Abstract
Extended-spectrum-β-lactamase (ESBL)-producing pathogens are associated with extensive morbidity and mortality and rising health care costs. Scant data exist on the impact of antimicrobial therapy on clinical outcomes in patients with ESBL bloodstream infections (BSI), and no large studies have examined the impact of cefepime therapy. A retrospective 3-year study was performed at the Detroit Medical Center on adult patients with BSI due to ESBL-producing Klebsiella pneumoniae or Escherichia coli. Data were collected from the medical records of study patients at five hospitals between January 2005 and December 2007. Multivariate analysis was performed using logistic regression. One hundred forty-five patients with BSI due to ESBL-producing pathogens, including K. pneumoniae (83%) and E. coli (16.5%), were studied. The mean age of the patients was 66 years. Fifty-one percent of the patients were female, and 79.3% were African-American. Fifty-three patients (37%) died in the hospital, and 92 survived to discharge. In bivariate analysis, the variables associated with mortality (P < 0.05) were presence of a rapidly fatal condition at the time of admission, use of gentamicin as a consolidative therapeutic agent, and presence of one or more of the following prior to culture date: mechanical ventilation, stay in the intensive care unit (ICU), and presence of a central venous catheter. In multivariate analysis, the predictors of in-hospital mortality included stay in the intensive care unit (odds ratio [OR], 2.17; 95% confidence interval [CI], 0.98 to 4.78), presence of a central-line catheter prior to positive culture (OR, 2.33; 95% CI, 0.77 to 7.03), presence of a rapidly fatal condition at the time of admission (OR, 5.13; 95% CI, 2.13 to 12.39), and recent prior hospitalization (OR, 1.92; 95% CI, 0.83 to 4.09). When carbapenems were added as empirical therapy to the predictor model, there was a trend between empirical carbapenem therapy and decreased mortality (OR, 0.61; 95% CI, 0.26 to 1.50). When added to the model, receipt of empirical cefepime alone (n = 43) was associated with increased mortality, although this association did not reach statistical significance (OR, 1.66; 95% CI, 0.71 to 3.87). The median length of hospital stay was shorter for patients receiving empirical cefepime than for those receiving empirical or consolidated carbapenem therapy. In multivariate analysis, empirical therapy with cefepime for BSI due to an ESBL-producing pathogen was associated with a trend toward an increased mortality risk and empirical carbapenem therapy was associated with a trend toward decreased mortality risk.
INTRODUCTION
Pathogens containing extended-spectrum β-lactamase (ESBL) have become increasingly problematic in both health care and community settings (8, 10). These pathogens are associated with enormous morbidity and mortality and rising health care costs (11, 12, 16). Carbapenems are currently considered the optimal therapy for serious ESBL infections and are particularly effective if administered during the early stages of bacteremia (8, 11, 17, 20). Due to concerns regarding the emergence of resistance, carbapenems are often avoided as empirical therapy for hospitalized patients.
Cefepime, a “fourth-generation” cephalosporin, is frequently used as first-line empirical therapy for health care-associated infections, including those caused by suspected Gram-negative pathogens (5, 13). Cefepime has activity against most Gram-negative pathogens, including Enterobacteriaceae, due in part to its relatively low susceptibility to degradation by chromosomal and plasmid-mediated extended-spectrum AmpC β-lactamases and ESBLs compared to that of other cephalosporins. However, MICs of cefepime for Gram-negative organisms that produce AmpC β-lactamase and ESBLs are often increased compared to those for organisms that do not produce these β-lactamases (5, 9).
There is a concern that the effectiveness of cefepime is compromised in ESBL-producing organisms because of the increased MIC mediated by ESBL production (7, 15). Cefepime has performed suboptimally in the treatment of bacteremia due to ESBL-producing pathogens (5, 15). Although antimicrobial resistance to cefepime among Enterobacteriaceae was defined by the Clinical and Laboratory Standards Institute (CLSI) in 2010 as an MIC of >8 μg/ml, studies have demonstrated that as the MIC of cefepime increases to >2 μg/ml in ESBL-producing Enterobacteriaceae, standard cefepime doses may not be clinically effective (5, 15). In fact, two small studies of infections due to ESBL-producing pathogens that were “susceptible” to cefepime reported a statistically significant increase in bacteriologic failure and/or mortality as the MIC of cefepime increased (7, 14). However, there have been no large clinical studies to date reporting outcomes for patients with infections due to ESBL-producing pathogens treated with cefepime.
