Abstract
The Escherichia coli LolA protein is a lipoprotein-specific chaperone that carries lipoproteins from the inner to the outer membrane. A dominant negative LolA mutant, LolA(I93C/F140C), in which both 93Ile and 140Phe are replaced by Cys, binds tightly to the lipoprotein-dedicated ABC transporter LolCDE complex on the inner membrane and therefore inhibits the detachment of outer membrane-specific lipoproteins from the inner membrane. We found that the expression of this mutant strongly induced lolA gene transcription. The depletion of the LolA or LolB protein also triggered lolA gene transcription, indicating that the inhibition of outer membrane lipoprotein transport triggers lolA transcription. To elucidate the mechanism, we isolated mutants that are unable to induce lolA transcription using the lacZ gene fused to the lolA promoter as a reporter and found that the Rcs phosphorelay system directly regulates lolA transcription. An outer membrane lipoprotein, RcsF, was essential for this activation, while the coactivator RcsA was dispensable. Taking the observation that an RcsF mutant localized in the inner membrane constitutively activated the Rcs phosphorelay system into consideration, the results shown here strongly suggest that correct lipoprotein sorting to the outer membrane is monitored by RcsF, which plays a key role in the Rcs stress response system.
INTRODUCTION
In the envelope of Escherichia coli, there are over 90 different species of lipoproteins, most of which are assumed to be localized to the inner leaflet of the outer membrane (41). Their N-terminal Cys residues are modified by lipid moieties, which anchor lipoproteins to membranes. The Lol system, composed of five Lol proteins, is responsible for the sorting and transport of lipoproteins to the outer membrane (31). After translocation across the inner membrane by the Sec translocon, lipoprotein precursors with signal peptides sequentially undergo modification with lipids and cleavage by enzymes on the periplasmic surface of the inner membrane. Mature lipoproteins destined for the outer membrane are then detached from the inner membrane through the action of LolCDE, an ATP-binding-cassette transporter, and bind to LolA, a lipoprotein-specific chaperone in the periplasm. A water-soluble LolA-lipoprotein complex travels through the periplasm to the outer membrane, where lipoproteins are transferred from LolA to the lipoprotein-specific acceptor LolB anchored to the periplasmic surface of the outer membrane. Finally, LolB embeds lipoproteins in the inner leaflet of the outer membrane.
LolA is composed of 11 antiparallel β-strands and three α helices, which form an incomplete β-barrel structure with a cavity inside (37). The cavity is covered by a lid comprising short α helices. The inside of the cavity is hydrophobic and binds to the acyl chains of lipoproteins (30). To study the importance of the opening and closing of the lid covering the cavity, we constructed a LolA mutant protein having two mutations, I93C and F140C (43). This mutant is fixed in the closed-lid conformation by the disulfide bridge formed between the two Cys residues in the absence of a reductant. LolA(I93C/F140C) revealed that the opening and closing of the lid are essential for continuous lipoprotein transfer from LolCDE to LolA (29, 43). This mutant stably bound to LolCDE on the inner membrane and therefore severely inhibited the growth of cells even in the presence of wild-type LolA. Furthermore, this mutant triggered the envelope stress response via the Cpx system (40), which is one of the five envelope stress response systems in E. coli (4).
During the study of this Cpx envelope stress response, we found that the expression of LolA(I93C/F140C) increased the expression of LolA. We show here that the transcription of the lolA gene is induced by the expression of LolA(I93C/F140C) in a manner dependent on the Rcs phosphorelay system, another stress response system comprised of RcsA, RcsB, RcsC, RcsD, and RcsF (21). These rcs genes were initially identified as activators of capsular polysaccharide synthesis in E. coli (15). Later, they were shown to regulate the transcriptions of genes involved in various cellular processes, mostly cell surface-related functions (4, 17, 21). RcsC and RcsD are inner membrane kinases involved in the phosphorylation of the cytoplasmic response regulator RcsB. Under certain growth conditions, the conserved His residue of the sensor kinase RcsC is autophosphorylated, followed by the transfer of the phosphate to the Asp residue in the C-terminal receiver domain of RcsC. The phosphate is then further transferred to the conserved His residue in the phosphotransfer domain of RcsD and finally to the Asp residue in the receiver domain of RcsB. Phosphorylated RcsB induces the transcription of target genes. A subset of target promoters requires the RcsA coactivator protein for transcriptional activation. RcsF, an outer membrane lipoprotein, is considered to function as a sensor and to transfer a signal to the RcsC sensor kinase under certain environmental conditions (7, 22).
MATERIALS AND METHODS
Bacterial strains, plasmids, and media.
The bacterial strains and plasmids used in this study are listed in Table 1. MY201 is an ara+ revertant of MC4100 (40) and was used as the parental strain. Strains carrying the specified rcs gene, which had been disrupted by a Kmr insertion, were obtained from the Keio Collection single-gene-deletion library (1). When required, the Kmr cassette sequence was removed by transforming the cells with pCP20 (10). pSW77 and pSWIF encode wild-type LolA tagged with FLAG at the C terminus and LolA(I93C/F140C), respectively (43). A strain carrying the lolA-lacZ fusion gene was constructed as follows. A DNA fragment encompassing the promoter region of the lolA gene (between bases −346 and +4 with respect to the translational initiation codon) was amplified by PCR using chromosomal DNA of MC4100 as a template and a pair of primers, lolAp5 and lolAp3 (Table 2). The amplified fragment was digested with EcoRI and BamHI and cloned into the same sites of a lac operon fusion vector, pRS551 (35). The resulting fusion plasmid was linearized with SalI and then transformed into TE2680 (12), which carries a DNA sequence homologous to the fusion vector. This allowed recombination between the transfected plasmid and the chromosome, generating a stable single-copy lolA-lacZ fusion gene at the trp locus on the chromosome. The single-copy fusion gene thus constructed on the chromosome of TE2680 was transduced into wild-type strain MY201 by P1 transduction, generating MY301. Strains carrying other lacZ promoter fusions were constructed similarly.
Table 1.
Bacterial strains and plasmids
| Strain or plasmid | Description | Reference |
|---|---|---|
| E. coli strains | ||
| MC4100 | Δ(argF-lac)U169 araD139 rpsL150 thiA1 relA1 flbB5301 deoC1 ptsF25 rbsR | 6 |
| MY201 | ara+ revertant of MC4100 | 40 |
| MY301 | MY201 lolA-lacZ | This study |
| MY302 | MY201 lolB-lacZ | This study |
| MY303 | MY201 lolC-lacZ | This study |
| MY304 | MY301 cpxA::spc | This study |
| MY418 | MY201 rprA-lacZ | This study |
| MY419 | MY201 wza-lacZ | This study |
| MY3011 | MY301 rcsF1::mini-Tn10 | This study |
| MY3012 | MY301 rcsC2::miniTn10 | This study |
| MY3018 | MY301 rcsF8::mini-Tn10 | This study |
| MY412 | MY301 ΔrcsA | This study |
| MY413 | MY301 ΔrcsB | This study |
| MY414 | MY301 ΔrcsC | This study |
| MY415 | MY301 ΔrcsD | This study |
| MY416 | MY301 ΔrcsF | This study |
| MY425 | MY301 Pbad-lolA 6×His lolA::Cm | This study |
| MY427 | MY301 Pbad-lolB lolB::Cm | This study |
| Plasmids | ||
| pRS551 | lacZ operon fusion vector; Apr Kmr | 35 |
| pCP20 | Thermoinducible FLP recombinase; Apr Camr | 10 |
| pKD32 | Template plasmid for Cmr cassette amplification | 10 |
| pKD119 | oriR101 repA101(Ts) Pbad-gam-bet-exo Tetr | 10 |
| pBAD18 | Pbad expression vector; Apr | 16 |
| pAM201 | lolA 6×His | 27 |
| pSW77 | Pbad-lolA Cmr | 43 |
| pSWIF | Pbad-lolA(I93C/F140C) Cmr | 43 |
| pMY114 | pBAD18 Pbad-lolA 6×His | This study |
| pMY117 | pBAD18 Pbad-lolB | This study |
Table 2.
