Abstract
Eighteen out of 45 children were reported to have a respiratory illness during an outbreak at a temporary dormitory in a nursery school in China in 2011. To study the outbreak and to determine the risk factors for infection, an epidemiological investigation was performed. A standardized questionnaire was completed for a total of 45 children with the help of their guardians and parents. In addition, acute- and convalescent-phase serum samples and throat swabs from the children were taken for laboratory diagnosis. The diagnosis of a Mycoplasma-like illness was based on the following clinical criteria. The criteria were onset of illness after 31 May 2011, characterized by a cough, fever(>37.5°C), or at least 3 of the following symptoms: fever, sore throat, cough or expectoration, and runny or stuffy nose. PCR-restriction fragment length polymorphism (PCR-RFLP), determination of MICs, and sequencing were performed to determine the genotype, antibiotic resistance, and sequence polymorphisms of the isolated strains, respectively. The paired sera revealed that 15 patients were infected with Mycoplasma pneumoniae. Epidemiology confirmed that this was a point source outbreak, characterized by a short incubation period, a high secondary attack rate, and a long period of hospitalization. PCR-RFLP analysis revealed that the 12 isolated strains of M. pneumoniae shared the same subtype P1 gene, and 23S rRNA sequence analysis showed that these strains harbored two macrolide-resistant gene-related point mutations at position 2063 and 2617. In this outbreak, the major risk factor was the distance between the bed of the first patient and the beds of close contacts (beds less than three meters apart). The strains isolated in this study were found to harbor two point mutations conferring macrolide resistance, indicating the importance of pathogen and drug resistance surveillance systems.
INTRODUCTION
Mycoplasma pneumoniae is a common cause of acute respiratory tract infections, especially in school-age children. Although some infections can be fatal, most cases attributed to this bacterium are relatively mild, and pneumonia caused by Mycoplasma rarely results in hospitalization (4, 15). Outbreaks can occur in closed surroundings, such as military camps and schools (9). We investigated an outbreak of acute respiratory disease that occurred in a nursery school in Beijing, China, in the summer of 2011. The analysis of paired serum and swab cultures indicated that the outbreak was caused by M. pneumoniae infection. Furthermore, PCR-restriction fragment length polymorphism (PCR-RFLP) analysis and sequence analysis showed that the M. pneumoniae strain responsible for the outbreak was a subtype 1 strain, harboring point mutations at positions 2063 and 2617, as first detected in China.
MATERIALS AND METHODS
A total of 45 children were included in this study. Approval for the study was obtained from the ethics committee of the Institute of Disease Control and Prevention, Academy of Military Medical Sciences, according to the medical research regulations of the Ministry of Health, China. The informed consent of all participants was obtained prior to enrollment. We defined Mycoplasma-like illness (MLI) as a combination of the following symptoms: fever (>37.5°C), sore throat, cough, and expectoration; we included those patients that visited the pediatric clinic between 31 May to 31 June 2011, for a respiratory condition. The roommates who had played with a child who developed MLI for over half an hour in 1 day and/or whose beds were located less than three meters from the bed of the first patient in the dormitory to fall ill were defined as close contacts in this outbreak. Controls were defined as the children without any respiratory tract symptoms in this nursery school. Based on the questionnaire completed by the guardians and parents of the children, a case control study was conducted to further investigate potential risk factors. Data entry and Fisher's exact test were performed with EpiInfo (version 3.4.1; Centers for Disease Control and Prevention, Atlanta, GA).
Acute-phase and convalescent-phase blood specimens were obtained from the children visiting the clinic with a respiratory illness on or after 31 May 2001, which was the date the first patient was hospitalized, and were followed up after 4 weeks. Serological testing for M. pneumoniae-specific IgM was performed using an enzyme immunoassay (EIA) kit (Remel, Lenexa, KS) (1), and Legionella (18), parainfluenza virus (7), and respiratory syncytial virus (7) were also tested for by standard methods (3).
