Abstract
The citrate transporter CitP of lactic acid bacteria catalyzes electrogenic precursor-product exchange of citrate versus l-lactate during citrate-glucose cometabolism. In the absence of sugar, l-lactate is replaced by the metabolic intermediates/end products pyruvate, α-acetolactate, and acetate. In this study, the binding and translocation properties of CitP were analyzed systematically for a wide variety of mono- and dicarboxylates of the form X-CR2-COO−, where X represents OH (2-hydroxy acid), O (2-keto acid), or H (acid) and R groups differ in size, hydrophobicity, and composition. It follows that CitP is a very promiscuous carboxylate transporter. A carboxylate group is both essential and sufficient for recognition by the transporter. A C-2 atom is not essential, formate is a substrate, and C-2 may be part of a ring structure, as in benzoate. The R group may be as bulky as an indole ring structure. For all monocarboxylates of the form X-CHR-COO−, the hydroxy (X = OH) analogs were the preferred substrates. The preference for keto (X = O) or acid (X = H) analogs was dependent on the bulkiness of the R group, such that the acid was preferred for small R groups and the 2-ketoacid was preferred for more bulky R groups. The C4 to C6 dicarboxylates succinate, glutarate, and adipate were also substrates of CitP. The broad substrate specificity is discussed in the context of a model of the binding site of CitP. Many of the substrates of CitP are intermediates or products of amino acid metabolism, suggesting that CitP may have a broader physiological function than its role in citrate fermentation alone.
INTRODUCTION
The citrate transporter CitP functions in citrate fermentation by lactic acid bacteria (LAB) such as Lactococcus lactis and Leuconostoc mesenteroides (16, 18). Internalized citrate is split into acetate and oxaloacetate, after which the latter is decarboxylated, yielding pyruvate. During cometabolism with glucose, pyruvate is reduced to l-lactate (9, 12, 14). CitP catalyzes uptake of divalent citrate (Hcit2−) in exchange with monovalent l-lactate (lac−) (precursor-product exchange), which results in generation of a membrane potential (Δψ) (13, 14, 15). Together with proton consumption in decarboxylation reactions in the citrate metabolic pathway, which results in a transmembrane pH gradient (ΔpH), the pathway generates proton motive force (PMF) (10, 11, 16). Recognition by CitP of two structurally related but different substrates, i.e., the tricarboxylate citrate and the monocarboxylate l-lactate, suggests an inherent broad substrate specificity of the transporter.
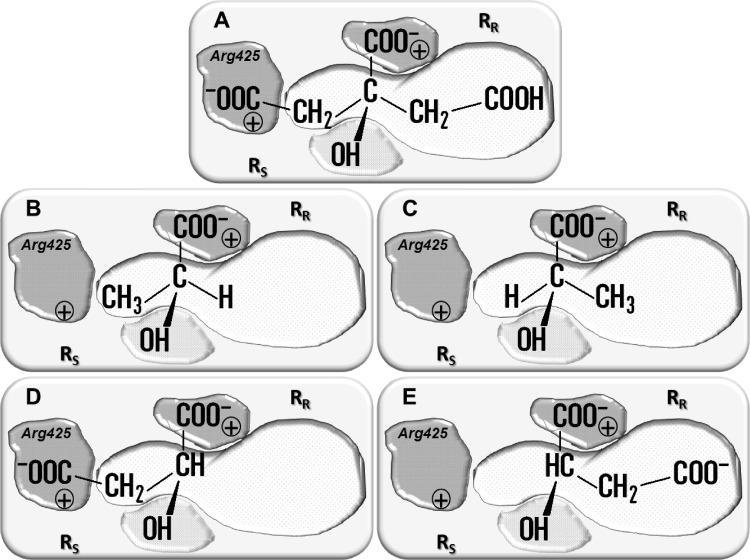
In vitro citrate transport studies using right-side-out (RSO) membrane vesicles derived from L. lactis demonstrated that CitP has affinity for 2-hydroxycarboxylates of the form HO-CR2-COO−, where the R group ranges from a hydrogen atom in glycolate to a phenyl group in mandalate and acetyl groups in malate and citrate (2). The transporter was shown to discriminate between high-affinity substrates that contain a second carboxylate group in one of the R substituents, such as citrate and malate, and low-affinity substrates, i.e., monocarboxylates such as lactate, suggesting an important role of the second carboxylate group in the interaction with the protein. Based on these experiments, a model of the binding site of CitP was proposed (Fig. 1) in which the carboxylate and hydroxyl groups of the 2-hydroxycarboxylate motif present in all substrates interact with specific sites on the protein (2, 3). This fixes the orientation of the substrate in the binding pocket and defines two separate sites in the binding pocket (RS and RR) (Fig. 1) for optional interactions with the R groups of the substrates, including the interaction with a second carboxylate in the RS site that results in high-affinity binding. In agreement with this model, the (S)-enantiomers of chiral dicarboxylate substrates such as malate were bound with high affinity, and the (R)-enantiomers were bound with low affinity, whereas both enantiomers of monocarboxylates such as lactate were low-affinity substrates (3). Site-directed mutagenesis of CitP of L. mesenteroides identified the conserved Arg425 residue as the site specifically interacting with the second carboxylate present in (S)-divalent substrates (4). Additionally, it was observed that increasing binding affinity of monocarboxylates with increasing hydrophobicity of the R groups suggested a hydrophobic nature of the RR and RS sites. Evidence was put forward that at least part of these sites is located in the C-terminal 46 residues (3).
Fig 1.
Schematic models of the substrate binding pocket of CitP. Substrates depicted in the pocket are citrate (A), (S)-lactate (B), (R)-lactate (C), (S)-malate (D), and (R)-malate (E). The interactions between the carboxylate groups and the hydroxyl group of the substrate and the protein are indicated by gray surfaces, and the hydrophobic interaction site (RR) is indicated by a white surface. The RS and RR sites bind the side chains of the (S)- and (R)-enantiomers of monosubstituted 2-hydroxycarboxylates (HO-CHR-COO−), respectively. Residue Arg425, responsible for binding of a carboxylate in the R side chain, is indicated in the RR site when present.
While the carboxylate group of the 2-hydroxycarboxylate motif was essential for the interaction of CitP with a substrate, transport studies with RSO membranes showed that the hydroxyl group could be replaced to some extent by keto groups, i.e., in oxaloacetate and pyruvate (2). Experiments with whole cells of L. lactis expressing the citrate fermentative pathway confirmed the affinity of CitP for these two metabolic intermediates of the pathway and also demonstrated its physiological relevance. In the absence of l-lactate, CitP catalyzes uptake of citrate in exchange with oxaloacetate and/or pyruvate when these accumulate in the cytoplasm. In addition to these two intermediates, citrate could also be taken up in exchange with acetate, an end product of the pathway, showing that CitP can bind and translocate substrates in which the hydroxyl group at the C-2 atom is replaced by a keto group or a hydrogen atom (18, 19).