The issue of decreased efficacy for cefepime in the treatment of ESBL-producing pathogens has increased in importance since the CLSI breakpoints for several cephalosporins (but not cefepime) were decreased to make ESBL-producing Enterobacteriaceae easier to detect by standard susceptibility testing, and routine testing for ESBL production is no longer recommended. Since the breakpoints for cefepime have not been decreased and because ESBL testing is no longer recommended, ESBL-producing pathogens might be reported as “susceptible” to cefepime (4). Thus, clinicians might be prompted to use cefepime to treat an invasive infection due to an ESBL-producing pathogen, even though the efficacy for cefepime in these settings is largely unknown (3).
The efficacy of empirical therapy is a critical factor in determining outcomes for patients with bloodstream infection (BSI). There have been a number of studies examining optimal management for infections due to ESBL-producing organisms. However, few data exist regarding empirical cefepime therapy for ESBL-producing organisms (15, 20). Such data are important in determining whether or not empirical therapy with agents that have a broader spectrum of activity than cefepime, such as carbapenems, might be warranted among patients at risk for invasive infection due to ESBL-producing pathogens. However, there needs to be a balance between implementing effective empirical therapy and promoting antimicrobial resistance by prescribing unnecessarily broad-spectrum empirical therapeutic agents, such as carbapenems (16). As health care providers and antimicrobial stewardship leadership try to find the balance between providing appropriate empirical therapy and limiting the emergence of antimicrobial resistance, more data are needed to improve treatment decisions and antimicrobial policies. This study describes a large cohort of patients with BSI due to ESBL-producing Escherichia coli and Klebsiella pneumoniae and analyzes the impact of antimicrobial therapy, with a focus on cefepime, on clinical outcomes. Our study also examines associations between MICs of cefepime for ESBL-producing bloodstream pathogens and mortality.
MATERIALS AND METHODS
Study design.
The Detroit Medical Center (DMC) health care system consists of 8 hospitals, has more than 2,200 inpatient beds, and serves as a tertiary referral hospital for metropolitan Detroit and southeastern Michigan. A retrospective cohort study was conducted at the Detroit Medical Center, and data were collected from five hospitals from 1 January 2005 to 31 December 2007. Institutional review boards at Wayne State University and DMC approved the study before its initiation.
Study population.
Patients with blood cultures positive for ESBL-producing K. pneumoniae or E. coli were included in this study. All pediatric patients younger than 18 years of age were excluded from this study. Clinical data, including those related to demographics, laboratory data, recent exposure to antibiotics, comorbid conditions, McCabe score (1), Charlson score (2), recent exposure to health care-associated environments or devices, immunosuppressive conditions, acute-illness indices, empirical and consolidative antimicrobial therapy usage, and time to initiation of appropriate therapy, were retrieved from medical records by use of a case record form. All risk factors were captured 30 days prior to positive culture. Empirical antibiotics were defined as antibiotics initiated during the time spanning from 2 days before initial positive blood culture to 3 days after culture. Consolidative therapy was defined as antibiotic therapy administered between day 4 and day 7 after the date of initial positive blood culture. The outcomes were abstracted from each patient chart and included in-hospital mortality rate, duration of hospitalization following initial culture (number of days from culture to discharge), and number of hospital readmissions within 30 days following culture.
Microbiology.
DMC has a single centralized clinical microbiology laboratory, which processes ∼500,000 samples annually. Multiple outpatient facilities in southeast Michigan utilize DMC's laboratory services on a routine basis. Bacteria are identified to the species level, and susceptibilities are determined for predefined antimicrobials on the basis of an automated broth microdilution system (MicroScan; Siemens AG, Germany) and in accordance with the CLSI criteria (3).
ESBL-producing E. coli and K. pneumoniae were identified first using the MicroScan Walk-Away system to screen for ceftazidime and cefpodoxime resistance, with subsequent confirmation by demonstration of synergy between these two antibiotics and clavulanic acid on disc synergy testing (9).
Statistical analysis.
All analyses were performed using the SAS software program (version 9.2; SAS Institute, Cary, NC). The t test and the Wilcoxon rank sum test were used to analyze continuous variables, the chi-square and Fisher exact tests were used for bivariate analyses, and logistic regression was used for multivariate analyses. For multivariate model building, variables with P values of <0.10 in the bivariate analyses were included as candidate variables. Logistic regression with backwards selection was used to select for variables in the final model. Final models included variables with adjusted P values of <0.05. All candidate variables that were not selected for final model inclusion were checked for confounding. Confounders were defined as variables that changed the β-coefficients of selected variables by >10% when added back to the model. Confounding variables were incorporated into the final model. When less than 5% of data were missing, missing values for continuous variables were imputed to the mean and those for categorical variables were imputed to the mode. All P values were two sided.