To identify these cases whether they were infected by the same source of infection, the strains of M. pneumoniae in this outbreak were classified by PCR-RFLP. Twelve strains of M. pneumoniae were isolated and cultured in PPLO (pleuropneumonialike organism) medium at 37°C, and DNA was then isolated using the QIAamp DNA minikit (Qiagen, Hilden, Germany) according to the manufacturer's recommendations. This DNA was used for genotyping of the M. pneumoniae P1 gene by PCR-RFLP (13). A nested PCR was used to amplify part of the RepMp2/3 element of the P1 gene of M. pneumoniae, and a 401-bp region was sequenced and compared to the published P1 sequences of M. pneumoniae strain M129 (subtype 1) and strain 1842 (subtype 2). Sequence analysis was performed using DNAMAN version 5.2.10 (Lynnon Corporation, USA).
The MICs of MLS (macrolide-lincosamide-streptogramin B) antibiotics were determined according to the method of the National Committee for Clinical Laboratory Standards. To detect the point mutations A2063G, A2063C, A2064G, and A2617G in domain V of the 23S rRNA gene, previously reported primers were used (10). The DNA sequences of the obtained PCR products were compared with the sequences of M. pneumoniae strain M129 (GenBank accession no. X68422) and strain 1842 (GenBank accession no. AF290002) using BLAST analysis ((http://blast.ncbi.nlm.nih.gov/Blast.cgi).
RESULTS
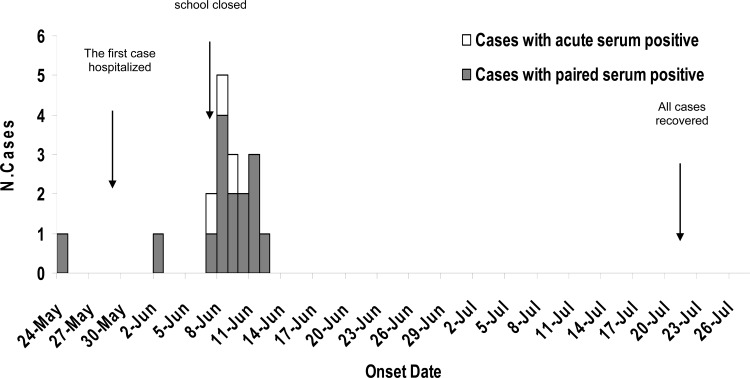
Forty-five children (30 boys and 15 girls) attending the same preschool completed a risk factor questionnaire with the assistance of their parents and guardians. Because some classrooms in the nursery school were being renovated, 45 children in one class were moved to a temporary room, which was a semiclosed setting at the north end of the building, on 10 May 2011. The first child developed acute respiratory disease on 24 May 2011 and continued to attend school for 2 days. This child, who had a high fever (40.0°C), cough, and sore throat, received a febrifuge (such as acetaminophen and ibuprofen) but showed no improvement before being admitted to the General Hospital of Beijing Military Region, Beijing, China, on 27 May 2011. Following hospitalization of the first patient, MLI syndromes appeared one after another in 17 children. Of these 17 children, one child was admitted to the hospital with radiological evidence of pneumonia on 2 June 2011, and the other 16 children developed similar symptoms between 7 June and 12 June 2011 (Fig. 1). The median incubation period was 15 days (range was from 9 to 19 days), and the secondary attack rate was 38.6% (17/44). All 17 patients had a fever (>38.0°C), sore throat, and expectoration. Sixteen of the patients had a cough and X-ray evidence of pneumonia, and 13 of these displayed a serious cough (Table 1). None of the parents or teachers of these patients exhibited similar symptoms. All of the children were treated with erythromycin (ERY), but most showed no improvement in symptoms, and they remained hospitalized up to 4 weeks. All patients recovered after the use of leucomycin (stereomycin) administered three times a day by July 20. The school was closed on 7 June, and there were no other children with respiratory symptoms in this nursery school during a retrospective investigation in August and September 2011. Further investigations showed that the most affected children had shared a dormitory with the first patient. The 45 children slept in proximity to each other. The bed belonging to the first patient was located at the center of the dormitory, and the distance between the first patient's bed and the beds of the other 17 patients were less than three meters. The other 27 children whose beds were located more than three meters from the bed of the first patient remained healthy during the outbreak. By comparing the healthy children with the sick children, we were able to determine that close contact with the first patient was the most important risk factor for MLI in this outbreak, and statistical analysis showed a significant difference between these two groups (odds ratio [OR], 14.86; 95% confidence interval [95% CI], 1.61 to 343.77; P < 0.05) (Table 2).
