Abstract
The bacterial pathogens Mannheimia haemolytica and Pasteurella multocida are major etiological agents in respiratory tract infections of cattle. Although these infections can generally be successfully treated with veterinary macrolide antibiotics, a few recent isolates have shown resistance to these drugs. Macrolide resistance in members of the family Pasteurellaceae is conferred by combinations of at least three genes: erm(42), which encodes a monomethyltransferase and confers a type I MLSB (macrolide, lincosamide, and streptogramin B) phenotype; msr(E), which encodes a macrolide efflux pump; and mph(E), which encodes a macrolide-inactivating phosphotransferase. Here, we describe a multiplex PCR assay that detects the presence of erm(42), msr(E), and mph(E) and differentiates between these genes. In addition, the assay distinguishes P. multocida from M. haemolytica by amplifying distinctive fragments of the 23S rRNA (rrl) genes. One rrl fragment acts as a general indicator of gammaproteobacterial species and confirms whether the PCR assay has functioned as intended on strains that are negative for erm(42), msr(E), and mph(E). The multiplex system has been tested on more than 40 selected isolates of P. multocida and M. haemolytica and correlated with MICs for the veterinary macrolides tulathromycin and tilmicosin, and the newer compounds gamithromycin and tildipirosin. The multiplex PCR system gives a rapid and robustly accurate determination of macrolide resistance genotypes and bacterial genus, matching results from microbiological methods and whole-genome sequencing.
INTRODUCTION
Respiratory tract diseases of cattle are a major cause of animal morbidity in feedlots and result in economic losses presently estimated to be well over $3 billion per annum (18). The causes of bovine respiratory disease are multiple and complex but generally involve stress from transportation, fatigue, anxiety, or viral infections combined with one or more bacterial pathogens. The main bacterial agents are Pasteurella multocida, Mannheimia haemolytica, and Histophilus somni (15, 19), all of which are members of the family Pasteurellaceae. Treatments can involve the use of one of several groups of antibiotics, including macrolides, and are generally successful in curing the disease. During the last few years, however, strains of M. haemolytica and P. multocida that are resistant to several veterinary antibiotics have appeared, and most recent reports have included strains resistant to macrolides (9, 10, 14, 19). The resistance determinants have been well documented for most of the drugs (9), although the macrolide resistance mechanisms (3, 4, 8) and the means of dissemination of the resistance determinants (10) have only just been characterized.
We reported that field isolates of M. haemolytica and P. multocida display patterns of resistance to 14-, 15-, and 16-membered macrolides that fall into three distinct classes (4). Whole-genome sequencing of strains revealed that these phenotypes were caused by combinations of at least three different macrolide resistance mechanisms. In the first class of isolates, the erythromycin resistance methyltransferase gene erm(42) is the determinant and confers the MLSB (macrolide, lincosamide, and streptogramin B) type I phenotype with high resistance to lincosamides and low to moderate resistance to macrolide and streptogramin B antibiotics. The erm(42) gene encodes a monomethyltransferase that adds a single methyl group to 23S rRNA nucleotide A2058 (Escherichia coli rRNA numbering system) (3); this type of resistance is generally found only in drug-producing actinomycetes (1). Although erm(42) is related to other members of the erm family (12, 20), its sequence is phylogenetically distinct and is unique in the Pasteurellaceae (3). A second class of isolates possesses neither an erm gene nor methylation at nucleotide A2058 and is macrolide resistant without concomitant lincosamide resistance. The class 2 isolates contain two resistance genes, msr(E) and mph(E), which are arranged in tandem and expressed from the same promoter, and encode a macrolide efflux pump and a macrolide-inactivating phosphotransferase, respectively (4). A third class of isolates is highly resistant to a comprehensive set of macrolides and contains all three determinants, erm(42), msr(E), and mph(E) (4, 8).