In this report, a systematic study of the substrate specificity of the citrate transporter CitP of lactic acid bacteria is presented. A wide range of analogous substrates with different substitutions at the C-2 atom and with various R groups, including both monocarboxylates and dicarboxylates, were included. It follows that CitP is a remarkably promiscuous transport protein. Many of the substrates of CitP are secondary metabolites derived from amino acid metabolism and important as flavor compounds or precursors thereof in food fermentations.
MATERIALS AND METHODS
Chemicals.
2-Hydroxy-4-methylthiobutyrate, 2-keto-4-methylthiobutyrate, 4-methylthiobutyrate, acetate, adipate, benzoate, formate, fumarate, glutarate, glycolate, glyoxylate, indole-3-lactate, indole-3-propionate, indole-3-pyruvate, isocaproate, isovalerate, l-lactate, l-malate, maleate, malonate, oxalate, oxaloacetate, phenylacetate, phenylglycolate, phenylglyoxylate, phenyllactate, phenylpropionate, phenylpyruvate, propionate, pyruvate, succinate, α-hydroxyisocaproate, α-hydroxyisovalerate, α-ketoisocaproate, and α-ketoisovalerate were obtained from Sigma-Aldrich. l-Lactate dehydrogenase (l-LDH), l-malate dehydrogenase (l-MDH), and citrate lyase (CL) were obtained from Roche Applied Science. 2′,7′-Bis-(2-carboxyethyl)-5(and -6)-carboxyfluorescein (BCECF; acid form) and 3,3′-dipropylthiocarbocyanine iodide (DiSC3) probes were obtained from Invitrogen Molecular Probes.
Bacterial strain and growth conditions.
Lactococcus lactis strain IL1403(pFL3) was used in this study. Plasmid pFL3 harbors the lactococcal CRL264 citP gene under the control of the Streptococcus pneumoniae polA promoter (12). Neither expression nor plasmid copy number is under the control of citrate or pH in this strain (8). Precultures were grown overnight at 30°C in M17 broth medium supplemented with 0.5% (wt/vol) glucose (M17G) and 5 μg ml−1 of tetracycline. Cells were grown in M17G medium with the initial pH adjusted to 7.0. Growth was performed in 100-ml serum bottles without agitation and at 30°C. Growth was followed by measuring the optical density at a wavelength of 660 nm (OD660). Cells were harvested at mid-exponential growth phase, when the optical density was 0.6, by spinning for 10 min at 3,000 rpm. Cells were washed two times with 50 mM potassium phosphate buffer, pH 5.8, and then suspended in the same buffer at 4°C.
Citrate consumption by resting cells of L. lactis IL1403(pFL3).
Resting cells at an OD660 of 1.5 in 50 mM potassium phosphate buffer, pH 5.8, were incubated at 30°C without agitation for 10 min. The assay was performed in a total volume of 1.5 ml. At time zero, citrate was added at a concentration of 2 mM. When indicated, additional substrates were added from 10-fold stock solutions set at pH 5.8, together with citrate. The pH of the suspension was monitored after the experiment and never changed by more than 0.1 unit. Samples of 100 μl were taken every 5 or 10 min and immediately centrifuged for 0.5 min at maximum speed in a tabletop centrifuge. The supernatant was stored on ice until further analysis by enzymatic assays. Initial rates of citrate consumption were calculated from the decrease in citrate concentration within the first 10 min, assuming zero-order kinetics.
Enzymatic assays.
Citrate, oxaloacetate, and pyruvate were measured as described before (18), using the commercially available enzymes CL, l-MDH, and l-LDH. Briefly, an aliquot of 30 μl of sample was added to 50 mM glycine-glycine buffer, pH 7.8, containing NADH and l-MDH. Under these conditions, oxaloacetate in the sample is converted to l-malate at the expense of NADH. Subsequently, pyruvate in the same sample was measured by addition of l-LDH, which results in the conversion of pyruvate to l-lactate at the expense of NADH. Subsequent addition of CL converts citrate in the sample to oxaloacetate (and pyruvate), resulting in an additional decrease in the NADH concentration equivalent to the citrate concentration present in the samples. The assay was performed in 96-well microtiter plates. The decrease in NADH concentration was measured spectroscopically at 340 nm. Standard deviations were calculated for data from three independent experiments.
Measurements of changes in internal pH (ΔpH) and membrane potential (ΔΨ).
The components of the proton motive force were measured as described before (18). To measure ΔpH, resting cells resuspended to a high density (typically containing 50 mg/ml of protein) in 50 mM potassium phosphate buffer, pH 5.8, were loaded with BCECF. Fluorescence measurements were performed in 1-cm cuvettes containing 50 mM potassium phosphate buffer, pH 5.8, equilibrated at 30°C, and cells loaded with BCECF. The cuvette was stirred with a magnetic stirring bar. Fluorescence was measured using excitation and emission wavelengths of 502 and 525 nm, respectively, with slit widths of 4 and 16 nm, respectively. The fluorescence signal was sampled every second. Opening of the measurement compartment caused a loss of data during the first 5 to 6 s after an addition to the cuvette was made. The cytoplasmic pH was calculated as described previously (17).
Membrane potential was measured qualitatively with the fluorescent probe DiSC3 (22). A decrease in fluorescence intensity correlates with an increase in electrical potential across the membrane. DiSC3 was added from a stock solution to a final concentration of 2 μM to quartz cuvettes containing 50 mM potassium phosphate buffer, pH 5.8, and cells. The system was left to equilibrate for 5 min at 30°C. Fluorescence measurements were performed using excitation and emission wavelengths of 500 and 705 nm, respectively, and a slit width of 8 nm.
RESULTS
Substrate specificity assay of CitP.
