REPLY
We thank Dr. Thomson for his critical review of our manuscript entitled “Double-carbapenem therapy for carbapenemase-producing Klebsiella pneumoniae” (1). In his letter to the editor, Dr. Thomson attempts to attribute the enhanced activity that was seen with the combination regimen of ertapenem plus doripenem to the ability of ertapenem to substantially reduce the inoculum, thereby allowing enhancements in the activity of the second carbapenem. This conclusion is based upon the results of our in vitro chemostat model in which we showed that ertapenem alone achieved a rapid decrease in bacterial density of the carbapenemase-producing K. pneumoniae isolate (doripenem MIC of 4 μg/ml; ertapenem MIC of 64 μg/ml), with regrowth beginning at 4 h. Based upon the results of the chemostat model, the conclusion was that the efficacy that was seen in the in vivo murine thigh infection model was due to ertapenem causing an initial decrease in the bacterial burden of the animals and not due to ertapenem acting as a suicide substrate for the carbapenemase. Dr. Thomson theorizes that ertapenem caused an initial decrease in the inoculum density in the murine thighs to such a level as would have been manageable by doripenem alone.
While we agree that our theory of ertapenem acting as a suicide substrate has not yet been fully elucidated and additional studies are under way to provide more clarity on this topic, we are not in full agreement with Dr. Thomson's rationale for the enhanced efficacy seen in the animals. Although we did not show the efficacy of ertapenem alone in the initial immunocompetent murine thigh infection model, subsequent data generated in our laboratory support our theory. As shown in Fig. 1, in duplicate studies in which the human-simulated dosing regimen of ertapenem at 1 g every 24 h was administered to immunocompetent mice infected with one of three different carbapenemase-producing K. pneumoniae isolates (doripenem and ertapenem MIC values of 8 and >64 μg/ml, 16 and >64 μg/ml, and 32 and >64 μg/ml, respectively), this dosing regimen was able to achieve only net bacterial stasis compared to the 24-hour growth control. If the theory that Dr. Thomson postulates were true, we would have expected to see a much more marked decrease in the bacterial burden at 24 h in the animals exposed to the humanized concentration-time profile of ertapenem. As such, we do not believe that the initial exposure profile of ertapenem is sufficient in and of itself to reduce the in vivo bacterial inoculum and fully explain the efficacy that has been observed with the combination regimen.
Fig 1.
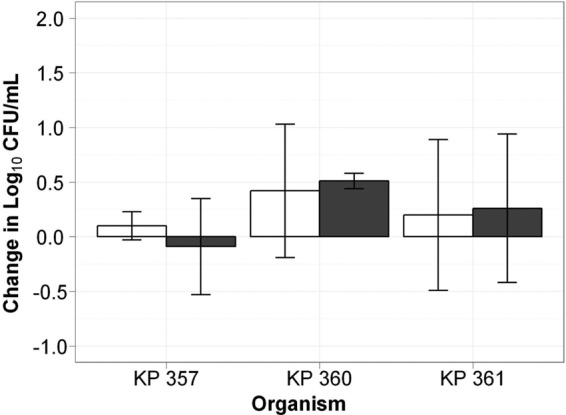
Comparative efficacies of ertapenem at 1 g every 24 h (gray bars) against the carbapenemase-producing K. pneumoniae isolates KP 357, KP 360, and KP 361 and of the growth control (white bars) in the in vivo murine thigh infection model.
In conclusion, we appreciate the theory that Dr. Thomson has put forward to help describe the efficacy seen with the double-carbapenem therapy against carbapenemase-producing K. pneumoniae. While we do not believe that a reduction in the in vivo inoculum due to ertapenem is the sole explanation for the beneficial effect of the double-carbapenem therapy against this organism, it is entirely possible that some reduction in bacterial density does occur and that this contributes to the enhanced activity of the combination regimen. Although elucidating the mechanism(s) responsible for our observation is important, the fact that humanized exposures of the double-carbapenem regimen enhanced the in vivo efficacy compared to that of monotherapy suggests a potential clinical role of this regimen in the management of this increasingly prevalent and highly drug-resistant carbapenemase-producing pathogen.
Contributor Information
Catharine C. Bulik, Translational Medicine Institute for Clinical Pharmacodynamics Latham, New York, USA
David P. Nicolau, Center for Anti-Infective Research and Development Hartford Hospital Hartford, Connecticut, USA
REFERENCE
- 1. Bulik CC, Nicolau DP. 2011. Double-carbapenem therapy for carbapenemase-producing Klebsiella pneumoniae. Antimicrob. Agents Chemother. 55:3002–3004 [DOI] [PMC free article] [PubMed] [Google Scholar]