Oligonucleotides used in this study
| Primer | Sequence |
|---|---|
| lolAp5 | TAGAATTCCGTTGTTCGATCAGGCGG |
| lolAp3 | CTGGATCCTCATTATTCCTCAAATTACGTC |
| lolBp5 | TAGAATTCTTCTCGCAGCGATACAGGTG |
| lolBp3 | CTGGATCCAGTGATGACAAGTCCTTGAGA |
| lolCp5 | TAGAATTCGGATAAAAGGTAATGACCGAG |
| lolCp3 | CTGGATCCGTCTGGTTGCTGTAGCAAAG |
| wzap5 | TAGAATTCATTTAAGAAATATCGCATGTGG |
| wzap3 | ATGGATCCTTTCCAATAATGCAGGAGGAAG |
| rprAp5 | TAGAATTCCAGCTGGTAGTACCTGTCG |
| rprAp3 | ATGGATCCACCGTGAGCTAATAGTAGGC |
| lolAEco | TAGAATTCTTTGAGGAATAATGATGAA |
| lolA6His-rv | ATGGTACCTTAATGATGATGATGATGATGC |
| lolB-Ec | GCGAATTCCTAGCATTAAGGGTTATAACTG |
| lolB-Sp | TTGCATGCACTAACGCGTCTTATCTGGCC |
| lolA-f | GGAACTCCCGATCGGGAGTGACGTAATTTGAGGAATAATGGTGTAGGCTGGAGCTG |
| lolA-r | GTTGAAAAGTATTATCCGAAAAATCGAGCGACAGATTGCTCATATGAATATCCTCCT |
| lolB-f | GGGTTATAACTGCAACGTATCTCAAGGACTTGTCATCACTGTGTAGGCTGGAGCTGCTTC |
| lolB-r | AAAACAGATTAAGTTTTGCCGGAGAGGGCCACTGTGTCCGCATATGAATATCCTCCTTA |
| lolARv | GCCCAAACGCTGCTTGCTACTAAGCTTGAGAG |
Cells were routinely grown in LB medium (26) at 37°C with aeration. MacConkey lactose plates were used to screen for mutants defective in lolA transcription activation. Chloramphenicol and kanamycin were each added to a final concentration of 25 μg/ml, when required. Ampicillin was added to 100 μg/ml.
Depletion of Lol proteins.
A DNA fragment carrying the lolA gene tagged with 6×His at its C terminus was amplified by PCR using pAM201 (27) as a template and a pair of primers, lolAEco and lolAHis-rv (Table 2). The amplified fragment was digested with EcoRI and KpnI and then cloned into the same sites of pBAD18 (16). The resulting plasmid, pMY114, was placed onto the chromosome of MY301 by using the λInCh recombination system, as described previously (3), yielding strain MY422, which carries lolA under the control of the araBAD promoter. A chloramphenicol resistance cassette was amplified by PCR using pKD32 as a template and a pair of primers, lolA-f and lolA-r, having sequences homologous to those flanking the lolA gene and 20-nucleotide priming sequences for pKD32 (10). To disrupt the authentic lolA gene, the amplified DNA fragment was electroporated into MY422 cells harboring pKD119 (10). Cells were plated onto LB agar containing chloramphenicol and then incubated at 37°C. Several chloramphenicol-resistant colonies were selected and examined for a disruption of the lolA gene by PCR using primers specific for the inserted chloramphenicol-resistant cassette and the sequences surrounding lolA. One of the clones with a correct chromosomal lolA deletion was chosen and named MY425. The LolB-depleting strain was constructed in a similar manner, except that the untagged LolB protein was expressed from the araBAD promoter. In brief, the coding region of lolB was amplified by PCR using MC4100 chromosomal DNA as a template and a pair of primers, lolB-Ec and lolB-Sp, followed by cloning into the EcoRI-SphI sites of pBAD18. The resulting plasmid was placed onto the chromosome of MY301 as described above, generating MY426. The coding region of the authentic lolB gene in MY426 was replaced by the chloramphenicol-resistant cassette as described above, resulting in the construction of MY427.
Cells carrying the lol genes under the control of the araBAD promoter were grown overnight in the presence of 0.2% arabinose. Cells were harvested by centrifugation and resuspended in the original volume of LB medium without arabinose. Cells were then diluted 100-fold with fresh LB medium containing 0.2% arabinose or 0.2% glucose and grown for 3 h. After the 100-fold dilution was repeated once more, cells were grown for a further 3 h. The cells in the presence of glucose ceased to grow by the end of this period. The β-galactosidase activity expressed from the lolA-lacZ fusion gene was measured as described previously (26). The depletion of the LolA and LolB proteins was confirmed by Western blotting. Antisera against LolA and LolB were described previously (24, 25).
Screening of mutants defective in lolA transcription activation.
MY301 was randomly mutagenized with mini-Tn10 using λ1098 as described previously (26). Mutagenized cells were transformed with pSWIF and plated onto MacConkey lactose plates containing 0.02% arabinose to moderately express LolA(I93C/F140C). Colonies exhibiting reduced color development were purified on the same plates. The inability to induce lolA transcription upon LolA(I93C/F140C) expression was examined in cells grown in liquid medium. The linkage between transposon insertions and phenotypes was confirmed after the transfer of the mini-Tn10 insertions into MY301 by P1 transduction. Clones with mutations of the lolA-lacZ reporter itself were discarded by examining the link between the Kmr (linked to lolA-lacZ) marker and the phenotype. The chromosomal locations of the mini-Tn10 insertions were determined by the sequencing of the DNA fragments of the junctions between the ends of mini-Tn10 and the chromosome, which were amplified by inverse PCR as described previously (19).
Primer extension analysis of lolA transcripts.
Total RNA was prepared from cells expressing or lacking LolB with RNAprotect bacterial reagent and an RNeasy minikit (Qiagen). Ten micrograms of total RNA was hybridized to a 32P-labeled primer specific for lolA (lolA-Rv [1 pmol]), followed by extension with Primescript reverse transcriptase (TaKaRa Shuzo). Extension products were analyzed on a 6% denaturing polyacrylamide gel, along with the sequencing reaction products with the same primer. A DNA fragment containing the lolA promoter region was amplified by PCR with a pair of primers (lolAp5 and lolARv) and MC4100 chromosomal DNA as a template and then used as a template for the sequencing reaction. Gels were dried, exposed to an imaging plate, and visualized with an FLA9000 image analyzer (Fujifilm).