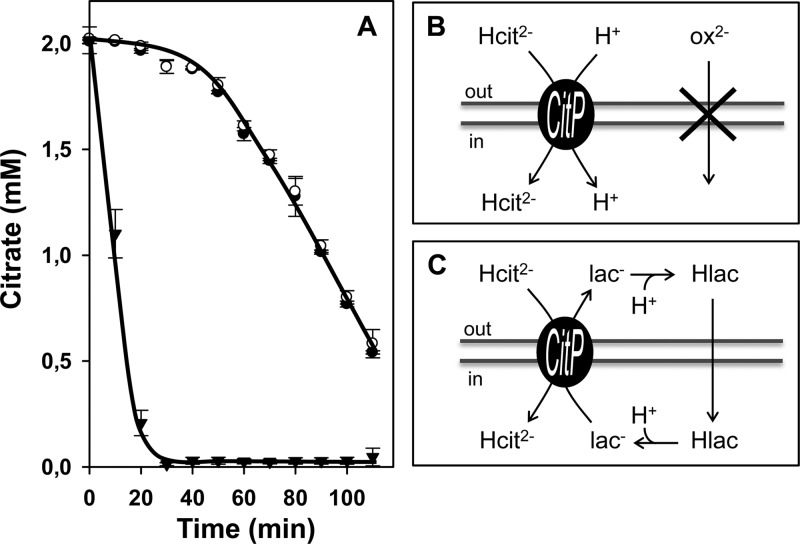
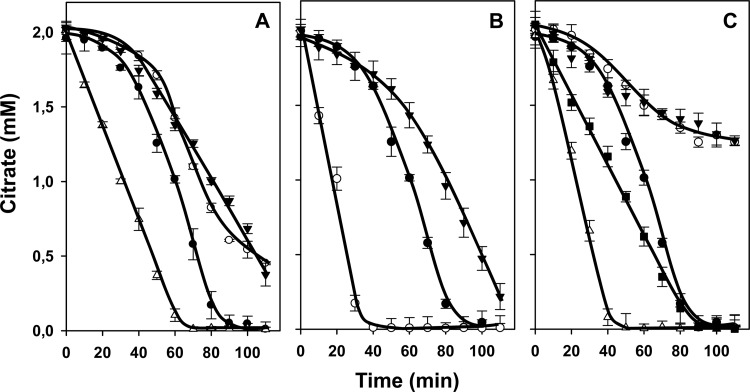
L. lactis strain IL1403(pFL3) harbors plasmid pFL3, which contains the citrate transporter gene citP under the control of the constitutive Streptococcus pneumoniae polA promoter (12). The genes encoding the metabolic enzymes of the citrate fermentation pathway are present on the chromosome of the IL1403 host strain (5). Cells of L. lactis IL1403(pFL3) grown in M17 broth medium supplemented with 0.5% glucose until mid-exponential growth phase (OD660 = 0.6) were resuspended in potassium phosphate buffer, pH 5.8, to an OD660 of 1.5. Following addition of 2 mM citrate, the consumption of citrate was shown to be biphasic (Fig. 2A) (18). The first phase represents slow uptake of citrate coupled to H+ (H+-Hcit2− symport) (Fig. 2B). The second phase represents fast uptake of citrate in exchange with intermediates/end products of citrate metabolism in the cytoplasm, i.e., pyruvate, α-acetolactate, and acetate, which accumulate in the cell during the first phase. Both modes of transport are catalyzed by the citrate transporter CitP (18). In the presence of 0.2 mM l-lactate, a physiological substrate of CitP, the first, slow phase is skipped and citrate is taken up in fast exchange with internal l-lactate from the beginning (Fig. 2A). The mechanism of uptake was described before as a shuttle mechanism (14, 18) (Fig. 2C). The acceleration of citrate consumption by the addition of a compound can be used to identify substrates of CitP when the following two conditions are met: (i) recognition of the substrate by CitP in the cytoplasm and (ii) high permeation of the substrate through the membrane. The latter requirement was demonstrated when l-lactate was replaced by oxaloacetate, another substrate of CitP (19). No acceleration of consumption was observed because oxaloacetate cannot enter the cell (Fig. 2A and B). In addition, oxaloacetic acid does not compete with citrate outside the cell because CitP has a much higher affinity for the latter (19). Monocarboxylates are weak acids that are permeative in the protonated state, which makes them good candidates for the substrate specificity assay. Dicarboxylates may not be able to permeate the membrane at physiological pH, and substrates of CitP are detected only when an appropriate transporter is present in the membrane to support a rate of uptake that can maintain the exchange reaction catalyzed by CitP. Apparently, the membrane of L. lactis does not contain such a transporter for oxaloacetate.
Fig 2.
Substrate specificity assay of CitP. (A) Citrate consumption by resting cells of L. lactis IL1403(pFL3) in the presence of 0.2 mM l-lactate (▼), 2 mM oxaloacetate (○), or no further additions (●). (B) Schematic of transport of citrate by CitP operating in the H+-symport mode in the presence of nonpermeative dicarboxylates. Oxaloacetate (ox2−) cannot enter the membrane by passive diffusion but competes with citrate (Hcit2−) outside the cell. (C) Schematic of the shuttle mechanism in the presence of permeative monocarboxylates. l-Lactate added outside the cells allows CitP to operate in the fast Hcit2−-lac− exchange mode by reentering the cell in the permeative protonated state (Hlac).
C-2-substituted monocarboxylates.
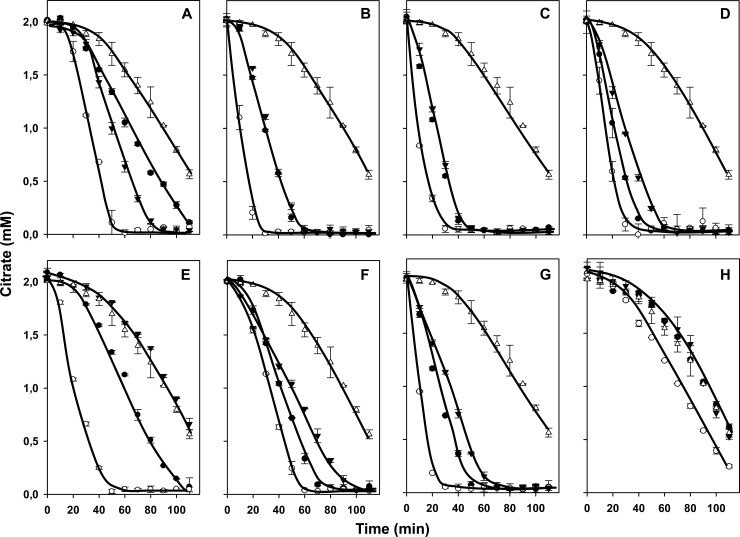
A set of 24 C-2-substituted monocarboxylates of the form X-CHR-COO− were selected, containing 8 different R groups and, for each of these, an X group of either OH (hydroxy), O (keto), or H (acid). The 8 R groups corresponded to the side chains of the 7 natural amino acids (X = NH3+), i.e., glycine, alanine, valine, leucine, methionine, phenylalanine, and tryptophan, and the unnatural amino acid phenylglycine. The side chains differed in composition, size, and hydrophobicity (see Table S1 in the supplemental material). The selection resulted in 8 groups of C-2-substituted analogs, e.g., glycolate, glyoxylate, and acetate, derived from glycine, and phenyllactate, phenylpyruvate, and phenylpropionate, derived from phenylalanine. All compounds were added at a concentration of 2 mM in the substrate specificity assay for CitP (Fig. 3).
Fig 3.
