Abstract
Monoclonal antibodies are successful biologics in treating a variety of diseases, including the prevention or treatment of viral infections. CL184 is a 1:1 combination of two human monoclonal IgG1 antibodies (CR57 and CR4098) against rabies virus, produced in the PER.C6 human cell line. The two antibodies are developed as replacements of human rabies immune globulin (HRIG) and equine rabies immune globulin (ERIG) in postexposure prophylaxis (PEP). The rapid fluorescent focus inhibition test (RFFIT) is a cell-based virus neutralization assay which is usually performed to determine the biological potency of a vaccine and to measure the levels of protection against rabies in humans and animals. In order to confirm the suitability of this assay as a pharmacodynamic assay, we conducted a validation using both HRIG- and CL184-spiked serum samples and sera from vaccinated donors. The validation results met all analytical acceptance criteria and showed that HRIG and CL184 serum concentrations can be compared. Stability experiments showed that serum samples were stable in various suboptimal conditions but that rabies virus should be handled swiftly once thawed. We concluded that the assay is suitable for the measurement of polyclonal and monoclonal rabies neutralizing antibodies in clinical serum samples.
INTRODUCTION
Rabies occurs worldwide, and more than 3 billion people live in areas in which the disease is enzootic. Every year about 55,000 people die from rabies, with more than 50% in Asia (3, 16). Postexposure prophylaxis (PEP) against rabies exposure consists of thorough washing of the wound, passive immunization with rabies immune globulin (RIG) administered in and around the wound, and active immunization with vaccine (12). The administration of RIG soon after exposure is essential to inhibit viral spread in the interval before sufficient immunity is developed in response to vaccination. Currently, human rabies immune globulin (HRIG) and equine rabies immune globulin (ERIG) are used in PEP. These plasma-derived, polyclonal products are obtained from rabies-vaccinated human donors or horses and can be produced only in limited amounts. Furthermore, the variable quality, low activity, and potential danger of contamination with adventitious pathogens warrant replacement with a more optimized product (18). Therefore, the World Health Organization (WHO) strongly encourages the development of alternative products to meet the global demand (17). We have developed an antibody cocktail, CL184, comprising of two monoclonal antibodies that target distinct nonoverlapping epitopes of the rabies virus glycoprotein (1, 5, 10). The CL184 antibody cocktail is currently being tested in clinical trials as a replacement for HRIG in PEP (2).
An important requirement of the CL184 antibody combination is that it confers similar rabies neutralizing activity as the comparator HRIG. The rapid fluorescent focus inhibition test (RFFIT) was selected as the pharmacodynamic marker assay. This assay is regarded as the standard rabies virus neutralization assay in diagnostic laboratories, vaccine and biotherapeutic characterization, and rabies-related clinical studies (9). To demonstrate that this assay is equally well suited for measurement of both polyclonal HRIG and the monoclonal CL184 combination in clinical serum samples, we conducted an assay validation as described below. The validation plan was based on the regular requirements as stated in the FDA Guidance for Industry (4) and ICH Q2(R1) guidelines (7), taking into account the limitations and variability of cell-based virus neutralization assays. This validation of the assay confirms the suitability and validity of this methodology for the intended purpose.
MATERIALS AND METHODS
RFFIT protocol.
The RFFIT procedure (13) is utilized to measure the level of rabies virus neutralizing antibody activity (RVNA) against the challenge virus standard 11 (CVS-11) strain of rabies virus in human serum samples. Five-fold serial dilutions of heat-inactivated serum samples were incubated with the CVS-11 strain in 8-well tissue culture chamber slides for 90 min at 37°C. Baby hamster kidney (BHK)-21 cells were then added to the serum-virus mixture and incubated for an additional 20 to 24 h at 37°C with 2 to 5% CO2. Slides were then acetone fixed and stained with an anti-rabies N-FITC conjugate.
Twenty distinct microscopic fields per well were examined using a fluorescence microscope at ×160 magnification to score the virus-infected cells (foci). The number of positive fields with rabies-infected cells per well was recorded. The neutralization endpoint titer was defined as the highest sample dilution at which 50% of the observed microscopic fields contain one or more infected cells. The RVNA titers are mathematically interpolated using the Reed and Muench method or a Reed and Muench chart for assigning a RFFIT titer (6).
The endpoint neutralization titer of the test serum is then transformed into international units (IU)/ml values by calibration against the endpoint neutralization titer of the U.S. Standard Rabies Immune Globulin (SRIG) (lot R-3, 59 IU; first WHO International Standard), which was measured in the same assay run, with an assigned potency value of 2.0 IU/ml.
RFFIT validation.
The validation plan was based on the FDA Guidance for Industry (4) and ICH Q2(R1) guidelines (7), taking into account the limitations and variability of cell-based virus neutralization assays. The validation parameters and acceptance criteria are listed in Table 1. In particular, the following validation parameters were considered.
Table 1.
