Abstract
Amiodarone, a commonly used antiarrhythmic, is also a potent and selective anti-Trypanosoma cruzi agent. Dronedarone is an amiodarone derivative in which the 2,5-diiodophenyl moiety of the parental drug has been replaced with an unsubstituted phenyl group aiming to eliminate the thyroid toxicity frequently observed with amiodarone treatment. Dronedarone has been approved by the Food and Drug Administration (FDA), and its use as a safe antiarrhythmic has been extensively documented. We show here that dronedarone also has potent anti-T. cruzi activity, against both extracellular epimastigotes and intracellular amastigotes, the clinically relevant form of the parasite. The 50% inhibitory concentrations against both proliferative stages are lower than those previously reported for amiodarone. The mechanism of action of dronedarone resembles that of amiodarone, as it induces a large increase in the intracellular Ca2+ concentration of the parasite, which results from the release of this ion from intracellular storage sites, including a direct effect of the drug on the mitochondrial electrochemical potential, and through alkalinization of the acidocalcisomes. Our results suggest a possible future repurposed use of dronedarone for the treatment of Chagas' disease.
INTRODUCTION
Trypanosoma cruzi is the causative agent of Chagas' disease, a chronically debilitating infection that to date has no approved efficient treatment in its chronic stages. However, it is now well established that amiodarone, a commonly used antiarrhythmic drug, has also potent and specific activity against the proliferative stages of T. cruzi (4) as well as Leishmania mexicana (22, 23) and Leishmania amazonensis (16). Recently, the added effect of amiodarone on recovering myocardial contractility in T. cruzi-infected cardiac myocytes through a direct action over F-actin fibrils and gap junction proteins such as connexin43 has been reported (1). Notably, amiodarone efficacy has also been demonstrated in at least one clinical case of human Chagas' disease (18) and in a case of cutaneous leishmaniasis (17). Amiodarone also supports host survival during malaria episodes (6), opening a whole new perspective for an alternative use of this drug. It has been also found that the combination of amiodarone with posaconazole, another potential option for treating Chagas' disease already in final clinical trials (24), and with other analogs has potent synergistic effects (4). Posaconazole has recently been found to effect successful treatment in humans with T. cruzi infection (20), and also in a case of cutaneous leishmaniasis (19).
Despite its extensive use in humans, the presence of a 2,5-diiodophenyl moiety in the structure of amiodarone, which has been associated with significant undesirable side effects related to antithyroid activity, makes its prolonged use in Chagas' disease a difficult therapeutic task for the clinician. Dronedarone, a derivative of amiodarone, has been recently synthesized with several significant structural modifications, including replacement of the 2,5-diiodophenyl with an unsubstituted phenyl group and the incorporation of a methyl sulfonyl group, aimed at reducing the thyroid toxicity and lipophilicity of the parental drug (Fig. 1). This new drug has already been FDA approved for use as an antiarrhythmic in humans and has begun to replace amiodarone as the drug of choice due to its improved safety profile and apparent absence of associated thyroid or pulmonary toxicity, therefore resulting in fewer treatment discontinuations and reduced mortality (21).
Fig 1.

Chemical structure of dronedarone and amiodarone.
In this report, we provide evidence that dronedarone also has activity against extracellular epimastigotes of T. cruzi, which represent the proliferative stage equivalent to that present in the parasite's insect vector and the clinically relevant intracellular amastigote form. Dronedarone and amiodarone act through the same mechanisms of action, by altering the intracellular Ca2+ homeostasis of the parasite as a consequence of a direct effect of the drug on the mitochondrial electrochemical potential and also by alkalinization of the acidocalcisomes.
MATERIALS AND METHODS
Chemicals.
Dronedarone was extracted from commercial tablets (Multaq), based on the fact that this compound is fully soluble in methanol. Dronedarone powder was obtained by grinding the tablets in a mortar, after which the powder was dissolved in methanol by vigorous mixing. The insoluble material was discarded by centrifugation. Amiodarone {(2-butyl-3-benzofuranyl)-[4-[2-(diethylamino)-ethoxi] 3,5-diiodophenyl] methanone hydrochloride}, EGTA, digitonin, fluorocarbonylcyanide P-(trifluoromethoxy) phenylhydrazone (FCCP), bafilomycin A, and nigericin were from Sigma (St. Louis, MO). Fura 2-acetoxymethyl ester (Fura 2-AM), rhodamine 123, rhod 2-AM, and acridine orange were from Molecular Probes (Eugene, OR).