Transport of hydroxy, keto, and acid analogs of amino acids by CitP. Citrate consumption by resting cells of L. lactis IL1403(pFL3) is shown in the absence (△) and presence of a 2 mM concentration of the OH (○), O (●), and H (▼) analogs of glycine, i.e., glycolate (○), glyoxylate (●), and acetate (▼) (A); alanine, i.e., l-lactate (○), pyruvate (●), and propionate (▼) (B); valine, i.e., α-hydroxyisovalerate (○), α-ketoisovalerate (●), and isovalerate (▼) (C); leucine, i.e., α-hydroxyisocaproate (○), α-ketoisocaproate (●), and isocaproate (▼) (D); methionine, i.e., 2-hydroxy-4-methylthiobutyrate (○), 2-keto-4-methylthiobutyrate (●), and 4-methylthiobutyrate (▼) (E); phenylglycine, i.e., phenylglycolate (○), phenylglyoxylate (●), and phenylacetate (▼) (F); phenylalanine, i.e., phenyllactate (○), phenylpyruvate (●), and phenylpropionate (▼) (G); and tryptophan, i.e., indole-3-lactate (○), indole-3-pyruvate (●), and indole-3-propionate (▼) (H) (see Table S1 in the supplemental material).
All 8 hydroxy analogs increased the rate of citrate consumption significantly, and in all cases, it was more than that observed with the keto and acid analogs (Fig. 3). Surprisingly, exchange of citrate and the OH analogs of valine (α-hydroxyisovalerate), leucine (α-hydroxyisocaproate), and phenylalanine (phenyllactate) was as fast as that observed for the physiological substrate l-lactate, the OH analog of alanine (Fig. 3C, D, G, and B, respectively). These substrates supported the depletion of 2 mM citrate in 20 to 30 min. The OH analogs of glycine (glycolate), methionine (2-hydroxy-4-methylthiobutyrate), and phenylglycine (phenylglycolate) resulted in a two times slower consumption, with depletion within 50 to 60 min (Fig. 3A, E, and F, respectively), and the kinetics was biphasic. The OH analog of tryptophan clearly showed the smallest effect, but the stimulation was significant, indicating that indole-3-lactate is also a substrate of CitP (Fig. 3H). Seven of eight keto analogs and six of eight acid analogs were positively identified as substrates of CitP in the assay. Both the O analog and the H analog of tryptophan (indole-3-pyruvate and indole-3-propionate, respectively) and the H analog of methionine (4-methylthiobutyrate) did not affect the citrate consumption pattern (Fig. 3H and E, respectively). The H analog resulted in faster consumption of citrate than the O analog for the glycine analogs acetate and glyoxylate (Fig. 3A), while the rates were the same for the alanine (pyruvate and propionate) and valine (α-ketoisovalerate and isovalerate) analogs (Fig. 3B and C). However, in most cases, exchange with the O analogs was faster than that with the H analogs (Fig. 3D, E, F, G, and H).
Relative activities with OH, O, and H analogs.
The differences between the hydroxy (OH), keto (O), and acid (H) analogs were further detailed by measuring the consumption rates of citrate in the presence of a range of concentrations (0 to 20 mM) of the three leucine analogs, i.e., α-hydroxyisocaproic acid, α-ketoisocaproic acid, and isocaproic acid. At a concentration of 2 mM, the differences were relatively small (Fig. 3D). The initial rates of citrate consumption at increasing external concentrations showed the same pattern for the three analogs (Fig. 4). The rates increased to reach a maximum value, followed by a decrease of the rates at higher concentrations. The initial rise shows the consequence of the increased rate of uptake of citrate in exchange with the analog, the maximum represents the maximal rate of the shuttle, consisting of the diffusion of the analog through the membrane and the citrate-analog exchange steps, and the inhibition relates to competition of citrate and the analog at the outer face of the membrane. The rates of the initial rise were in the order H < O < OH, suggesting that the highest affinity of the cytoplasmic binding site of CitP was for the OH analog, followed by the O analog and the H analog. Similarly, the maximal consumption rates were 0.051, 0.073, and 0.092 mM min−1 for the H, O, and OH analogs, respectively, suggesting the same order (H < O < OH) of the maximal exchange rates catalyzed by CitP. Also, the inhibition at higher concentrations increased in the order H < O < OH, suggesting an increasing affinity for the analogs of the external binding site of CitP, in the same order. It follows that for the leucine analogs, α-hydroxyisocaproate was the best substrate of CitP with respect to both turnover in the citrate-analog exchange mode and the affinity of the transporter at the two sides of the membrane. Next best was the keto analog, α-ketoisocaproate, followed by the acid analog, isocaproate.
Fig 4.
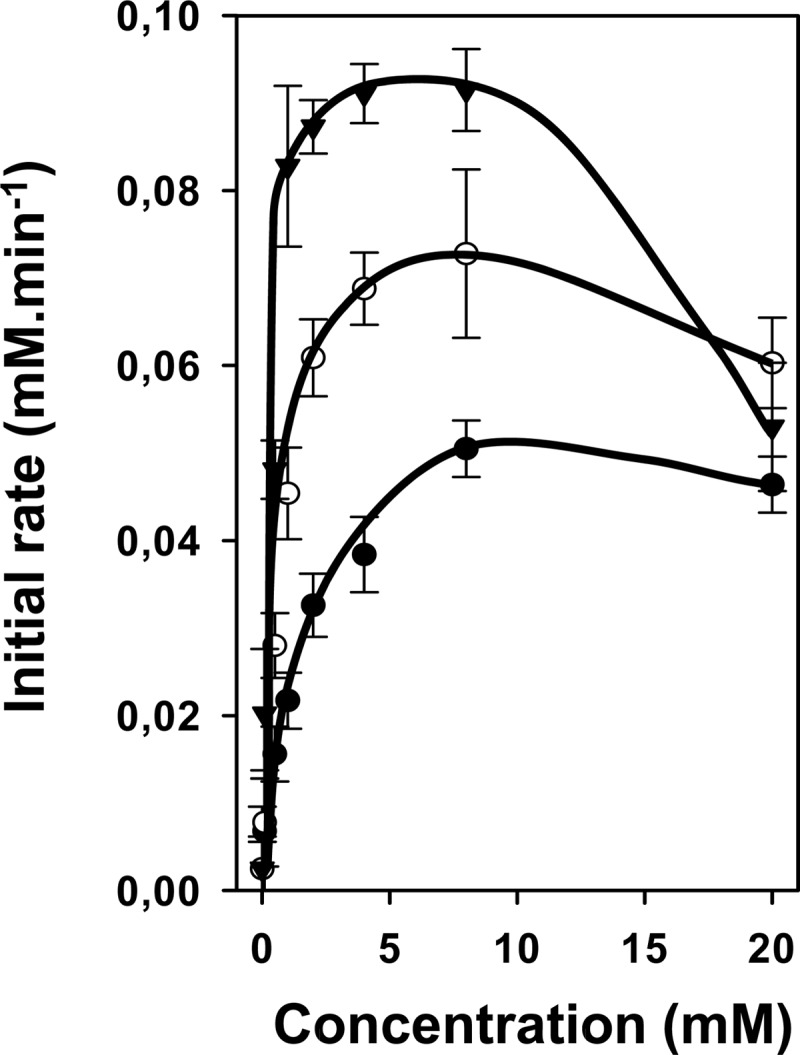
Effects of C-2 substituents on transport via CitP in L. lactis IL1403(pFL3). Rates of citrate consumption in the presence of α-hydroxyisocaproic acid (▼), α-ketoisocaproic acid (○), and isocaproic acid (●) at concentrations ranging from 0 to 20 mM are shown.