Validation parameters and acceptance criteria
| Validation parameter | Acceptance criterion | Remark |
|---|---|---|
| Specificity | Nonblocked/blocked ratio ≥ 4 | |
| Matrix effect | Effect of matrix ≤ 30% | Criteria adjusted to cell-based assay performance |
| Linearity | 0.7 < 90% CI of slope < 1.3 | |
| Repeatability | CV ≤ 30% | Criteria adjusted to cell-based assay performance |
| Intermediate precision | CV ≤ 30% | Criteria adjusted to cell-based assay performance |
| LOQ | For information purposes only | |
| LOD | For information purposes only | |
| Accuracy | Difference between HRIG and CL184 ≤ 30% | Accuracy is measured as concordance between HRIG and CL184 |
| Stability | Difference between stability sample and comparator sample ≤ 30% | Criteria adjusted to cell-based assay performance |
(i) Precision.
FDA and ICH guidelines recommend a percent coefficient of variation (CV) of 15% to 20% as acceptance criteria for precision and accuracy in analytical method validation, whereas in the literature, a CV of 20% to 25% is recommended for ligand binding assays such as the enzyme-linked immunosorbent assay (ELISA) (14). However, cell-based assays are expected to have a much higher CV, as acknowledged by the WHO (18). The RFFIT is a bioassay, using biological materials such as BHK-21 cells and rabies virus, which induces more variation; thus, typically, a greater CV limit criterion is accepted for viral neutralization assays. For this RFFIT validation, a CV of <30% was implemented.
Since the data obtained in the RFFIT usually displays a log-normal distribution, RVNA activity data (IU/ml) were first log10 transformed to achieve a normal distribution. Subsequently, the standard deviation was calculated from the square root of the mean squared error generated by analysis of variance (ANOVA). In order to express precision in the percent CV, the formula was used to translate the standard deviation on a log scale into a percent CV that can be interpreted and compared with the acceptance criteria.
(ii) Accuracy.
Accuracy is generally measured by using an international standard reference sample. For the RFFIT, SRIG is used as an international standard, which was included in all assay runs. Specifically for the purpose of our rabies virus-antibody combination, we investigated the concordance between results obtained with HRIG and CL184 samples. As was done for precision, we implemented a bias of 30% as acceptance criterion for accuracy.
(iii) Robustness.
Kansas State Veterinary Diagnostic Laboratory (KSVDL) participates in an annual proficiency evaluation program for serum neutralization assays of rabies antibodies from ANSES (formerly AFSSA), the French agency for food, environmental, and occupational health. ANSES is an OIE Reference Laboratory, WHO Collaborating Centre, and an EU National and Community Reference Laboratory. Currently, this program includes more than 50 international laboratories. Because the assay has been in use continuously for over 20 years at KSVDL and its consistency is routinely confirmed by the lab's participation in the proficiency program, robustness was not included as a parameter in this validation.
Validation samples.
CL184 comprises two antibodies, CR4098 and CR57, in a 1:1 protein ratio. CR57 and CR4098 are fully human monoclonal IgG1 antibodies directed against different rabies virus glycoprotein epitopes (antigenic site I and III, respectively) and are capable of neutralizing rabies virus (1, 10). HRIG is a human polyclonal immune globulin product containing a specific level of rabies neutralizing activity (Imogam; Sanofi Pasteur). The products' potencies were determined by RFFIT using lot R-3 (DMPQ/CBER/FDA) as a reference standard for CL184 and the second International Standard (NIBSC/WHO) for Imogam.
All experiments were performed using normal human serum spiked with either HRIG (>150 IU/ml, lot no. D0578-9 from Sanofi Pasteur) or CL184 (500 IU/ml, lot no. 07L12403-01A from Crucell). Pooled normal human serum was spiked with different concentrations of HRIG or CL184 to obtain a range of internal control (IC) samples (Table 2). Serial dilutions of HRIG and CL184 in serum were calculated based on the clinical dosages as specified on the labels, i.e., 500 IU/ml (CL184) and 150 IU/ml (HRIG). At the start of the study, a pool of each IC sample was prepared, aliquoted, and frozen at −80°C until it was used. To assess the serum background, a nonspiked serum sample was included with the IC samples (IC0).
Table 2.
Validation test samplesa
| Sample type | Sample spiked with RVNA (IU/ml) of: |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 0 | 0.025 | 0.05 | 0.1 | 0.2 | 0.5 | 1 | 2.5 | 5 | 7.5 | 10 | |
| HRIG spiked | IC0 | HIC1 | HIC2 | HIC3 | HIC4 | HIC5 | HIC6 | HIC7 | HIC8 | HIC9 | HIC10 |
| CL184 spiked | IC0 | CIC1 | CIC2 | CIC3 | CIC4 | CIC5 | CIC6 | CIC7 | CIC8 | CIC9 | CIC10 |
| SRIG spiked | IC0 | N/A | N/A | N/A | N/A | N/A | SIC1 | N/A | N/A | N/A | SIC2 |
| RVNA positive serum (from vaccinated donors) | N/A | N/A | N/A | 09R3 | N/A | N/A | N/A | 09R2 | N/A | 09R1 | |
Normal human serum spiked with HRIG, CL184, or SRIG was used as IC samples. H, HRIG; C, CL184; S, SRIG; N/A, not available.