Culture of epimastigotes of Trypanosoma cruzi and determination of susceptibility to dronedarone.
T. cruzi epimastigotes (CL Brener strain) were grown in LIT (liver infusion-tryptose) medium supplemented with 10% fetal bovine serum at 29°C, with strong agitation (100 rpm) as reported previously (4), in the absence or presence of either dronedarone or amiodarone at different concentrations. Live parasites were counted daily using a Neubauer chamber. The initial parasite concentration was 106 parasites/ml. Either the drug or vehicle (dimethyl sulfoxide [DMSO]) was added after 24 h under each condition. At least 3 independent experiments were performed for each drug and dose, and the 50% effective concentration (EC50) was determined using Prisma GraphPad 5.0.
Amastigote growth inhibition assay.
Amastigotes were cultured in Vero cells maintained in Dulbecco's minimal essential medium, supplemented with 1% fetal bovine serum and incubated at 37°C in humidified 95% air–5% CO2 (4). For the assays, Vero cells were placed in a 6-well plate and infected with trypomastigotes (ratio, 1:10) for 12 h. After infection, cells were washed three times to remove noninteriorized trypomastigotes, and culture medium was added either with or without dronedarone at different concentrations. The infected cells were incubated for 96 h under conditions previously described (4). At 96 h, the cells were fixed with methanol and stained with Giemsa stain to determine the percentages of infected cells.
Determination of the intracellular Ca2+ concentration.
To evaluate the effect of dronedarone on intracellular Ca2+ concentration, we loaded T. cruzi epimastigotes with the fluorescent ratiometric indicator Fura 2. Briefly, 2 × 108 parasites were collected by centrifugation at 600 × g for 2 min and washed twice in phosphate-buffered saline (PBS) plus 1% glucose. Since epimastigotes possess low levels of esterases, which make the development of Fura 2 in the cytoplasm difficult, we found it appropriate to load the parasites overnight with Fura 2-AM (6 μM) with probenecid (12 μM) and pluronic acid (12 μM) in PBS plus 1% glucose at 29°C, in the dark with continuous agitation. This procedure enabled sufficient loading of the parasites with the Ca2+ fluorophore to allow precise measurement of the intracellular Ca2+ changes after addition of either dronedarone or amiodarone. The Fura 2-loaded parasites were washed by centrifugation twice and resuspended in Tyrode buffer in the presence or absence of Ca2+. For Fura 2 measurements, these parasites were placed in a cuvette with continuous stirring and thermostated at 29°C in a Perkin Elmer 510 spectrofluorimeter. The conditions of measurement were as follows: dual excitation wavelength (Ex), 340 nm and 380 nm, and emission wavelength (Em), 510 nm. Intracellular Ca2+ concentration has been calculated as described by Grynkiewicz et al. (15), by applying the following equation: Kd × [(R − Rmin)/(Rmax − R)] × [Fmin(380)/Fmax(380)], where Kd is the dissociation constant of Fura 2 (244 nM); R is the ratio of the fluorescence emission obtained after excitation at 340 nm to that obtained after excitation at 380 nm; Rmax and Fmax are the ratio of excitation fluorescence at 340 nm to the excitation fluorescence at 380 nm and the fluorescence of Fura 2 at 380 nm, respectively, under saturated Ca2+ concentrations; and Rmin and Fmin are the ratio of excitation fluorescence at 340 nm/excitation fluorescence at 380 nm and the fluorescence of Fura 2 at 380 nm, respectively, in the absence of Ca2+. Maximum and minimum values were obtained after the addition of 30 μM digitonin, which allows the flow of Ca2+ to the interior of the cell. Then, 8 mM EGTA was added to chelate all the remaining Ca2+ (9).
Mitochondrial membrane potential.
To evaluate the effect of dronedarone on the mitochondrial membrane potential of T. cruzi epimastigotes, we used the fluorescent dye rhodamine 123, as reported previously (22). The parasites were collected by centrifugation at 600 × g for 2 min and washed in PBS buffer plus 1% glucose. Then, the cells were resuspended in the same buffer and loaded with 10 μM rhodamine 123 for 40 min at 29°C with mild agitation. The loaded parasites were washed twice and resuspended in the same buffer plus 2 μM probenecid and 2 μM pluronic acid. Measurements (excitation λ [λext], 488 nm; emission λ [λem], 530 nm) were made in a Hitachi 7000 spectrofluorimeter under stirring at 29°C. FCCP (1 μM) was added as a positive control.
