Abstract
Mammalian cells of innate immunity respond to pathogen invasion by activating proteins that generate a burst of oxidative and nitrosative stress. Pathogens defend themselves from the toxic compounds by triggering a variety of detoxifying enzymes. Escherichia coli flavorubredoxin is a nitric oxide reductase that is expressed under nitrosative stress conditions. We report that in contrast to nitrosative stress alone, exposure to both nitrosative and oxidative stresses abolishes the expression of flavorubredoxin. Electron paramagnetic resonance (EPR) experiments showed that under these conditions, the iron center of the flavorubredoxin transcription activator NorR loses the ability to bind nitric oxide. Accordingly, triggering of the NorR ATPase activity, a requisite for flavorubredoxin activation, was impaired by treatment of the protein with the double stress. Studies of macrophages revealed that the contribution of flavorubredoxin to the survival of E. coli depends on the stage of macrophage infection and that the lack of protection observed at the early phase is related to inhibition of NorR activity by the oxidative burst. We propose that the time-dependent activation of flavorubredoxin contributes to the adaptation of E. coli to the different fluxes of hydrogen peroxide and nitric oxide to which the bacterium is subjected during the course of macrophage infection.
INTRODUCTION
In order to control infection, mammalian phagocytes express NADPH oxidase (Nox), which produces superoxide that undergoes spontaneous dismutation to hydrogen peroxide, and inducible nitric oxide synthase (iNOS), which generates nitric oxide (NO) (25). However, microorganisms possess a diverse range of defense mechanisms for sensing and responding to these stresses that are crucial for survival and virulence. To detoxify reactive oxygen species (ROS), microbes utilize an array of enzymes that include scavengers of superoxide and hydrogen peroxide, such as superoxide dismutases or reductases, peroxidases, and catalases (19, 22, 29). Nitric oxide detoxification is achieved by NO dioxygenases and reductases, which are widespread in denitrifying bacteria, nitrate-dissimilating fungi, pathogenic bacteria, and protozoa (2, 32, 46).
Escherichia coli contains three NO-detoxifying enzymes, namely, the cytochrome c nitrite reductase (NrfA), flavohemoglobin (Hmp), and a flavodiiron protein known as flavorubredoxin (FlRd; encoded by the norV gene) (32, 43, 45). NrfA is a periplasmic enzyme with high NO reductase activity (41), but its role in vivo is still under debate (45). Hmp acts as an NO dioxygenase or reductase, but the latter activity is low (11, 14). In contrast, FlRd seems to be dedicated to scavenging NO under anaerobic conditions, with significant activity (16). While most studies have focused on nonpathogenic E. coli strains, the hmp and norV genes are also present in uropathogenic, enteropathogenic, and enterohemorrhagic E. coli strains as well as in closely related pathogens such as Salmonella and Shigella. For E. coli, strains deleted in the hmp and norV genes have higher sensitivities to NO under aerobic and anaerobic conditions, respectively (11, 13). Although the NO reduction rates of single mutants defective in hmp or norV are similar to those of parental strains, a double hmp norV mutant exhibits a clear defect in the ability to metabolize NO anaerobically (21, 44).
The expression of hmp is highly induced by NO under aerobic and anaerobic conditions, through a complex regulation that involves at least three regulators, namely, FNR, MetR, and the NO-sensitive repressor NsrR (3, 9, 30). The transcription of the norV gene is strongly upregulated in cells cultured anaerobically and exposed to NO, through the activation of the nitric oxide sensor NorR (12, 21). The norR gene is transcribed divergently from the norVW operon, which encodes FlRd and its redox partner, the NADH-flavorubredoxin reductase (NorW) (4, 12). Induction of norVW occurs upon ligation of NO to NorR and binding of the regulator to three motifs present in the promoter region of norVW (20). NorR is a σ54-dependent transcription factor formed by three domains: an N-terminal regulatory GAF domain harboring a mononuclear iron site that binds NO, a central AAA+ domain responsible for ATPase activity and interaction with the σ54 subunit of RNA polymerase, and a C-terminal DNA binding domain that interacts with enhancer sequences (6, 12, 20). The binding of NO to the ferrous iron center stimulates the ATPase activity of NorR and enables NorR to activate the transcription of norV (6).
In this work, we addressed the behavior of E. coli FlRd in the presence of the combined effects of NO and hydrogen peroxide, having analyzed norV gene transcription and protein expression profiles. Furthermore, the survival of the E. coli norV mutant strain in activated macrophages was also studied.
MATERIALS AND METHODS
Reagents and bacterial strains.