RESULTS
LolA(I93C/F140C) induces lolA expression.
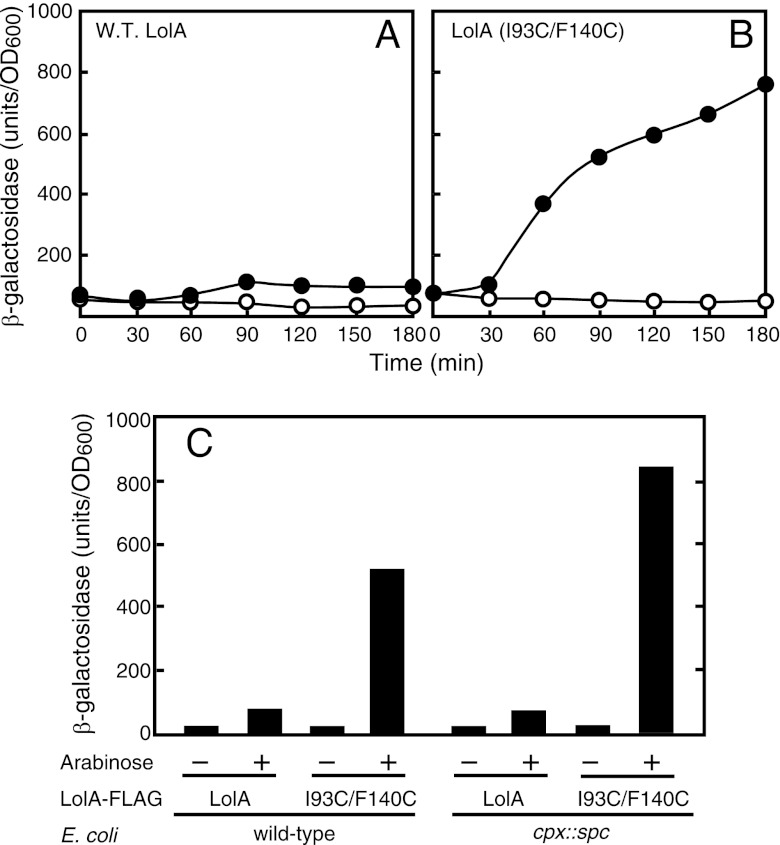
In our previous study (40), we noticed that the amount of LolA increased upon the overexpression of LolA(I93C/F140C). To quantitatively assess the expressions of lol genes, we fused the promoter regions of the respective lol genes to the coding region of lacZ, and each resulting fusion gene was then placed onto the chromosome as a single copy. The strains carrying the lol promoter-lacZ fusion gene were transformed with a plasmid encoding wild-type LolA or LolA(I93C/F140C) under the control of the araBAD promoter. Cells were grown to the early logarithmic phase of growth, and the expressions of the wild type and LolA(I93C/F140C) were induced with arabinose (0.2%).
The expression of LolA(I93C/F140C) markedly increased the activity of β-galactosidase encoded by the lolA-lacZ fusion gene (Fig. 1). The increase in β-galactosidase activity upon the induction of wild-type LolA was small but reproducible and seemed to be important, as discussed below (see Discussion). We previously reported that LolA(I93C/F140C) induced the Cpx-dependent envelope stress response (40). To determine whether or not the triggering of lolA expression is dependent on the Cpx system, we introduced the cpxR::spc mutation into a strain carrying lolA-lacZ and measured β-galactosidase activity. The cpx mutation did not decrease the lolA expression level induced by the expression of wild-type LolA or LolA(I93C/F140C) (Fig. 1C), indicating that the triggering of lolA transcription observed here is not dependent on the Cpx envelope stress response.
Fig 1.
Induction of lolA expression by LolA(I93C/F140C). (A and B) MY301 cells harboring either pSW77 (A) or pSWIF (B) were grown to the early logarithmic phase of growth (optical density at 600 nm [OD600] of 0.2), and the expressions of LolA and LolA(I93C/F140C) were then induced with 0.2% arabinose at time zero (closed circles) or not induced (open circles). Samples were taken periodically to measure the β-galactosidase activity expressed from the lolA-lacZ fusion gene. (C) MY301 (wild-type [W.T.]) and MY304 (cpx::spc) cells harboring either pSW77 or pSWIF were grown as described above, and the expression of the plasmid-encoded LolA protein was induced with 0.2% arabinose. β-Galactosidase activity was measured after 2 h of cultivation.
The expressions of lolB and lolC were examined in a similar manner with strains carrying the lolB-lacZ and lolC-lacZ fusion genes, respectively. In contrast to that of lolA gene expression, the triggering of lolB or lolC gene expression by LolA(I93C/F140C) was only marginal, and wild-type LolA had no effect on the expressions of lolB and lolC (data not shown). Therefore, we focused on the transcriptional regulation of lolA here.
Malfunction of the outer membrane transport of lipoproteins induces lolA transcription.
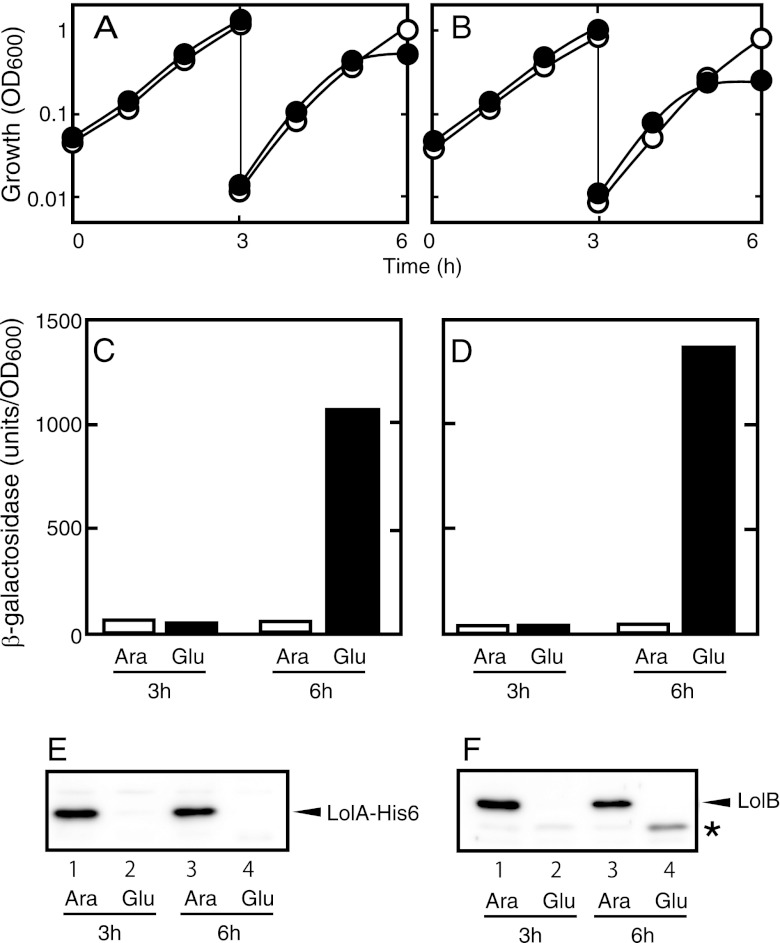
We previously showed that LolA(I93C/F140C) formed a stable complex with LolCDE on the inner membrane (40) and therefore severely inhibited both lipoprotein transfer from LolCDE to LolA and cellular growth. To determine whether lolA transcription is triggered by the inhibition of lipoprotein transport to the outer membrane or by the aberrant structure of oxidized LolA(I93C/F140C), we depleted LolA or LolB.