SRIG (lot R-3, 59 IU; first WHO International Standard) was spiked at two concentration levels (1 IU/ml and 10 IU/ml) to assess the effect of normal human serum matrix on the accuracy of the assay.
All tested sera were stored at −80°C (nominal), similar to the storage temperature of the clinical sample, unless stated otherwise.
The RFFIT was used to evaluate RVNA activity in sera of subjects receiving both rabies neutralizing antibodies, HRIG and CL184. Subjects also received rabies vaccine as part of PEP. Therefore, serum of rabies-vaccinated subjects was included in the study. Serum with RVNA activity levels around 0.2 IU/ml, 5 IU/ml, and 10 IU/ml was included in the precision experiments (see Table 2).
RESULTS
Validation experiments.
Six experiments that assessed specificity, matrix effect, linearity, repeatability, intermediate precision, limit of quantitation (LOQ), limit of detection (LOD), accuracy, and stability were performed on different days by 2 operators. The experiment outline is shown in Table 3.
Table 3.
RFFIT validation experiment outline and plan
| Expt | Day | Operator | CP#a | Data collection parameter |
|---|---|---|---|---|
| 1 | 1 | 1 | Early | Repeatability, intermediate precision, accuracy, linearity, LOQ/LOD, bench top stability |
| 2 | 2 | 2 | Late | Repeatability, intermediate precision, accuracy, linearity, LOQ/LOD, stability at −20°C |
| 3 | 3 | 1 | Late | Repeatability, intermediate precision, accuracy, linearity, LOQ/LOD |
| 4 | 4 | 2 | Early | Repeatability, intermediate precision, accuracy, linearity, LOQ/LOD, specificity |
| 5 | 5 | 1 | Early | Repeatability, intermediate precision, accuracy, linearity, LOQ/LOD, freeze-thaw stability |
| 6 | 6 | 2 | Late | Repeatability, intermediate precision, accuracy, linearity, LOQ/LOD |
CP#, cell passage number.
Specificity.
Specificity of the RFFIT was tested by blocking HRIG or CL184 with inactivated rabies virus. Inactivated rabies virus CVS-11 was incubated for 1 h at 37°C with a fixed amount of HRIG and CL184. Additionally, the same treatment was performed using SRIG. Subsequently, virus-antibody mixtures were tested in the RFFIT assay along with nontreated samples (incubated only with medium). As a negative control, an irrelevant virus was used (vesicular stomatitis virus, strain Indiana Lab V-520-001-522). Eight replicates per condition were performed. Blocked and nonblocked conditions were compared. The decrease was determined by calculating the ratio between the geometric means of nonblocked and blocked RVNA activities, and specificity has been confirmed when the ratio between nonblocked and blocked condition is greater than or equal to 4.
The blocking experiments showed that the ratio of the mean nonblocked and blocked values was 17-fold for CL184, 8-fold for HRIG, and 21-fold for SRIG (Table 4). Thus, a >4-fold decrease has been demonstrated for CL184, HRIG, and SRIG and the acceptance criteria for specificity have been met.
Table 4.
Specificity analysisa
| Sample | Concn (IU/ml) after addition of: |
Medium/CVS-11 ratio | Medium/VSV ratio | ||
|---|---|---|---|---|---|
| Medium | CVS-11 | VSV | |||
| CL184 | 0.77 ± 0.10 | 0.05 ± 0.01 | 1.19 ± 0.18 | 16.59 | 0.64 |
| HRIG | 0.41 ± 0.08 | 0.05 ± 0.01 | 0.37 ± 0.06 | 7.88 | 1.13 |
| SRIG | 0.69 ± 0.07 | 0.03 ± 0.01 | 0.67 ± 0.08 | 20.52 | 1.04 |
Reported data are the means of 8 measurements ± standard deviations. VSV, vesicular stomatitis virus.
Matrix effect.
To monitor the effect of human serum matrix on the accuracy of the assay, SRIG was spiked in normal human serum at two different levels (see Table 2) and was tested three times in six independent assay runs. The means of the log titers were compared to the nominal values of 0 (log 1.0 = 0) and 1.0 (log 10 = 1) with a zone of indifference of ±0.114, which corresponds to 30% on a linear scale, and ≤30% was defined as the acceptance criterion.
The 90% confidence intervals (CI) of the difference of the mean log titers and 0 were −0.0305 to 0.0672 for the 1.0-IU/ml sample and −0.0443 to 0.0270 for the 10.0-IU/ml sample, both of which are contained within the zone of indifference; thus, equivalence between the expected and observed titers has been demonstrated (P < 0.05).
