarized in Table 1. Concentrations of LO and laninamivir in ELF were measurable in all samples examined, though relatively large interindividual differences were observed at an early time point. The Cmax values for LO and laninamivir in ELF from BAL1 were 102.40 and 8.57 μg/ml, respectively, and both LO and laninamivir concentrations in ELF decreased with long t1/2 values (Table 2).
Fig 1.
Mean and individual concentrations of LO (left panels) and laninamivir (right panels) in ELF, calculated using the first BAL fluid (BAL1; closed symbols) and pooled BAL fluid aliquots (BAL2; open symbols) after a single inhaled administration of 40 mg of LO to healthy subjects. Each symbol represents an individual concentration, and the line shows the mean concentration.
Table 1.
LO and laninamivir concentrations in ELF (CELF) and AM (CAM) at each time point, evaluated via BAL1 and BAL2
| BAL fluid | Time (h) | LO concna |
Laninamivir concna |
||
|---|---|---|---|---|---|
| CELF (μg/ml) | CAM (μg/ml) | CELF (μg/ml) | CAM (μg/ml) | ||
| BAL1 | 4 | 102.40 ± 210.86 | 694.1 ± 452.5 | 8.57 ± 15.53 | 26.2 ± 11.5 |
| 8 | 21.64 ± 23.82 | 2,085.0 ± 725.8 | 3.86 ± 2.54 | 125.1 ± 56.3 | |
| 24 | 2.61 ± 3.80 | 680.6 ± 924.3 | 1.12 ± 0.86 | 74.6 ± 80.9 | |
| 48 | 0.26 ± 0.20 | 471.0 ± 399.3 | 0.68 ± 0.49 | 94.4 ± 66.4 | |
| 72 | 0.11 ± 0.09 | 124.0 ± 61.2 | 0.54 ± 0.52 | 54.1 ± 30.6 | |
| 168 | 0.27 ± 0.46 | 165.5 ± 243.6 | 0.70 ± 0.67 | 79.4 ± 121.4 | |
| 240 | 0.08 ± 0.06 | 51.1 ± 30.9 | 0.56 ± 0.27 | 62.2 ± 52.6 | |
| BAL2 | 4 | 7.25 ± 10.34 | 556.4 ± 413.0 | 2.40 ± 3.74 | 54.6 ± 44.4 |
| 8 | 3.27 ± 3.15 | 1,088.0 ± 813.9 | 1.10 ± 0.47 | 152.3 ± 107.4 | |
| 24 | 0.57 ± 0.54 | 232.2 ± 248.3 | 0.39 ± 0.31 | 46.4 ± 44.3 | |
| 48 | 0.08 ± 0.03 | 335.5 ± 505.9 | 0.30 ± 0.10 | 82.7 ± 81.0 | |
| 72 | 0.07 ± 0.09 | 50.8 ± 36.0 | 0.23 ± 0.25 | 41.0 ± 42.5 | |
| 168 | 0.10 ± 0.12 | 31.0 ± 22.1 | 0.18 ± 0.14 | 26.1 ± 17.4 | |
| 240 | 0.01 ± 0.01 | 13.6 ± 4.7 | 0.14 ± 0.06 | 28.3 ± 16.4 | |
Data are means ± SD (n = 5).
Table 2.
Pharmacokinetic parameters of LO and laninamivira
| Drug | Sample type | BAL fluid | Cmax (μg/ml) | Tmax (h) | AUClast (μg·h/ml) | t1/2 (h) |
|---|---|---|---|---|---|---|
| LO | Plasma | 0.162 (0.013) | 0.3 | 0.705 (0.045) | 2.6 | |
| ELF | BAL1 | 102.40 (94.3) | 4.0 | 716.5 (393.8) | 41.7 | |
| ELF | BAL2 | 7.25 (4.62) | 4.0 | 87.7 (24.3) | 41.3 | |
| AM | BAL1 | 2,085 (325) | 8.0 | 71,719 (13,585) | 69.5 | |
| AM | BAL2 | 1,088 (364) | 8.0 | 31,942 (7,060) | 89.9 | |
| Laninamivir | Plasma | 0.025 (0.001) | 3.5 | 0.826 (0.041) | 45.7 | |
| ELF | BAL1 | 8.57 (6.94) | 4.0 | 222.9 (42.7) | 141 | |
| ELF | BAL2 | 2.40 (1.67) | 4.0 | 69.4 (11.5) | 241 | |
| AM | BAL1 | 125.1 (25.2) | 8.0 | 17,271 (4,824) | 1029 | |
| AM | BAL2 | 152.3 (48.0) | 8.0 | 10,325 (1,715) | 142 |
Pharmacokinetic parameters were estimated using the sparse sampling option in WinNonlin and are presented as means (SE) for Cmax and AUClast.