Hydrogen peroxide (Carl-Roth), spermine NONOate (Cayman Chemical) prepared in 0.01 M NaOH (herein named an NO donor), and pure NO-saturated anaerobic water solution (∼2 mM) (1) were used as stress inducers.
The strains and plasmids utilized in this study are described in Table 1.
Table 1.
Bacterial strains and plasmids used in this work
| E. coli strain or plasmid | Description or genotype | Source or reference |
|---|---|---|
| Strains | ||
| XL2-Blue | F′ proAB lacIqZΔM15 Tn10 Tetr endA1 supE44 thi-1 recA1 gyrA96 relA lac | Lab stock |
| BL21Gold(DE3) | F− ompT hsdS(rB− mB−) dcm+ Tetr gal λ(DE3) endA Hte | Stratagene |
| Wild type | E. coli K-12 ATCC 23716 | ATCC |
| RK4353 | lacZ mutant strain; (argF-lac)U169 | 39 |
| ΔnorV strain | LMS2710; K-12 norV mutant; ΔnorV::Cmr | 21 |
| Δhmp strain | LMS2552; K-12 hmp mutant; Δhmp::Kmr | 21 |
| ΔnorV Δhmp strain | LMS5262; K-12 hmp and norV double mutant; Δhmp::Kmr ΔnorV::Cmr | 21 |
| Plasmids | ||
| pAA182-PnorV | Plasmid pAA182 carrying the entire norV promoter fused to lacZ; Ampr | 20 |
| pET24a | T7-based expression vector; Kmr | Novagen |
| pME2337 | pET24a carrying the norV coding region | 47 |
| pET28a | T7-based expression vector; Kmr | Novagen |
| pET28a-norR | pET28a with the norR coding region cloned into NdeI and EcoRI sites and expressing an N-terminal His6-tag fusion | This work |
| pFLAG-CTC | Vector for protein expression under the influence of the tac promoter; Ampr | Sigma |
| pFLAG-norV | pFLAG-CTC carrying norV coding region subcloned into NdeI and HindIII sites from pME2337 | This work |
Immunoblotting assays.
E. coli K-12 ATCC 23716 cells were grown anaerobically in Luria-Bertani (LB) medium at 37°C and 150 rpm to the early exponential phase (optical density at 600 nm [OD600] of ∼0.3) and collected after 45 min of treatment with NO (50 μM), hydrogen peroxide (3 mM), or NO (50 μM) plus H2O2 (3 mM). Cells were disrupted in a French pressure cell (Thermo Electron Corporation), the cell extracts were cleared by centrifugation (30 min at 12,000 × g and 4°C), and their protein concentrations were determined by the bicinchoninic acid (BCA) method (36). Protein samples (75 μg) were separated by SDS-PAGE, transferred to nitrocellulose membranes, and detected with polyclonal antibodies raised against E. coli FlRd and Hmp as previously described (21).
β-Galactosidase activity assays.
Cultures of E. coli RK4353 carrying pAA182-PnorV (20) were grown anaerobically in LB at 37°C and 150 rpm, to an OD600 of ∼0.3. At this point, cells were treated for 25 min with NO (50 μM), spermine NONOate (25 μM), hydrogen peroxide (3 mM or 25 μM), NO (50 μM) plus H2O2 (3 mM), or spermine NONOate (25 μM) plus H2O2 (25 μM). Double treatments were also done sequentially, with the second chemical added 10 min after the first and the cells incubated for another 15 min, for a total of 25 min. Cells were lysed and assayed for β-galactosidase activity as previously described (20). At least three independent cultures were analyzed in duplicate.
Macrophage assays.
J774A.1 murine macrophages (LGC Promochem) were maintained at 37°C in a 5% CO2-air atmosphere in Dulbecco's modified Eagle medium (DMEM) supplemented as previously described (26). For infection studies, macrophages were seeded at 5 × 105 cells/well and incubated for 24 h prior to being activated for 16 h with 0.3 μg/ml gamma interferon (Sigma) and 1.6 μg/ml lipopolysaccharide (Sigma). The medium was then changed to DMEM without antibiotics, and macrophages were infected with bacterial suspensions at a multiplicity of infection (MOI) of 20 for 30 min at 37°C. Bacterial suspensions were prepared from cells of E. coli K-12 wild-type, ΔnorV, and Δhmp strains grown aerobically in LB to an OD600 of ∼0.3, harvested, washed three times with phosphate-buffered saline (PBS), and resuspended in DMEM. Noninternalized bacteria were killed upon incubation in DMEM supplemented with penicillin-streptomycin (Gibco-Invitrogen), and internalized bacteria were further incubated in macrophages for up to 48 h. At the indicated times, macrophages were lysed with 2% saponin and intracellular bacterial content assessed by CFU counting of viable cells.