Both LolA and LolB are essential for E. coli growth (36, 38). The depletion of LolA and LolB causes the accumulation of outer membrane-specific lipoproteins in the inner membrane (36) and in both the periplasm and inner membrane (38), respectively. We constructed mutants carrying the respective lol genes under the tightly controlled araBAD promoter on the chromosome. The chromosomal lolA gene was disrupted by homologous recombination to construct MY425. A lolB-disrupted strain, MY427, was constructed in a similar manner. These strains did not grow on LB plates in the absence of arabinose, while they grew normally in the presence of 0.2% arabinose (data not shown). In liquid cultures, these strains grew continuously in the presence of 0.2% arabinose, whereas they stopped growing after about 6 h in medium containing glucose instead of arabinose (Fig. 2A and B). The levels of both LolA and LolB were below the detection limit at the end of the first 3 h of growth in the absence of arabinose (Fig. 2E and F). The β-galactosidase activity was not increased at either 3 or 6 h in the presence of arabinose, whereas it was strongly induced at 6 h in the absence of arabinose. The level of β-galactosidase activity at 3 h in the absence of arabinose did not increase, even though the amount of LolA or LolB was greatly reduced, indicating that small amounts of LolA and LolB can support cellular growth, as described previously (36, 38). Taken together, these results indicate that the triggering of lolA transcription occurs when outer membrane lipoprotein transport malfunctions.
Fig 2.
Induction of lolA expression by depletion of LolA or LolB. MY425 (A, C, and E) and MY427 (B, D, and F) were grown overnight in LB medium containing 0.2% arabinose and then diluted 100-fold with fresh medium containing 0.2% arabinose (open circles and bars) or 0.2% glucose (closed circles and bars). After 3 h, the cultures were again diluted 100-fold with the same medium and grown for an additional 3 h. At 3 and 6 h after the start of cultivation, samples were taken for measurements of β-galactosidase activity (C and D) and for Western blot analysis (E and F). Cellular growth was monitored by measuring the OD600 of the cultures (A and B). Judging from the OD600 of the cultures, equivalent amounts of protein were loaded onto each lane for Western blot analysis. The asterisk in panel F indicates an uncharacterized material that seemed to be induced by LolB depletion and to cross-react with the anti-LolB antiserum.
Isolation of mutants unable to induce lolA transcription.
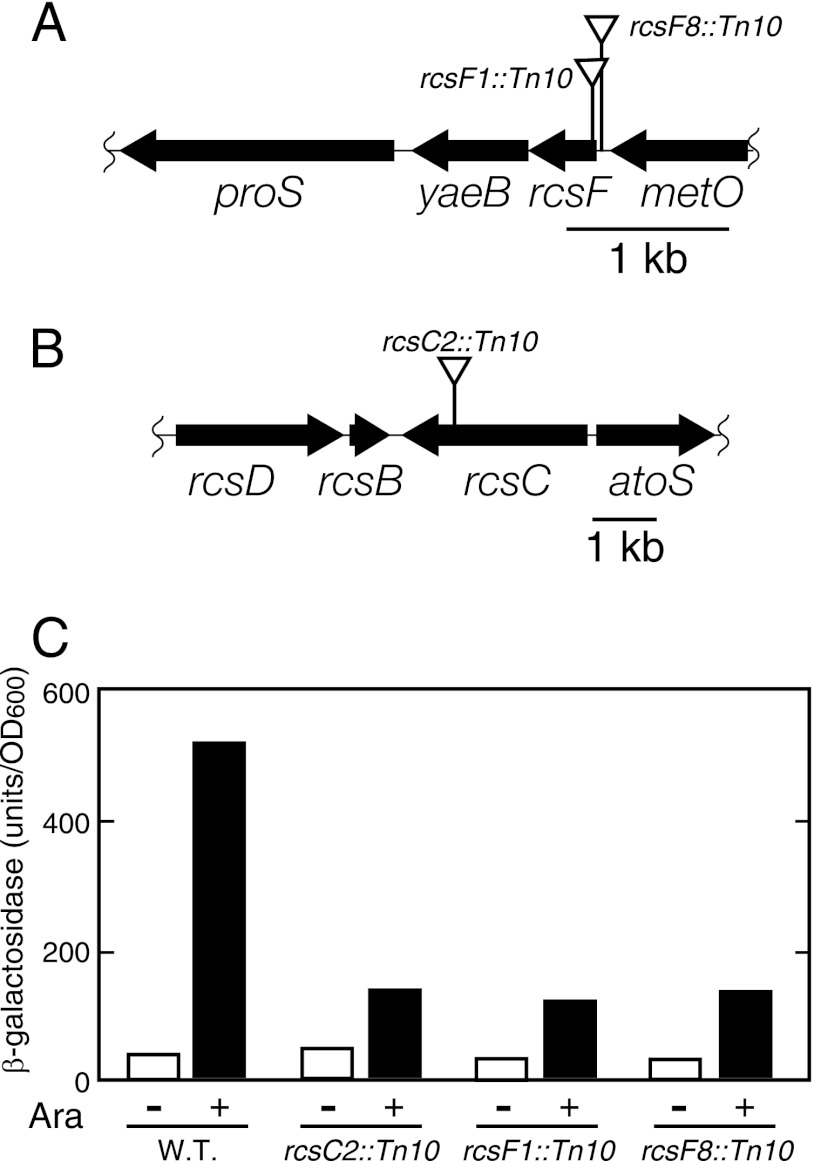
To elucidate the molecular mechanism underlying the triggering of lolA transcription, we isolated mutants defective in the LolA(I93C/F140C)-dependent triggering of lolA transcription using the lolA-lacZ fusion as a reporter gene. The wild-type strain carrying the lolA-lacZ fusion gene was randomly mutagenized with the mini-Tn10 transposon. A plasmid encoding LolA(I93C/F140C) was transformed into the pool of mutagenized cells, followed by cultivation on MacConkey lactose plates containing 0.02% arabinose. At this arabinose concentration, LolA(I93C/F140C) was moderately expressed and caused a slight growth inhibition with a concomitant weak induction of lolA-lacZ transcription. Under these conditions, wild-type cells harboring pSWIF formed slightly smaller colonies than those formed in the absence of arabinose, and the center of the colonies was red, whereas colonies in the absence of arabinose were entirely white. We then subjected cells to transposon insertion mutagenesis and sought colonies that exhibited reduced color development in the presence of 0.02% arabinose.
