Abstract
We analyzed the evolution of viral tropism after 8 days of maraviroc monotherapy, i.e., we used the maraviroc clinical test (MCT), in 21 patients with and 14 without virological response to the drug (MCT+ and MCT− patients, respectively). No increases in CXCR4 inferred viral loads (X4IVLs) were observed in MCT+ patients, while X4IVLs increased only in MCT− patients, with X4IVLs of >2 log10 HIV RNA copies/ml. These results shed light on the evolution of viral tropism under a CCR5 antagonist in vivo.
TEXT
Determining HIV-1 coreceptor usage is essential before prescribing the CCR5 (R5) antagonist maraviroc (MVC) to patients (6). As an alternative to phenotypic (8) and genotypic (7) tropism tests, our group has developed a new strategy before prescribing MVC, called the maraviroc clinical test (MCT) (3). This strategy is not a tropism test but an in vivo drug sensitivity assay based on an 8-day-long period of MVC monotherapy. This is a unique approach to analyze HIV-1 tropism evolution under the selective pressure of an R5 antagonist in vivo.
The blockage of the R5 coreceptor by MVC could lead to an increase of CXCR4 (X4)-tropic strains. This phenotype usually emerges late in the course of the disease and has been associated with a poor clinical prognosis (5). Studies analyzing the effect of short-term MVC monotherapy have been scarce and have demonstrated that the emergence of X4-tropic strains was rare and due to the expansion of a preexisting X4-tropic reservoir (10). However, patients included in that study were prescreened for R5-tropic virus only; the possible selection of X4-tropic strains in vivo in non-R5 patients is poorly understood. Furthermore, MCT is used to implement a subsequent combined antiretroviral therapy (cART), which includes MVC if the patient achieves a virological response (MCT+ patient) or omits MVC if the patient does not (MCT− patient). No immunodeleterious effects of a cART started after MCT have been reported (1, 2). This fact could be partially explained by the viral tropism evolution after MCT, which is currently unknown. The aim of the present work was to analyze the evolution of viral tropism during MVC monotherapy in a group of patients who harbored viruses with different coreceptor preferences, by using a quantitative phenotypic tool, tropism coreceptor assay information (TROCAI) (4).
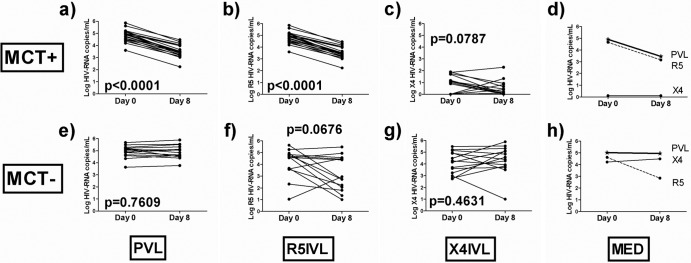
TROCAI was performed on 35 patients on day 0 and after 8 days of the MCT (see the supplemental material). Patients or their guardians (for those patients under 18 years of age) provided written informed consent, and the ethics committee of the hospital approved the study. Characteristics of the patients are shown in Table S1 in the supplemental material. This analysis was restricted to 8 days of MVC monotherapy because it is the minimum time needed to determine sensitivity to MVC in the MCT. Consequently, all the results presented here must be interpreted according to this model. Once we know the sensitivity of the patient to the drug, a new cART is started; to go on with MVC monotherapy would be clinically unacceptable. It is unknown whether results presented here would have changed over a longer period of time on MVC monotherapy. MCT+ patients (n = 21) experienced a median plasma viral load (PVL) change of −1.35 (range, −1.48 to −1.07) log10 HIV RNA copies/ml (Fig. 1a). There was a significant decrease