Here we present a multiplex PCR assay to detect and track these resistance determinants rapidly and accurately. As described here, the system is specifically designed for use on Pasteurellaceae isolates, but it can easily be adapted to other bacterial species. Most field isolates of M. haemolytica and P. multocida are susceptible to macrolides, and therefore the assay has a built-in control reaction to verify whether the PCR has functioned as intended for the strains that are genuinely negative for erm(42), msr(E), and mph(E). This control reaction amplifies a distinctive region of the 23S rRNA gene rrl from members of the class Gammaproteobacteria and makes a further differentiation within the Pasteurellaceae family so that P. multocida is distinguished from M. haemolytica. The multiplex system was tested on more than 40 resistant field strains, and the PCR patterns were compared with macrolide MIC measurements, with sequence data for individual genes and complete genomes of members of the Pasteurellaceae, and with P. multocida and M. haemolytica species determination by classic microbiological methods. The multiplex PCR assay consistently provided a rapid and reliable picture of the macrolide resistance determinants while confirming the bacterial genus and at the same time eliminating false-negative results.
MATERIALS AND METHODS
Design of primers.
Oligodeoxynucleotide primers for multiplex PCR screening of the macrolide resistance genes are listed in Table 1. These primers were designed from the erm(42) sequence (GenBank accession number HQ888763) (3) and the msr(E) and mph(E) sequences (GenBank accession number JF769133) (4). The priming sites correspond to sequences that are unique in Pasteurellaceae genomes and, in the case of erm(42), that are distinct from erm resistance genes found in other bacterial groups. The primers were spaced within the targeted genes to give PCR fragments with lengths that are easily distinguishable: 173 bp for erm(42), 271 bp for mph(E), and 395 bp for msr(E).
Table 1.
Oligodeoxynucleotide primers in the multiplex PCR
| Primer | Sequence (5′–3′) | Direction | Functiona | PCR fragment size (bp)b |
|---|---|---|---|---|
| p64 | TGCACCATCTTACAAGGAGT | Forward | Screening for erm(42) | 173 |
| p66 | CATGCCTGTCTTCAAGGTTT | Reverse | Screening for erm(42) | |
| p67 | ATGCCCAGCATATAAATCGC | Forward | Screening for mph(E) | 271 |
| p68 | ATATGGACAAAGATAGCCCG | Reverse | Screening for mph(E) | |
| p70 | TATAGCGACTTTAGCGCCAA | Forward | Screening for msr(E) | 395 |
| p71 | GCCGTAGAATATGAGCTGAT | Reverse | Screening for msr(E) | |
| p84 | GACGGAAAGACCCCGTGAACCT | Forward | Screening for Gammaproteobacteria rrl sequence from G2053 to T2074 | |
| p85 | GGCAAGTTTCGTGCTTAGAT | Reverse | Screening for Gammaproteobacteria rrl sequence from A2753 to C2772 | 720c |
| p86 | GGAGCAGCCCCAATCAATCA | Reverse | Screening for P. multocida rrl sequence from T2633 to C2652 | 600c |
Nucleotide positions for the 23S rRNA (rrl) genes are defined by the standard E. coli rRNA numbering system.
The sizes of the fragments generated from DNA from macrolide-resistant P. multocida and M. haemolytica strains are given for each primer combination.
The rrl gene fragments are obtained in combination with the p84 primer.
Three additional oligonucleotides (p84, p85, and p86) prime at specific locations in the 23S rRNA (rrl) genes of P. multocida (NCBI accession number NC_002663.1), M. haemolytica, and other members of the Gammaproteobacteria. The sequence of Mannheimia succiniciproducens 23S rRNA (NCBI accession number NC_006300.1) was used where the published M. haemolytica sequence was incomplete, and the sequence was verified by reverse transcriptase sequencing on M. haemolytica 23S rRNA (16). Primers p84 and p85 are complementary to the 23S rRNA nucleotides G2053 to U2074 and A2753 to C2772, respectively, that are conserved in Gammaproteobacteria (Table 1) and give a PCR product of 720 bp. Sequence analyses indicate that no such fragment would be produced from bacteria that are phylogenetically more distant, such as Gram-positive bacteria or even the Gram-negative members of the Betaproteobacteria. The p86 primer is complementary to the sequence from T2633 to C2652 of a few members of the Pasteurellaceae, including P. multocida and Haemophilus influenzae, but is mismatched in the rrl sequence of M. haemolytica. Thus, the p84/p86 primer combination produces a PCR fragment of 600 bp from P. multocida DNA, but not from M. haemolytica.
Bacterial strains, growth, and DNA preparation.