Out of approximately 20,000 mutagenized colonies, we obtained six candidates that exhibited decreased color development on MacConkey lactose plates containing arabinose. They formed colonies with a similar color on MacConkey lactose plates containing 0.02% arabinose and exhibited reduced levels of β-galactosidase activity when LolA(I93C/F140C) was expressed in liquid culture (Fig. 3C).
Fig 3.
Locations of mini-Tn10 insertions and phenotypes of the mutants. (A and B) The locations of the mini-Tn10 insertions are indicated by triangles above the maps. Cells with the indicated mini-Tn10 insertions were tested for the induction of β-galactosidase activity expressed from the lolA-lacZ fusion gene upon the induction of LolA(I93C/F140C). Exponentially growing cells (OD600 of 0.2) were induced for LolA(I93C/F140C) expression by 0.2% arabinose. (C) After 2 h, β-galactosidase activity was measured.
To determine the locations of the transposon insertions in these mutants, DNA fragments around the inserted transposon were amplified by PCR and sequenced. Three different mutations were found in six mutants: two mutations were located around the rcsF coding region, and the third one was found in the coding region of the rcsC gene (Fig. 3A and B). One mutation, found in two mutants, was mapped at 5 nucleotides upstream of the rcsF initiation codon. Three mutants had an insertion mutation 34 nucleotides downstream of the initiation codon of rcsF that disrupted codon 12. The former mutation is likely to affect the expression of rcsF upon insertion into the immediate upstream region. The insertion into rcsC was 2,438 nucleotides from the initiation codon and disrupted codon 813. Taken together, these results suggest that the Rcs phosphorelay system (21) is responsible for the induction of lolA transcription in the absence of lipoprotein transport.
Activation of the Rcs stress response system increases lolA transcription.
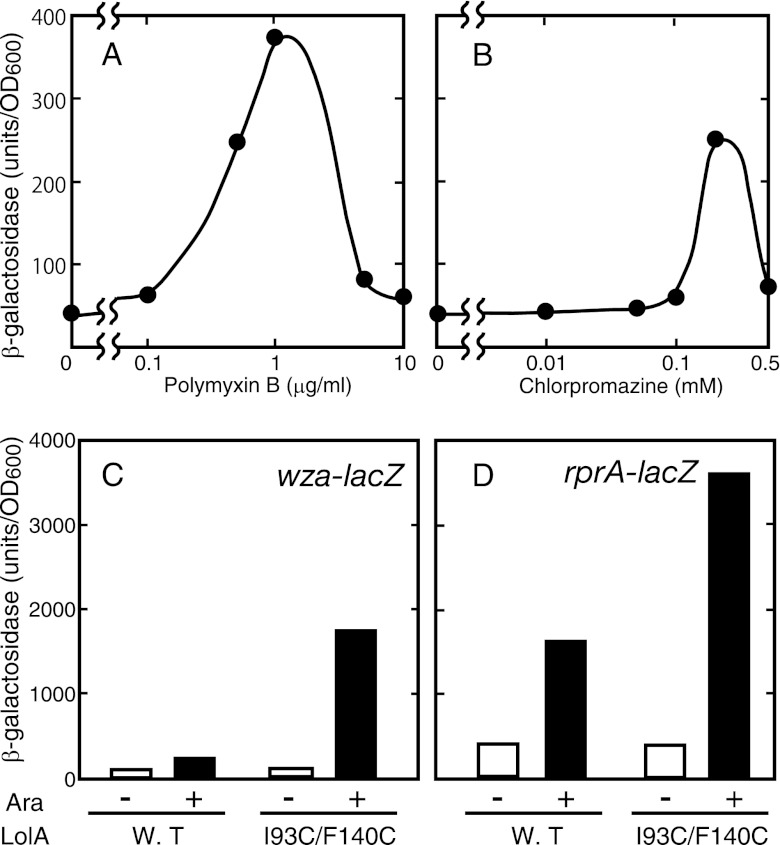
Chlorpromazine is a cationic amphipathic drug that intercalates into the membrane lipid bilayer and changes the membrane properties (32). Polymyxin B is a cationic amphipathic antimicrobial peptide that binds to outer membrane lipopolysaccharides, which leads to membrane perturbation (42). Both compounds were shown previously to activate the Rcs phosphorelay system (9, 13, 14). If lolA is under the control of the Rcs system, these compounds may induce the transcription of lolA. Indeed, chlorpromazine and polymyxin B triggered lolA transcription depending on their concentrations (Fig. 4A and B). The highest level of induction was observed at concentrations where growth inhibition became nearly maximum.
Fig 4.
Induction of lolA by Rcs-activating compounds, and activation of Rcs regulon genes by LolA(I93C/F140C). (A and B) MY301 cells were grown to the early logarithmic phase, and chlorpromazine (A) and polymyxin B (B) were added to the culture at the indicated concentrations. (C and D) MY419 (wza-lacZ) (C) and MY418 (rprA-lacZ) (D) cells harboring either pSW77 or pSWIF were grown to the early logarithmic phase, and the expression of LolA(I93C/F140C) was induced with 0.2% arabinose. After a further 2 h of growth, β-galactosidase activity was measured.
We then performed the converse experiment; i.e., we examined whether or not the expression of LolA(I93C/F140C) activates the Rcs regulon genes. Promoters of the wza and rprA genes were chosen as representative Rcs regulons (21). When LolA(I93C/F140C) was expressed in a strain carrying the wza-lacZ or rprA-lacZ fusion, the β-galactosidase activity increased about 20-fold or 10-fold, respectively, whereas wild-type LolA was activated about 3-fold in both strains (Fig. 4C and D). Taken together, these results indicate that LolA(I93C/F140C) activates the Rcs phosphorelay system and that the lolA gene is a member of the Rcs regulon genes.
Identification of the Rcs-regulated lolA promoter.
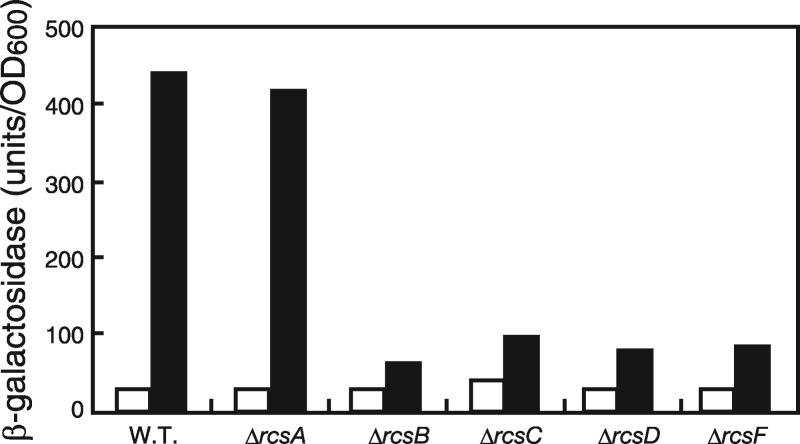
We next examined whether or not all the rcs genes are required for the triggering of lolA transcription in response to LolA(I93C/F140C) expression in mutants lacking various rcs genes (Fig. 5). As expected, neither the ΔrcsF nor the ΔrcsC mutant triggered lolA transcription. Likewise, the ΔrcsB and ΔrcsD mutants were unable to trigger lolA transcription in response to LolA(I93C/F140C). In contrast, the lolA transcription level induced in the ΔrcsA mutant was similar to that in the wild-type strain, indicating that the lolA promoter belongs to a group which does not require RcsA as a coactivator protein. Taken together, these results establish that lolA expression is regulated by the Rcs phosphorelay system.