Fig 1.
Graph showing cases of an outbreak of M. pneumoniae in a nursery school in China in 2011. The number of cases is shown on the y axis, and the date of onset of illness in 2011 is shown on the x axis.
Table 1.
Characteristics of patients during an outbreak of M. pneumoniae in a nursery school
| Patient no. | Gender | Age (yr) | Onset date (day/mo/yr) | Clinical symptoms and examination |
Test IgM antibody titer in: |
Code no. of strain | Type PCR-RFLP of the P1 gene | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Fever (max temp) (°C) | Cougha | Sore throat | Expectoration | WBCb count (mean no. of cells) (× 103) | X-ray examination of pneumonia | Acute-phase serum | Convalescent-phase serum | ||||||
| 1 | Male | 6 | 24/05/11 | 40.0 | + | + | + | 7.7 | + | <1:160 | ≥1:1,280 | 1 | 1 |
| 2 | Male | 6.5 | 02/06/11 | 40.5 | ++ | + | + | 7.1 | + | <1:80 | ≥1:1,280 | ||
| 3 | Female | 6.5 | 07/06/11 | 41.5 | ++ | + | + | 5.2 | + | <1:40 | ≥1:1,280 | 2 | 1 |
| 4 | Male | 6 | 07/06/11 | 39.0 | + | + | 5.9 | + | ≥1:1,280 | ||||
| 5 | Male | 6 | 08/06/11 | 41.5 | ++ | + | + | 6.1 | + | <1:80 | ≥1:1,280 | 3 | |
| 6 | Female | 5 | 08/06/11 | 40.5 | ++ | + | + | 5.9 | + | <1:160 | ≥1:1,280 | 4 | 1 |
| 7 | Female | 4.5 | 08/06/11 | 38.5 | + | + | + | 3.9 | ≥1:1,280 | ||||
| 8 | Male | 6 | 08/06/11 | 41.0 | ++ | + | + | 7.5 | + | <1:80 | ≥1:1,280 | 5 | 1 |
| 9 | Female | 5.5 | 08/06/11 | 40.0 | ++ | + | + | 6.4 | + | <1:80 | ≥1:1,280 | 6 | 1 |
| 10 | Male | 6 | 09/06/11 | 41.0 | ++ | + | + | 7.2 | + | ≥1:1,280 | |||
| 11 | Male | 6 | 09/06/11 | 40.5 | ++ | + | + | 5.3 | + | <1:40 | ≥1:1,280 | 7 | 1 |
| 12 | Male | 6.5 | 09/06/11 | 41.0 | ++ | + | + | 7.9 | + | <1:80 | ≥1:1,280 | 8 | 1 |
| 13 | Male | 6 | 10/06/11 | 40.0 | ++ | + | + | 4.6 | + | <1:160 | ≥1:1,280 | ||
| 14 | Female | 5 | 10/06/11 | 38.5 | + | + | 4.8 | <1:160 | ≥1:1,280 | 9 | 1 | ||
| 15 | Male | 5.5 | 11/06/11 | 40.0 | ++ | + | 6.6 | + | <1:80 | ≥1:1,280 | |||
| 16 | Male | 6 | 11/06/11 | 39.0 | + | + | + | 5.8 | + | <1:40 | ≥1:1,280 | 10 | 1 |
| 17 | Female | 6.5 | 11/06/11 | 40.0 | ++ | + | + | 6.9 | + | <1:160 | ≥1:1,280 | 11 | 1 |
| 18 | Male | 5 | 12/06/11 | 40.0 | ++ | + | + | 7.1 | + | <1:80 | ≥1:1,280 | 12 | 1 |
Coughs were scored as follows: +, mild; ++, severe.
