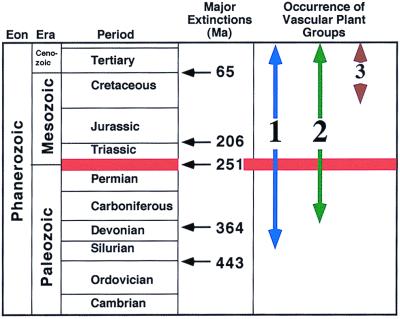
A large part of the biomass on Earth is sequestered as terrestrial vegetation. Thus, we have to study land plants if we want to understand large-scale processes during periods of rapid floristic and faunal turnover. In this issue of PNAS, Looy et al. (1) have done just that for the time interval after the ecological crisis that occurred at the Permian-Triassic boundary, about 251 million years ago. This is the largest of the six major extinctions and has been called “The Mother of Mass Extinctions” by Erwin (2). The five major crises of the Phanerozoic occurred at the Ordovician-Silurian boundary, within the Late Devonian, at the Permian-Triassic boundary, the Triassic-Jurassic boundary, and the Cretaceous-Tertiary boundary (Fig. 1). During the last four of these crises, the vascular land plants contained the majority of the biomass. We have to find out what happened during these dramatic changes to be able to evaluate and perhaps predict the potential dangers for our current terrestrial ecosystems.
Figure 1.
Geologic time scale showing major mass extinctions and the occurrence in time of the main groups of vascular plants on land. 1 = spore-bearing plants including lycopsids and ferns; 2 = gymnosperms; 3 = angiosperms (numerical ages from ref. 1 and Geologic Time Scale, Geological Society of America, http://www.geosociety.org/pubs/index.htm). Ma, millions of years. Red line indicates time discussed by Looy et al. (1).
Looy et al. (1) present, for the first time, quantitative data for land plants during the “recuperation” period after the major crisis. In the European part of the mega-continent Pangaea, conifer forests were dominant during the latest Permian in a tropical setting. These forests became extinct during the crisis and for 4–5 million years small lycopsids experienced an adaptive radiation and dominated the landscapes. The replacement of the lycopsids by newly evolved conifers itself took another 0.5 million years. These changes represent an extremely serious disturbance of vegetation and landscape that was more serious and lasted longer than after other mass extinctions.
The ultimate cause(s) for the five crises are not known except for the one at the Cretaceous-Tertiary boundary. The actual processes that produced the extinction of certain life forms, but not others also are not yet well understood. Elucidating the patterns of extinctions and recuperations for different climatic belts is the first step in understanding what happened. The fact that Looy et al. (1) present quantitative data for this time interval is especially noteworthy. It is a bleak picture indeed that they paint of a tropical world in which the forests vanish to be replaced for millions of years by unbranched 1- to 2-m high lycopsids that look essentially like poles with leaves, not unlike bottle brushes, with a spore-bearing cone at the tip. Other components of the flora were minor constituents, for instance pteridosperms, cycads, and even herbaceous gymnosperms.
The mass extinction at the Permian-Triassic boundary can be described as a major environmental disturbance. As such it can be seen as an extreme point in a continuum of disturbances that occurred and occur at nearly any scale of space and frequency. It has been recognized by ecologists for some time that most environments, even if they appear to be very stable, consist of a patchwork of former disturbances. Such a disturbance may be a single fallen tree that creates a light gap in the forest allowing new growth. It also could be a larger area measured in tens or hundreds of square kilometers destroyed by a forest fire. In each case, the environment will bounce back and re-establish the previous plant community although several intermediate steps and many years may be needed to achieve this condition. If the disturbance is small in area and does not encompass all there is of a given environment it is obvious that plants will re-establish themselves through the supply of propagules from the surrounding vegetation. Thus, looking at a time when the environment was changed for 5 million years over a large area the questions include: How large (or how severe) does a disturbance have to be so that the area will not return to its previous state rapidly? Related questions are: How long will the recuperation period last? Will the same plants come back or will they be replaced by others in the restoration of the ecosystem?
Not surprisingly, there is no simple answer. Every disturbance results in extinctions. These extinctions may be local, regional, continental, or global. Whether an extinction is permanent depends on other factors. Most ecosystems re-establish themselves after disturbance. It is worthwhile to look at examples. If landslides occur in tropical forests, for instance in Puerto Rico’s Luquillo mountains, the landslide scar is stripped of vegetation and soil. Nevertheless the forest will re-establish itself within about 60 years, recreating new soil in the process (3, 4). The area of the New River Gorge National Park in West Virginia is now a wilderness area that is heavily forested and visited by tourists who would like to experience unspoiled nature. A hundred years ago the same valley was a coal-mining district with a large number of underground mines and mining towns. All the timber had been removed for use in the mines. While doing geological field research in the area I was surprised to hear about this development. I did not believe it until I was shown foundations of former houses totally overgrown and hidden in the forest. Thus, in the moist climate of West Virginia the forest will recuperate completely from a major disturbance by humans within a hundred years. Larger disturbances are of greater interest in a comparison with the one analyzed by Looy et al. (1).