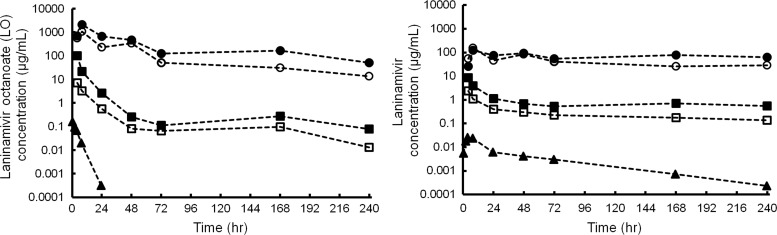
Mean concentrations of LO and laninamivir in plasma, ELF, and AM are shown in Fig. 2. LO appeared rapidly in plasma, with a Tmax of 0.3 h, and decreased with a t1/2 of 2.6 h (Table 2). The peak plasma concentrations of laninamivir occurred 3.5 h after inhalation and decreased with a t1/2 of 45.7 h. The mean AUC0–3.5 for LO and laninamivir did not differ among the times of BAL (307 to 445 ng·h/ml for LO and 48.3 to 63.0 ng·h/ml for laninamivir). Both LO and laninamivir concentrations in ELF and AM were much higher than those in plasma and lasted for 240 h after dosing, with a longer t1/2 than that in plasma. The concentration-time profile for LO or laninamivir in ELF from BAL1 was almost parallel to each obtained for ELF from BAL2. The Cmax and AUClast of LO in ELF from BAL1 were 14- and 8.2-fold higher, respectively, than those for ELF from BAL2, and those of laninamivir in ELF from BAL1 were 3.6- and 3.2-fold higher, respectively, than those for ELF from BAL2 (Table 2).
Fig 2.
Mean concentrations of LO (left) and laninamivir (right) in plasma (triangles), ELF (squares), and AM (circles) after a single inhaled administration of 40 mg of LO to healthy subjects. ELF and AM concentrations were calculated using the first BAL fluid (BAL1; closed symbols) and pooled BAL fluid aliquots (BAL2; open symbols), respectively.
Safety.
All of the AEs that occurred during the study were mild and transient. Among the total of 36 participants who received LO, 26 participants experienced AEs. No AEs were judged to be a drug-related event. The following AEs considered to be related to BAL were reported: C-reactive protein (CRP) elevation (20 participants), leukocytosis (10 participants), neutrophilia (8 participants), and lymphopenia (5 participants). Among the 20 participants who experienced mild CRP elevation, 12 participants were dosed with antibiotics and recovered. Others recovered without any treatment. No notable mean changes from the baseline were recorded for the vital signs or clinical laboratory variables, and none of the individual participant values outside the laboratory reference ranges were considered to be clinically significant.
DISCUSSION
This study clearly demonstrated that a single inhaled administration of 40 mg of LO achieved a relatively high intrapulmonary concentration of laninamivir for 10 days after dosing. High-level accumulation of laninamivir in the lung, with a long t1/2, was observed after intranasal or intratracheal administration of LO to mice or rats (14, 15). PK studies of humans also showed a long t1/2 of laninamivir in plasma (10, 11), which is reflected by the slow release from retaining tissues to plasma and by the slow formation of laninamivir from LO in ELF and AM. These PK profiles provide support for the characteristics of laninamivir as a long-acting neuraminidase inhibitor.