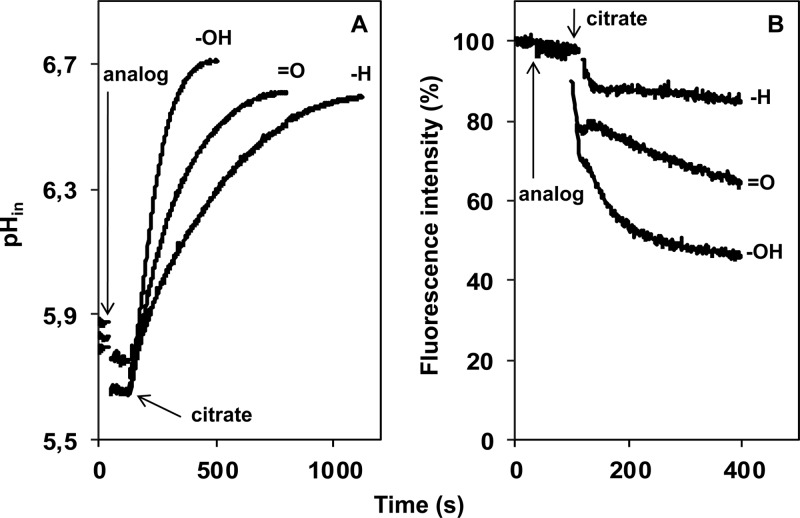
Flux through the citrate metabolic pathway generates proton motive force via both a pH gradient (ΔpH) and the membrane potential (ΔΨ) (14, 18). The transmembrane pH gradient, evaluated via the internal pH inferred by use of the fluorescent dye BCECF (17), and the membrane potential, measured qualitatively by use of the potentiometric probe DiSC3 (22), were measured in the presence of the three leucine analogs in potassium phosphate buffer, pH 5.8. Previously, it was shown that the pH and membrane potential probes had an inhibitory effect on flux through the citrate pathway in L. lactis IL1403(pFL3), which could be compensated for in part by increasing the concentration of the exchanged substrate (18). Addition of 2 mM citrate in the presence of 8 mM α-hydroxyisocaproic acid, the best leucine analog, resulted in the same ΔpH (0.9 to 0.95 unit) as that observed in the presence of a 2 mM concentration of the physiological substrate l-lactate (Fig. 5A) (18). Similarly, using the same conditions, a ΔΨ value of comparable magnitude was generated (Fig. 5B) (18). In the presence of both 2-hydroxy-monocarboxylates, the pH gradient and membrane potential developed during the first 5 min of citrate consumption (Fig. 5), which proceeded thereafter at more or less the same rate (Fig. 4 and 6C). The O and H analogs of leucine, i.e., α-ketoisocaproic acid and isocaproic acid, resulted in similar pH gradients, of 0.85 to 0.9 unit, but the steady state was reached later, correlating with the lower consumption rates in the presence of these two analogs (Fig. 4 and 5A). In contrast, the extent of the membrane potential seemed to decrease in the order OH > O > H (Fig. 5B). It follows that the energetics of citrate consumption was the same in the presence of the three leucine analogs and the physiological substrate l-lactate. The PMF increased in the order H < O < OH. Moreover, it seems that the extent of the membrane potential is more sensitive than the pH gradient to the flux through the pathway.
Fig 5.
Effects of C-2 substituents on generation of ΔpH (A) and Δψ (B) by citrate metabolism in L. lactis IL1403(pFL3). The internal pH of cells loaded with BCECF (see Materials and Methods) was monitored continuously over time (A), and changes in the membrane potential were evaluated qualitatively from the quenching of the potentiometric probe DiSC3 (see Materials and Methods) (B). At the times indicated by the arrows, 8 mM α-hydroxyisocaproic acid (-OH), α-ketoisocaproic acid (=O), or isocaproic acid (-H) was added. Citrate was added at a concentration of 2 mM.
Fig 6.
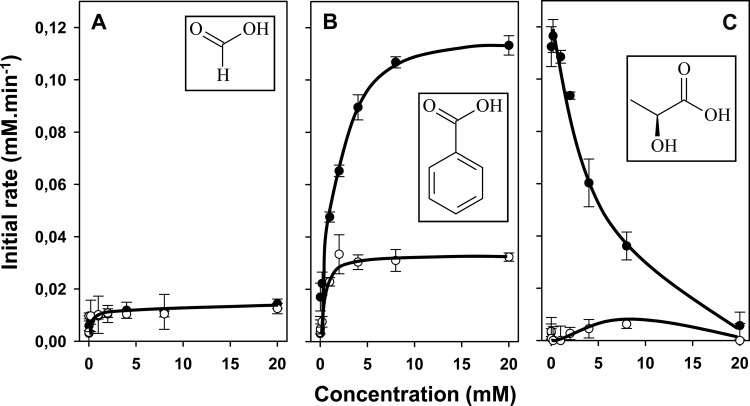
Formate and benzoate are substrates of CitP. Rates of citrate consumption (●) and pyruvate production (○) by cells of L. lactis IL1403(pFL3) in the presence of formic acid (A), benzoic acid (B), or lactic acid (C) at concentrations ranging from 0 to 20 mM are shown.
Activity with the monocarboxylates formic acid and benzoic acid.