Extinction of terrestrial ecosystems over large parts of a continent occurred in North America during the Late Carboniferous. The ocean flooded significant parts of the continent, from Texas to central Pennsylvania, during interglacial times and receded when the ice caps on Gondwana grew again. This repeated itself for more than 25 million years. A very clear record is preserved in the Carbondale Formation in Illinois where about 10 marine layers are alternating with terrestrial coal beds and sedimentary rocks of fluvial origin, representing an interval of approximately 1 million years. One would expect major changes in the flora if it was wiped out over most of the lowlands of the continent again and again. However, the same plants migrated back into the area, forming the same ecosystems over and over (5). This finding indicates that plants and ecosystems are astonishingly resilient and probably have “emergent” properties that result from the interaction of coexisting species leading to the re-establishment of identical or nearly identical plant communities even after significant disturbances (6).
The other side of the coin is disturbances after which recuperation is slow or results in a different vegetation. These differences can be expressed taxonomically but also are expressed in dominance and diversity patterns. In the previous paragraph the persistence of Late Carboniferous tropical vegetation was mentioned during glacially driven climatic and sea-level fluctuations (5). However, at the top of the Carbondale Formation one finds the record of a large extinction, mostly of tree-lycopsids, and a complete rearrangement of ecosystems (6). The reason was a climatic change toward a generally drier tropical climate (7). This shift to a drier climate represented a process where the ecological threshold of the environment and its constituents was surpassed.
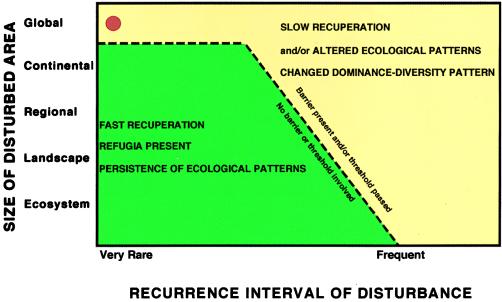
The rapid re-establishment of plant communities in northern North America at the end of the last glacial interval was caused by habitat restoration and migration (8). Glacial-interglacial cycles had not led to significant extinctions but rather to a shift in plant ranges and vegetation belts from north to south and vice versa. However, if we consider the entire late Cenozoic glacial age, the situation is different. During the Tertiary the northern continents, North America, Europe, and eastern Asia, had a rather similar flora of high diversity. The current floras of these three areas are quite distinct, which is especially obvious if one considers diversity of tree species (9). The lowest diversity exists in Europe. North America has a distinctly higher diversity, but southern China has by far the largest number of species. The best interpretation is that the north-south migration of species at the beginning of the late Cenozoic glacial age did run up against east-west ranging mountain chains in Europe that served as barriers to migration. Extinction was the consequence for all species that could not migrate over the elevated terrain or survive north of it. In North America the area for migration was larger but ultimately was limited by the Gulf of Mexico and the Mexican mountain chains. However, in China a multitude of different habitats had a large north-south range and allowed migration with minimal extinction (9). Thus, many floras from southern China today look quite similar to Late Tertiary floras in Europe or North America. To recreate a paleo-botanical garden for the Tertiary in Europe or North America one has to go to China to find closely related species with similar ecological tolerances (10). In this particular case extinctions were selectively triggered by a combination of climate change and barriers to migration. Obviously thresholds exist beyond which a species cannot survive. If these thresholds are surpassed a turnover occurs, which can happen at any scale, but will be most common for major disturbances that are global in nature (Fig. 2).
Figure 2.
Schematic diagram showing the recuperation of ecosystems after a disturbance as a function of size and recurrence interval of disturbance. Another factor is the presence or absence of one or several barriers or the passing of thresholds. Red dot indicates approximate position of Permian-Triassic boundary disturbance (1).
If an extinction kills every individual of a dominant species, this particular species cannot participate in the recolonization. If this particular species was the only one in its genus (or even family) it will not be replaced by a related taxon but rather by an ecological convergent species that has a taxonomically different position. A species for an ecological vicarious replacement often does not exist but has to evolve before it can migrate into the area to occupy the same niche. Such an evolutionary replacement will require a longer time period if a larger amount of genetic diversity has been permanently removed from the system. Such an extreme case occurred at the Permian-Triassic boundary.