Fig 5.
Induction of lolA expression by LolA(I93C/F140C) in the rcs mutants. Cells harboring pSWIF were grown to the early logarithmic phase of growth (OD600 of 0.2), and the expression of LolA(I93C/F140C) was induced with 0.2% arabinose (closed bars) or not induced (open bars). After a 2-h incubation, β-galactosidase activity was measured.
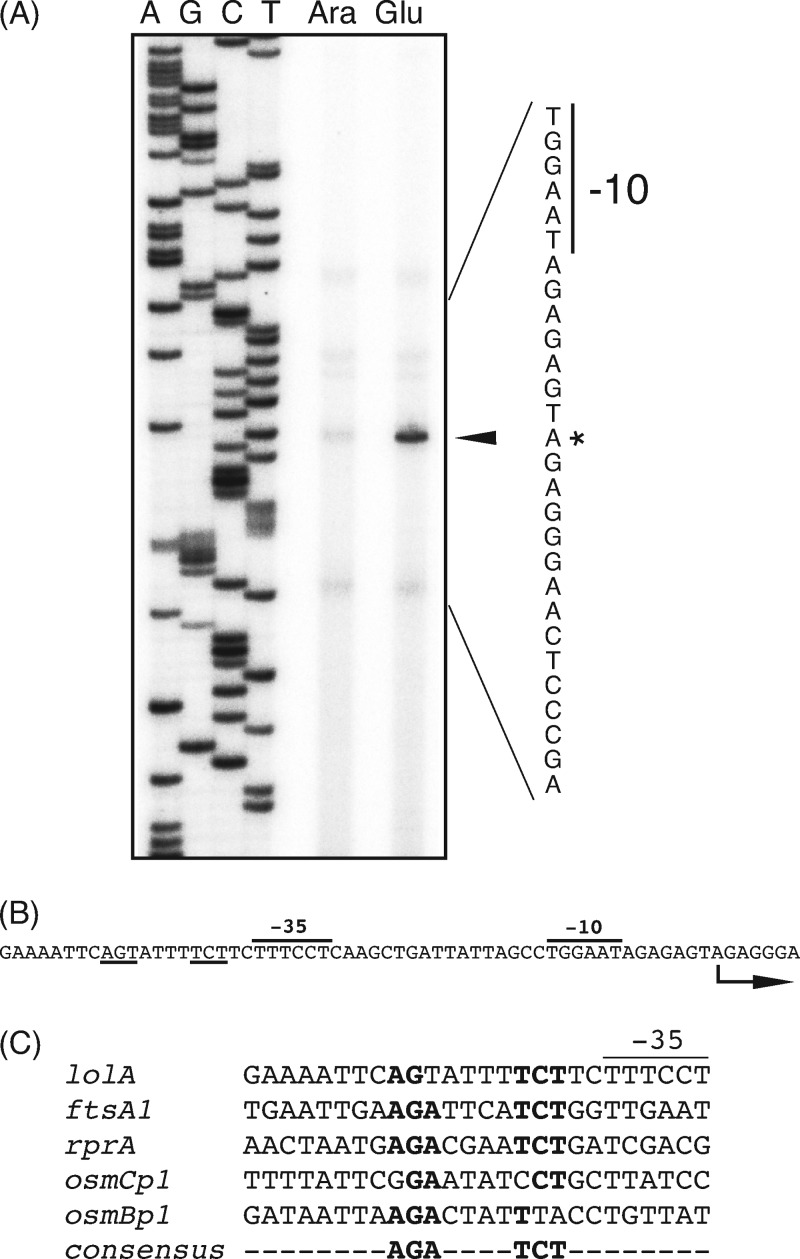
To locate the Rcs-regulated lolA promoter, we determined the transcription start site by means of primer extension experiments. Total RNA was isolated from cells expressing or lacking LolB and then subjected to primer extension reactions with a primer specific to lolA, followed by analysis on a denaturing polyacrylamide gel. A transcript induced under LolB-depleting conditions was observed and was mapped to the A residue 41 nucleotides upstream of the translational start site of lolA (Fig. 6A). Putative promoter −35 and −10 sequences were found for this transcript (Fig. 6B). Immediately upstream of the −35 element, a nucleotide sequence similar to the RcsB-binding consensus sequence (2, 5, 11, 23) was present (Fig. 6C). This is consistent with previous findings that the RcsB homodimer binds upstream of the −35 sequence of RcsA-independent promoters, whereas the RcsA-RcsB heterodimer binds far upstream of the transcription start sites of RcsA-dependent promoters (21). Overall, we concluded that lolA expression is directly regulated by the Rcs phosphorelay system rather than indirectly controlled via Rcs-regulated transcriptional regulators.
Fig 6.
Identification of the lolA promoter and the RcsB-binding motif. (A) Primer extension analysis of the lolA transcript. Total RNA was isolated from cells grown as described in the legend of Fig. 2B. The 32P-labeled lolA-specific oligonucleotide primer was hybridized to RNA isolated from LolB-expressing (lane Ara) or LolB-lacking (lane Glu) cells and extended by reverse transcriptase. Extension products were analyzed on a 6% polyacrylamide gel containing 8 M urea. Sequencing reaction products (lanes A, G, C, and T) of the lolA promoter region with the same primer were electrophoresed alongside. The arrowhead indicates the extension product specific for lolA. The nucleotide sequence of the corresponding region is shown on the right. The asterisk indicates the transcription start site. (B) Nucleotide sequence of the lolA promoter region. The promoter −35 and −10 sequences are indicated, and the RcsB-binding consensus sequences are underlined. The arrow indicates the transcription start site. (C) Sequence similarities among RcsA-independent promoters. The nucleotide sequences around the proposed RcsB-binding sites are shown. The consensus sequence for the RcsB recognition site is also shown at the bottom.
DISCUSSION
The molecular mechanisms by which lipoproteins are sorted to the outer membrane by the Lol system have been extensively studied in E. coli (31). However, little is known about the regulation of Lol protein expression, although lipopolysaccharide-deficient Acinetobacter baumannii was very recently reported to exhibit increased expression levels of genes involved in lipoprotein transport, such as LolA, LolB, LolE, and LolD, to various extents (18). No clear homolog of LolC was identified in this gammaproteobacterium. It is not yet known what kind of regulatory system is responsible for this increased expression of Lol proteins.
It was found previously for Salmonella enterica that the outer membrane lipoprotein RcsF is essential for the activation of the Rcs system by an antimicrobial peptide (14). RcsF is a small (14-kDa) outer membrane-specific lipoprotein facing the periplasm and was proposed previously to be a sensor for environmental signals (7, 22). RcsF is assumed to transfer signals to the RcsC sensor kinase on the inner membrane, although how this spatially separated transfer took place was not determined. Since RcsF mutants localized to the periplasm and inner membrane were more active than wild-type RcsF (14, 33), it was suggested previously that RcsF present in the outer membrane somehow interacts directly with RcsC in the inner membrane. The RcsF-independent activation of RcsC is also known (21).