RESULTS
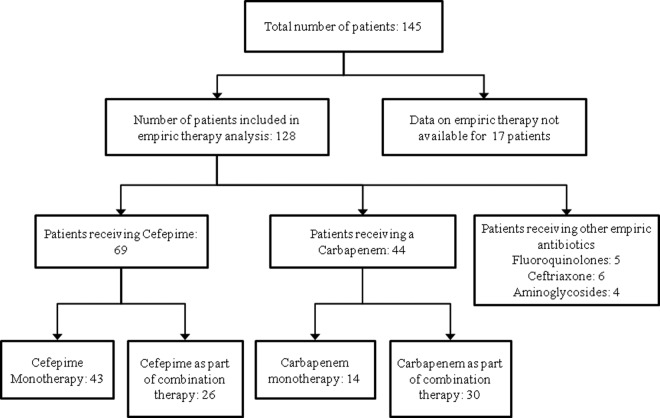
One hundred forty-five patients with BSI due to ESBL-producing organisms were identified at DMC during the 3-year study period; 83% of the patients were infected with K. pneumoniae, and 16.5% were infected with E. coli (Table 1). The mean age of the patients was 66 years, 51% of the patients were female, and 79.3% were African-American. Cefepime was utilized as an empirical antibiotic in 69 (48%) patients (as a single agent in 43 patients and in combination with another agent active against the pathogen in 26 patients) (Table 2). Carbapenems were used as empirical therapy in 44 (30.3%) patients (as monotherapy in 14 patients and in combination with another active agent in 30 patients). Of the 44 patients receiving empirical therapy with carbapenems, more than 75% received meropenem. Other empirical antibiotics utilized included fluoroquinolones (n = 5; 8%), ceftriaxone (n = 6; 11.3%), and aminoglycosides (n = 4; 7.5%) (Fig. 1).
Table 1.
Bivariate predictors of mortality among patients with bloodstream infection due to ESBL-producing Enterobacteriaceae
| Characteristic | Value for patientsa |
OR | 95 % CI | P | |
|---|---|---|---|---|---|
| Dead | Alive | ||||
| Demographics | |||||
| Male sex | 30 (56.60) | 41 (44.57) | 1.62 | 0.82–3.20 | 0.17 |
| African-American ethnicity | 38 (71.70) | 77 (83.70) | 0.49 | 0.21–1.11 | 0.09 |
| Age (mean ± SD) | 66.86 ± 14.88 | 65.45 + 16.24 | 0.42 | ||
| Severity of illness and comorbid conditions | |||||
| Hospitalization within 30 days prior to admission | 37 (69.81) | 51 (55.43) | 1.86 | 0.90–3.80 | 0.11 |
| Median duration of hospitalization within 30 days prior to positive culture (IQR) | 7 (0–19) | 2 (0–15) | 0.1 | ||
| Median Charlson scoreb (IQR) | 3 (0–6) | 4 (0–7) | 0.98 | ||
| Median McCabe score on admission (IQR) | 2 (1–2) | 2 (2–3) | <0.0001 | ||
| McCabe score of 1c | 25 (47.2) | 11 (2) | 6.57 | 2.86–15.06 | <0.0001 |
| Diabetes mellitus | 22 (41.51) | 48 (52.17) | 0.65 | 0.32–1.28 | 0.23 |
| Chronic obstructive lung diseases | 13 (24.53) | 20 (21.74) | 1.17 | 0.52–2.59 | 0.68 |
| Congestive heart failure | 22 (41.51) | 38 (41.30) | 1.00 | 0.50–2.00 | 1.00 |
| Cerebrovascular accident | 12 (8.28) | 32 (34.78) | 0.54 | 0.25–1.18 | 0.13 |
| Human immunodeficiency virus infection | 1 (1.89) | 1 (1.09) | 1.75 | 0.10–28.56 | 1.00 |
| Hemiplegia | 3 (5.66) | 13 (14.13) | 0.36 | 0.01–1.34 | 0.17 |
| Peptic ulcer disease | 6 (11.32) | 10 (10.87) | 1.04 | 0.35–3.06 | 1.00 |
| Peripheral vascular disease | 8 (15.09) | 24 (26.09) | 0.50 | 0.21–1.21 | 0.15 |
| Metastatic solid tumor | 6 (11.32) | 7 (7.61) | 1.55 | 0.50–4.88 | 0.55 |
| Moderate to severe liver disease | 8 (15.09) | 9 (9.78) | 1.64 | 0.59–4.54 | 0.42 |
| Myocardial infarction | 11 (20.75) | 19 (20.65) | 1.01 | 0.44–2.31 | 1.00 |
| Lymphoma | 0 (0.00) | 3 (3.30) | 0.62 | 0.549–0.70 | 0.29 |
| Leukemia | 2 (3.77) | 2 (2.17) | 1.76 | 0.24–12.90 | 0.62 |