The resistant strains of M. haemolytica and P. multocida (Table 2) are field isolates obtained from nasal swabs of cattle in the United States; the susceptible P. multocida strain 4407 was isolated in France. All strains were procured from the MSD Animal Health (Intervet) Culture Collection. Strains were plated onto agar containing brain heart infusion broth (Oxoid) and grown at 37°C overnight to form individual colonies for direct PCR testing. Purified DNA for control experiments was extracted from M. haemolytica and P. multocida strains as previously described (3, 4). The DNA pellet was dissolved, and a portion was examined on agarose gels to estimate the average fragmentation size pattern (≥50,000 bp), and the rest was stored in aliquots at −20°C.
Table 2.
P. multocida and M. haemolytica strains used in this studya
| Lane no.b | Strain | Bacterial species | MIC (μg/ml)c |
Presence or absence of the following macrolide resistance gene: |
|||||
|---|---|---|---|---|---|---|---|---|---|
| TIP | TUL | TIL | GAM | erm(42) | msr(E) | mph(E) | |||
| 1 | 11949 | P. multocida | >128 | >128 | >128 | 64 | + | + | + |
| 2 | 11952 | P. multocida | >128 | 8 | >128 | 8 | + | − | − |
| 3 | 11953 | P. multocida | >128 | 4 | 128 | 4 | + | − | − |
| 4 | 11955 | P. multocida | >128 | 4 | 128 | 4 | + | − | − |
| 5 | 11956 | P. multocida | >128 | 4 | 128 | 4 | + | − | − |
| 6 | 11957 | P. multocida | >128 | >128 | 128 | 128 | + | + | + |
| 7 | 12591 | P. multocida | 1 | 0.5 | 32 | 0.5 | − | − | − |
| 8 | 12593 | P. multocida | 2 | >128 | 32 | 64 | − | + | + |
| 9 | 12594 | P. multocida | 2 | >128 | 32 | 64 | − | + | + |
| 10 | 12595 | P. multocida | 4 | >128 | 32 | 64 | − | + | + |
| 11 | 12596 | P. multocida | 4 | >128 | 32 | 64 | − | + | + |
| 12 | 12599 | P. multocida | 1 | 1 | 32 | 0.5 | − | − | − |
| 13 | 12600 | P. multocida | 1 | 1 | 32 | 0.5 | − | − | − |
| 14 | 12601 | P. multocida | 1 | 0.5 | 32 | 0.5 | − | − | − |
| 15 | 12602 | P. multocida | 4 | >128 | 32 | 32 | − | + | + |
| 16 | 12604 | P. multocida | 1 | 0.5 | 32 | 0.5 | − | − | − |
| 17 | 12606 | P. multocida | >128 | >128 | >128 | 128 | + | + | + |
| 18 | 12608 | P. multocida | >128 | 8 | >128 | 16 | + | − | − |
| 19 | 3358 | P. multocida | 128 | >128 | >128 | 128 | + | + | + |
| 20 | 3361 | P. multocida | 2 | 64 | 32 | 32 | − | + | + |
| 21 | 4407 | P. multocida | 1 | 0.5 | 4 | 0.5 | − | − | − |
| 22 | 6052 | P. multocida | >128 | 8 | >128 | 8 | + | − | − |
| 23 | 11933 | M. haemolytica | 128 | 8 | 64 | 4 | + | − | − |
| 24 | 11934 | M. haemolytica | 128 | 64 | 128 | 64 | + | + | + |
| 25 | 11935 | M. haemolytica | 0.5 | 2 | 4 | 0.25 | − | − | − |
| 26 | 11937 | M. haemolytica | 0.5 | 2 | 8 | 0.5 | − | − | − |
| 27 | 11938 | M. haemolytica | 128 | 128 | 64 | 128 | + | + | + |
| 28 | 12540 | M. haemolytica | 1 | 2 | 32 | 0.5 | − | − | − |
| 29 | 12548 | M. haemolytica | 0.5 | 128 | 32 | 64 | − | + | + |
| 30 | 12553 | M. haemolytica | 1 | 128 | 32 | 128 | − | + | + |
| 31 | 12554 | M. haemolytica | 1 | 128 | 32 | 128 | − | + | + |
| 32 | 12557 | M. haemolytica | 1 | 128 | 32 | 128 | − | + | + |
| 33 | 12558 | M. haemolytica | 2 | >128 | 32 | 128 | − | + | + |
| 34 | 12565 | M. haemolytica | 2 | 2 | 32 | 1 | − | − | − |
| 35 | 12568 | M. haemolytica | 2 | 2 | 32 | 1 | − | − | − |
| 36 | 12580 | M. haemolytica | >128 | 16 | 128 | 8 | + | − | − |
| 37 | 12581 | M. haemolytica | >128 | 16 | 128 | 8 | + | − | − |
| 38 | 12582 | M. haemolytica | >128 | 16 | 128 | 8 | + | − | − |
| 39 | 12583 | M. haemolytica | >128 | 16 | 128 | 8 | + | − | − |