Linearity.
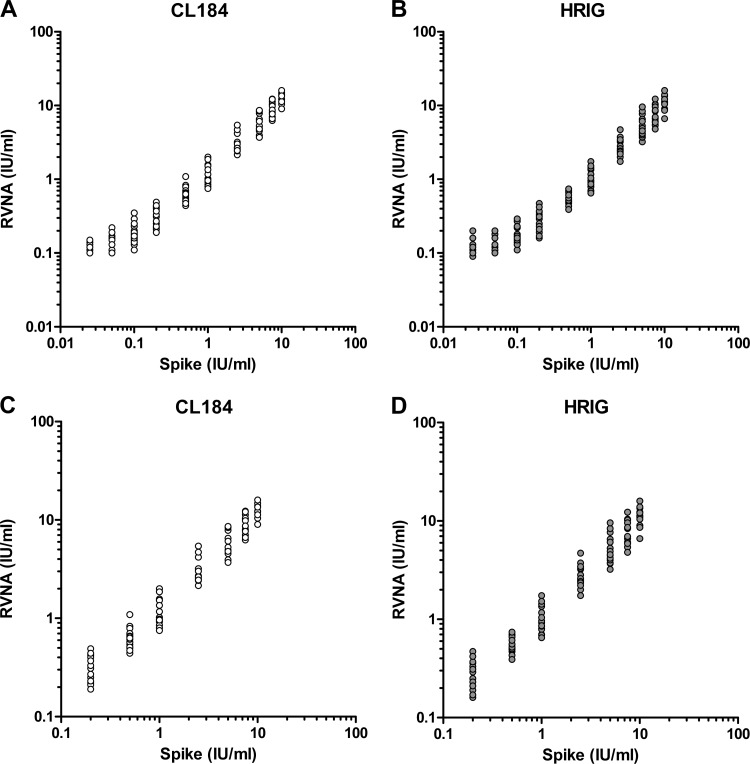
For the determination of linearity, the data obtained from all 6 experiments were used. Linearity was analyzed for HRIG and CL184 separately using a linear regression model, and the assumptions were assessed via analysis of the residuals. A regression line was fitted through the observed RVNA activity data points using the least-squares method. The correlation coefficient, y intercept, slope, and residual sum of squares were calculated. The 90% confidence interval of the slope of the regression analysis should be within 0.7 to 1.3. The two main assumptions of a linear regression are the normality of the residuals and the homogeneity of variance. The residuals were plotted in a normal probability plot and a histogram in order to assess normality (data not shown). The homogeneity of variance assumption was assessed via a residual versus predicted scatterplot (data not shown). The range where the data follows a linear regression model and has a slope equivalent to 1 constitutes the linear range of the assay. In Fig. 1A and B, including all data, it is clear that for both HRIG- and CL184-spiked ICs, the data curves at the lower concentrations. Regression analysis using all spike levels (IC1 to IC10) resulted in a slope of 0.81 and 0.82 for HRIG and CL184, respectively, of which the confidence intervals are within the acceptance limits. However, a curvature starting at a spike of 0.2 IU/ml (IC4) was observed. Since the slope should be as close as possible to 1, the lowest spike levels were excluded in order to achieve a more optimal slope (Fig. 1C and D). Regression analysis with a range of 0.2 IU/ml and higher resulted in slopes of 0.966 (0.935, 0.997) and 0.95 (0.921, 0.979) for HRIG and CL184, respectively.
Fig 1.
Linearity analysis of serum spiked with CL184 (A) and HRIG (B) from 0.025 international units (IU)/ml to 10 IU/ml. Linearity analysis of serum spiked with CL184 (C) and HRIG (D) from 0.2 IU/ml to 10 IU/ml. Expected rabies virus neutralization activity (RVNA) is shown on the x axis, and the measured results (n = 18 per spike level) are shown on the y axis.
Repeatability (intra-assay precision).
Since the linear range was defined from RVNA levels of 0.2 IU/ml to 10 IU/ml, data of spike levels below 0.2 IU/ml (IC1 to IC3) were excluded from the precision analysis. To assess the repeatability of the assay, the two sets (HRIG and CL184) of 7 IC samples (IC4 to IC10) were measured three times per experiment in 6 independent experiments executed over 6 different days (see Table 2). Data generated using the IC0 sample were not included in the analysis and are provided for information only. Data of all 6 independent experiments (n = 7 spikes × 3 replicates × 6 experiments = 126) were used to determine the repeatability. Sera of subjects vaccinated against rabies were included to assess repeatability of the assay when sera containing vaccine-induced RVNA were tested. Three replicates were used at every RNVA level per experiment. This resulted in 54 data points (n = 3 RVNA levels × 3 replicates × 6 experiments) to determine repeatability.