| Low albumin level | 47 (88.68) | 71 (77.71) | 2.31 | 0.87–6.16 | 0.12 |
| ADLsd at time of admission | 20 (39.22) | 37 (39.36) | 0.99 | 0.00 | 1.00 |
| Need for assistance with bathing | 37 (72.55) | 70 (74.47) | 0.90 | 0.06 | 0.84 |
| Need for assistance with feeding | 32 (62.75) | 55 (58.51) | 1.19 | 0.24 | 0.72 |
| Need for assistance with ambulation | 37 (72.55) | 71 (75.53) | 0.85 | 0.15 | 0.69 |
| Bowel incontinence | 15 (28.3) | 26 (28.3) | 1.0 | 0.47–2.1 | 1.00 |
| Urine incontinence | 36 (70.59) | 65 (69.15) | 0.93 | 0.03 | 1.00 |
| Admission information and hospital exposures prior to culture | |||||
| Admission from home | 16 (31.37) | 38 (40.43) | 0.67 | 1.15 | 0.36 |
| Presence of ventilation | 28 (52.83) | 26 (28.26) | 2.84 | 1.40–5.75 | 0.004 |
| Admission to intensive care unit | 31 (58.49) | 29 (31.52) | 3.06 | 1.51–6.17 | 0.001 |
| Exposure to Foley catheter | 25 (47.10) | 46 (50.00) | 0.89 | 0.45–1.75 | 0.863 |
| Exposure to central-line catheter | 48 (90.57) | 61 (66.30) | 4.87 | 1.76–13.49 | 0.001 |
| Receipt of total parenteral nutrition | 4 (7.55) | 15 (16.30) | 0.42 | 0.13–1.33 | 0.20 |
| Admission to intensive care unit prior to culture | 23 (45.10) | 14 (14.89) | 4.69 | 15.87 | 1.19 |
| Organism(s) | |||||
| Escherichia coli | 8 (15.69) | 16 (17.02) | 0.2 | ||
| Klebsiella pneumoniae | 42 (81) | 79 (83) | |||
| Klebsiella pneumoniae and Escherichia coli | 1 (1.96) | 0 (0.00) | |||
| Empirical therapy | |||||
| Cefepime | 26 (49.06) | 443 (46.7) | 1.09 | 0.55–2.15 | 0.86 |
| Carbapenem | 15 (28.30) | 25 (27.17) | 1.06 | 0.49–2.24 | 1.00 |
| Cefotaxime | 1 (1.89) | 0 (0.00) | 0.36 | 0.30–132.09 | 0.36 |
| Ceftazidime | 0 (0.00) | 1 (1.09) | 0.63 | 0.55–0.71 | 1.00 |
| Cefoxitin | 0 (0.00) | 3 (3.26) | 0.63 | 0.55–0.711 | 0.30 |
| Ceftrioxone | 6 (11.32) | 15 (16.30) | 0.66 | 0.23–1.80 | 0.47 |
| Tobramycin | 4 (7.55) | 15 (16.30) | 0.42 | 0.13–1.33 | 0.20 |
| Ciprofloxacin | 5 (9.43) | 6 (6.52) | 1.50 | 0.43–5.15 | 0.53 |
| Consolidative therapy | |||||
| Cefepime alone | 3 (5.66) | 6 (6.52) | 0.86 | 0.20–3.59 | 1.00 |
| Cefazolin | 0 (0.00) | 3 (3.26) | 0.62 | 0.55–0.71 | 0.29 |
| Cefotaxime | 0 (0.00) | 1 (1.09) | 0.63 | 0.55–0.71 | 1.00 |
| Carbapenem alone | 12 (22.64) | 21 (22.83) | 0.98 | 0.44–2.21 | 1.00 |
| Gentamicin | 3 (5.66) | 0 (0.00) | 0.35 | 0.28–0.44 | 0.05 |
| Cefoxitin | 0 (0.00) | 1 (1.09) | 0.63 | 0.55–0.71 | 1.00 |
| Ceftrixone | 3 (5.66) | 6 (6.52) | 0.86 | 0.20–3.59 | 1.00 |
| Cefoxime | 5 (9.43) | 8 (8.70) | 1.09 | 0.33–3.53 | 1.00 |
| Piperacillin-tazobactam | 7 (13.21) | 11(11.96) | 1.12 | 0.40–3.09 | 0.80 |
| Tigecycline | 3 (5.66) | 5 (5.43) | 1.04 | 0.23–4.55 | 1.00 |
| Colistin | 2 (3.77) | 4 (4.35) | 0.86 | 0.15–4.87 | 1.00 |
| Amikacin | 4 (7.55) | 9 (9.78) | 0.75 | 0.22–2.57 | 0.76 |
| Amoxicillin | 1 (1.89) | 4 (4.35) | 0.42 | 0.04–3.88 | 0.65 |
| Ampicillin | 0 (0.00) | 1 (1.09) | 0.63 | 0.55–0.71 | 1.00 |
| Total | 53 (36.5) | 92 (63.5) | |||
Table 2.