WBC, white blood cell.
Table 2.
Risk factor analysis for Mycoplasma pneumoniae in an outbreak in a nursery school
| Risk factor | No. of children |
OR (95% CI) | P valuea | |
|---|---|---|---|---|
| With M. pneumoniae | Without M. pneumoniae | |||
| Close contactb with the first patient | 16 | 14 | 14.86 (1.61–343.77) | 0.0038 |
| Not close contact with the first patient | 1 | 13 | ||
| Total (all children) | 17 | 27 | ||
Fisher's exact test.
Close contact means the roommates who played with the first patient who became ill more than a half hour a day and/or whose beds were within 3 m of the bed of the first patient in the dormitory.
Furthermore, these 45 children, including 18 patients in the nursery school, resided in a temporary and small room which was a semiclosed setting at the north end of the building. The 45 children were crowded together, and their beds were located in close proximity. The humid environment and poor ventilation conditions in this room were noted. Thus, we suppose that poor ventilation also contributed to this outbreak. After some children were hospitalized, the nursery school was closed and the classroom and dormitory were disinfected with UV light for about 30 min every day until June 20.
Of the 18 children displaying MLI syndrome, 15 (83%) were serologically positive for M. pneumoniae, which was defined as a >4-fold increase in the antibody titers between the acute- and convalescent-phase serum samples. The other three children showed a high titer of antibodies of ≥1,280 in their acute-phase serum samples (Table 1). Only 12 strains of M. pneumoniae were isolated from throat swabs of 15 patients whose paired serum samples tested positive. None of the children tested positive for Legionella, parainfluenza virus, or respiratory syncytial virus.
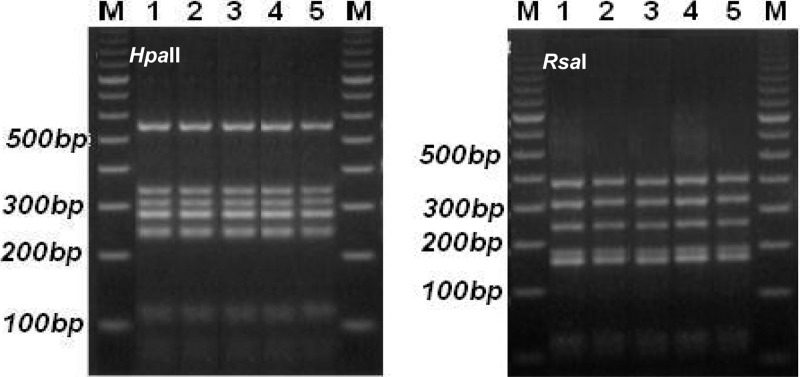
From PCR-RFLP analysis, the 12 strains of M. pneumoniae isolated from throat swabs of 15 patients whose sera tested positive were found to share the same subtype of P1 gene (Fig. 2). Sequencing of the nested PCR products of the RepMp2/3 element of the P1 gene revealed that the 12 isolates from the outbreak showed the highest level of sequence similarity with M. pneumoniae strain M129 (subtype 1, GenBank accession number M18639; similarity of 99 to 100% [the sequences of 5 strains were 99% similar, and the sequences of 7 strains were 100% identical]), and that these sequences were different from that of strain 1842 (subtype 2, GenBank accession number AF290002; similarity of 89%). These results indicated that the strain of M. pneumoniae associated with the outbreak could be assigned to subtype 1.
Fig 2.
PCR-RFLP patterns of two PCR fragments of the P1 gene of M. pneumoniae isolates digested with two endonucleases, HpaII (left panel) and RsaI (right panel). Lanes 1, P1 subtype reference strains of M. pneumoniae; lanes 2 to 5, randomly selected M. pneumoniae strains numbered 1, 3, 7, and 10 isolated from throat swabs and sputum samples of patients 1, 5, 11, and 16; lanes M, molecular size markers.