RVNA activity data (IU/ml) were first log10 transformed, and the overall standard deviation was calculated from the square root of the mean squared error generated by analysis of variance (ANOVA) with experiment, IC sample or RVNA activity level, operator, and cell passage number as cofactors. The intra-assay variability, expressed as percent CV, was calculated using the formula . The percent CV for HRIG, CL184, and positive serum ICs were calculated to be 26%, 18%, and 25%, respectively, which was within the acceptance limit of ≤30%.
Intermediate precision (interassay precision).
The same data set as mentioned above was used to determine the intermediate precision. Here, the data were analyzed by ANOVA with only the IC sample as a cofactor. The CV for HRIG, CL184, and positive serum ICs were calculated to be 28%, 26%, and 30%, respectively, which was within the acceptance limit of ≤30%.
LOQ.
The limit of quantitation (LOQ) is set by the IC sample with the lowest concentration of CL184 or HRIG within the linear range that has shown acceptable precision in the intermediate precision experiments. Following the above-mentioned results, the LOQ is 0.2 IU/ml.
Estimation of the LOD.
During the validation experiments of the RFFIT assay using CL184 and HRIG spiked at different levels, a nonspiked pooled serum sample (IC0) was taken along as well. Using this sample, a total of 36 data points were obtained during 6 experiments. Ideally, to establish a more precise limit of detection (LOD), individual sera should be tested in order to provide statistical rigor; here, we instead opted to estimate the LOD using a pooled serum sample. The LOD determined here is therefore an estimate and should be used for information purposes only. To determine the LOD, the mean value of the 36 data points is taken and 3 times the standard deviation is added to this mean value. The LOD was estimated to be 0.118 IU/ml.
Concordance between CL184 and HRIG (accuracy).
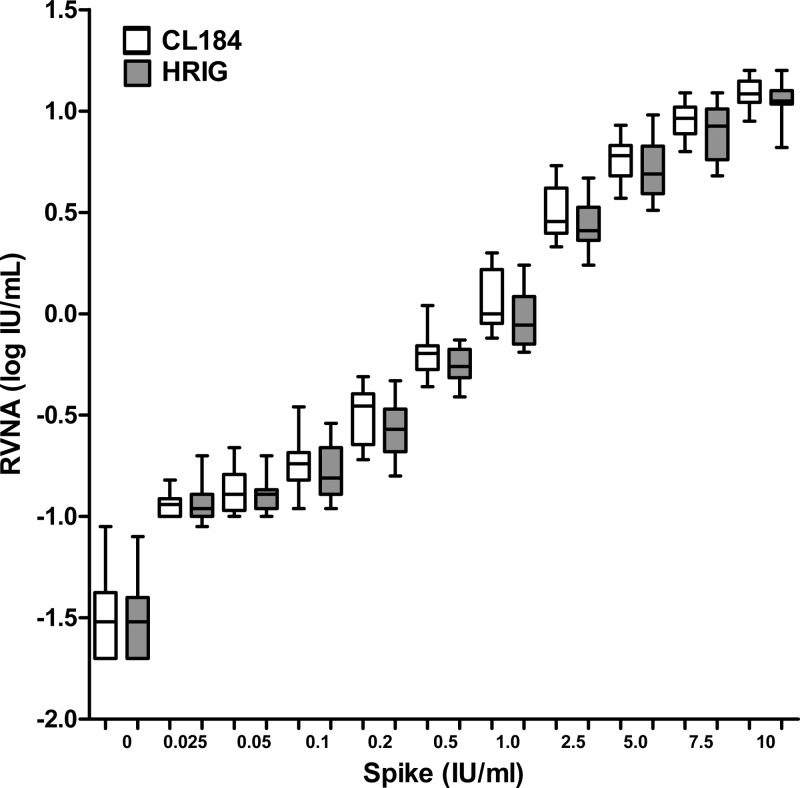
CL184 is being tested in clinical studies as a replacement for HRIG. Comparison of the two spike reagents would verify whether the RFFIT performs equally well against both CL184 and human rabies immune globulin, which enables comparison of clinical trial arms that comprise either HRIG or CL184 treatment. For each IC, the mean RVNA activities of the log10-transformed data were calculated based on all 6 intermediate precision experiments (see Table 2). Mean RVNA activities from CL184 ICs were compared to mean RVNA activities of HRIG ICs at each spike level (Fig. 2). The percentage difference between CL184 and HRIG IC was calculated. Concordance between CL184 and HRIG was demonstrated by applying a two-way ANOVA model using SAS software with the spike and sample concentrations as fixed factors. An equivalence test was performed on the overall difference between CL184 and HRIG after adjusting the spike level with a zone of indifference of ±0.114, corresponding to 30% on a linear scale. The 90% CI of the difference of the mean log titer value of CL184 and HRIG was −0.063 to −0.024, which is within the zone of indifference of ±0.114.
Fig 2.