Types of antimicrobial therapy and impacts on outcome
| Treatment and outcome (no. of patients) | No. (%) of patients with outcome: |
Pa | ORa | 95% CIa | |
|---|---|---|---|---|---|
| Present | Absent | ||||
| Empirical therapy | |||||
| Cefepime alone (43) | |||||
| Mortality | 17 (40) | 26 (60.5) | 0.7 | 1.19 | 0.57–2.49 |
| Readmission | 13 (30) | 30 (70) | 0.8 | 1.14 | 0.52–2.50 |
| Cefepime alone or in combination (69) | |||||
| Mortality | 26 (38) | 43 (62.3) | 0.86 | 1.09 | 0.55–2.15 |
| Readmission | 17 (25) | 52 (75.4) | 0.3 | 0.70 | 0.34–1.47 |
| Carbapenem alone (14) | |||||
| Mortality | 5 (36) | 9 (64.3) | 1.0 | 0.96 | 0.30–3.03 |
| Readmission | 2 (14.3) | 12 (86) | 0.3 | 0.39 | 0.08–1.83 |
| Carbapenem alone or in combination (40) | |||||
| Mortality | 15 (38) | 25 (63) | 1.0 | 1.05 | 0.49–2.24 |
| Readmission | 7 (17.5) | 33 (80.5) | 0.09 | 0.44 | 0.17–1.13 |
| Consolidative Therapy | |||||
| Cefepime alone (9) | |||||
| Mortality | 3 (33.3) | 6 (67) | 1.00 | 0.86 | 0.20– 3.59 |
| Readmission | 5 (56) | 4 (44) | 0.12 | 3.47 | 0.88–13.64 |
| Cefepime alone or in combination (31) | |||||
| Mortality | 9 (29) | 22 (71) | 0.40 | 0.65 | 0.27–1.54 |
| Readmission | 9 (29) | 22 (71) | 1.00 | 1.04 | 0.43–2.5 |
| Carbapenem alone (33) | |||||
| Mortality | 12 (36.4) | 21 (64) | 1.00 | 0.98 | 0.44–2.21 |
| Readmission | 9 (27) | 24 (73) | 1.00 | 0.93 | 0.39–2.23 |
| Carbapenem alone or in combination (78) | |||||
| Mortality | 25 (32) | 53 (68) | 0.23 | 0.65 | 0.33–1.29 |
| Readmission | 21 (27) | 57 (73) | 0.71 | 0.86 | 0.41–1.78 |
These analyses were conducted by comparing patients who had the antibiotic exposure variable to patients who did not have the antibiotic exposure variable.
Fig 1.
Flow chart describing the different types of empirical antibiotic therapy used in patients suspected of having bloodstream infections due to ESBL-producing Enterobacteriaceae.
Thirty-one patients received consolidative therapy with an agent other than cefepime or carbapenem that was not active against the ESBL-producing pathogen, and 23 patients did not receive any consolidative therapy, due to a variety of reasons, including early death or discharge. The consolidative antimicrobial therapy regimens included a carbapenem alone (n = 33; 23%), piperacillin-tazobactam (n = 18; 12.4%), combination therapy with cefepime and a carbapenem (n = 16; 11%), ciprofloxacin (n = 13; 9%), amikacin (n = 13; 9%), cefepime alone (n = 9; 6.2%), and tigecycline (n = 8; 5.5%) (Table 2). Of the 78 patients who received consolidative therapy with a carbapenem, 45 received meropenem, 22 imipenem, and 11 ertapenem.
Mortality.