| 40 | 12584 | M. haemolytica | >128 | 128 | 128 | 128 | + | + | + |
| 41 | 12585 | M. haemolytica | >128 | 16 | 128 | 8 | + | − | − |
| 42 | 12587 | M. haemolytica | >128 | >128 | >128 | 128 | + | + | + |
Screening for the erm(42), msr(E), and mph(E) genes was carried out using multiplex PCR in all cases. Strains 3358 and 3361 have been characterized by whole-genome sequencing (3, 4). Class 1 strains with only the erm(42) gene are shown in italic type. Class 2 strains with the msr(E) and mph(E) genes are shown on a gray background. Class 3 strains with the erm(42), msr(E), and mph(E) genes are shown in boldface type on a gray background. Strains containing none of the three macrolide resistance genes are not highlighted by italic or boldface type or by a gray background.
The lane numbers are the same as for the gels in Fig. 1.
MICs for tildipirosin (TIP), tulathromycin (TUL), tilmicosin (TIL), and gamithromycin (GAM) are shown. The MICs were measured according to CSLI standards (2); E. coli ATCC 25922 (TIP MICs, 8 to 16 μg/ml; TUL MICs, 4 to 16 μg/ml; TIL MICs, 128 to >128 μg/ml; GAM MICs, 8 to 16 μg/ml) and Staphylococcus aureus ATCC 29213 (TIP MICs, 8 to 16 μg/ml; TUL MICs, 4 to 8 μg/ml; TIL MICs, 1 to 2 μg/ml; GAM MICs, 1 to 2 μg/ml) were included as quality control reference strains.
Additional bacterial pathogens linked with bovine and swine respiratory diseases were tested and included Actinobacillus pleuropneumoniae strain 11608, Bordetella bronchiseptica 11887, Haemophilus parasuis 11883, and Histophilus somni 12207, all of which were grown on chocolate agar. The specificity of the assay was tested by including Listeria monocytogenes FSL J1-175 and Streptococcus mutans UA140 grown on brain heart infusion agar, the latter under anaerobic conditions, and Bacillus subtilis 168 and E. coli strain DH1 grown on Luria-Bertani agar (13). The strains were grown under standard growth conditions at 37°C.
PCRs.
Each reaction mixture contained 200 μM each of dATP, dCTP, dGTP, and dTTP, 1.0 U Taq polymerase (VWR International), 10 pmol each of the six primers complementary to the erm(42), msr(E), and mph(E) genes, and 1 to 3 pmol of each of the three primers for the 23S rRNA genes (Table 1) in 20-μl total volume of 10 mM Tris-HCl (pH 8.3), 50 mM KCl, 1.5 mM MgCl2, and 0.1% Triton X-100. Purified DNA was initially used as the template, although the assay was shown to work equally well by transferring small amounts of bacterial colonies on toothpicks to PCR tubes. Gene amplification was carried out in a Mastercycler Personal apparatus (Eppendorf) with a denaturation step of 5 min at 94°C, followed by 25 cycles, with each cycle consisting of 30 s at 94°C, 30 s at 68°C, and 45 s at 72°C, with the final cycle concluding after 5 min at 72°C. PCR fragments were analyzed on 2% agarose gels, and the sizes were estimated from a Gene-Ruler 100-bp DNA ladder (Fermentas).
MIC determination.
The macrolide antibiotics used were tilmicosin (Sigma), tildipirosin (MSD Animal Health), and gamithromycin and tulathromycin, which were extracted and purified from Zactran (Merial) and Draxxin (Pfizer), respectively. Tildipirosin, gamithromycin, and tulathromycin were obtained as colorless powders, and their structures were verified by liquid chromatography/mass spectrometry and nuclear magnetic resonance.
MICs were determined for the M. haemolytica and P. multocida isolates according to CSLI standards (2) using microtiter plates with macrolides in 2-fold dilution steps between 0.5 μg/ml and 128 μg/ml.