Concordance between CL184 and HRIG spiked in human normal serum. Nominal rabies virus neutralization activity (RVNA) titers expressed in international units (IU)/ml after spiking with CL184 or HRIG is shown on the x axis. Box plots indicate the quartiles of 18 measurements.
To test for proportional bias, a Passing-Bablok regression model (8) was fitted to the data in the linear range only (i.e., spike levels in the range 0.2 IU/ml to 10 IU/ml) using SAS software. The estimated slope was 0.88 with a 95% CI of 0.80 to 0.96, indicating a maximal bias of 20%. The estimated intercept was 0.16 with a 95% CI of 0.12 to 0.17. In the ideal fit with no bias, the intercept should be 0, and the estimated intercept of 0.16 suggests that the bias contribution is predominantly in the lower range. When considering the data from the linear range, only the values close to spike level 0.2 have a greater influence, whereas the data from the entire range tested support a conclusion of much smaller bias and, thus, greater agreement between the values of CL184 and HRIG. In summary, concordance between HRIG and CL184 has been established, with a maximum bias of 20% occurring predominantly in the lower range of the assay.
Serum sample stability.
Since antibodies in serum are generally very stable, it is assumed that RVNA activities generated from serum samples stored at −80°C are also very stable. Stability may be jeopardized during handling of the samples at room temperature (RT) during the setup of the assay, when samples are stored at −20°C, in which case no −80°C freezer is available, or when samples undergo reanalysis, thus undergoing freeze-thaw cycles. To allow detection of a significant statistical difference between stability samples and comparator samples of more than 0.114 log units (corresponding with 30% on the original scale), 21 observations per sample type were needed. This generates a power of 80% at a two-sided significance level with an α level of 0.05. Therefore, data of CL184 and HRIG IC samples spiked at 3 concentration levels were combined for each product and used in the statistical analysis.
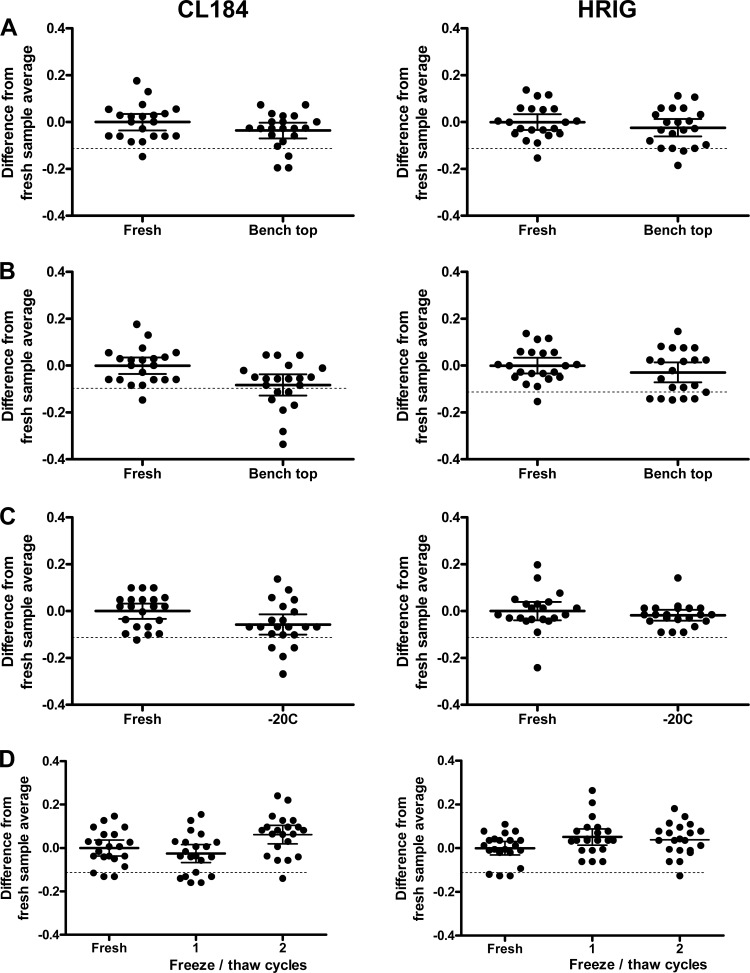
Bench top stability.
For bench top stability, 7 aliquots of HRIG and CL184 IC2, -3, and -4 (0.2, 0.5, and 1 IU/ml, respectively) were retrieved from the −80°C freezer and kept for 4 h at RT and allowed to thaw naturally (stability sample). After 4 h, another 7 aliquots of the same ICs were retrieved (comparator sample) and all samples were tested in the RFFIT assay. The RVNA activities (IU/ml) for each IC stability sample, obtained in triplicate, were log10 transformed and compared with the log10-transformed comparator samples by ANOVA, which were corrected for differences in concentration. In total, 21 data points of stability samples and 21 data points of the comparator samples were used in the statistical analysis (data of IC2, -3, and -4 are combined).