Of the 145 patients with BSI due to ESBL-producing organisms, 53 patients (37%) died in the hospital and 92 survived to discharge (Table 1). In bivariate analysis, the variables associated with mortality (P < 0.05) were McCabe score at admission, use of gentamicin as a consolidative therapeutic agent, and presence of one or more of the following prior to culture date: mechanical ventilation, stay in the intensive care unit (ICU), and presence of central venous catheter. When patients with vascular catheter-related BSI due to ESBL-producing pathogens (n = 122) were compared to patients with a source of BSI other than a vascular catheter (n = 23), the differences in mortality between the two groups were not found to reach statistical significance (34% versus 48%; P = 0.24; odds ratio [OR], 0.5; 95% confidence interval [CI], 0.23 to 1.40). There were no significant associations between antimicrobial therapy and mortality. Forty percent of patients who received cefepime monotherapy died, compared to 35% of patients who did not receive cefepime monotherapy (P = 0.7) (Table 2). Thirty-eight percent of patients who received carbapenem therapy (alone or in combination) died, compared to 36% of patients who did not receive a carbapenem (P = 1.0). Neither cefepime nor carbapenem consolidative therapy was associated with in-hospital mortality (Table 2).
In multivariate analysis, the predictor model for in-hospital mortality included prior admission to the intensive care unit (OR, 2.17; 95% CI, 0.98 to 4.78), presence of a central-line catheter prior to positive culture (OR, 2.33; 95% CI, 0.77 to 7.03), presence of a rapidly fatal condition at the time of admission (OR, 5.13; 95% CI, 2.13 to 12.39), and prior hospitalization (OR, 1.92; 95% CI, 0.83 to 4.09) (Table 3). The independent impact of empirical antimicrobial agents was analyzed by adding different antimicrobial agents to the predictor model. When added to the model, receipt of empirical cefepime alone (n = 43) was associated with increased mortality, although this association did not reach statistical significance (OR, 1.66; 95% CI, 0.71 to 3.87). When empirical carbapenems were added to the model, there was a trend between empirical carbapenem therapy and decreased mortality (OR, 0.61; 95% CI, 0.26 to 1.50).
Table 3.
Independent predictors of in-hospital mortality
| Variable | Odds ratio | 95% CI |
|---|---|---|
| Admission to ICU prior to culture | 2.17 | 0.98–4.78 |
| Presence of central-line catheter prior to culture | 2.33 | 0.77–7.03 |
| Prior hospitalization | 1.92 | 0.83–4.09 |
| McCabe score of 1a | 5.13 | 2.12–12.4 |
A McCabe score of 1 at the time of hospital admission, indicating the presence of a rapidly fatal underlying condition (1).
The independent impact of consolidative antimicrobial was analyzed by adding different consolidative antimicrobial agents to the predictor model. In multivariate analysis, neither cefepime (OR, 0.8; 95% CI, 0.34 to 2.29) nor carbapenem (OR, 0.5; 95% CI, 0.25 to 1.21) therapy was significantly associated with mortality. Additional data regarding consolidative therapy and mortality are shown in Table 2.
To further analyze the impact of cefepime therapy and the role of MIC on mortality, a subanalysis limited to the 43 patients who were treated with empirical cefepime alone was performed (Table 4). There was no association between increasing MIC of cefepime and mortality. Thirteen patients on empirical cefepime therapy had cefepime MICs of ≤2. Five of the patients (39%) died (Table 4). Due to the small number of patients in this group, no further analyses were conducted. Due to restrictions imparted by use of automated panel breakpoints, we were unable to compare patients with an infection due to an ESBL producer with a cefepime MIC of ≤1 to patients with an infection due to an ESBL producer with a cefepime MIC of >1 and ≤2.
Table 4.
Cefepime MIC and mortality among patients who received empirical therapy with cefepime alone
| Cefepime MIC (μg/ml) | In-hospital mortality rate (no. of deaths/total no. of patients) (%) |
|---|---|
| ≤2 | 5/13 (39) |
| 4 | 1/4 (25) |
| 8 | 1/2 (50) |
| ≥16 | 10/24 (42) |
Readmission to the hospital.
The rate of readmission among surviving patients was 39%. There were no significant associations between type of empirical or consolidative antimicrobial regimen administered and readmission rate (Table 2).
Length of stay after culture.
The median length of hospital stay after positive culture for the cohort was 10 days (interquartile range [IQR], 5 to 16 days). Length of stay was shorter for patients receiving empirical cefepime (7 days [IQR, 4 to 11 days]) and was longer for patients receiving empirical (15 days [IQR, 9 to 20 days]) or consolidative carbapenem (12 days [IQR, 9 to 16 days]) therapy. Length of stay was shorter for patients who were directly admitted to the ICU than for those who had histories of hemiplegia or peripheral vascular disease (P < 0.05).