RESULTS AND DISCUSSION
Primer design for the multiplex PCR system.
The priming sites within erm(42), msr(E), and mph(E) target regions that are unique to these genes and produce relatively short PCR fragments that are easily distinguishable by gel electrophoresis. The proteins encoded by these resistance genes possess relatively low amino acid identity to their closest orthologs. The closest ortholog of erm(42) is erm(Q) from Clostridium perfringens (GenBank accession number AAC36915) with 30% amino acid identity over the whole length of the protein encoded by the gene. Sequences identical to msr(E) and mph(E) have been observed in other bacterial groups (6, 7, 11, 24), although they are distinct from their nearest known relatives with 61% amino acid identity to msr(D) from Streptococcus pneumoniae (GenBank accession number ZP_02708480) and 37% identity to mph(A) from E. coli (GenBank accession number D16251). The primers (Table 1) showed high specificity for erm(42), mph(E), and msr(E) and generated PCR products of 173, 271, and 395 bp, respectively (Fig. 1A).
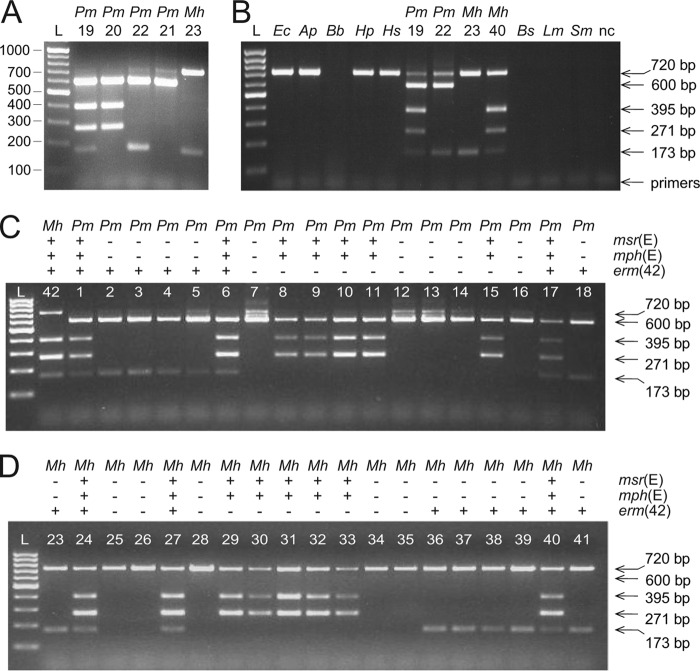
Fig 1.
Gene identification using the multiplex PCR system. (A) The multiplex PCR system was tested on DNA purified from four previously characterized P. multocida (Pm) strains (lanes 19 to 22) and a newly isolated M. haemolytica (Mh) strain (lane 23). PCR fragment sizes can be seen against the 100-bp/step GeneRuler DNA ladder (lane L). (B) Multiplex PCR applied directly to colonies of E. coli (Ec), Actinobacillus pleuropneumoniae (Ap), Bordetella bronchiseptica (Bb), Haemophilus parasuis (Hp), Histophilus somni (Hs), P. multocida, M. haemolytica, Bacillus subtilis (Bs), Listeria monocytogenes (Lm) and Streptococcus mutans (Sm). Templates that gave no products with the multiplex PCR were tested and shown to function in control reactions with species-specific primers (not shown). The 600-bp fragment, which here verifies the presence of P. multocida, is also generated from the DNA of another Pasteurellaceae member, Haemophilus influenzae (not shown). nc, negative control without DNA template. (C) Reactions performed directly on colonies of P. multocida (Pm) strains. None of the strains had been previously genetically characterized. A single M. haemolytica (Mh) strain is included to show species differentiation by formation of the 720-bp, but not the 600-bp, fragment. (D) Reactions performed directly on colonies of M. haemolytica (Mh) strains, none of which had previously been characterized genetically. Species classification and the presence (+) or absence (−) of the erm(42), msr(E), and mph(E) genes are tabulated above the gel lanes. Strain annotations and MIC profiles are given in Table 2.