For complementation studies, the norV gene was excised from plasmid pME2337 (47) and cloned into NdeI-HindIII-digested pFLAG-CTC, yielding pFLAG-norV. E. coli K-12 wild-type and ΔnorV Δhmp cells carrying empty pFLAG or pFLAG-norV were grown aerobically for 16 h in LB medium with 100 μg/ml ampicillin and 1 mM isopropyl β-d-1-thiogalactopyranoside (IPTG), harvested, washed twice with PBS, and resuspended in DMEM.
Production of recombinant E. coli NorR protein, ATPase activity assays, and electron paramagnetic resonance (EPR) studies.
For production of recombinant E. coli NorR, the DNA coding region was cloned into NdeI-EcoRI-digested pET28a and expressed in E. coli BL21Gold(DE3) cells as previously reported (5, 6). NorR was isolated from the soluble fraction of E. coli cells and purified under anaerobic conditions in a Coy model A-2463 anaerobic chamber. Cell extracts were incubated with 1 mM MgATP and Fe(NH4)2(SO4)2 at room temperature for 1 h (5) and then loaded into a HisTrap HP column (GE Healthcare) equilibrated in 20 mM Tris-HCl, pH 7.4, 500 mM NaCl, and 20% glycerol (buffer A). The fraction containing NorR was eluted with 250 mM imidazole, concentrated in an Amicon ultrafiltration cell (Millipore), and desalted in a Superdex 30 column (GE Healthcare) equilibrated with buffer A. The protein purity was evaluated by SDS-PAGE, and the concentration and iron content were determined by BCA and 2,4,6-tripyridyl-1,2,3-triazine (TPTZ) methods, respectively (10, 36). The purified NorR protein contained ∼0.9 Fe atom per monomer.
For ATPase activity assays (27), the reaction mixtures contained 30 mM ATP (Sigma), 1 mM phosphoenolpyruvate (PEP; Sigma), a 5 nM concentration of a 320-bp DNA fragment that spans the entire norV promoter (20), 7 U pyruvate kinase (Roche Applied Science), 23 U lactate dehydrogenase (Roche Applied Science), 2 mM MgCl2, and 300 nM E. coli NorR in 50 mM Tris-HCl, pH 8.0, plus 100 mM KCl. Activities were evaluated at 37°C for 20 min, upon addition of 0.3 mM NADH and following its oxidation at 340 nm (ε = 6.22 × 103 M−1 cm−1). For these assays, NorR was left untreated or incubated for 5 min with 20 μM NO, 100 μM hydrogen peroxide, or mixtures of both chemicals.
EPR spectra were acquired on a Bruker EMX spectrometer equipped with an Oxford Instruments continuous-flow helium cryostat and recorded at 9.38 MHz at a temperature of 6 to 7 K. For whole-cell EPR, E. coli BL21Gold(DE3) cells carrying pT7.7-NorR (20) were grown for 16 h at 28°C in LB supplemented with 50 μM IPTG. The cells were washed twice and resuspended in 1/100 of the culture volume in 20 mM Tris-HCl, pH 7.5. Aliquots (300 μl) were treated at room temperature with 150 μM NO, 4 mM H2O2, or both, added simultaneously or sequentially, with an interval of 5 min.
The purified NorR protein (75 μM) was incubated for 5 min at room temperature with 2 equivalents of H2O2, potassium ferricyanide, and NO before being transferred to EPR tubes and frozen in liquid nitrogen. All manipulations and incubations were performed in an anaerobic chamber.
RESULTS
Expression of E. coli FlRd is reduced under the combined effect of oxidative and nitrosative stress.
In the present work, we compared the expression of FlRd in cells exposed to nitric oxide, H2O2, and a combination of the two.