DISCUSSION
There has been recent debate in the literature as to whether the use of cefepime is an effective therapy for the treatment of infections due to ESBL-producing pathogens (5, 9). While much focus has been directed toward the use of cefepime in febrile neutropenia and for treatment of infection with relatively susceptible Gram-negative bacilli (6), no studies have focused on the impact of cefepime therapy for treatment of infections with ESBL-producing organisms. This study describes outcomes in a large urban health care system where cefepime was used as a “workhorse” agent for both empirical and consolidative treatment of infections due to health care-associated Gram-negative bacilli. This is the first study to analyze the impact of cefepime therapy on outcomes for patients with bloodstream infections due to ESBL-producing Enterobacteriaceae. Although this study did not identify statistically significant differences in mortality between patients receiving carbapenem therapy and those receiving cefepime therapy in multivariate analysis, trends were noted between empirical cefepime therapy and increased risk of mortality and between carbapenem therapy and decreased risk of mortality. These trends might be related to the greater in vitro activity of carbapenems than of cefepime against ESBL producers and the greater stability of carbapenems to degradation by ESBLs. The association between empirical carbapenem therapy and a trend toward improved survival is consistent with recommendations for treatment of invasive infections due to ESBL producers with a carbapenem. This study was not able to demonstrate an association between increased MIC of cefepime and mortality among patients receiving cefepime monotherapy but might have been underpowered to identify an association.
This study identified other predictors of mortality that have been reported by other investigators and that are markers for increased severity of illness or exposure to health care-associated pathogens. These included presence of a rapidly fatal condition at admission (1), history of central-line-catheter insertion, admission to the intensive care unit, and prior hospitalization (19).
Empirical carbapenem therapy was associated with a longer duration of hospitalization than cefepime therapy. The explanation for this association is unclear but might be related to an increased level of severity of illness among patients receiving empirical carbapenem therapy.
In any observational study of antimicrobial therapy, the issue of confounding by indication is an inherent challenge. This type of bias pertains to differential antimicrobial-prescribing practices by physicians based on how acutely or chronically ill their patients are (e.g., patients receiving empirical carbapenem therapy might have a higher level of severity of illness than patients receiving alternative antimicrobials). Although this study attempted to address this bias by controlling for severity of illness in multivariate analysis, there might have been residual confounding that biased results toward underestimating the true effect of antimicrobial therapeutic choices on outcomes. The carbapenems in this study were almost exclusively type 2 carbapenems (predominantly meropenem, which provides reliable coverage against Pseudomonas aeruginosa, Acinetobacter spp., and enterococci) and not type 1 carbapenems such as ertapenem (which provides no reliable coverage against P. aeruginosa, Acinetobacter spp., or enterococci).
Few patients in this study received piperacillin-tazobactam, an antimicrobial frequently used for empirical therapy in hospitalized patients. A recent study suggested that amoxicillin-clavulanic acid and piperacillin-tazobactam were suitable alternatives to carbapenems for treating patients with bloodstream infection due to ESBL-producing Escherichia coli originating from urinary and biliary sources (18). Due to its observational nature, this recent study had many of the same limitations as the present study. Future studies should focus on the role of type 1 carbapenems, amoxicillin-clavulanic acid, and piperacillin-tazobactam in the treatment of BSI due to ESBL-producing pathogens.
The results from this study support the continued use of cefepime for empirical therapy of suspected bloodstream infection with Gram-negative organisms among hospitalized patients. Patients at increased risk for infection with an ESBL-producing pathogen and for mortality, including those with recent ICU exposure and rapidly fatal underlying conditions, might benefit from empirical carbapenem therapy. For patients who have confirmed bacteremia due to an ESBL-producing pathogen, carbapenems should remain the antimicrobial agents of choice.
ACKNOWLEDGMENTS
This study was supported in part by a research grant from the Investigator-Initiated Studies Program of Merck Sharp & Dohme Corporation. Keith S. Kaye was supported in part by the National Institutes of Health (protocol number 10-0065).
The opinions expressed in this paper are those of the authors and do not necessarily represent those of Merck Sharp & Dohme Corporation.