Acidocalcisome alkalinization.
The effects of dronedarone on the alkalinization of acidocalcisomes were measured using the fluorescence properties of acridine orange as previously described (23). Epimastigotes (109 cells/ml) were collected and washed and then loaded with 2 μM acridine orange in PBS for 5 min at 29°C with constant stirring. Measurements were performed at λext at 488 nm and λem at 530 nm at 29°C in a Hitachi 7000 spectrofluorimeter under continuous stirring. Nigericin (1 μM) was used as a positive control.
RESULTS
We first determined the antiproliferative effects of dronedarone and amiodarone on T. cruzi epimastigotes grown in axenic LIT medium at 29°C. As can be seen in Fig. 2, there is a dose-dependent parasiticidal effect of dronedarone. Interestingly, the 50% inhibitory concentration (IC50) for dronedarone (4.6 μM) was somehow lower than for amiodarone (7.9 μM). Since the clinically relevant stage of the T. cruzi life cycle is the amastigote form growing inside mammalian host cells, we determined the effect of dronedarone on the proliferation of these parasites inside Vero cells cultivated at 37°C. As shown in Fig. 3, there was a clear dose-dependent response to dronedarone, leading to the total disappearance of infected cells at a drug concentration of 2 μM. The IC50 was 0.75 μM, which again was lower than that obtained previously for amiodarone, 2.7 μM (4).
Fig 2.

Susceptibility of Trypanosoma cruzi epimastigotes to dronedarone. Cultures of T. cruzi epimastigotes were exposed to different concentrations of dronedarone (Dron) and amiodarone (Amio), as indicated. Each point represents the mean ± standard deviation of at least triplicate experiments.
Fig 3.

Effects of dronedarone against intracellular amastigotes of T. cruzi. Vero cells infected with T. cruzi amastigotes were exposed to different concentrations of dronedarone. The percentages of infected Vero cells (filled squares) and the numbers of noninfected Vero cells per field (filled circles) were determined at 72 h after the addition of the drug. Experiments were carried out in triplicate (at least) for each experimental condition.
It has previously been shown that amiodarone is able to induce a large increase in the intracellular Ca2+ concentration in T. cruzi epimastigotes (4). Furthermore, it was found that such an effect results from the release of Ca2+ from intracellular compartments, since the phenomenon was observed in the presence or absence of extracellular calcium (4). As shown in Fig. 4, dronedarone also induced an increase in the intracellular Ca2+ concentration in these parasites, in the presence (Fig. 4A) or absence (Fig. 4B) of extracellular Ca2+. Interestingly, a biphasic effect on the intracellular Ca2+ is clearly discernible upon addition of dronedarone under both conditions, probably accounting for the induction of the release of Ca2+ from two different intracellular compartments, namely, the mitochondrion and the acidocalcisomes, as previously found for the action of amiodarone in Leishmania mexicana (22). Thus, amiodarone is now known to cause the death of different trypanosomatids primarily through disruption of intracellular Ca2+ homeostasis, although it also blocks the de novo synthesis of sterols at the level of oxidosqualene synthase (4). Specifically, in previous studies it was found that amiodarone acts directly on the parasites' large mitochondrion, inducing the rapid collapse of the electrochemical proton gradient and leading to the release of Ca2+ to the cytoplasm (4). The electrochemical potential is the driving force for the accumulation of Ca2+ in the mitochondrion (2, 3, 5, 26) through a calcium electrophoretic uniporter whose molecular structure has been recently identified (10) and has been found to be very conserved throughout evolution (10, 14). For this reason, we next studied the effect of dronedarone on the mitochondrial membrane potential using rhodamine 123, a mitochondrion-specific cationic dye that allows the visualization of the state of the electrochemical potential of this organelle, since this dye is distributed between the internal mitochondrion and the cytoplasm according to the magnitude of the electrochemical potential (4, 22). As can be seen in Fig. 5, dronedarone (10 μM) induced a rapid release of rhodamine 123. Addition of FCCP, an uncoupler protonophore (22), did not produce any further effect, thus indicating that dronedarone induced the total collapse of the mitochondrial electrochemical potential. Conversely, when FCCP is added before dronedarone (Fig. 5B), the uncoupler was not able to fully abolish the electrochemical potential, since the further addition of dronedarone induced further release of rhodamine 123. Another interesting result obtained from this experiment was that the rate of release of rhodamine 123 upon addition of amiodarone (10 μM) was somehow slower than after addition of dronedarone. This was also observed in the experiment in which FCCP was added before these drugs (Fig. 5A and B).