Formate does not have a C-2 atom, and the C-2 atom of benzoate is part of the aromatic phenyl ring. The cell membrane is very permeable to the protonated forms of both, which have pK values of 3.8 and 4.2, respectively. Titration of the citrate consumption rate with increasing concentrations of formate in the range of 0 to 20 mM revealed only a small maximal acceleration of the initial rate (to 0.014 mM min−1), obtained at relatively low concentrations (<1 mM) (Fig. 6A). For comparison, at a 0.2 mM concentration of the physiological substrate l-lactate, the rate was 10 times higher (0.12 mM min−1) (Fig. 6C). This demonstrates that while CitP has affinity in the submillimolar range for cytoplasmic formate, the translocation rate of citrate-formate exchange is relatively low. Benzoic acid proved to be a much better substrate of CitP (Fig. 6B). With increasing concentrations of benzoic acid added, the rate of citrate consumption increased to up to 0.11 mM min−1, very close to the maximal rate of 0.12 mM min−1 observed with l-lactate. However, the maximum rate of uptake in the presence of benzoate was observed at a 1,000-fold higher concentration than was the case for l-lactate, i.e., at 20 mM versus 0.02 mM (Fig. 6B and C) (also see reference 18). It follows that while the rate of citrate-benzoate exchange is high, the affinities of CitP for the benzoate anion are low on both sides of the membrane. The different affinities affect the product profile of the citrate pathway in cells. The high affinity of CitP for l-lactate ensures that the transporter remains in the citrate–l-lactate exchange mode during complete consumption. The intermediate pyruvate is not excreted (Fig. 6C). In contrast, CitP has a higher affinity for pyruvate than for benzoate, and the transporter switches rapidly from citrate-benzoate exchange in the initial stages to citrate-pyruvate exchange once enough cytoplasmic pyruvate is produced. Pyruvate shows up as a major product under these conditions (Fig. 6B).
Transport of dicarboxylates by CitP.
The C4-dicarboxylates l-malate, oxaloacetate, and succinate represent a series of analogs of the hydroxy (OH), keto (O), and acid (H) forms (see Table S2 in the supplemental material), respectively, as introduced above for the monocarboxylates. Significant effects on citrate consumption by cells were observed only at higher concentrations of these compounds. The presence of 2 mM citrate and 32 mM l-malate or oxaloacetate resulted in a slight inhibition of the consumption rate due to inhibition of citrate uptake by the analogs outside the membrane, i.e., due to competition between citrate and l-malate and between citrate and oxaloacetate (Fig. 7A; also see Fig. 2B). Surprisingly, the H analog succinate enhanced the consumption of citrate significantly (Fig. 7A), identifying succinate as a substrate of CitP. The high concentration necessary to see the acceleration may reflect a low affinity of CitP for internal succinate but is more likely related to the low ability of succinate to permeate the membrane, resulting in a low cytoplasmic concentration (Fig. 2C). Transport of succinate into the cell probably relies on a transporter protein in the membrane. The double bond between C-2 and C-3 in the geometric isomers maleic acid (cis) and fumaric acid (trans) fixes the relative positions of the two carboxylate groups of succinate in space. In the presence of a 32 mM concentration of the cis isomer maleate, the consumption rate was higher than that in the presence of the same concentration of succinate and approached the rate observed with a 2 mM concentration of the physiological substrate l-lactate. In marked contrast, a 32 mM concentration of the trans isomer fumarate did inhibit citrate consumption (Fig. 7B). The length of the carbon chain in dicarboxylates appears to play an important role in the ability of the substrate to enhance citrate metabolism (see Table S2). Oxalic acid (C2) and malonic acid (C3) inhibited the rate of citrate consumption to some extent, while succinate (C4), glutaric acid (C5), and especially adipic acid (C6) increased the rate (Fig. 7C). It follows that the dicarboxylates used here affect the rate of citrate consumption by either inhibition (malate, oxaloacetate, fumarate, oxalate, and malonate) or acceleration (succinate, maleate, glutarate, and adipate) and therefore are recognized by CitP.
Fig 7.
Transport of dicarboxylates by CitP. (A) Effects of C-2 substituents in dicarboxylates on transport via CitP. Citrate consumption by resting cells of L. lactis IL1403(pFL3) is shown in the absence (●) or presence of a 32 mM concentration of the C4-dicarboxylates l-malate (○), oxaloacetate (▼), and succinate (△) (A); 32 mM fumaric acid (▼) and maleic acid (○) (B); and 32 mM oxalic acid (○), malonic acid (▼), glutaric acid (△), and adipic acid (■) (C).
DISCUSSION
Substrate specificity of CitP.
The citrate transporter CitP is a member of the 2-hydroxycarboxylate transporter (2-HCT) family (transporter classification [TC] 2.A.24) (21), in which the malate-lactate exchanger MleP of lactic acid bacteria, the Na+-citrate symporter CitS of Klebsiella pneumoniae, the H+-malate/citrate symporter CimH of Bacillus subtilis, and the H+-malate symporter MalP of Streptococcus bovis are also found (24). Substrates of these transporters share the 2-hydroxycarboxylate motif, i.e., HO-CR2-COO−, hence the name of the family (2). The functionally characterized symporters in the family have a narrow substrate specificity, while the exchangers have a much broader specificity which is inherent to their physiological function of exchanging a precursor and product of a metabolic pathway. Previous and present studies have demonstrated that substrates of CitP are not restricted to 2-hydroxycarboxylates, even though these appear to be the best substrates. CitP is best characterized as a very promiscuous carboxylate transporter. A C-2 atom is not essential (formate) or may be part of a delocalized ring structure (benzoate). Most substrates of CitP take the form X-CR2-COO−, in which X is either OH, O, or H but not NH3+ (amino acids). CitP is very tolerant toward the R groups. At the lower size limit, H atoms are accepted, making glycolate, glyoxylate, and acetate suitable substrates. At the higher size limit, the binding pocket seems to discriminate between hydrophilic and hydrophobic R groups, allowing more bulky groups in case of the latter. In that case, isocitrate is not a substrate, while the 5- plus 6-ringed indole group is (2) (Fig. 3H). A number of substrates other than 2-hydroxycarboxylates have been shown to be of physiological importance in the citrate fermentation pathway (18, 19). In addition, the broad specificity may be related to a function of the transporter in degradative amino acid pathways (see below).
CitP specificity assay.
Previously reported transport studies using RSO membranes determined the (kinetic) affinity of CitP for substrates at the external face of the membrane in the exchange reaction (4). The citrate consumption studies using resting cells reported here identified substrates recognized by CitP at the cytoplasmic face of the membrane. The substrate, i.e., l-lactate, enters the cell by a CitP-independent mechanism, after which the substrate is recognized at the inside by CitP and extruded again in exchange for citrate. While a substrate that is translocated from outside to inside is necessarily also translocated in the reverse direction, the affinities for the substrate at the two sides of the membrane may be very different. The best example is the affinity of CitP for lactate, which is in the millimolar range on the outside (3) and in the micromolar range on the cytoplasmic side of the membrane (18) (Fig. 6C). Similarly, substrates such as acetate, propionate, glyoxylate, and succinate, which were readily identified as substrates of CitP in the citrate consumption assay (Fig. 3A and B and 7A), were not identified as substrates in transport assays, most likely because of too low an affinity of the externally facing binding site.