Footnotes
Published ahead of print 30 April 2012
REFERENCES
- 1. Bion JF, Edlin SA, Ramsay G, McCabe S, Ledingham IM. 1985. Validation of a prognostic score in critically ill patients undergoing transport. Br. Med. J. (Clin. Res. Ed.) 291:432–434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Charlson ME, Pompei P, Ales KL, MacKenzie CR. 1987. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J. Chronic Dis. 40:373–383 [DOI] [PubMed] [Google Scholar]
- 3. CLSI 2009. Performance standards for antimicrobial susceptibility testing; 19th informational supplement. CLSI document M100-S19. Clinical and Laboratory Standards Institute, Wayne, PA [Google Scholar]
- 4. CLSI 2010. Performance standards for antimicrobial susceptibility testing; 21st informational supplement. CLSI document M100-S21. Clinical and Laboratory Standards Institute, Wayne, PA [Google Scholar]
- 5. Endimiani A, Perez F, Bonomo RA. 2008. Cefepime: a reappraisal in an era of increasing antimicrobial resistance. Expert Rev. Anti Infect. Ther. 6:805–824 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Freifeld AG, et al. 2011. Clinical practice guideline for the use of antimicrobial agents in neutropenic patients with cancer: 2010 update by the Infectious Diseases Society of America. Clin. Infect. Dis. 52:e56–e93 doi:10.1093/cid/cir073 [DOI] [PubMed] [Google Scholar]
- 7. Goethaert K, et al. 2006. High-dose cefepime as an alternative treatment for infections caused by TEM-24 ESBL-producing Enterobacter aerogenes in severely-ill patients. Clin. Microbiol. Infect. 12:56–62 [DOI] [PubMed] [Google Scholar]
- 8. Goossens H, Grabein B. 2005. Prevalence and antimicrobial susceptibility data for extended-spectrum beta-lactamase- and AmpC-producing Enterobacteriaceae from the MYSTIC Program in Europe and the United States (1997–2004). Diagn. Microbiol. Infect. Dis. 53:257–264 [DOI] [PubMed] [Google Scholar]
- 9. Jones RN, Marshall SA. 1994. Antimicrobial activity of cefepime tested against Bush group I beta-lactamase-producing strains resistant to ceftazidime. A multilaboratory national and international clinical isolate study. Diagn. Microbiol. Infect. Dis. 19:33–38 [DOI] [PubMed] [Google Scholar]
- 10. Kaye KS, Engemann JJ, Fraimow HS, Abrutyn E. 2004. Pathogens resistant to antimicrobial agents: epidemiology, molecular mechanisms, and clinical management. Infect. Dis. Clin. North Am. 18:467–511 [DOI] [PubMed] [Google Scholar]
- 11. Lee CC, et al. 2010. Bacteremia due to extended-spectrum-β-lactamase-producing Enterobacter cloacae: role of carbapenem therapy. Antimicrob. Agents Chemother. 54:3551–3556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Lee SY, Kotapati S, Kuti JL, Nightingale CH, Nicolau DP. 2006. Impact of extended-spectrum beta-lactamase-producing Escherichia coli and Klebsiella species on clinical outcomes and hospital costs: a matched cohort study. Infect. Control Hosp. Epidemiol. 27:1226–1232 [DOI] [PubMed] [Google Scholar]
- 13. Masterton R, Drusano G, Paterson DL, Park G. 2003. Appropriate antimicrobial treatment in nosocomial infections-the clinical challenges. J. Hosp. Infect. 55(Suppl. 1):1–12 [DOI] [PubMed] [Google Scholar]
- 14. Paterson DL, et al. 2001. Outcome of cephalosporin treatment for serious infections due to apparently susceptible organisms producing extended-spectrum beta-lactamases: implications for the clinical microbiology laboratory. J. Clin. Microbiol. 39:2206–2212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Paterson DL, et al. 2004. Antibiotic therapy for Klebsiella pneumoniae bacteremia: implications of production of extended-spectrum beta-lactamases. Clin. Infect. Dis. 39:31–37 [DOI] [PubMed] [Google Scholar]
- 16. Pena C, et al. 2008. Infections due to Escherichia coli producing extended-spectrum beta-lactamase among hospitalised patients: factors influencing mortality. J. Hosp. Infect. 68:116–122 [DOI] [PubMed] [Google Scholar]
- 17. Ramphal R, Ambrose PG. 2006. Extended-spectrum beta-lactamases and clinical outcomes: current data. Clin. Infect. Dis. 42(Suppl. 4):S164–S172 [DOI] [PubMed] [Google Scholar]
- 18. Rodriguez-Bano J, Navarro MD, Retamar P, Picon E, Pascual A. 2012. beta-Lactam/beta-lactam inhibitor combinations for the treatment of bacteremia due to extended-spectrum beta-lactamase-producing Escherichia coli: a post hoc analysis of prospective cohorts. Clin. Infect. Dis. 54:167–174 [DOI] [PubMed] [Google Scholar]
- 19. Tumbarello M, et al. 2007. Predictors of mortality in patients with bloodstream infections caused by extended-spectrum-beta-lactamase-producing Enterobacteriaceae: importance of inadequate initial antimicrobial treatment. Antimicrob. Agents Chemother. 51:1987–1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Zanetti G, et al. 2003. Cefepime versus imipenem-cilastatin for treatment of nosocomial pneumonia in intensive care unit patients: a multicenter, evaluator-blind, prospective, randomized study. Antimicrob. Agents Chemother. 47:3442–3447 [DOI] [PMC free article] [PubMed] [Google Scholar]