Fig 4.
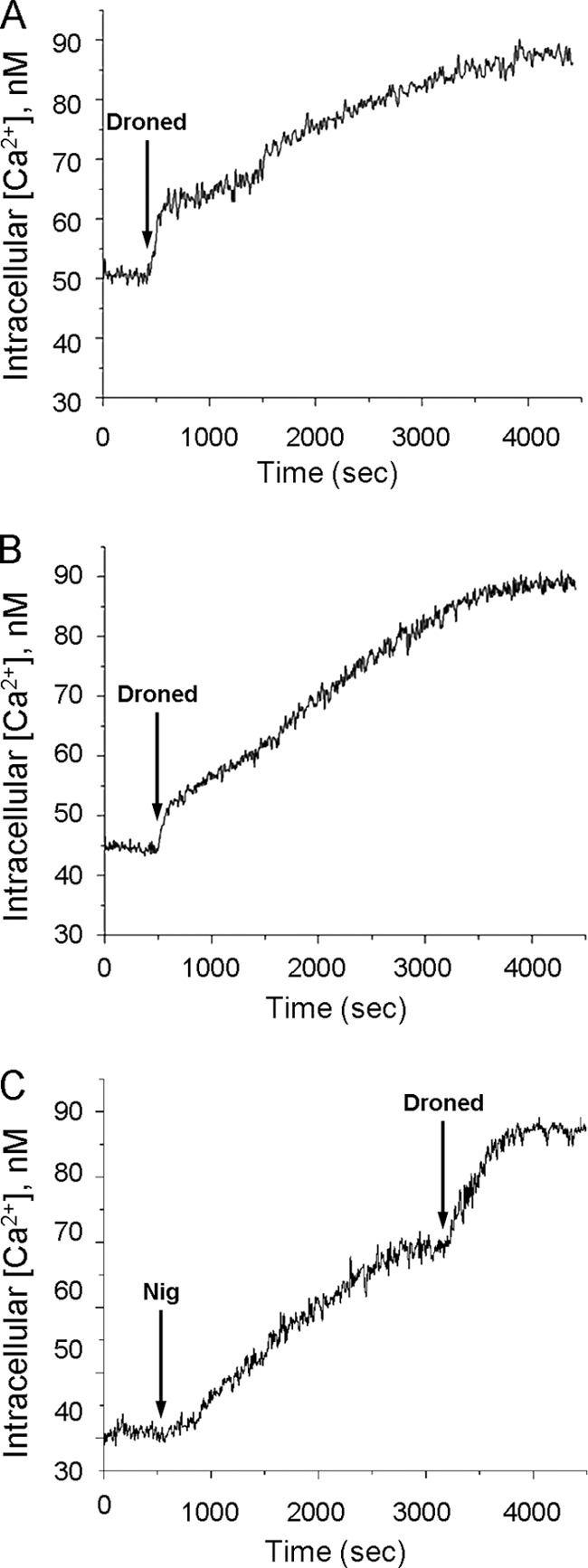
Effects of dronedarone on the intracellular Ca2+ concentration of T. cruzi epimastigotes. Epimastigotes of T. cruzi were loaded overnight with Fura 2-AM (6 μM) with probenecid (12 μM) and pluronic acid (12 μM) in PBS buffer plus 1% glucose at 29°C, in the dark and with continuous agitation, and intracellular Ca2+ was calculated as described in Materials and Methods. (A) Effect of 10 μM dronedarone (arrow) on the parasite cytoplasmic Ca2+ concentration in the presence of 2 mM Ca2+. (B) Effect of 10 μM dronedarone (arrow) on epimastigotes loaded with Fura 2 in the absence of external Ca2+ (EGTA). (C) Effects of 2 μM nigericin (first arrow) and 10 μM dronedarone (second arrow) on the parasite cytoplasmic Ca2+ concentration in the presence of 2 mM Ca2+. Droned, dronedarone; Nig, nigericin.