Since LO is a prodrug of laninamivir, LO in the intrapulmonary compartment is important for supplying laninamivir to ELF. Higher LO concentrations than laninamivir concentrations in ELF were achieved at the early BAL time points, and they decreased faster than laninamivir concentrations in ELF, with a t1/2 of 41.3 h in BAL2 (Table 2). Laninamivir in ELF is derived from this distributed LO and from that metabolized to laninamivir in the lung, as the hydrolysis of LO to laninamivir was observed substantially in S9 (supernatant fraction obtained from human lung homogenate by centrifugation at 9,000 × g) human lungs in vitro (K. Koyama, M. Takahashi, D. Nakai, N. Kobayashi, and T. Izumi, unpublished data). Moreover, the accumulated LO and laninamivir in AM could serve as a source to supply laninamivir to ELF. The calculated values of AUClast for LO and laninamivir in AM were 77 to 364 times higher than those for the drugs in ELF (Table 2). High-level accumulation in AM was also observed in several human BAL studies after the oral administration of antibiotics (3, 5, 8, 18, 22). The uptake mechanism of clarithromycin and azithromycin to AM was explored using cultured AM cells (NR8383 cells), and it was found that the uptake was partly reduced by ATP depletors (21). In addition to the nonspecific distribution of those basic compounds to acidic organelle compartments, an active transporter(s) might contribute to the high-level accumulation of those compounds in AM. Though the contribution of a transporter has not been clarified for LO and laninamivir, LO in AM achieved considerably high concentrations and decreased to less than 10% of the Cmax by 72 h after dosing. These data indicate that LO in AM partly supplies ELF with LO, even though the total amount of LO in AM might be limited. On the other hand, once absorbed into the systemic circulation and then returned to ELF, LO contributes minimally to LO and laninamivir concentrations in ELF, because the plasma AUClast values for LO and laninamivir were 84 to 1,016 times lower than those for the drugs in ELF and because LO in ELF lasted even after the plasma concentration had diminished. Collectively, the majority of laninamivir in ELF must be derived from LO directly and locally distributed in ELF, AM, and the lung by inhalation.
The individual concentrations of laninamivir (molecular weight [MW], 346.34) in ELF at all time points examined notably exceeded the median in vitro 50% inhibitory concentrations (IC50s) for influenza viral neuraminidases (2.09, 14.2, and 15.9 nM for subtype A/H1N1, H3N2, and B neuraminidases, respectively) (24). Since the plasma protein binding of laninamivir was <0.1% (10) and the albumin concentration in ELF was >10-fold lower than that in plasma (17), the observed laninamivir concentration in ELF could be deemed to be similar to the unbound laninamivir concentration in ELF. The mean laninamivir concentration in ELF calculated from BAL2 240 h after the inhalation was 0.14 μg/ml, which represents 25 to 193 times the IC50s for influenza virus neuraminidases mentioned above. Similarly, the concentration of zanamivir in ELF following i.v. and twice-daily oral inhalation was evaluated, and it was found that the zanamivir concentration in ELF also achieved remarkably higher values than the IC50s for influenza virus neuraminidases of various influenza virus subtypes, including the subtype A/H1N1, A/H3N2, and B neuraminidases (19). These data support the doses used in a phase III study to evaluate the efficacy and safety of i.v. zanamivir twice daily in patients (ClinicalTrials.gov identifier NCT01231620). Interestingly, the median zanamivir concentrations in ELF 12 h after oral inhalation were 891 ng/ml (BAL1) and 326 ng/ml (BAL2), which correspond approximately to the mean laninamivir concentrations in ELF at 24 h postdosing (i.e., 1.12 μg/ml [BAL1] and 0.39 μg/ml [BAL2]) or later. In addition, the MW of zanamivir (332.31) is similar to that of laninamivir, and the amount of plasma protein binding of zanamivir is also negligible (<10%) (6). Considering that (i) both laninamivir and zanamivir show efficacy for treatment of patients suffering from influenza, (ii) there is no great difference in neuraminidase inhibitory activity between laninamivir and zanamivir in vitro (24, 25), and (iii) after inhaled dosing of each therapeutic dosage regimen, the mean laninamivir concentration in ELF at 24 h postdosing or later corresponded approximately to the median zanamivir trough concentration in ELF, a relatively high concentration in the pulmonary compartment compared with the IC50s might be necessary to treat patients suffering from influenza virus. Since the relationship between the intrapulmonary PK of a neuraminidase inhibitor and its efficacy in humans has not been understood fully, these results need further investigation.