Substrates of CitP are identified when the initial rate of citrate consumption is higher in the presence than in the absence of the substrate. The latter condition represents the symport mode of uptake of citrate by CitP, which is rate limited by the isomerization of the “empty” binding site. The observed initial rate reflects the recycling rate of the substrate over the membrane, determined by the activity of CitP and the influx into the cell by passive diffusion or the action of a transporter other than CitP (Fig. 2B and C). Passive permeation depends on the hydrophobicity of the molecule, because the molecule has to pass the hydrophobic interior of the lipid membrane, and on the pK of the carboxylate group(s), because this value determines the fraction in the permeative protonated form. In addition, the size of the side chain may play a role. The hydrophobicities of the R groups of the amino acid analog monocarboxylate substrates used in this study are high (see Table S1 in the supplemental material) (7), and it may be expected that the citrate consumption rate is determined by the activity of CitP. Therefore, the order of preference of CitP for OH > O > H groups at the C-2 atom in case of, e.g., the analogs of leucine, appears to be a justified conclusion. Dicarboxylates are more hydrophilic by nature, but their pKs are higher, and therefore they are not less permeative a priori. The pK values, together with increasing hydrophobicity with chain length, suggest an increase in membrane permeability in the order oxalate (C2) < malonate (C3) < succinate (C4) < glutarate (C5) < adipate (C6) (see Table S2). The last three accelerated the citrate consumption rate, indicating rapid entry into the cell and recognition by CitP. Of the three C4-dicarboxylates, succinate and maleate entered the cell and were exchanged with citrate, while fumarate was not. Since maleate is probably the least permeative form (lowest pK1) (see Table S2), most likely a transporter is responsible for the uptake of the former two. So far, a C4-dicarboxylate transporter gene has not been identified in the genome of L. lactis IL1403 (5).
In principle, the citrate consumption assay identifies substrates at the external face of the membrane as well, since they compete with external citrate. For most substrates, this is not observed because citrate, a high-affinity substrate, is present in excess and/or the acceleration of the rate by an internal substrate is dominant. In the absence of the latter, e.g., if the substrate was not permeative, high concentrations of dicarboxylates, i.e., oxalate, malonate, malate, oxaloacetate, and fumarate, were shown to inhibit the consumption rate to some extent, demonstrating that CitP has affinity for these compounds as well.
Substrate recognition by CitP.
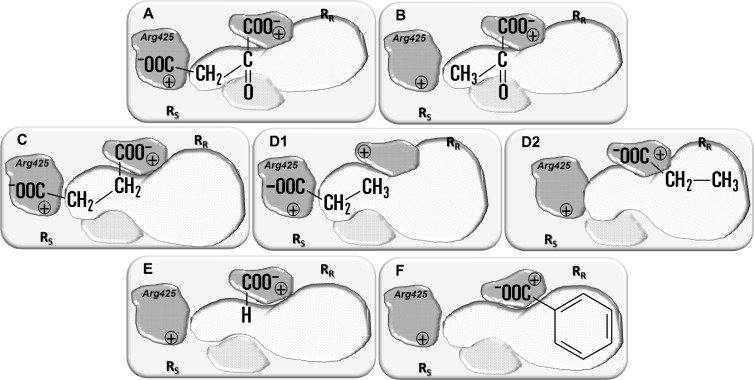
The substrate binding pocket of CitP contains sites that interact specifically with a substrate (Fig. 1). The interactions contribute to the affinity of the protein for the substrate and affect the transition between the inside- and outside-facing binding sites of the transporter. The latter is slow in the absence of a bound substrate and fast when a substrate is bound, which is the basis of the assay used to identify substrates of the protein (see above). In view of the broad substrate specificity of CitP, not all interactions have to be satisfied. The interaction with a single carboxylate group on the substrate (formate) is both essential and sufficient (Fig. 6A and 8E). Interaction of the Arg425 residue in the RS site with a second carboxylate group on the substrate is not essential, which allows the transporter to exchange mono- and divalent substrates, e.g., Hcit2−-lac− exchange, which is the basis of membrane potential generation (Fig. 1). The interaction with the 2-hydroxyl group is also not essential but results in a better overall interaction than that with a 2-keto group or the acids. With a 2-keto group, the nature of the interaction may be the same, and monocarboxylate 2-keto acids would be oriented in the pocket in the same way as the 2-hydroxy acids (Fig. 1 and 8A and B). Replacement of the hydroxy group with H results in loss of the interaction, and monocarboxylates possibly have two binding modes, with the carboxylate interacting with either of the carboxylate binding sites on the protein, depending on the R group (Fig. 8D1 and D2). Remarkably, the preference of CitP for keto or acid analogs of X-CHR-COO− (X is O or H, respectively) appears to depend on the bulkiness of the side chain (R). The acid is preferred for the glycine (X = NH3+) analogs, no preference is observed for alanine and valine analogs, and the keto acids are preferred for the leucine, phenylglycine, and phenylalanine analogs (Fig. 3; see Table S1 in the supplemental material). Dicarboxylates such as succinate are likely to interact with both carboxylate binding sites on the protein (Fig. 8C). Finally, the different interactions between substrate and protein in the binding pocket seem to have different contributions to affinity for the substrate and to lowering of the activation energy of the isomerization transition. Formate, which interacts only with a carboxylate binding site on the protein, stimulates isomerization only poorly but binds with a relatively high affinity (Fig. 6A and 8E). It may be noted that the binding pocket with bound formate, the smallest possible substrate, is most reminiscent of the empty pocket, which shows the slowest isomerization. In contrast, the affinity for benzoic acid is considerably lower, but the isomerization is as fast as that observed for lactate (Fig. 6B and C). Apparently, the interaction with the hydrophobic phenyl group stimulates isomerization but negatively affects affinity (Fig. 8F).
Fig 8.
Substrate binding modes of CitP. Substrates depicted in the binding pocket are oxaloacetate (A), pyruvate (B), succinate (C), propionate (D1 and D2), formate (E), and benzoate (F). For further explanation, see the legend to Fig. 1 and the text.
Physiology of CitP substrates.
The citrate transporter CitP transports a broad range of mono- and dicarboxylates that are found in the metabolome of LAB and play an important role in flavor perception of dairy products (23). These compounds are used in the food industry as flavor enhancers or food additives (20) (see Tables S1 and S2 in the supplemental material). Products of citrate and sugar fermentation and lipolysis, such as acetate and propionate, give a pungent, sour milk flavor, and products of amino acid metabolism, such as isovalerate or phenylacetate, are important in the flavor and taste of cheddar cheese (6). The first step in degradative amino acid pathways is transamination yielding the corresponding keto acid, e.g., α-ketoisocaproate from leucine, α-ketoisovalerate from valine, phenylpyruvate from phenylalanine, and 2-keto-4-methylthiobutyrate from methionine. Formation of keto acids from branched-chain amino acids, aromatic amino acids, and methionine in L. lactis IL1403 is catalyzed by two transaminases, BcaT and AraT. The keto acids produced in this way may be reduced to the corresponding hydroxy acids by the activity of 2-hydroxy acid dehydrogenases (HA-DH) at the expense of NADH. Genome analysis of L. lactis IL1403 revealed a potential HA-DH-encoding gene, the l-2-hydroxyisocaproate dehydrogenase gene hicD (5, 23) (see Table S1). All of these compounds were shown to be substrates of CitP in this study and are flavor compounds or precursors thereof. Furthermore, naturally occurring compounds that play an important role in plant growth as auxins, i.e., phenylacetate (PAA) and indole-3-lactate (ILA), the precursor of indole-3-acetate (IAA), are produced by metabolism of tryptophan in soil bacteria (1). IAA can be produced from indole-3-pyruvate (IPA; keto-Trp) by oxidative decarboxylation initiated by the decarboxylase IPA-DC, encoded in the genome of L. lactis IL1403 (5, 23). It is possible that CitP plays a role in exporting these compounds out of the cell and has a much broader physiological function than uptake of citrate in the citrate fermentation pathway alone. The encoding of CitP on an endogenous plasmid while the metabolic enzymes are chromosomally encoded in L. lactis strains supports this view.