Fig 5.

Action of amiodarone on the mitochondrial electrochemical potential of T. cruzi epimastigotes. Parasites were incubated in the presence of rhodamine 123 (10 μg/ml) for 30 min at room temperature, as indicated in Materials and Methods. (A) Effects of dronedarone (10 μM) followed by the addition of FCCP (1 μM) on the mitochondrial electrochemical potential (upper black line). Effects of amiodarone (10 μM) followed by the addition of FCCP (1 μM) on the mitochondrial electrochemical potential (lower gray line). (B) Effects of FCCP (1 μM) followed by the addition of dronedarone (10 μM) on the mitochondrial electrochemical potential (upper black line). Effects of FCCP (1 μM) followed by the addition of amiodarone (10 μM) on the mitochondrial electrochemical potential (lower gray line). Arrows indicate the different additions. Amiod, amiodarone; Droned, dronedarone.
We also performed experiments on noninfected Vero cells to investigate whether dronedarone affects mammalian cells as well. While dronedarone was not able to exert any effect on the intracellular Ca2+ levels of Vero cells, even at the maximal concentration used in this work (2 μM), a small but reproducible effect on the electrochemical mitochondrial potential with 2 μM dronedarone, as measured by the release of rhodamine 123, was observed (results not shown).
Acidocalcisomes are acidic organelles, also involved in Ca2+ homeostasis in trypanosomatids, having a larger overall capacity of accumulation of this ion than does the mitochondrion (6, 9, 10). Amiodarone targets the acidocalcisomes in Leishmania mexicana (22), inducing their fast alkalinization. Thus, we tested if dronedarone and amiodarone could also affect the acidocalcisomes from T. cruzi. The release of acridine orange in the acidocalcisomes was used as a probe for alkalinization, as reported by Docampo et al. (11). As depicted in Fig. 6A (gray trace) the addition of 10 μM amiodarone generated a rapid alkalinization of the parasite acidocalcisomes. Additionally, upon adding nigericin, an electroneutral K+/H+ exchanger known to alkalinize the acidocalcisomes of these parasites (11), a further alkalinization response was observed. The same general overall response was observed when dronedarone (10 μM) was added to the preparation (Fig. 6, black trace). These results strongly suggested that acidocalcisomes also contribute to the increase in the intracellular Ca2+ concentration induced by both drugs in T. cruzi. As was the case for the action of dronedarone on the mitochondrial electrochemical potential, the alkalinization induced by dronedarone occurs more rapidly than that induced by amiodarone.
Fig 6.

Effects of dronedarone on acidocalcisomes from T. cruzi epiomastigotes. Parasites were loaded with acridine orange (2 μM) as described in Materials and Methods. The excitation wavelength was 488 nm, and emission was at 530 nm. (A) The upper black trace shows the effects of dronedarone (10 μM) followed by the addition of nigericin (2 μM) on the acidic level of acidocalcisomes. The lower gray trace shows the effects of amiodarone (10 μM) and then nigericin (2 μM) on the acidic level of acidocalcisomes. (B) The black trace shows the effects of nigericin (2 μM) followed by the addition of dronedarone (10 μM) on the acidic level of acidocalcisomes. The gray trace shows the effects of nigericin (2 μM) and then amiodarone (10 μM) on the acidic level of acidocalcisomes. Amiod, amiodarone; Droned, dronedarone; Nig, nigericin.
Additionally, we performed experiments in which nigericin was added before dronedarone or amiodarone (Fig. 6B). In both cases, the addition of the drugs after the ionophore induced a further release of acridine orange. Since nigericin completely disrupts acidocalcisome function, this further effect of the drugs probably indicates that another separate compartment is also involved in their action.
We also studied the effect of nigericin by itself on the intracellular Ca2+ concentration (Fig. 4C), showing that this ionophore induced a large Ca2+ increase. Addition of dronedarone after nigericin induced a further Ca2+ augmentation. These experiments support the notion that acidocalcisomes are the main intracellular Ca2+ compartment in Trypanosoma cruzi, but the mitochondrion also appears to contribute.