Another observation presented in the paper by Looy et al. (1) is that lycopsids, i.e., a group of spore-bearing plants, became the dominant plant form over a large area and for a long time during a time interval in which most ecosystems were dominated by gymnosperms. Spore-bearing vascular plants had originated in the Late Silurian about 60 million years before gymnosperms appear in the Late Devonian. During this time interval and for some time afterward lycopsids, sphenopsids (horse-tails), and ferns dominated all, most, or at least major environments. By mid-Carboniferous times several pteridosperm families (early gymnosperms that are extinct) were dominant in many environments. Even precursors of the conifers (Cordaitales) dominated a few areas but were rare in most. The Late Permian and the end Permian crisis were actually the time when many Late Paleozoic spore-bearing taxa became extinct. However, finding them as dominants after the Permian-Triassic crises is less surprising when we look at disturbed sites in the tropics today. Lycopsids (Fig. 3) and ferns (personal observations in the Merida Andes and the Orinoco Delta, Venezuela; ref. 11) are often the pioneer species that take over disturbed sites. Also in the Late Cretaceous ferns were still in some sites the dominant ground cover (12). A direct comparison is possible with the crisis at the Cretaceous-Tertiary boundary. A “fern-spike” has been observed after the crisis indicating that ferns took over after the extensive destruction of forests (15). Thus, spore-bearing vascular plants are superior to seed plant under certain circumstances, in effect preparing a site after a disturbance for the recolonization by seed plants later in the succession.
Figure 3.

Modern tropical lycopsids growing on a disturbed site, a road cut in the foothills of the Merida Andes, Venezuela (photograph by author). Each plant is about 50 cm high. This lycopsid serves as the pioneer species on a disturbed site similar to the lycopsids (belonging to a different order) that became dominant after the Permian-Triassic boundary crisis (1).
Looy et al. (1) have quantitatively demonstrated the completeness of destruction of a tropical forest ecosystem during the major mass extinction of the Phanerozoic and the extremely long pathway of habitat restoration, migration, and evolutionary processes that were necessary to return to a comparable ecosystem. This investigation is an important step in understanding the sudden major changes of life on Earth. These results will become part of the data that form the basis of comparing mass extinctions, climate change thresholds, and other major disturbances of the environment.
Footnotes
See companion article on page 13857.
References
- 1.Looy C V, Brugman W A, Dilcher D L, Visscher H. Proc Natl Acad Sci USA. 1999;96:13857–13862. doi: 10.1073/pnas.96.24.13857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Erwin D H. The Great Paleozoic Crisis: Life and Death in the Permian. New York: Columbia Univ. Press; 1993. [Google Scholar]
- 3.Guariguata M R. J Ecol. 1990;78:814–832. [Google Scholar]
- 4.Zarin D J, Johnson A H. Soil Sci Soc Am J. 1995;59:1444–1452. [Google Scholar]
- 5.DiMichele W A, Pfefferkorn H W, Phillips T L. Palaeogeogr Palaeoclim Palaeoecol. 1996;125:105–128. [Google Scholar]
- 6.DiMichele W A, Phillips T L. In: Effects of Past Global Change on Life. Stanley S M, Knoll A H, Kennett J P, editors. Washington, DC: Natl. Acad. Press; 1995. pp. 134–155. [Google Scholar]
- 7.Pfefferkorn H W, Thomson M C. Geology. 1982;10:641–644. [Google Scholar]
- 8.Delcourt H R, Delcourt P A. Quaternary Ecology: A Paleoecological Perspective. London: Chapman & Hall; 1991. [Google Scholar]
- 9.Latham R E, Ricklefs R E. In: Species Diversity in Ecological Communities. Ricklefs R E, Schluter D, editors. Chicago: Univ. of Chicago Press; 1993. pp. 294–317. [Google Scholar]
- 10.Kirchheimer F. Palaeontologische Zeitschrift. 1953;27:11. [Google Scholar]
- 11.Walker L R. J Veg Sci. 1994;5:525–532. [Google Scholar]
- 12.Wing S L, Hickey L J, Swisher C C. Science. 1993;363:342–344. [Google Scholar]
- 13.Tschudy R H, Pillmore C L, Orth C J, Gilmore J S, Knight J D. Science. 1984;225:1030–1032. doi: 10.1126/science.225.4666.1030. [DOI] [PubMed] [Google Scholar]