Abstract
Multidrug-resistant clinical isolate Klebsiella pneumoniae BM4686 was highly resistant to 4,6-disubstituted 2-deoxystreptamines and to fortimicin. Resistance was due to the presence, on the 40-kb non-self-transferable plasmid pIP849, of the rmtF gene which was cotranscribed with the upstream aac(6′)-Ib gene. The deduced RmtF protein had 25 to 46% identity with members of the N7 G1405 family of aminoglycoside resistance 16S rRNA methyltransferases.
TEXT
Aminoglycosides are used for the treatment of severe infections caused by Gram-negative organisms. They interfere with bacterial 16S rRNA function by binding at the A site where codon-anticodon accuracy is assessed (10). In Gram-negative pathogens, resistance to aminoglycosides is mediated primarily by enzymes that modify the drugs and less commonly by other mechanisms, including efflux (7). Substitution or methylation of bases involved in the binding of aminoglycosides to 16S rRNA can lead to loss of affinity and to resistance of the host (7, 9, 11). Methylation was initially observed in the actinomycete and streptomycete producers of aminoglycosides (1). Recently, 16S rRNA N7 G1405 methyltransferases which confer high-level resistance to the 4,6-disubstituted 2-deoxystreptamines and to fortimicin have emerged and disseminated among Gram-negative human pathogens. The first gene for this type of resistance was named armA, for aminoglycoside resistance methyltransferase (5). Reports followed of five other methyltransferases: RmtA (rRNA methyltransferase A), RmtB, RmtC, RmtD, and RmtE (4). In most instances, these genes are parts of large conjugative plasmids, together with quinolone resistance determinants and genes for extended-spectrum β-lactamases (6).
Klebsiella pneumoniae BM4686 was isolated from a patient in the Hôpital Sud, city of Saint-Pierre, La Réunion Island, in 2011. Antibiotic susceptibility was tested by disk diffusion on Mueller-Hinton agar according to the Comité de l'Antibiogramme de la Société Française de Microbiologie standards (3). MICs of antimicrobial agents were determined by dilution in Mueller-Hinton agar with 104 CFU per spot after 24 h of incubation. The strain was resistant to all β-lactams due to the presence of the genes blaOXA-1 and blaNDM-1; no PCR products were obtained with primers internal to the genes for the PER, VEB, GES, KPC, VIM, IMP1, IMP2, and CTX-M β-lactamases. K. pneumoniae BM4686 was also resistant to all aminoglycosides, to fluoroquinolones, to rifampin, and to chloramphenicol but remained susceptible to the tetracyclines and colistin (Table 1; data not shown).
Table 1.
MICs of antimicrobial agents for the strains used in this study
| Strain | MIC (μg/ml)a |
||||
|---|---|---|---|---|---|
| AMI | GEN | TOB | APR | RIF | |
| K. pneumoniae BM4686 | >256 | >256 | >256 | 2 | >256 |
| E. coli | |||||
| TOP10 | 1 | 0.5 | 0.5 | 2 | 12 |
| TOP10(pIP849) | >256 | 128 | 256 | 2 | >256 |
| TOP10(pAT860) | >256 | 256 | 256 | 2 | 12 |
| TOP10(pAT861) | 256 | 128 | 128 | 2 | 12 |
Abbreviations: AMI, amikacin; GEN, gentamicin; TOB, tobramycin; APR, apramycin; RIF, rifampin.
High-level resistance to aminoglycosides was indicative of the presence of a 16S rRNA methyltransferase, but attempts to amplify known genes were unsuccessful. Resistance could not be transferred to Escherichia coli J53 by conjugation, but plasmid DNA extracted from BM4686 and introduced by electrotransformation into E. coli TOP10 gave rise to cells growing on arbekacin (500 μg/ml). The transformants harbored ca. 40-kb plasmid pIP849, which conferred high-level resistance to the kanamycin and gentamicin classes, to fortimicin, and to rifampin (Table 1; data not shown). Plasmid pIP849 was nontypeable by PCR-based replicon typing (2). DNA from pIP849 and pUC19 was digested with EcoRI, mixed, ligated, and introduced by transformation into E. coli TOP10 with selection on arbekacin. The smallest plasmid in the transformants, pAT860, contained a ca. 4.5-kb insert which was sequenced.