To study whether the effect of dronedarone on epimastigotes was trypanolytic or trypanostatic, we measured epimastigote viability through a much shorter time course (1 to 5 h), demonstrating that during this period there is not any discernible effect on cell viability. The trypanocidal action appeared only after 24 h, showing that 10 μM dronedarone was able to reduce the parasite population by about 25% after this period. Thus, dronedarone does not appear to induce a rapid trypanosome death (data not shown). These experiments demonstrate that rapid Ca2+ elevation by itself is not able to induce a rapid parasite death. However, this was not surprising, since it has been previously demonstrated that the rapid lethal effect of amphotericin B in Leishmania braziliensis was not due to a Ca2+ entry, since the addition of A-23187, a Ca2+ ionophore, albeit also inducing a fast intracellular Ca2+ increase, failed to induce the rapid parasiticidal effect observed with amphotericin B (8). In conclusion, the drug seems to be trypanostatic, at least on epimastigotes over short periods of time. On the other hand, dronedarone must be trypanolytic on amastigotes inside Vero cells, since the parasites faded away from the infected cells after treatment with the drug.
DISCUSSION
Amiodarone, a commonly used antiarrhythmic, has recently emerged as a potential drug candidate for the treatment of Chagas' disease, either as monotherapy or in combination with other drugs such as the azole-based antifungals posaconazole (4) and itraconazole (19). Nevertheless, and mainly due to the presence of a 2,5-diiodophenyl moiety in its structure, amiodarone exhibits a spectrum of undesirable side effects, causing mainly thyroid, gastrointestinal, and pulmonary toxicity, as well as less toxic side effects such as cutaneous pigmentation. Dronedarone, an amiodarone derivative developed to overcome these limitations, has a similar pharmacological profile, potent antiarrhythmic activity, and multichannel blocking properties, including blocking of Na+ channels, K+ currents, and L- and T-type Ca2+ channels. However, dronedarone has fewer unwanted side effects and a decreased lipophilicity, which improves its pharmacokinetic properties by lowering its elimination half-time. In this work, we studied the effects of dronedarone on T. cruzi in order to assess whether this modified drug retained the trypanocidal properties of amiodarone. Our results indicate that dronedarone has a dose-dependent effect on the growth of epimastigotes and on intracellular amastigotes, which are in the clinically relevant parasitic stage. Additionally, we observed that the IC50s of dronedarone against both forms were lower than that of amiodarone. This difference is higher for the amastigote form than for the epimastigote (0.75 μM versus 2.7 μM). It was also found that dronedarone acted more rapidly than amiodarone against its intracellular targets, as depicted in the experiments performed on the mitochondrial membrane potential and also on the alkalinization induced by these drugs on the acidocalcisomes.
Similarly to amiodarone, dronedarone induced an increase of intracellular Ca2+ concentration. The scarcity of intracellular esterases, which are essential for the breakdown of the acetoxymethyl esters present in the precursor form of Fura 2, in the epimastigote form of T. cruzi, has been a limitation in previous studies. We successfully addressed this problem by incubating the parasites overnight with the precursor. It is noteworthy that in these experiments it was found that incubation of epimastigotes with amiodarone led to a biphasic response of the intracellular Ca2+ levels, suggesting that this drug induces the release of the divalent cation from two different intracellular compartments, the mitochondrion and the acidocalcisomes.
We have previously demonstrated that amiodarone is able to induce alkalinization of acidocalcisomes of promastigotes from Leishmania mexicana (22). In the present study, we found that this effect is also observed in T. cruzi. The interior of these organelles is highly acidic due to the presence of a vacuolar H+-ATPase that pumps protons from the cytoplasm, inducing in turn the uptake of large amounts of Ca2+ by the combination of numerous ionic transporters, including a Ca2+-ATPase and several channels and exchangers (12, 13). These organelles are essential in trypanosomatids, since they contribute to the internal pH regulation and osmoregulation and also constitute the main reservoir of pyrophosphate (and polyphosphates), a known alternative source of energy, besides ATP, in these parasites (12, 13). We found that dronedarone targets the acidocalcisomes of T. cruzi, inducing a rapid alkalinization, which is enhanced by nigericin, an ionophore that exchanges K+ and H+, equilibrating the pH between the interior of the organelle and the cytoplasm (12).