in the R5 inferred viral load (R5IVL) after MVC exposure (Fig. 1b). In addition, a decreasing trend was also observed for the X4 inferred viral load (X4IVL) after MVC exposure (Fig. 1c). However, decreases in the PVL were mainly due to decreases in the R5IVL (Fig. 1d). These decreases in the PVL were accompanied by significant increases in the CD4+ T-cell counts (median increase, 88 [range, 7 to 173] cells/mm3) (P = 0.0010) (Table 1). Although the X4IVLs were negligible, we observed a significant decrease in 9/21 patients (P = 0.0350), whereas only four patients experienced a nonsignificant increase (P = 0.1250). In the remaining 8/17 patients, the X4IVL was not detectable either at the beginning or at the end of the MCT (Table 1). This absence of expansion of X4-tropic strains in MCT+ patients is in agreement with previous findings (10) and has several possible explanations. First, we cannot exclude the possibility that 8 days of MVC monotherapy is insufficient time to induce an increase in X4-tropic strains. Second, we can hypothesize that adverse immunological conditions, such as high CD4+ T-cell levels, may also prevent the expansion of X4-tropic viruses, and third, the small amount and/or low diversity of X4-tropic viruses could prevent an increase of these strains. In fact, in MCT− patients (n = 14) the expansion of the X4-tropic pool occurred only at baseline X4IVLs of >2 log10 HIV RNA copies/ml in all cases (Table 1). These data show for the first time in non-R5 patients that increases in X4-tropic strains after the R5 antagonist pressure are primarily due to the expansion of a preexisting X4-tropic pool. However, PVLs in MCT− patients remained unchanged (median, 0.03 [range, −0.21 to 0.23] log10 HIV RNA copies/ml) (Fig. 1e), and we found a decreasing trend in the R5IVL after MVC exposure (Fig. 1F). This pattern could be explained by the presence of dual mixed strains with an R5 coreceptor preference that were affected by the presence of MVC (9). However, no change was observed in the X4IVL after MVC exposure (Fig. 1g). The median distributions of PVL, R5IVL, and X4IVL are represented in Fig. 1h. Because the PVLs from the MCT− patients remained unchanged during the MCT (Fig. 1e), it was surprising to find a decreasing trend in the R5IVL (Fig. 1f) that was not accompanied by an increase in the X4IVL (Fig. 1g). In analyzing R5IVL and X4IVL changes in each MCT− patient (see Fig. S1 in the supplemental material), it was observed that in 7/14 patients, R5IVL decreases were accompanied by X4IVL increases. Then, overall, the R5IVL decrease was accompanied by an X4IVL expansion into the vacant niche (see Fig. S1).These X4-tropic strain expansions in some MCT− patients do not seem to have any clinical implications. A cART implemented after MCT has displayed no immunovirologically deleterious effect (1). This result is not surprising because in MCT− patients, a cART omitting MVC is implemented, which has an antiviral effect independent of viral tropism and, thus, will not exert a selective pressure on X4-tropic strains. In fact, in previous studies the X4-tropic virus reverted to R5 tropism once MVC was removed (10).
Fig 1.
Viral load evolution during the MCT. (a to d) MCT+ patients; (e to h) MCT− patients. (a and e) Plasma viral load (PVL) evolution; (b and f) R5-tropic inferred viral load (R5IVL) evolution; (c and g) X4-tropic inferred viral load (X4IVL) evolution; (d and h) median viral load evolution (MED). The dashed lines represent the R5IVL, the solid lines represent the X4IVL, and the thick lines represent the PVL, which coincides with the R5IVL in panel d.