All 12 strains of M. pneumoniae showed resistance to macrolide antibiotics. The in vitro activities of the MLS antibiotics against the macrolide-resistant clinical isolates of M. pneumoniae were as follows: >256 μg/ml for erythromycin (ERY), >256 μg/ml for clarithromycin (CLR), >256 μg/ml for lincomycin (LCM), >256 μg/ml for oleandomycin (OL), and 64 μg/ml for azithromycin (AZM). The results of nucleotide sequence analysis of the domain V region of the 23S rRNA gene showed that the strains harbored two point mutations of A to G and C to G at positions 2063 and 2617, respectively.
DISCUSSION
The highest incidence rates of pneumonia caused by M. pneumoniae are among children from 5 to 9 years old, and children show a higher infection rate with this bacterium than adults. M. pneumoniae accounts for a low proportion of all cases of pneumonia in children in the United States (5). High rates of transmission have been documented in families in the United States, with a high proportion of secondary cases involving lower respiratory tract infections (2). In a study of community spread, transmission of M. pneumoniae within schools was relatively low compared with the spread of this bacterium within families and infections among neighborhood playmates in the United States (6). Although outbreaks of M. pneumoniae in China have been reported (8, 11, 14), this is the first report of a point source outbreak caused by a rare macrolide-resistant strain of M. pneumoniae in China, and this outbreak attracted great attention from local government and public health departments.
In this outbreak, the risk of infection in the 30 patients who had close contact with the first patient was 7.47 times higher than that of the other 14 healthy children in the same temporary classroom and similar respiratory tract symptoms, showing that this outbreak was a point source outbreak. The time (9 to 19 days) between the onset of illness in the first child and the appearance of MLI symptoms in the rest of the small cluster of children supports this conclusion. The median incubation period (15 days) was shorter than that of a previous report, which was reported to be about 3 weeks (5). The high secondary attack rate (38.6%) and the fact that no family members were infected during this outbreak indicated that children in the school were more susceptible to infection than other family members during the M. pneumoniae outbreak. In addition, the poor ventilation in the temporary classroom and the close contact among these children, whose beds were located within 3 meters of each other, may have promoted this outbreak. Increasing the distances between the beds may decrease the spread of infections by respiratory droplets (12), but in this outbreak the school was closed when some children were hospitalized, so the effectiveness of this measure is not known. This highlights the importance of suitable environmental and sanitation conditions for the prevention and control of respiratory tract infectious disease outbreaks.
In this outbreak, the severe clinical symptoms, the long period of hospitalization of all patients, and the macrolide-resistant phenotype of the infective strain suggested that the outbreak strain may have mutated. The isolated strains were therefore analyzed by PCR-RFLP, and the P1 gene was sequenced in each case. The results showed that the 12 isolated strains were all of the same type (subtype 1) and were macrolide resistant, a result that differed from a previous report (16). We also confirmed transversions of C to G and A to G at positions 2617 and 2063 in domain V of the P1 gene in all 12 clinical isolates, which again differed from the point mutations at positions 2063 and 2064 reported previously (15). The strain isolated in this study therefore harbored a rare point mutation conferring macrolide resistance, and these findings warrant close attention by the Chinese government. It is of utmost importance that pathogen and drug resistance surveillance systems are established to prevent and control such mutated strains of M. pneumoniae from causing future outbreaks or epidemics in China.
ACKNOWLEDGMENTS
We are grateful to Xue-Jie Yu of the Department of Pathology, University of Texas Medical Branch (Galveston, TX) for reviewing the manuscript.
This work was funded by Major-Project of Science and Technology Research of China (2012ZX10004-215, 2012ZX10004-801, and 2012ZX10004-401), the National Natural Science Foundation of China (grant 81001267), and the Beijing Science and Technology Nova program (grant 2010B067). We had no conflicts of interest.