It has been reported that the first aliquot of BAL fluid is more related to bronchi and that the others are more related to the more distal parts of the lung, including bronchioles and alveoli (7, 12). In this study, the distribution of LO and laninamivir in the lung was explored by comparison of the concentrations in the first BAL fluid and those in the remaining aliquots recovered, as performed in a study of zanamivir (19). The Cmax and AUClast of LO in ELF from BAL1 were 14- and 8.2-fold higher, respectively, than those for BAL2 (Table 2). In contrast, a relatively small difference in laninamivir compared with LO in ELF was observed between BAL1 and BAL2. The Cmax and AUClast of laninamivir in ELF from BAL1 were 3.6- and 3.2-fold higher, respectively, than those for BAL2. On the other hand, the Cmax and AUClast of LO in AM from BAL1 were 1.9- and 2.2-fold higher, respectively, than those for BAL2. Moreover, the Cmax of laninamivir in AM was lower for BAL1 than for BAL2, and the AUClast of laninamivir in AM was 1.7-fold higher for BAL1 than for BAL2. Such nonuniform distributions and PK of LO and laninamivir in the lung can be explained partly by the distribution of particle sizes of LO in a dry powder formulation, regional differences in membrane permeability, differences in the enzymatic activity to metabolize LO to laninamivir, and so forth. Zanamivir disposition following an inhaled administration was evaluated directly using radiolabeled zanamivir in positron emission tomography (2). However, it is difficult to evaluate laninamivir distribution and to clarify its long-lasting PK by this method, because laninamivir is administered as a prodrug and has a longer t1/2 in the body than that of the radioactive nuclides used in positron emission tomography. Although the analysis of BAL1 versus BAL2 may not give a quantitative conclusion with absolute precision, the evaluation of BAL1 provides some indication of the distribution of the topical drug in the proximal region of the bronchoscope, as one cannot easily evaluate its PK characteristics through other methods.
Laninamivir was distributed and lasted in the lung for 10 days after a single inhaled dose of LO. The laninamivir concentration profile in ELF supports its long-lasting effect for treatment of patients with influenza virus infection by a single inhaled administration.
ACKNOWLEDGMENTS
This study was sponsored by Daiichi Sankyo Co., Ltd.
We acknowledge the technical assistance of Saiko Yamada, Kayo Matsumoto, Yukio Hori, Noriko Goto, Shinichi Miyaji, Saeko Fukushima, Rumi Wada, Ryoko Yokota, and Tsunenori Nakazawa.
Hitoshi Ishizuka, Kaoru Toyama, Satoshi Yoshiba, and Hiromi Okabe are employees of Daiichi Sankyo Co., Ltd.
Footnotes
Published ahead of print 23 April 2012
REFERENCES
- 1. Baldwin DR, Wise R, Andrews JM, Gill M, Honeybourne D. 1993. Comparative bronchoalveolar concentrations of ciprofloxacin and lomefloxacin following oral administration. Respir. Med. 87:595–601 [DOI] [PubMed] [Google Scholar]
- 2. Bergstrom M, et al. 1999. Deposition and disposition of [11C]zanamivir following administration as an internal spray. Clin. Pharmacokinet. 36:33–39 [DOI] [PubMed] [Google Scholar]
- 3. Conte JE, Jr, DeVoe C, Little E, Golden JA. 2010. Steady-state intrapulmonary pharmacokinetics and pharmacodynamics of posaconazole in lung transplant recipients. Antimicrob. Agents Chemother. 54:3609–3613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Conte JE, Jr, et al. 1996. Single-dose intrapulmonary pharmacokinetics of azithromycin, clarithromycin, ciprofloxacin, and cefuroxime in volunteer subjects. Antimicrob. Agents Chemother. 40:1617–1622 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Conte JE, Jr, et al. 2000. Single-dose intrapulmonary pharmacokinetics of rifapentine in normal subjects. Antimicrob. Agents Chemother. 44:985–990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Daniel M, Barnett J, Pearson B. 1999. The low potential for drug interactions with zanamivir. Clin. Pharmacokinet. 36(Suppl 1):41–50 [DOI] [PubMed] [Google Scholar]
- 7. Davis GS, Giancola MS, Costanza MC, Low RB. 1982. Analyses of sequential bronchoalveolar lavage samples from healthy human volunteers. Am. Rev. Respir. Dis. 126:611–616 [DOI] [PubMed] [Google Scholar]
- 8. Furuie H, Saisho Y, Yoshikawa T, Shimada J. 2010. Intrapulmonary pharmacokinetics of S-013420, a novel bicyclolide antibacterial, in healthy Japanese subjects. Antimicrob. Agents Chemother. 54:866–870 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Imberti R, et al. 2010. Steady-state pharmacokinetics and BAL concentration of colistin in critically ill patients after iv colistin methanesulfonate administration. Chest 138:1333–1339 [DOI] [PubMed] [Google Scholar]