Table 1.
Viral and CD4+ T-cell dynamics in patients (n = 35)a
| MCT result and patient no. | PVL0 | PVL8 | X4IVL0 | X4IVL8 | R5IVL0 | R5IVL8 | CD4_0 | CD4_8 | CD4_8-0 |
|---|---|---|---|---|---|---|---|---|---|
| Positive | |||||||||
| 1 | 5.21 | 4.17 | 1.91 | 0.95 | 5.21 | 4.47 | 106 | 206 | 100 |
| 2 | 5.18 | 3.65 | 1.18 | 0.00 | 5.18 | 3.65 | 100 | 267 | 167 |
| 3 | 4.77 | 3.41 | 1.07 | 0.11 | 4.77 | 3.41 | 39 | 85 | 46 |
| 4 | 4.49 | 3.42 | 1.09 | 0.20 | 4.49 | 3.42 | 458 | 574 | 116 |
| 5 | 4.98 | 3.44 | 0.98 | 0.00 | 4.98 | 3.44 | 264 | 265 | 1 |
| 6 | 5.09 | 3.97 | 1.09 | 0.68 | 5.09 | 4.20 | 383 | 506 | 123 |
| 7 | 4.19 | 3.11 | 0.00 | 0.00 | 4.19 | 3.11 | 221 | 659 | 438 |
| 8 | 4.45 | 3.02 | 0.00 | 0.00 | 4.45 | 3.02 | 361 | 541 | 180 |
| 9 | 5.62 | 4.47 | 0.00 | 0.00 | 5.62 | 4.47 | 125 | 207 | 82 |
| 10 | 5.12 | 3.32 | 0.00 | 0.00 | 5.12 | 3.32 | 298 | 262 | −36 |
| 11 | 3.60 | 2.23 | 0.00 | 0.00 | 3.60 | 2.23 | 302 | 503 | 201 |
| 12 | 4.35 | 3.24 | 0.00 | 0.00 | 4.35 | 3.24 | 388 | 575 | 187 |
| 13 | 4.88 | 3.48 | 0.00 | 0.00 | 4.88 | 3.48 | 117 | 189 | 72 |
| 14 | 4.58 | 3.18 | 0.00 | 0.39 | 4.58 | 3.18 | 466 | 467 | 1 |
| 15 | 5.86 | 4.31 | 1.86 | 2.30 | 5.86 | 4.31 | 433 | 714 | 281 |
| 16 | 5.07 | 4.05 | 0.00 | 0.82 | 5.07 | 4.05 | 275 | 174 | −101 |
| 17 | 4.71 | 3.45 | 0.00 | 1.33 | 4.71 | 3.45 | 369 | 532 | 163 |
| 18 | 4.59 | 3.54 | 0.89 | 0.00 | 4.59 | 3.54 | 375 | 344 | −31 |
| 19 | 4.95 | 3.23 | 0.00 | 0.00 | 4.95 | 3.23 | 402 | 490 | 88 |
| 20 | 4.91 | 3.54 | 1.69 | 0.00 | 4.91 | 3.54 | 131 | 144 | 13 |
| 21 | 4.98 | 3.97 | 1.76 | 0.92 | 4.98 | 3.97 | 460 | 512 | 52 |
| Median value | 4.91 | 3.45 | 0.00 | 0.00 | 4.91 | 3.45 | 302 | 467 | 88 |
| Negative | |||||||||
| 22 | 4.60 | 4.39 | 2.98 | 1.00 | 4.59 | 4.39 | 41 | 39 | −2 |
| 23 | 5.15 | 4.48 | 4.95 | 3.50 | 4.72 | 4.44 | 16 | 18 | 2 |
| 24 | 5.07 | 5.06 | 4.91 | 4.39 | 4.56 | 4.95 | 77 | 99 | 22 |
| 25 | 4.35 | 4.61 | 4.26 | 3.93 | 3.62 | 4.51 | 162 | 185 | 23 |
| 26 | 5.17 | 4.84 | 5.15 | 4.84 | 3.67 | 1.00 | 49 | 37 | −12 |
| 27 | 5.49 | 5.53 | 5.48 | 5.53 | 3.58 | 2.23 | 9 | 8 | −1 |
| 28 | 3.63 | 3.77 | 3.62 | 3.70 | 1.04 | 2.95 | 503 | 445 | −58 |
| 29 | 5.22 | 5.32 | 5.22 | 5.32 | 2.33 | 1.80 | 134 | 130 | −4 |
| 30 | 5.28 | 5.51 | 3.70 | 4.26 | 5.27 | 5.49 | 149 | 214 | 65 |
| 31 | 4.87 | 5.07 | 4.16 | 5.07 | 4.78 | 2.73 | 4 | 7 | 3 |
| 32 | 4.85 | 4.65 | 2.80 | 3.84 | 4.84 | 4.58 | 187 | 184 | −3 |
| 33 | 4.66 | 4.57 | 3.24 | 4.57 | 4.64 | 1.35 | 337 | 312 | −25 |
| 34 | 5.67 | 5.89 | 4.48 | 5.89 | 5.64 | 2.73 | 24 | 22 | −2 |
| 35 | 4.96 | 5.11 | 2.73 | 5.11 | 4.95 | 2.06 | 8 | 6 | −2 |
| Median value | 5.01 | 4.95 | 4.21 | 4.48 | 4.61 | 2.84 | 63 | 69 | −2 |
Abbreviations: MCT, maraviroc clinical test; PVL0, plasma viral load at day 0; PVL8, plasma viral load at day 8; X4IVL0, X4-tropic inferred viral load at day 0; X4IVL8, X4-tropic inferred viral load at day 8; R5IVL0, R5-tropic inferred viral load at day 0; R5IVL8, R5-tropic inferred viral load at day 8; CD4_0, CD4+ T-cell counts (cells/mm3) at day 0; CD4_8, CD4+ T-cell counts (cells/mm3) at day 8; CD4_8-0, CD4+ T-cell counts (cells/mm3) increase between days 0 and 8. Viral load values are expressed as log10 HIV RNA copies/ml.