The three additional primers included in the reaction mixtures (p84, p85, and p86) provided an internal control that showed whether the PCR had functioned and also discriminated between P. multocida and M. haemolytica. The p84/p85 primers are complementary to 23S rRNA genes in all members of the Gammaproteobacteria and are shown here to produce a fragment of 720 bp on DNA from P. multocida, M. haemolytica, E. coli, A. pleuropneumoniae, H. parasuis, and H. somni. No such PCR band is produced from betaproteobacterium DNA, exemplified here by B. bronchiseptica, or from Gram-positive DNA, as shown here for Bacillus, Listeria, and Streptococcus species (Fig. 1B). In the case of P. multocida, an additional fragment of 600 bp is generated by nested priming of p86 within the 720-bp fragment. This leads to a higher fold enrichment of the 600-bp fragment in assays with P. multocida DNA, where the 720-bp fragment consequently appears weak. The p86 primer does not amplify the rrl genes from M. haemolytica or from the other Gammaproteobacteria tested here, and in these cases, only the 720-bp fragment is produced (Fig. 1B).
The multiplex PCR system was initially tested on purified DNA from strains that had previously been genetically characterized by genome sequencing (3, 4). Gel analysis showed that the PCR fragments of 600 bp and 720 bp clearly distinguish P. multocida and M. haemolytica (Fig. 1A). Furthermore, the PCR patterns clearly discriminated between the susceptible P. multocida 4407 strain (sample 21), the class 1 strains with erm(42) (P. multocida 6052 [sample 22] and M. haemolytica 11933 [sample 23]), class 2 with msr(E) and mph(E) (P. multocida 3361 [sample 20]), and class 3 with all three resistance determinants (P. multocida 3358 [sample 19]). No false-positive or nonspecific PCR product was observed.
Direct PCR screening of bacterial colonies.
The multiplex system was used to screen larger sets of P. multocida (18 field isolates) and M. haemolytica (20 isolates) that had been identified as having an elevated MIC to at least one veterinary macrolide. Reactions were performed directly on bacterial colonies without purifying DNA. Five of the P. multocida strains were shown to contain none of the three macrolide resistance genes (Fig. 1C), while combinations of erm(42) and/or msr(E)-mph(E) were evident in the other 13 P. multocida strains. Analysis of the M. haemolytica isolates revealed five strains without any of the three macrolide resistance genes (Fig. 1D), while all the other 15 strains harbored combinations of resistance determinants.
Strains with msr(E) or mph(E) always possessed both genes as a tandem pair, as previously observed in P. multocida 3361 (4) and in an independently isolated P. multocida strain (8). The 600-bp and 720-bp fragments discriminated between P. multocida and M. haemolytica isolates (Fig. 1) and were fully consistent with the microbiological characterization of these strains (Table 2).
Phenotypes of M. haemolytica and P. multocida isolates.
Our recent proposal that macrolide-resistant P. multocida and M. haemolytica field isolates can be categorized into three groups was based on a smaller number of field isolates (4). In the first class, five additional P. multocida isolates and six M. haemolytica isolates have the type I MLSB resistance phenotype conferred by erm(42) as the sole macrolide resistance determinant (Table 2). Isolates in this first class show greatly elevated MICs for the 16-membered macrolides tildipirosin and tilmicosin, while smaller MIC increases were seen for the 15-membered drugs tulathromycin and gamithromycin.
Members of the second class lack erm(42) but contain msr(E) and mph(E), as seen here for an additional five P. multocida isolates and five M. haemolytica isolates (Table 2). Single-fragment PCR amplifications using the upstream msr(E) and downstream mph(E) primers (p67 and p71 [Table 1]) showed that the genes were present in the same tandem arrangement and are presumably expressed from a common promoter as seen in strain 3361 (4). These two genes were associated with large increases in MICs for tilmicosin, tulathromycin, and gamithromycin but not for tildipirosin. This pattern is consistent with these types of efflux and phosphotransferase (4, 12, 21) conferring resistance to the 14-membered macrolide erythromycin and derivatives (such as tulathromycin and gamithromycin) and generally being ineffective against 16-membered macrolides (tildipirosin). However, this does not explain the resistance seen to tilmicosin.
The third class of isolates exhibit high resistance to all of the macrolides tested. From the more recently isolated strains, three P. multocida isolates and four M. haemolytica isolates fall into this third class and contain all three erm(42), msr(E), and mph(E) macrolide resistance determinants. The erm(42), msr(E), and mph(E) genes have been shown to be carried on the bacterial chromosome and intermingled with other exogenous genes, many of which appear to have been transferred from other members of the Pasteurellaceae (4).