Regarding the critical reagents, SRIG and virus stability were tested in the same way. In this case, SRIG was incubated 4 h at RT. Since virus is normally retrieved from the freezer directly before use, the RT incubation time for this reagent was 20 min, which is more realistic than 4 h. Subsequently, 7 aliquots of IC2, -3, and -4 were thawed and incubated with the reagents.
The results are shown in Fig. 3A. A two-way ANOVA with spike level and stability condition as fixed factors was performed. An equivalence test was performed on the stability conditions after adjusting for spike level. The post hoc testing was done using a Dunnett's adjustment. All the test conditions were equivalent (P < 0.05) except for the stability of the virus in the CL184 IC sample testing, where the difference of the mean log titers is 0.082 and the 90% CI is −0.135 to −0.030, which falls outside the zone of indifference of ±0.114. This underscores the importance in the RFFIT protocol of retrieving the CVS-11 aliquot from frozen storage and thawing immediately before the virus working dilution is prepared and added to the serum dilutions.
Fig 3.
Stability experiments. (A) Bench top stability of quality control (QC) samples. (B) Bench top stability of virus stock. (C) Stability at −20°C. (D) Freeze-thaw stability. Fresh aliquots of QC samples 4, 6, and 8 are compared with stability samples that underwent specific stability conditions. International units (IU) titers were log transformed and normalized for the difference between the measurement and the average of the fresh sample titer. Solid lines represent the mean; dotted lines represent the indifference limit of 0.114 log IU/ml.
Stability at −20°C.
For stability at −20°C, 7 aliquots of HRIG and CL184 IC2, -3, and -4 were retrieved from the −80°C freezer and kept for 2 weeks at −20°C (stability samples). After 2 weeks, another 7 aliquots of the same ICs were retrieved from the −80°C freezer (comparator samples) and all samples were tested in the RFFIT assay. The RVNA activities (IU/ml) for each IC stability sample were log10 transformed and compared with the log10-transformed comparator samples by ANOVA, which was corrected for differences in concentration. In total, 21 data points of stability samples and 21 data points of the comparator samples were used in the statistical analysis (data of IC2, -3, and -4 are combined).
The results are shown in Fig. 3B. A two-way ANOVA with spike level and stability condition as fixed factors was performed. For HRIG, the difference in the means of the log titers is 0.017 with a 90% CI of −0.020 to 0.055, and for CL184, it was 0.057 with a 90% CI of 0.014 to 0.100, which are both within the zone of indifference. Stability at −20°C has been proven for at least 2 weeks.
Freeze-thaw stability.
For freeze-thaw stability, 14 aliquots of HRIG and CL184 IC2, -3, and -4 were retrieved from the −80°C freezer, thawed completely and unassisted at RT, and frozen again overnight at −80°C. To test two freeze-thaw cycles, 7 of the 14 aliquots were thawed again and frozen overnight at −80°C. The stability sample aliquots were retrieved from the −80°C freezer together with 7 untreated aliquots of the same ICs, and all samples were tested in the RFFIT assay (Fig. 3C).
The two-way ANOVA resulted in confidence intervals within the zone of indifference of ±0.114 for both HRIG (−0.003, 0.079) and CL184 (0.009, 0.1138) samples for two freeze-thaw cycles. This demonstrates the acceptable use of one aliquot of a clinical sample up to 2 freeze-thaw cycles in case a determination should be repeated.
DISCUSSION
The RFFIT method has been recommended by the U.S. Centers for Disease Control and Prevention (CDC) and the WHO (9, 15) as the standard assay to measure antibody levels against rabies virus in order to determine whether persons at risk for rabies exposure need a booster vaccination. Despite the availability of alternatives, this method remains the standard method for measuring rabies-specific antibodies (11). Therefore, this test has been adopted as a pharmacodynamic marker in the clinical development of a rabies antibody cocktail. Determination of the pharmacodynamics is of key importance for the development of therapeutic or prophylactic monoclonal antibodies. Because pharmacodynamic markers should reflect the biological mechanism as close as possible, pharmacodynamic assays are usually cell-based assays that indicate the biological activity of the drug. An inherent problem with cell-based assays is that the biological processes increase the variance of the assay readout. Considering the inclusion of BHK-21 cells and live rabies virus as critical reagents in the RFFIT, it is reassuring that the overall precision and accuracy did not exceed 30%. It must be noted that at the first step of the assay, the serum dilutions are performed using a pipetting robot, which adds to the precision of the assay compared to manual pipetting.
Validation of an assay is considered critical in product development to ensure the accurate assessment of a given biological or pharmacological parameter. Although validation is a snapshot of assay performance at a given time, it does provide evidence that the assay will perform adequately when testing clinical samples and, in addition, provides useful information on the critical steps of the assay. The only aspect of the validation that did not meet the acceptance criteria was the 20-minute bench top stability of the rabies virus. This constrains the execution of the assay to a minimal amount of time when handling virus, but it also emphasizes this particular step as critical. Knowledge of critical steps in the assay will lead to better performance overall.