Footnotes
Published ahead of print 14 May 2012
REFERENCES
- 1. Balassanian N, Robbins FC. 1967. Mycoplasma pneumoniae infection in families. N. Engl. J. Med. 277:719–725 [DOI] [PubMed] [Google Scholar]
- 2. Barraza EM, Ludwig SL, Gaydos JC, Brundage JF. 1999. Reemergence of adenovirus type 4 acute respiratory disease in military trainees: report of an outbreak during a lapse in vaccination. J. Infect. Dis. 179:1531–1533 [DOI] [PubMed] [Google Scholar]
- 3. Centers for Disease Control and Prevention 1993. Outbreaks of Mycoplasma pneumoniae respiratory infection—Ohio, Texas, and New York, 1993. MMWR Morb. Mortal. Wkly. Rep. 42:931–939 [PubMed] [Google Scholar]
- 4. Foy HM. 1993. Infections caused by Mycoplasma pneumoniae and possible carrier state in a different population of patients. Clin. Infect. Dis. 17(Suppl. 1):37–46 [DOI] [PubMed] [Google Scholar]
- 5. Foy HM, Kenny GE, McMahan R, Kaiser G, Grayston JT. 1971. Mycoplasma pneumoniae in the community. Am. J. Epidemiol. 93:55–67 [DOI] [PubMed] [Google Scholar]
- 6. Foy HM, Grayston JT, Kenny GE, Alexander ER, McMahan R. 1966. Epidemiology of Mycoplasma pneumoniae infection in families. JAMA 197:859–866 [PubMed] [Google Scholar]
- 7. Herrman K, Erdman DD. 1995. Diagnosis by serologic assays, p 121–138 In Lennette EH, Lennette DA, Lennette ET. (ed), Diagnostic procedures for viral, rickettsial and chlamydial infections, 7th ed American Public Health Association, Washington, DC [Google Scholar]
- 8. Hosker HS, Tam JS, Chain CH, Lai CK. 1993. Mycoplasma pneumoniae infection in Hong Kong–clinical and epidemiological features during an epidemic. Respiration 60:237–240 [DOI] [PubMed] [Google Scholar]
- 9. Klausner JD, et al. 1998. Enhanced control of an outbreak of Mycoplasma pneumoniae pneumonia with azithromycin prophylaxis. J. Infect. Dis. 177:161–166 [DOI] [PubMed] [Google Scholar]
- 10. Liu CL, et al. 2009. An outbreak of Mycoplasma pneumoniae pneumonia in a kindergarten. Zhonghua Yu Fang Yi Xue Za Zhi 43:206–209 [PubMed] [Google Scholar]
- 11. Lucier TS, Heitzman K, Liu S-K, Hu P-C. 1995. Transition mutations in the 23S rRNA of erythromycin-resistant isolates of Mycoplasma pneumoniae. Antimicrob. Agents Chemother. 39:2770–2773 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Roberts L, et al. 2000. Effect of infection control measures on the frequency of upper respiratory infection in child care: a randomized, controlled trial. Pediatrics 105:738–742 [DOI] [PubMed] [Google Scholar]
- 13. Sasaki T, et al. 1996. Epidemiological study of Mycoplasma pneumoniae infections in Japan based on PCR-restriction fragment length polymorphism of the P1 cytadhesin gene. J. Clin. Microbiol. 34:447–449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Su Z, Zhou Z. 2002. Investigation of an outbreak of Mycoplasma pneumoniae. Dis. Surveill. 17:415–416 [Google Scholar]
- 15. Talkington DF, Thacker WL, Keller DW, Jensen JS. 1998. Diagnosis of Mycoplasma pneumoniae infection in autopsy and open lung biopsy tissues by nested PCR. J. Clin. Microbiol. 36:1151–1153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Vester B, Douthwaite S. 2001. Macrolide resistance conferred by base substitutions in 23S rRNA. Antimicrob. Agents Chemother. 45:1–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Reference deleted.
- 18. Wilkinson HW, Cruce DD, Broome CV. 1981. Validation of Legionella pneumophila indirect immunofluorescence assay with epidemic sera. J. Clin. Microbiol. 13:139–146 [DOI] [PMC free article] [PubMed] [Google Scholar]