We note that five P. multocida isolates and five M. haemolytica isolates contained none of the three resistance determinants and were susceptible to tildipirosin, gamithromycin, and tulathromycin with MICs of 0.5 to 2 μg/ml. These strains were included in the study because of their elevated tilmicosin MICs, which in several cases were as high as 32 μg/ml, and the cause of this tilmicosin-specific effect remains unclear. The mechanism behind the increased tilmicosin tolerance in these strains is presumably the same that is responsible for the elevated MICs to tilmicosin in the class 2 isolates discussed above.
Conclusions on the status of Pasteurellaceae isolates.
The recent identification of M. haemolytica and P. multocida field isolates with high MICs for macrolides prompted us to screen the resistance mechanisms in these strains. We note that at present the majority of M. haemolytica and P. multocida strains isolated from cattle with respiratory tract infections have remained susceptible to macrolide antibiotics. These strains are represented here by M. haemolytica 11935 and P. multocida 4407 that are susceptible to the macrolide drugs currently used in veterinary medicine (MICs from 0.5 to 4 μg/ml) and lack all the resistance determinants for which we tested. In contrast, the MIC values for the resistant isolates were higher by 8- to ≥254-fold and on the basis of their resistance patterns segregate into three distinct classes, as shown for P. multocida and M. haemolytica in Table 2.
Multiplex PCR assays have previously been shown to be valuable in the screening of, for example, other macrolide resistance genes in streptococci (5, 17) and beta-lactamases in Gram-negative bacteria (22, 23). In addition to detection of the erm(42), msr(E), and mph(E) resistance determinants, the multiplex PCR described here gives a rapid discrimination between P. multocida and M. haemolytica isolates, simultaneously ruling out false-negative results caused by PCR malfunction. We note that the multiplex PCR can be used for macrolide resistance screening and genus determination in other bacterial groups after the sequences of the rrl primers are adjusted.
ACKNOWLEDGMENTS
S.D. gratefully acknowledges support from Intervet Innovation GmbH, the Danish Research Agency (FNU-rammebevillinger 09-064292/10-084554), and the Nucleic Acid Center of the Danish Grundforskningsfond. Michael Linder, Heinz-Jörg Wennesheimer, Karl-Heinz Grimm, and colleagues at Intervet Innovation MSD Animal Health are thanked for strain characterization and macrolide drug purification.
Footnotes
Published ahead of print 7 May 2012
REFERENCES
- 1. Calcutt MJ, Cundliffe E. 1990. Cloning of a lincosamide resistance determinant from Streptomyces caelestis, the producer of celesticetin, and characterization of the resistance mechanism. J. Bacteriol. 172:4710–4714 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Clinical and Laboratory Standards Institute 2008. Performance standards for antimicrobial disk and dilution susceptibility tests for bacteria isolated from animals - third edition: approved standard M31-A3. Clinical and Laboratory Standards Institute, Wayne, PA [Google Scholar]
- 3. Desmolaize B, Rose S, Warrass R, Douthwaite S. 2011. A novel Erm monomethyltransferase in antibiotic resistance isolates of Mannheimia haemolytica and Pasteurella multocida. Mol. Microbiol. 80:184–194 [DOI] [PubMed] [Google Scholar]
- 4. Desmolaize B, Rose S, Wilhelm C, Warrass R, Douthwaite S. 2011. Combinations of macrolide resistance determinants in field isolates of Mannheimia haemolytica and Pasteurella multocida. Antimicrob. Agents Chemother. 55:4128–4133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Farrell DJ, Morrissey I, Bakker S, Felmingham D. 2001. Detection of macrolide resistance mechanisms in Streptococcus pneumoniae and Streptococcus pyogenes using a multiplex rapid cycle PCR with microwell-format probe hybridization. J. Antimicrob. Chemother. 48:541–544 [DOI] [PubMed] [Google Scholar]