The manufacturers of the two products in comparison have tested the potency of the products using different reference standards (see Materials and Methods). Since the first WHO International Standard/lot R-3 potency was observed to decrease compared to the second WHO International Standard potency (11), this could have influenced the concordance between the two products. However, the differences were not statistically significant to warrant a change in the product label for the reference standards. Additionally, if the difference in SRIG would have been significant, we would have expected to see a bias toward higher HRIG levels compared to CL184, which was not the case.
The primary objective of the validation was to assess assay performance for samples containing different sources of anti-rabies antibodies. The results presented here show that the assay behaves similar to the CL184 monoclonal antibody combination and polyclonal HRIG antibody preparations in human serum. This assay characteristic allows for the direct comparison of different treatment arms in clinical trials. The assay protocol as validated in this report will be used to assess pharmacodynamics of upcoming pivotal phase III studies, supporting the further development of our PER.C6-produced monoclonal antibody combination CL184.
ACKNOWLEDGMENTS
We thank Stephen Hildreth and Victor Hou of Sanofi Pasteur for advice on the validation plan and review of the manuscript.
Footnotes
Published ahead of print 30 April 2012
REFERENCES
- 1. Bakker AB, et al. 2005. Novel human monoclonal antibody combination effectively neutralizing natural rabies virus variants and individual in vitro escape mutants. J. Virol. 79:9062–9068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Bakker AB, et al. 2008. First administration to humans of a monoclonal antibody cocktail against rabies virus: safety, tolerability, and neutralizing activity. Vaccine 26:5922–5927 [DOI] [PubMed] [Google Scholar]
- 3. Dodet B. 2007. An important date in rabies history. Vaccine 25:8647–8650 [DOI] [PubMed] [Google Scholar]
- 4. FDA 2001. Guidance for industry. Bioanalytical method validation. Food and Drug Administration, Rockville, MD [Google Scholar]
- 5. Goudsmit J, et al. 2006. Comparison of an anti-rabies human monoclonal antibody combination with human polyclonal anti-rabies immune globulin. J. Infect. Dis. 193:796–801 [DOI] [PubMed] [Google Scholar]
- 6. Habel K. 1996. Habel test for potency, p 369–373 In Meslin FX, Kaplan MM, Koprowski H. (ed), Laboratory techniques in rabies, 4th ed World Health Organization, Geneva, Switzerland [Google Scholar]
- 7. ICH 2005. Validation of analytical procedures: text and methodology Q2(R1). ICH, Geneva, Switzerland [Google Scholar]
- 8. Johnson R. 2008. Assessment of bias with emphasis on method comparison. Clin. Biochem. Rev. 29(Suppl 1):S37–S42 [PMC free article] [PubMed] [Google Scholar]
- 9. Manning SE, et al. 2008. Human rabies prevention—United States, 2008: recommendations of the Advisory Committee on Immunization Practices. MMWR Recomm. Rep. 57:1–28 [PubMed] [Google Scholar]
- 10. Marissen WE, et al. 2005. Novel rabies virus-neutralizing epitope recognized by human monoclonal antibody: fine mapping and escape mutant analysis. J. Virol. 79:4672–4678 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Moore SM, Hanlon CA. 2010. Rabies-specific antibodies: measuring surrogates of protection against a fatal disease. PLoS Negl. Trop. Dis. 4:e595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Rupprecht CE, Gibbons RV. 2004. Clinical practice. Prophylaxis against rabies. N. Engl. J. Med. 351:2626–2635 [DOI] [PubMed] [Google Scholar]
- 13. Smith JS, Yager PA, Baer GM. 1973. A rapid reproducible test for determining rabies neutralizing antibody. Bull. World Health Organ. 48:535–541 [PMC free article] [PubMed] [Google Scholar]
- 14. Viswanathan CT, et al. 2007. Quantitative bioanalytical methods validation and implementation: best practices for chromatographic and ligand binding assays. Pharm. Res. 24:1962–1973 [DOI] [PubMed] [Google Scholar]
- 15. WHO 2011. The immunological basis for immunization series, module 17: rabies. World Health Organization, Geneva, Switzerland [Google Scholar]
- 16. WHO 2010. Rabies vaccines: WHO position paper—recommendations. Vaccine 28:7140–7142 [DOI] [PubMed] [Google Scholar]
- 17. WHO 2002. WHO consultation on a rabies monoclonal antibody cocktail for rabies post exposure treatment. World Health Organization, Geneva, Switzerland: http://www.who.int/rabies/vaccines/en/mabs_final_report.pdf [Google Scholar]
- 18. WHO 1997. WHO recommendations on rabies post-exposure treatment and the correct technique of intradermal immunization against rabies. WHO/EMC/ZOO/96.6. World Health Organization, Geneva, Switzerland: http://whqlibdoc.who.int/hq/1996/WHO_EMC_ZOO_96.6.pdf [Google Scholar]