E. coli cells that are unable to synthesize phosphatidylglycerol (PG) are viable if the cells lack the major outer membrane lipoprotein Lpp (20). The Rcs phosphorelay system was found previously to be activated in these cells (34). Moreover, it was found that an inhibitor of a lipoprotein-specific signal peptidase, globomycin, also caused the activation of the Rcs system (33). This activation could be suppressed by the overexpression of Lgt, which mediates the transfer of the diacylglyceryl moiety from PG to the N-terminal cysteine of lipoprotein precursors (33). It seems likely that the diacylglyceryl moiety is provided by lipids other than PG in the presence of a large amount of Lgt in PG-deficient E. coli cells. However, there is no evidence of a relationship between the activation of the Rcs system and lipoprotein transfer.
We show here that not only a dominant negative LolA derivative but also the depletion of the Lol system strongly activated the Rcs phosphorelay system, which then triggered the expression of LolA. Both the expression of LolA(I93C/F140C) (43) and the depletion of LolA (36) or LolB (38) cause the accumulation of outer membrane-specific lipoproteins, including RcsF, in the inner membrane. The results shown here and those reported previously by other groups (14, 33) altogether indicate that the mislocalization of RcsF to the inner membrane due to defective lipoprotein transport is critical for the activation of the Rcs system. It is likely that an interaction between RcsF and RcsC is possible under these conditions and causes the phosphorelay among RcsCDB proteins. We propose that an important function of RcsF is to monitor the proper operation of lipoprotein transport. However, it remains to be clarified whether or not all stresses that are known to activate the Rcs system in an RcsF-dependent manner cause the mislocalization of RcsF in the inner membrane. The disintegration of the outer membrane was proposed previously to render a direct interaction between RcsF and RcsC possible (14). However, this seems to be unlikely because such a severe disintegration of the outer membrane may be lethal to cells. It may be possible that RcsF has another function in the outer membrane. It should also be noted that various rcs gene disruptants exhibit increased β-galactosidase activity upon the expression of LolA(I93C/F140C) (Fig. 3C and 5), suggesting that another unknown system regulates lolA gene expression.
A recent comprehensive transcriptome analysis revealed that several outer membrane lipoproteins were upregulated during envelope stress responses (4). For example, bamD and osmB were strongly induced by σE and RcsB, respectively. In addition, the prolipoprotein diacylglycerol transferase Lgt was also upregulated by CpxR (4). These results suggest that lipoprotein synthesis increases under certain stress conditions and that the increased amounts of LolA might be required for the proper sorting of lipoproteins to the outer membrane in such situations.
Among the five Lol proteins, only LolA expression was activated by the Rcs system. The bulk accumulation of outer membrane-specific lipoproteins in the inner membrane is likely to cause a perturbation of the inner membrane. On the other hand, the accumulation of the LolA-lipoprotein complexes in the periplasm is not toxic, as observed previously in the case of the LolA(R43L) mutant, in which lipoproteins cannot be transferred to LolB (39). This seems to be why lolA gene expression is induced exclusively by a defect in the Lol system.
The function of RcsF, which monitors lipoprotein transport by the Lol system, resembles that of SecM, which is itself a secretory protein and monitors the proper operation of Sec-dependent protein secretion across the inner membrane. When the secretion of SecM is impaired, the expression of SecA, a critical secretion motor protein, is triggered (28).
We have identified a DNA sequence similar to the RcsB-binding consensus sequence immediately upstream of the promoter −35 element of lolA. However, it is still possible that the transcriptional activation of lolA is mediated by another transcription factor induced by RcsB under these conditions. Further studies are required to conclude definitely whether lolA is controlled directly or indirectly by RcsB.
Consistent with data from a previous report (8), the overproduction of wild-type LolA slightly induced lolA gene transcription. This was dependent on RcsF (our unpublished observations). It may be possible that the level of the periplasmic LolA-RcsF complex increased upon the overproduction of LolA, causing the interaction with RcsC.
ACKNOWLEDGMENTS
We thank NBRP (NIG, Japan) for providing the rcs gene disruptants.
This work was supported by grants to H.T. from the Ministry of Education, Science, Sports, and Culture of Japan.
Footnotes
Published ahead of print 4 May 2012
REFERENCES
- 1. Baba T, et al. 2006. Construction of Escherichia coli K-12 in-frame, single-gene knockout mutants: the Keio collection. Mol. Syst. Biol. 2:2006.0008 doi:10.1038/msb4100050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Boulanger A, et al. 2005. Multistress regulation in Escherichia coli: expression of osmB involves two independent promoters responding either to σs or to the RcsCDB his-asp phosphorelay. J. Bacteriol. 187:3282–3286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Boyd D, Weiss DS, Chen JC, Beckwith J. 2000. Towards single-copy gene expression systems making gene cloning physiologically relevant: lambda InCh, a simple Escherichia coli plasmid-chromosome shuttle system. J. Bacteriol. 182:842–847 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bury-Moné S, et al. 2009. Global analysis of extracytoplasmic stress signaling in Escherichia coli. PLoS Genet. 5:e1000651 doi:10.1371/journal.pgen.1000651 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Carballes F, Bertrand C, Bouche JP, Cam K. 1999. Regulation of Escherichia coli cell division genes ftsA and ftsZ by the two-component system rcsC-rcsB. Mol. Microbiol. 34:442–450 [DOI] [PubMed] [Google Scholar]
- 6. Casadaban MJ. 1976. Transposition and fusion of the lac genes to selected promoters in Escherichia coli using bacteriophage lambda and Mu. J. Mol. Biol. 104:541–555 [DOI] [PubMed] [Google Scholar]
- 7. Castanie-Cornet MP, Cam K, Jacq A. 2006. RcsF is an outer membrane lipoprotein involved in the RcsCDB phosphorelay signaling pathway in Escherichia coli. J. Bacteriol. 188:4264–4270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Chen M, et al. 2001. Characterization of the RcsC→YojN→RcsB phosphorelay signaling pathway involved in capsular synthesis in Escherichia coli. Biosci. Biotechnol. Biochem. 65:2364–2367 [DOI] [PubMed] [Google Scholar]