The other intracellular compartment affected by dronedarone is the unique mitochondrion present in these parasites. As mentioned, the release of rhodamine 123 was faster when this drug was used in comparison with amiodarone. Another interesting effect observed during this work is that the uncoupler FCCP was not able to fully collapse the electrochemical gradient, but if amiodarone was added after FCCP, full uncoupling action was attained. However, since we used whole intact parasites (not treated with the sterol-dependent detergent digitonin), it is possible that the plasma membrane barrier could limit the access of FCCP to the interior of the cell, thus curtailing its action. Alternatively, these results are consistent with an effect of dronedarone on a separate compartment, where FCCP would not have any effect.
The pharmacokinetics of dronedarone is driven by its lipophilicity, with the drug achieving concentrations that range from 0.01 to 5 μg/ml in plasma and that range from 0.02 to 500 μg/ml in myocardial tissue (in goats and dogs) (7, 25). Nevertheless, concentrations in cardiac tissue have not been assessed in humans to date. In this work, it is shown that the effective range of dronedarone concentration in amastigote-infected Vero cells varies from 0.75 μM (IC50) to 2 μM (MIC). That is approximately 0.4 to 1.1 μg/ml, about 2 orders of magnitude lower than the maximal level attained in cardiac tissue under experimental conditions. This reflects in all likelihood that under the reported antiarrhythmic conventional therapeutic doses, the concentration of dronedarone reached in infected cells would be large enough to exert an adequate parasiticidal effect at the intracellular level.
Based on the present results, dronedarone appears to be a promising option for the symptomatic and specific treatment of chagasic cardiomyopathy and other trypanosomatid infections (such as leishmaniasis), due to its intrinsic antiparasitic activity and improved overall safety profile over that of amiodarone. To verify this hypothesis, work in animal models of Chagas' disease is currently in preparation.
ACKNOWLEDGMENTS
We thank Emilia Mia Sordillo for critically reading the manuscript and Barbara Romeo for logistical support.
This work was supported by grants from the Consejo Nacional de Investigaciones Científicas y Tecnológicas (FONACIT) grant no. 2011000884, Venezuela, and the Consejo de Desarrollo Científico y Humanístico from the Universidad Central de Venezuela (C.D.C.H.-U.C.V) grant PI 03-00-7380-2008/2) to G.B.
Footnotes
Published ahead of print 16 April 2012
REFERENCES
- 1. Adesse D, Meirelles Azzam E, Meirelles MN, Urbina JA, Garzoni LR. 2011. Amiodarone inhibits Trypanosoma cruzi infection and promotes cardiac cell recovery with gap junction and cytoskeleton reassembly in vitro Antimicrob. Agents Chemother. 55:203–210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Benaim G, Bermúdez R, Urbina J. 1990. Ca2+ transport in isolated mitochondrial vesicles from Leishmania braziliensis promastigotes. Mol. Biochem. Parasitol. 39:61–68 [DOI] [PubMed] [Google Scholar]
- 3. Benaim G. 1996. Intracellular calcium signaling and regulation in Leishmania, p 89–106 In Tapia F, Caceres-Dittmar G, Sanchez MA. (ed), Molecular and immune mechanism in the pathogenesis of cutaneous leishmaniasis. R. G. Landes Co., Medical Intelligence Unit, Austin, TX [Google Scholar]
- 4. Benaim G, et al. 2006. Amiodarone has intrinsic anti-Trypanosoma cruzi activity and acts synergistically with posaconazole. J. Med. Chem. 49:892–899 [DOI] [PubMed] [Google Scholar]
- 5. Benaim G, Garcia CRS. 2011. Targeting calcium homeostasis as the therapy of Chagas' disease and leishmaniasis. Trop. Biomed. 28:471–481 [PubMed] [Google Scholar]
- 6. Bobbala D, et al. 2010. Protective effect of amiodarone in malaria. Acta Trop. 116:39–44 [DOI] [PubMed] [Google Scholar]
- 7. Bolderman RW, Hermans JJ, Maessen JG. 2009. Determination of the class III antiarrhythmic drugs dronedarone and amiodarone, and their principal metabolites in plasma and myocardium by high-performance liquid chromatography and UV-detection. J. Chromatogr. B 877:1727–1731 [DOI] [PubMed] [Google Scholar]