Supplementary Material
Footnotes
Published ahead of print 4 May 2012
Supplemental material for this article may be found at http://jb.asm.org/.
REFERENCES
- 1. Ali B, Sabri AN, Ljung K, Hasnain S. 2009. Auxin production by plant associated bacteria: impact on endogenous IAA content and growth of Triticum aestivum L. Lett. Appl. Microbiol. 48:542–547 [DOI] [PubMed] [Google Scholar]
- 2. Bandell M, Ansanay V, Rachidi N, Dequin S, Lolkema JS. 1997. Membrane potential generating malate (MleP) and citrate (CitP) transporters of lactic acid bacteria are homologous proteins. J. Biol. Chem. 272:18140–18146 [DOI] [PubMed] [Google Scholar]
- 3. Bandell M, Lolkema JS. 1999. Stereoselectivity of the membrane potential-generating citrate and malate transporters of lactic acid bacteria. Biochemistry 38:10352–10360 [DOI] [PubMed] [Google Scholar]
- 4. Bandell M, Lolkema JS. 2000. Arg-425 of the citrate transporter CitP is responsible for high affinity binding of di- and tricarboxylates. J. Biol. Chem. 275:39130–39136 [DOI] [PubMed] [Google Scholar]
- 5. Bolotin A, et al. 2001. The complete genome sequence of the lactic acid bacterium Lactococcus lactis ssp. lactis IL1403. Genome Res. 11:731–753 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Curioni PMG, Bosset JO. 2002. Key odorants in various cheese types as determined by gas chromatography-olfactometry. Int. Dairy J. 12:959–984 [Google Scholar]
- 7. Eisenberg D, Schwarz E, Komaromy M, Wall R. 1984. Analysis of membrane and surface protein sequences with the hydrophobic moment plot. J. Mol. Biol. 179:125–142 [DOI] [PubMed] [Google Scholar]
- 8. García-Quintáns N, Magni C, de Mendoza D, López P. 1998. The citrate transport system of Lactococcus lactis subsp. lactis biovar diacetylactis is induced by acid stress. Appl. Environ. Microbiol. 64:850–857 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Hugenholtz J. 1993. Citrate metabolism in lactic acid bacteria. FEMS Microbiol. Rev. 12:165–178 [Google Scholar]
- 10. Lolkema JS, Poolman B, Konings WN. 1995. Role of scalar protons in metabolic energy generation in lactic acid bacteria. J. Bioenerg. Biomembr. 27:467–473 [DOI] [PubMed] [Google Scholar]
- 11. Lolkema JS, Poolman B, Konings WN. 1996. Secondary transporters and metabolic energy generation, p 229–260 In Konings WN, Kaback HR, Lolkema JS. (ed), Handbook of biological physics. Elsevier, Amsterdam, The Netherlands [Google Scholar]
- 12. Magni C, Lopez de Felipe F, Sesma F, López P, de Mendoza D. 1994. Citrate transport in Lactococcus lactis subsp. lactis biovar diacetylactis. Expression of the citrate permease. FEMS Microbiol. Lett. 118:78–82 [Google Scholar]
- 13. Magni C, de Mendoza D, Konings WN, Lolkema JS. 1999. Mechanism of citrate metabolism in Lactococcus lactis: resistance against lactate toxicity at low pH. J. Bacteriol. 181:1451–1457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Marty-Teysset C, Lolkema JS, Schmitt P, Divies C, Konings WN. 1995. Membrane potential-generating transport of citrate and malate catalyzed by CitP of Leuconostoc mesenteroides. J. Biol. Chem. 270:25370–25376 [DOI] [PubMed] [Google Scholar]
- 15. Marty-Teysset C, et al. 1996. Proton motive force generation by citrolactic fermentation in Leuconostoc mesenteroides. J. Bacteriol. 178:2175–2185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Marty-Teysset C, Lolkema JS, Schmitt P, Divies C, Konings WN. 1996. The citrate metabolic pathway in Leuconostoc mesenteroides: expression, amino acid synthesis, and α-ketocarboxylate transport. J. Bacteriol. 178:6209–6215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Molenaar D, Abee T, Konings WN. 1991. Continuous measurement of the cytoplasmic pH in Lactococcus lactis with a fluorescence pH indicator. Biochim. Biophys. Acta 1115:75–83 [DOI] [PubMed] [Google Scholar]
- 18. Pudlik AM, Lolkema JS. 2011. Citrate uptake in exchange with intermediates of citrate metabolic pathway in Lactococcus lactis IL1403. J. Bacteriol. 193:706–714 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Pudlik AM, Lolkema JS. 2011. Mechanism of citrate metabolism by an oxaloacetate decarboxylase deficient mutant of Lactococcus lactis IL1403. J. Bacteriol. 193:4049–4056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Ricke SC. 2003. Perspectives on the use of organic acids and short chain fatty acids as antimicrobials. Poult. Sci. 82:632–639 [DOI] [PubMed] [Google Scholar]
- 21. Saier MH., Jr 2000. A functional-phylogenetic classification system for transmembrane solute transporters. Microbiol. Mol. Biol. Rev. 64:354–411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Sip M, Herman J, Plasek J, Hrouda V. 1990. Transmembrane potential measurement with carbocyanine dye diS-C3-(5): fast fluorescence decay studies. J. Photochem. Photobiol. B Biol. 4:321–328 [DOI] [PubMed] [Google Scholar]
- 23. Smit G, Smit BA, Engels WJ. 2005. Flavour formation by lactic acid bacteria and biochemical flavour profiling of cheese products. FEMS Microbiol. Rev. 29:591–610 [DOI] [PubMed] [Google Scholar]
- 24. Sobczak I, Lolkema JS. 2005. The 2-hydroxycarboxylate transporter family: physiology, structure, and mechanism. Microbiol. Mol. Biol. Rev. 69:665–695 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.