- 6. Golebiewski M, et al. 2007. Complete nucleotide sequence of the pCTX-M3 plasmid and its involvement in spread of the extended-spectrum beta-lactamase gene blaCTX-M-3. Antimicrob. Agents Chemother. 51:3789–3795 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Gonzalez-Zorn B, et al. 2005. Genetic basis for dissemination of armA. J. Antimicrob. Chemother. 56:583–585 [DOI] [PubMed] [Google Scholar]
- 8. Kadlec K, et al. 2011. Molecular basis of macrolide, triamilide, and lincosamide resistance in Pasteurella multocida from bovine respiratory disease. Antimicrob. Agents Chemother. 55:2475–2477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Katsuda K, Kohmoto M, Mikami O, Uchida I. 2009. Antimicrobial resistance and genetic characterization of fluoroquinolone-resistant Mannheimia haemolytica isolates from cattle with bovine pneumonia. Vet. Microbiol. 139:74–79 [DOI] [PubMed] [Google Scholar]
- 10. Michael GB, et al. 2012. ICEPmu1, an integrative conjugative element (ICE) of Pasteurella multocida: analysis of the regions that comprise 12 antimicrobial resistance genes. J. Antimicrob. Chemother. 67:84–90 [DOI] [PubMed] [Google Scholar]
- 11. Poirel L, Mansour W, Bouallegue O, Nordmann P. 2008. Carbapenem-resistant Acinetobacter baumannii isolates from Tunisia producing the OXA-58-like carbapenem-hydrolyzing oxacillinase OXA-97. Antimicrob. Agents Chemother. 52:1613–1617 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Roberts MC. 2008. Update on macrolide-lincosamide-streptogramin, ketolide, and oxazolidinone resistance genes. FEMS Microbiol. Lett. 282:147–159 [DOI] [PubMed] [Google Scholar]
- 13. Sambrook J, Russell DW. 2001. Molecular cloning: a laboratory manual, 3rd ed Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY [Google Scholar]
- 14. Schwarz S, Kehrenberg C, Salmon SA, Watts JL. 2004. In vitro activities of spectinomycin and comparator agents against Pasteurella multocida and Mannheimia haemolytica from respiratory tract infections of cattle. J. Antimicrob. Chemother. 53:379–382 [DOI] [PubMed] [Google Scholar]
- 15. Snowder GD, et al. 2007. Bovine respiratory disease in feedlot cattle: phenotypic, environmental, and genetic correlations with growth, carcass, and longissimus muscle palatability traits. J. Anim. Sci. 85:1885–1892 [DOI] [PubMed] [Google Scholar]
- 16. Stern S, Moazed D, Noller HF. 1988. Structural analysis of RNA using chemical and enzymatic probing monitored by primer extension. Methods Enzymol. 164:481–489 [DOI] [PubMed] [Google Scholar]
- 17. Sutcliffe J, Grebe T, Tait-Kamradt A, Wondrack L. 1996. Detection of erythromycin-resistant determinants by PCR. Antimicrob. Agents Chemother. 40:2562–2566 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. US Department of Agriculture 2000. Part I: Baseline reference of feedlot management practices, 1999. N327.0500. National Animal Health Monitoring System, Centers for Epidemiology and Animal Health, Veterinary Services, Animal and Plant Health Inspection Service, US Department of Agriculture, Fort Collins, CO [Google Scholar]
- 19. Watts JL, Sweeney MT. 2010. Antimicrobial resistance in bovine respiratory disease pathogens: measures, trends, and impact on efficacy. Vet. Clin. North Am. Food Anim. Pract. 26:79–88 [DOI] [PubMed] [Google Scholar]
- 20. Weisblum B. 1995. Erythromycin resistance by ribosome modification. Antimicrob. Agents Chemother. 39:577–585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Weisblum B. 1998. Macrolide resistance. Drug Resistance Updates 1:29–41 [DOI] [PubMed] [Google Scholar]
- 22. Woodford N. 2010. Rapid characterization of beta-lactamases by multiplex PCR. Methods Mol. Biol. 642:181–192 [DOI] [PubMed] [Google Scholar]
- 23. Woodford N, et al. 2006. Multiplex PCR for genes encoding prevalent OXA carbapenemases in Acinetobacter spp. Int. J. Antimicrob. Agents 27:351–353 [DOI] [PubMed] [Google Scholar]
- 24. Zarrilli R, et al. 2008. A plasmid-borne blaOXA-58 gene confers imipenem resistance to Acinetobacter baumannii isolates from a Lebanese hospital. Antimicrob. Agents Chemother. 52:4115–4120 [DOI] [PMC free article] [PubMed] [Google Scholar]