- 8. Cohen BE, Benaim G, Ruiz MC, Michelangeli F. 1990. Increased calcium permeability is not responsible for the rapid lethal effects of amphotericin B on Leishmania sp. FEBS Lett. 259:286–288 [DOI] [PubMed] [Google Scholar]
- 9. Colina C, et al. 2005. Ceramide-1-P induces Ca2+ mobilization in Jurkat T cells by elevation of Ins(1,4,5)-P3 and activation of a store-operated calcium channel. Biochem. Biophys. Res. Commun. 360:54–60 [DOI] [PubMed] [Google Scholar]
- 10. De Stefani D, Rafaello A, Teardo E, Szabo I, Rizzuto R. 2011. A forty-kilodalton protein of the inner membrane is the mitochondrial calcium uniporter. Nature 476:336–340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Docampo R, Scott D, Vercesi A, Moreno SNJ. 1995. Intracellular Ca2+ storage in acidocalcisomes of Trypanosoma cruzi. Biochem. J. 310:1005–1012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Docampo R, Moreno SNJ. 2001. The acidocalcisome. Mol. Biochem. Parasitol. 114:151–159 [DOI] [PubMed] [Google Scholar]
- 13. Docampo R, Ulrich P, Moreno SNJ. 2010. Evolution of acidocalcisomes and their role in polyphosphate storage and osmoregulation in eukaryotic microbes. Philos. Trans. R. Soc. B 365:775–784 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Docampo R, Lukes J. 2012. Trypanosomes and the solution to a 50-year mitochondrial calcium mystery. Trends Parasitol. 28:31–37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Grynkiewicz G, Poenie M, Tsien R. 1985. A new generation of Ca2+ indicators with greatly improved fluorescence properties. J. Biol. Chem. 260:3440–3450 [PubMed] [Google Scholar]
- 16. Macedo-Silva ST, Oliveira Silva TL, Urbina JA, Souza W, Rodrigues JC. 2011. Antiproliferative, ultrastructural, and physiological effects of amiodarone on promastigote and amastigote forms of Leishmania amazonensis. Mol. Biol. Int. doi:10.4061/2011/876021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Paniz-Mondolfi AE, et al. 2008. Concurrent Chagas' disease and borderline disseminated cutaneous leishmaniasis: the role of amiodarone as an antitrypanosomatidae drug. Ther. Clin. Risk Manag. 4:659–663 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Paniz-Mondolfi AE, Pérez-Álvarez AM, Lanza Márquez GE, Concepción JL. 2009. Amiodarone and itraconazole: a rational therapeutic approach for the treatment of chronic Chagas' disease. Chemotherapy 55:228–233 [DOI] [PubMed] [Google Scholar]
- 19. Paniz Mondolfi AE, et al. 2011. Successful treatment of Old World cutaneous leishmaniasis caused by Leishmania infantum with posaconazole. Antimicrob. Agents Chemother. 55:1774–1776 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Pinazo MJ, et al. 2010. Successful treatment with posaconazole of a patient with chronic Chagas disease and systemic lupus erythematosus. Am. J. Trop. Med. Hyg. 82:583–587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Prystowsky EN. 2010. Atrial fibrillation: dronedarone and amiodarone—the safety versus efficacy debate. Nat. Rev. Cardiol. 7:5–6 [DOI] [PubMed] [Google Scholar]
- 22. Serrano-Martín X, et al. 2009. Amiodarone destabilizes the intracellular Ca2+ homeostasis and the biosynthesis of sterols in Leishmania mexicana. Antimicrob. Agents Chemother. 53:1403–1410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Serrano-Martín X, et al. 2009. Amiodarone and miltefosine synergistically induce parasitological cure of mice infected with Leishmania mexicana. Antimicrob. Agents Chemother. 53:5108–5113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Urbina J, et al. 1998. Antiproliferative effects and mechanism of action of SCH 56592 against Trypanosoma (Schizotrypanum) cruzi: in vitro and in vivo studies. Antimicrob. Agents Chemother. 42:1771–1777 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Varró A, et al. 2001. Electrophysiological effects of dronedarone (SR 33589), a noniodinated amiodarone derivative in the canine heart: comparison with amiodarone. Br. J. Pharmacol. 133:625–634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Vercesi AE, Bernardes CF, Hoffman ME, Gadelha FR, Docampo R. 1991. Digitonin permeabilization does not affect mitochondrial function and allows the determination of the mitochondrial membrane potential of Trypanosoma cruzi in situ. J. Biol. Chem. 266:14431–14434 [PubMed] [Google Scholar]