In conclusion, using the MCT model we have shown that MVC did not induce an increase of X4-tropic strains in MCT+ patients. These increases occurred only in two-thirds of the MCT− patients with preexisting relatively high X4IVLs (>2 log10 HIV RNA copies/ml). These results explain, at least in part, the absence of immunodeleterious effects of a cART implemented after MCT (1, 2) and shed light on the evolution of viral tropism under the selective pressure of an R5 antagonist in vivo.
Supplementary Material
ACKNOWLEDGMENTS
This work was supported by Pfizer (project no. WS843473), Redes Telemáticas de Investigación Cooperativa en Salud (RETICS; 2006, RED RIS RD06/0006/0021, 2007-2010; RD06/0006/0035 and RD06/0006/1004), and Fondo de Investigaciones Sanitarias (INTRASALUD RD09/0076/00103, FIS 09/01595 and 10/2635). A.G.-S. has a grant from Fundación FISEVI. E.R.-M. and S.F.-M. have grants from Fondo de Investigaciones Sanitarias (CP08/00172 and CD10/00382, respectively).
We are grateful to all of the patients who participated in this study; José Manuel Lara Ruiz and Carmen Morales from the Immunology Service for their technical support; and Marien Sanchez, Francisca Cano, Magdalena Rodriguez, and Manuel Moyano for their clinical support. Patient samples were kindly provided by the HIV BioBank of the Spanish AIDS Research Network (RIS).
Regarding transparency declarations, M.L. has a grant from ViiV Healthcare.
Footnotes
Published ahead of print 30 April 2012
Supplemental material for this article may be found at http://aac.asm.org/.
REFERENCES
- 1. Genebat M, et al. 2011. Maraviroc clinical test (MCT) effectiveness for CCR5 prescription in clinical practice, abstr PE7.9/8. 13th Eur. AIDS Conf./EACS, Belgrade, Serbia [Google Scholar]
- 2. Genebat M, et al. 2010. Long-term immunovirological effect and tolerability of a maraviroc-containing regimen in routine clinical practice. Curr. HIV Res. 8:482–486 [DOI] [PubMed] [Google Scholar]
- 3. Genebat M, et al. 2009. Correlation between the Trofile test and virological response to a short-term maraviroc exposure in HIV-infected patients. J. Antimicrob. Chemother. 64:845–849 [DOI] [PubMed] [Google Scholar]
- 4. Gonzalez-Serna A, et al. 2010. TROCAI (tropism coreceptor assay information): a new phenotypic tropism test and its correlation with Trofile enhanced sensitivity and genotypic approaches. J. Clin. Microbiol. 48:4453–4458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Koot M, et al. 1993. Prognostic value of HIV-1 syncytium-inducing phenotype for rate of CD4R cell depletion and progression to AIDS. Ann. Intern. Med. 118:681–688 [DOI] [PubMed] [Google Scholar]
- 6. Lin NH, Kuritzkes DR. 2009. Tropism testing in the clinical management of HIV-1 infection. Curr. Opin. HIV AIDS 4:481–487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Raymond S, et al. 2008. Correlation between genotypic predictions based on V3 sequences and phenotypic determination of HIV-1 tropism. AIDS 22:F11–F16 [DOI] [PubMed] [Google Scholar]
- 8. Reeves JD, Coakley E, Petropoulos CJ, Whitcomb JM. 2009. An enhanced sensitivity Trofile HIV coreceptor tropism assay for selecting patients for therapy with entry inhibitors targeting CCR5: a review of analytical and clinical studies. J. Viral Entry 3:94–102 [Google Scholar]
- 9. Toma J, Whitcomb JM, Petropoulos CJ, Huang W. 2010. Dual-tropic HIV type 1 isolates vary dramatically in their utilization of CCR5 and CXCR4 coreceptors. AIDS 24:2181–2186 [DOI] [PubMed] [Google Scholar]
- 10. Westby M, et al. 2006. Emergence of CXCR4-using human immunodeficiency virus type 1 (HIV-1) variants in a minority of HIV-1-infected patients following treatment with the CCR5 antagonist maraviroc is from a pretreatment CXCR4-using virus reservoir. J. Virol. 80:4909–4920 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.