- 10. Ishizuka H, Yoshiba S, Okabe H, Yoshihara K. 2010. Clinical pharmacokinetics of laninamivir, a novel long-acting neuraminidase inhibitor, after single and multiple inhaled doses of its prodrug, CS-8958, in healthy male volunteers. J. Clin. Pharmacol. 50:1319–1329 [DOI] [PubMed] [Google Scholar]
- 11. Ishizuka H, Yoshiba S, Yoshihara K, Okabe H. 2011. Assessment of the effect of the pharmacokinetic profile of laninamivir, a novel neuraminidase inhibitor, after a single inhaled dose of its prodrug, CS-8958. J. Clin. Pharmacol. 51:243–251 [DOI] [PubMed] [Google Scholar]
- 12. Kelly CA, Kotre CJ, Ward C, Hendrick DJ, Walters EH. 1987. Anatomical distribution of bronchoalveolar lavage fluid as assessed by digital subtraction radiography. Thorax 42:624–628 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kiso M, et al. 2010. Efficacy of the new neuraminidase inhibitor CS-8958 against H5N1 influenza viruses. PLoS Pathog. 6:e1000786 doi:10.1371/journal.ppat.1000786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Koyama K, et al. 2010. Pharmacokinetics and disposition of CS-8958, a long-acting prodrug of the novel neuraminidase laninamivir in rats. Xenobiotica 40:207–216 [DOI] [PubMed] [Google Scholar]
- 15. Koyama K, et al. 2009. CS-8958, a prodrug of the novel neuraminidase inhibitor R-125489, demonstrates a favorable long-retention profile in the mouse respiratory tract. Antimicrob. Agents Chemother. 53:4845–4851 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Nguyen HT, Sheu TG, Mishin VP, Klimov AI, Gubareva LV. 2010. Assessment of pandemic and seasonal influenza A (H1N1) virus susceptibility to neuraminidase inhibition in three enzyme activity inhibition assays. Antimicrob. Agents Chemother. 54:3671–3677 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Rennard SI, et al. 1986. Estimation of volume of epithelial lining fluid recovered by lavage using urea as marker of dilution. J. Appl. Physiol. 60:532–538 [DOI] [PubMed] [Google Scholar]
- 18. Schüler P, et al. 1997. Penetration of sparfloxacin and ciprofloxacin into alveolar macrophages, epithelial lining fluid, and polymorphonuclear leucocytes. Eur. Respir. J. 10:1130–1136 [DOI] [PubMed] [Google Scholar]
- 19. Shelton MJ, et al. 2011. Zanamivir pharmacokinetics and pulmonary penetration into epithelial lining fluid following intravenous or oral inhaled administration to healthy adult subjects. Antimicrob. Agents Chemother. 55:5178–5184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Sugaya N, Ohashi Y. 2010. Long-acting neuraminidase inhibitor laninamivir octanoate (CS-8958) versus oseltamivir as treatment for children with influenza virus infection. Antimicrob. Agents Chemother. 54:2575–2582 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Togami K, Chono S, Morimoto K. 2011. Distribution characteristics of clarithromycin and azithromycin, macrolide antimicrobial agents used for treatment of respiratory infections, in lung epithelial lining fluid and alveolar macrophages. Biopharm. Drug Dispos. 32:389–397 [DOI] [PubMed] [Google Scholar]
- 22. Walsh TJ, et al. 2010. Intrapulmonary pharmacokinetics and pharmacodynamics of micafungin in adult lung transplant patients. Antimicrob. Agents Chemother. 54:3451–3459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Watanabe A, Chang SC, Kim MJ, Chi DW, Ohashi Y. 2010. Long-acting neuraminidase inhibitor laninamivir octanoate versus oseltamivir for treatment of influenza: a double-blind, randomized, noninferiority clinical trial. Clin. Infect. Dis. 51:1167–1175 [DOI] [PubMed] [Google Scholar]
- 24. Yamashita M. 2010. Laninamivir and its prodrug, CS-8958: long-acting neuraminidase inhibitors for the treatment of influenza. Antivir. Chem. Chemother. 21:71–84 [DOI] [PubMed] [Google Scholar]
- 25. Yamashita M, et al. 2009. CS-8958, a prodrug of the new neuraminidase inhibitor R-125489, shows long-acting anti-influenza virus activity. Antimicrob. Agents Chemother. 53:186–192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Yoshiba S, Okabe H, Ishizuka H. 2011. Pharmacokinetics of laninamivir after a single administration of its prodrug, laninamivir octanoate, a long-acting neuraminidase inhibitor, using an easy-to-use inhaler in healthy volunteers. J. Bioequiv. Availab. 3:1–4 [Google Scholar]