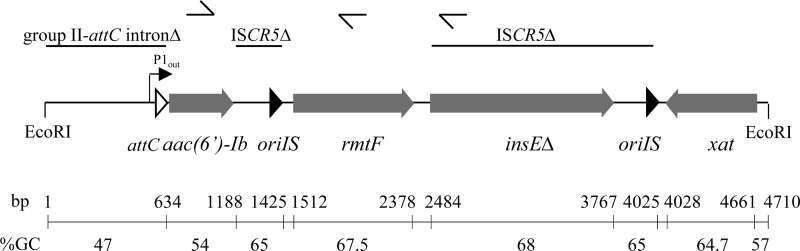
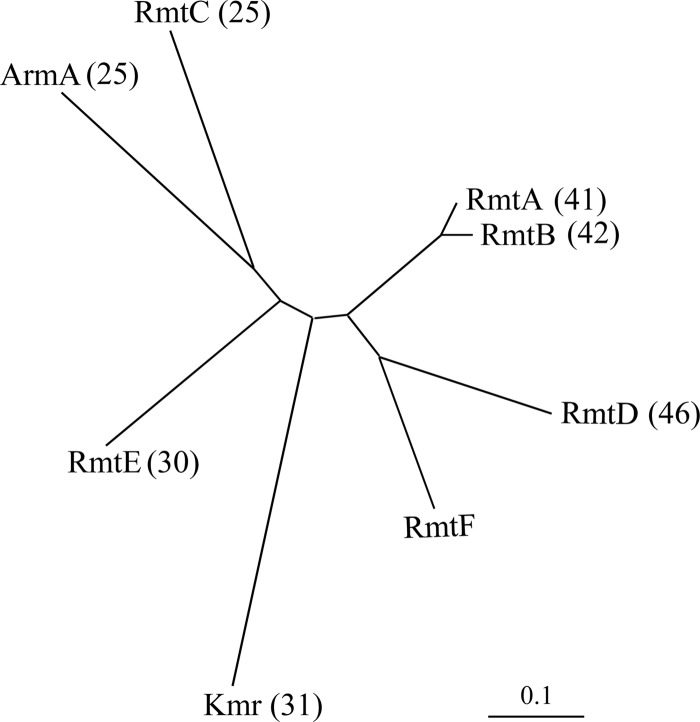
The insert contained four open reading frames (ORFs) (Fig. 1). Comparison with sequences in the GenBank database revealed homology of the deduced products from ORF1 (positions 634 to 1188) with aminoglycoside 6′-N-acetyltransferase-Ib (99% identity over 193 amino acids; accession number BAE66666), from ORF3 (positions 2484 to 3767) with transposase InsE ISCR5 of E. coli (95% identity over 429 amino acids; accession number YP_002891161), and from ORF4 (positions 4661 to 4028) with a putative xenobiotic acetyltransferase from Paracoccus denitrificans (74% identity over 155 amino acids; accession number YP_913929). ORF2 (positions 1512 to 2378) did not share homology with sequences in GenBank, but a BLASTx query of the deduced protein indicated similarity to G1405 16S rRNA methylases (25 to 46% identity over 254 amino acids) (Fig. 2) and it was designated RmtF (2). Within ORF2, a probable ribosome binding site, GGAGA (positions 1585 to 1589), was present 9 nucleotides upstream from a putative ATG initiation codon leading to a 777-bp coding sequence. In silico analysis of the sequence upstream from rmtF did not reveal any putative promoter. Synthesis of cDNA from total RNA of E. coli TOP10 harboring pIP849 was carried out using either a random hexamer (Thermoscientific RevertAid premium first-strand cDNA synthesis kit) or rmtF- and insEΔ-specific primers (Fig. 1). PCR products were obtained with the three cDNAs and primers specific for aac(6′)-Ib, rmtF, and insEΔ, indicating that the aac(6′)-Ib and rmtF genes are cotranscribed. A 5′ rapid amplification of cDNA ends experiment (Ambion/Life Technologies FirstChoice RLM-RACE kit) indicated that adenine 490 is the transcription initiation site for the aac(6′)-Ib-rmtF operon. This suggests the P1out promoter (−35 motif [TTGCCA] and −10 motif [TTTAAT], positions 455 to 483), which is part of the group IIC-attC intron (Fig. 1) (8), for expression of the genes. The 1.5-kb PCR fragment obtained with the 5′-ATTCGAGCGAACACGCAGTGA and 5′-AGAACCCGCGCTTCTTGCAGG primers encompassing rmtF was cloned into pCR-Blunt (Invitrogen) and resequenced, and the pAT861 recombinant plasmid conferred high-level resistance to 4,6-disubstituted 2-deoxystreptamines and to fortimicin on E. coli TOP10 (Table 1; data not shown).
Fig 1.
Schematic representation of the genetic environment of rmtF. Arrows indicate ORFs and directions of transcription. Closed arrowheads, oriIS of ISCR5. Open arrowhead, attC. Broken arrow, group IIC-attC P1out promoter. The genetic elements are indicated by thin lines. The primers used for reverse transcription and amplification are indicated by thin arrows at the top, and the G+C contents and scale are shown at the bottom.
Fig 2.