- 9. Conter A, Sturny R, Gutierrez C, Cam K. 2002. The RcsCB His-Asp phosphorelay system is essential to overcome chlorpromazine-induced stress in Escherichia coli. J. Bacteriol. 184:2850–2853 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Datsenko KA, Wanner BL. 2000. One-step inactivation of chromosomal genes in Escherichia coli K-12 using PCR products. Proc. Natl. Acad. Sci. U. S. A. 97:6640–6645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Davalos-Garcia M, Conter A, Toesca I, Gutierrez C, Cam K. 2001. Regulation of osmC gene expression by the two-component system rcsB-rcsC in Escherichia coli. J. Bacteriol. 183:5870–5876 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Elliott T. 1992. A method for constructing single-copy lac fusions in Salmonella typhimurium and its application to the hemA-prfA operon. J. Bacteriol. 174:245–253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Erickson KD, Detweiler CS. 2006. The Rcs phosphorelay system is specific to enteric pathogens/commensals and activates ydeI, a gene important for persistent Salmonella infection of mice. Mol. Microbiol. 62:883–894 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Farris C, Sanowar S, Bader MW, Pfuetzner R, Miller SI. 2010. Antimicrobial peptides activate the Rcs regulon through the outer membrane lipoprotein RcsF. J. Bacteriol. 192:4894–4903 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Gottesman S, Trisler P, Torrescabassa A. 1985. Regulation of capsular polysaccharide synthesis in Escherichia coli K-12: characterization of three regulatory genes. J. Bacteriol. 162:1111–1119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Guzman L, Belin D, Carson M, Beckwith J. 1995. Tight regulation, modulation, and high-level expression by vectors containing the arabinose P-bad promoter. J. Bacteriol. 177:4121–4130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Hagiwara D, et al. 2003. Genome-wide analyses revealing a signaling network of the RcsC-YojN-RcsB phosphorelay system in Escherichia coli. J. Bacteriol. 185:5735–5746 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Henry R, et al. 2012. Colistin-resistant, lipopolysaccharide-deficient Acinetobacter baumannii responds to lipopolysaccharide loss through increased expression of genes involved in the synthesis and transport of lipoproteins, phospholipids, and poly-β-1,6-N-acetylglucosamine. Antimicrob. Agents Chemother. 56:59–69 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Higashitani A, Higshitani N, Yasuda S, Horiuchi K. 1994. A general and fast method for mapping mutations on the Escherichia coli chromosome. Nucleic Acids Res. 22:2426–2427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kikuchi S, Shibuya I, Matsumoto K. 2000. Viability of an Escherichia coli pgsA null mutant lacking detectable phosphatidylglycerol and cardiolipin. J. Bacteriol. 182:371–376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Majdalani N, Gottesman S. 2005. The Rcs phosphorelay: a complex signal transduction system. Annu. Rev. Microbiol. 59:379–405 [DOI] [PubMed] [Google Scholar]
- 22. Majdalani N, Heck M, Stout V, Gottesman S. 2005. Role of RcsF in signaling to the Rcs phosphorelay pathway in Escherichia coli. J. Bacteriol. 187:6770–6778 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Majdalani N, Hernandez D, Gottesman S. 2002. Regulation and mode of action of the second small RNA activator of RpoS translation, RprA. Mol. Microbiol. 46:813–826 [DOI] [PubMed] [Google Scholar]
- 24. Matsuyama S, Tajima T, Tokuda H. 1995. A novel periplasmic carrier protein involved in the sorting and transport of Escherichia coli lipoproteins destined for the outer membrane. EMBO J. 14:3363–3372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Matsuyama S, Yokota N, Tokuda H. 1997. A novel outer membrane, LolB (HemH), involved in the LolA (p20)-dependent localization of lipoproteins to the outer membrane of Escherichia coli. EMBO J. 16:6947–6955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Miller JH. 1992. A short course in bacterial genetics: a laboratory manual and handbook for Escherichia coli and related bacteria. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY [Google Scholar]
- 27. Miyamoto A, Matsuyama S, Yokuda H. 2001. Mutant of LolA, a lipoprotein-specific molecular chaperone of Escherichia coli, defective in the transfer of lipoproteins to LolB. Biochem. Biophys. Res. Commun. 287:1125–1128 [DOI] [PubMed] [Google Scholar]
- 28. Nakatogawa H, Murakami A, Ito K. 2004. Control of SecA and SecM translation by protein secretion. Curr. Opin. Microbiol. 7:145–150 [DOI] [PubMed] [Google Scholar]
- 29. Oguchi Y, et al. 2008. Opening and closing of the hydrophobic cavity of LolA coupled to lipoprotein binding and release. J. Biol. Chem. 283:25414–25420 [DOI] [PubMed] [Google Scholar]
- 30. Okuda S, Tokuda H. 2009. Model of mouth-to-mouth transfer of bacterial lipoproteins through inner membrane LolC, periplasmic LolA, and outer membrane LolB. Proc. Natl. Acad. Sci. U. S. A. 106:5877–5882 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Okuda S, Tokuda H. 2011. Lipoprotein sorting in bacteria. Annu. Rev. Microbiol. 65:239–259 [DOI] [PubMed] [Google Scholar]
- 32. Sheetz MP, Painter RG, Singer SJ. 1976. Biological membranes as bilayer couples. III. Compensatory shape changes induced in membranes. J. Cell Biol. 70:193–203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Shiba Y, et al. 2012. Exploring the relationship between lipoprotein mislocalization and activation of the Rcs signal transduction system in Escherichia coli. Microbiology 158:1238–1248 [DOI] [PubMed] [Google Scholar]
- 34. Shiba Y, et al. 2004. Activation of the rcs signal transduction system is responsible for the thermosensitive growth defect of an Escherichia coli mutant lacking phosphatidylglycerol and cardiolipin. J. Bacteriol. 186:6526–6535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Simons RW, Houman F, Kleckner K. 1987. Improved single and multicopy lac-based cloning vectors for protein and operon fusions. Gene 53:85–96 [DOI] [PubMed] [Google Scholar]
- 36. Tajima T, Yokota N, Matsuyama S, Tokuda H. 1998. Genetic analyses of the in vivo function of LolA, a periplasmic chaperone involved in the outer membrane localization of Escherichia coli lipoproteins. FEBS Lett. 439:51–54 [DOI] [PubMed] [Google Scholar]
- 37. Takeda K, et al. 2003. Crystal structure of bacterial lipoprotein localization factors, LolA and LolB. EMBO J. 22:3199–3209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Tanaka K, Matsuyama SI, Tokuda H. 2001. Deletion of lolB, encoding an outer membrane lipoprotein, is lethal for Escherichia coli and causes accumulation of lipoprotein localization intermediates in the periplasm. J. Bacteriol. 183:6538–6542 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Taniguchi N, Matsuyama S, Tokuda H. 2005. Mechanisms underlying energy-independent transfer of lipoproteins from LolA to LolB, which have similar unclosed β-barrel structures. J. Biol. Chem. 280:34481–34488 [DOI] [PubMed] [Google Scholar]
- 40. Tao K, Watanabe S, Narita S, Tokuda H. 2010. A periplasmic LolA derivative with a lethal disulfide bond activates the Cpx stress response system. J. Bacteriol. 192:5657–5662 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Tokuda H, Matsuyama S, Tanaka-Masuda K. 2007. Structure, function, and transport of lipoproteins in Escherichia coli, p 67–79 In Ehrmann M. (ed), The periplasm. ASM Press, Washington, DC [Google Scholar]
- 42. Vaara M. 1992. Agents that increase the permeability of the outer membrane. Microbiol. Rev. 56:395–411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Watanabe S, Oguchi Y, Takeda K, Miki K, Tokuda H. 2008. Introduction of a lethal redox switch that controls the opening and closing of the hydrophobic cavity in LolA. J. Biol. Chem. 283:25421–25427 [DOI] [PubMed] [Google Scholar]