Phylogenetic relationships among 16S rRNA methyltransferases determined using CLUSTALW. The GenBank accession numbers of plasmid-borne 16S rRNA methyltransferase genes are as follows: ArmA, AY220558; RmtA, AB083212; RmtB, AB103506; RmtC, AB194779; RmtE, GU201947; RmtF, JQ808129. Kmr (accession no. Y15838) is a chromosomal 16S rRNA methyltransferase from aminoglycoside-producing Streptomyces kanamyceticus. The scale bar represents a 10% amino acid sequence difference. The values in parentheses are percentages of identity relative to RmtF.
The overall G+C contents of the rmtF gene (67.5 mol%), flanking oriIS ISCR5 (65 mol%), insE (68 mol%), and xat (64.7 mol%) were similar and significantly higher than that of the aac(6′)-Ib (54 mol%) and the adjacent upstream region (47 mol%) (Fig. 1). The G+C content of rmtF was significantly different from that of the genome of members of the family Enterobacteriaceae, which is ca. 50 mol%, but was close to those of the 16S rRNA methyltransferase structural genes from producers such as “Streptomyces tenebrius” (kgmB; 71 mol%) and Micromonospora purpurea (grmA; 65 mol%), favoring a direct and recent origin in the family Actinomycetaceae.
The rmtF gene was bracketed by a 3′ portion of ISCR5 including oriIS and by a 5′-truncated insE gene for the ISCR5 transposase together with oriIS (Fig. 1). This genetic organization is consistent with the hypothesis that rmtF was recruited through ISCR transposition or homologous recombination (13), as has been proposed from rmtD1-rmtD2 and ISCR14 (12). A transposition-associated deletion would then be responsible for the transcriptional fusion with the aac(6′)-Ib gene.
Nucleotide sequence accession number.
The nucleotide sequence of the insert in pAT860 has been deposited in the GenBank database and assigned accession number JQ808129.
ACKNOWLEDGMENTS
We thank S. Picot for the gift of strain BM4686.
This work was supported by an unrestricted grant from Reckitt-Benckiser.
Footnotes
Published ahead of print 30 April 2012
REFERENCES
- 1. Beauclerk AA, Cundliffe E. 1987. Sites of action of two ribosomal RNA methylases responsible for resistance to aminoglycosides. J. Mol. Biol. 193:661–671 [DOI] [PubMed] [Google Scholar]
- 2. Carattoli A, et al. 2005. Identification of plasmids by PCR-based replicon typing. J. Microbiol. Methods 63:219–228 [DOI] [PubMed] [Google Scholar]
- 3. Comité de l'Antibiogramme de la Société Française de Microbiologie 2012. Communiqué 2012. Comité de l'Antibiogramme de la Société Française de Microbiologie, Paris, France [Google Scholar]
- 4. Doi Y, Wachino J, Arakawa Y. 2008. Nomenclature of plasmid-mediated 16S rRNA methylases responsible for panaminoglycoside resistance. Antimicrob. Agents Chemother. 52:2287–2288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Galimand M, Courvalin P, Lambert T. 2003. Plasmid-mediated high-level resistance to aminoglycosides in Enterobacteriaceae due to 16S rRNA methylation. Antimicrob. Agents Chemother. 47:2565–2571 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Jiang Y, et al. 2010. Complete nucleotide sequence of Klebsiella pneumoniae multidrug resistance plasmid pKP048, carrying blaKPC-2, blaDHA-1, qnrB4, and armA. Antimicrob. Agents Chemother. 54:3967–3969 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Kotra LP, Haddad J, Mobashery S. 2000. Aminoglycosides: perspectives on mechanisms of action and resistance and strategies to counter resistance. Antimicrob. Agents Chemother. 44:3249–3256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Léon G, Quiroga C, Centrón D, Roy HR. 2010. Diversity and strength of internal outward-oriented promoters in group IIC-attC introns. Nucleic Acids Res. 38:8196–8207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Liou GF, Yoshizawa S, Courvalin P, Galimand M. 2006. Aminoglycoside resistance by ArmA-mediated ribosomal 16S methylation in human bacterial pathogens. J. Mol. Biol. 359:358–364 [DOI] [PubMed] [Google Scholar]
- 10. Moazed D, Noller HF. 1987. Interaction of antibiotics with functional sites in 16S ribosomal RNA. Nature 327:389–397 [DOI] [PubMed] [Google Scholar]
- 11. Schmitt E, Galimand M, Panvert M, Courvalin P, Mechulam Y. 2009. Structural bases for 16S rRNA methylation catalyzed by ArmA and RmtB methyltransferases. J. Mol. Biol. 388:570–582 [DOI] [PubMed] [Google Scholar]
- 12. Tijet N, et al. 2011. rmtD2, a new allele of a 16S rRNA methylase gene, has been present in Enterobacteriaceae isolates from Argentina from more than a decade. Antimicrob. Agents Chemother. 55:904–909 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Toleman MA, Bennett PM, Walsh TR. 2006. ISCR elements: novel gene capturing systems of the 21st century? Microbiol. Mol. Biol. Rev. 70:296–316 [DOI] [PMC free article] [PubMed] [Google Scholar]