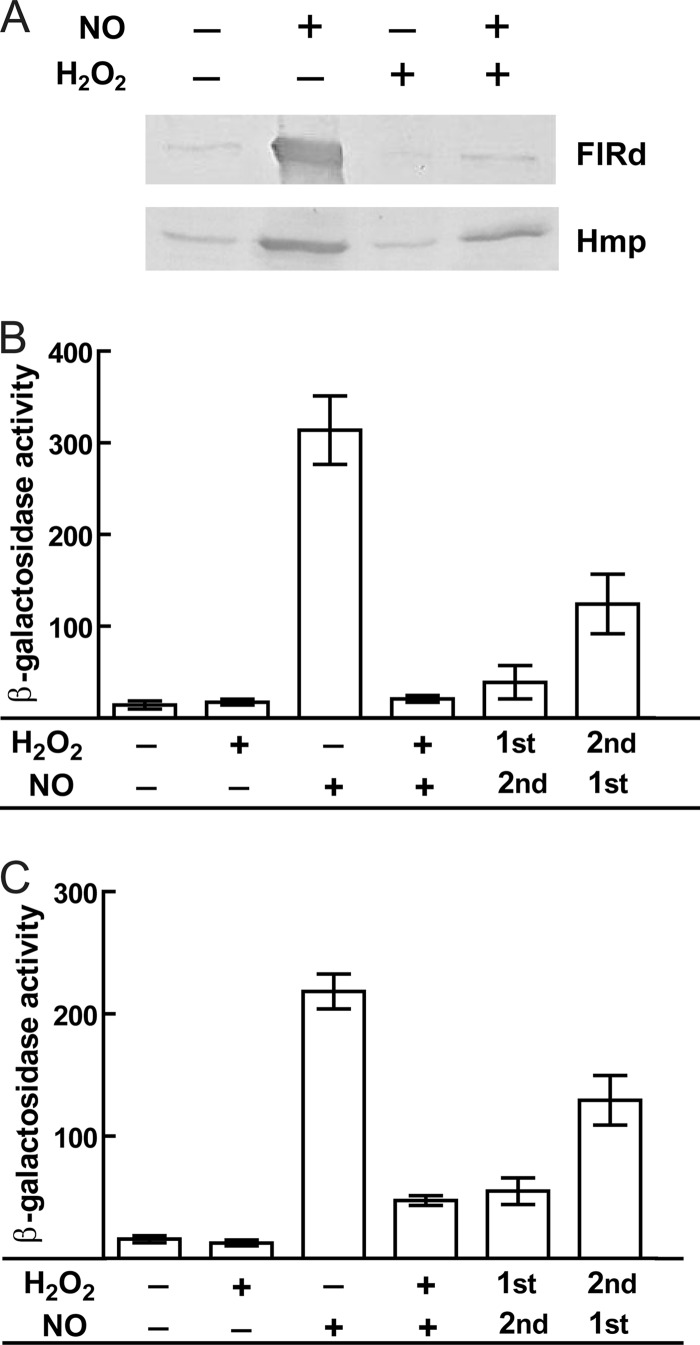
Immunoblotting assays revealed that NO-treated E. coli cell extracts contained increased amounts of FlRd, which is consistent with the reported induction of norV by NO (12, 21, 24). In contrast, cells exposed to hydrogen peroxide and to nitric oxide plus hydrogen peroxide displayed very low levels of FlRd (Fig. 1A). Although hydrogen peroxide was not expected to induce expression of FlRd, the markedly decreased amount of FlRd in cells treated with nitric oxide plus hydrogen peroxide was surprising. One possible explanation was that compounds that have no ability to trigger norV expression were being generated as a result of the chemical reaction between H2O2 and NO. However, this seems not to be the case, since the expression of the NO detoxifier Hmp was similar in E. coli cells treated with NO only and in those exposed to NO plus H2O2 (Fig. 1A).
Fig 1.
Analysis of FlRd expression in cells exposed to combined nitrosative and oxidative stress. (A) Immunoblotting analysis of E. coli K-12 cells grown anaerobically and exposed for 45 min to 50 μM NO and 3 mM H2O2, using antibodies against flavorubredoxin (FlRd) and flavohemoglobin (Hmp). (B and C) β-Galactosidase activities of E. coli RK4353 cells carrying plasmid pAA182-PnorV, which contains the norV promoter-lacZ fusion. Cells cultured anaerobically in LB were treated, at an OD600 of 0.3, with 50 μM NO and/or 3 mM H2O2 (B) or with 25 μM spermine NONOate and/or 25 μM H2O2 (C). All cultures were subjected to the stresses for a total of 25 min. Sequential exposures were done by a 10-min exposure to H2O2 followed by a 15-min treatment with NO/NO donor and by exposure for 10 min to NO/NO donor followed by 15 min of treatment with H2O2. Results are means ± standard errors (SE) for three independent cultures assayed in duplicate. Activities are expressed in nmol of o-nitrophenol/min/mg of bacterial dry mass.
To assess whether the smaller amount of FlRd was due to transcriptional alterations, the activation of the reporter fusion containing the norV promoter was evaluated. While the norV promoter was activated by NO, no activation occurred in cells treated with hydrogen peroxide or in cells exposed to NO plus H2O2 (Fig. 1B). When E. coli was first treated with H2O2 (10 min) and then with NO (15 min), the promoter activation was very low and almost comparable to that observed upon simultaneous exposure to the two stresses for 25 min (Fig. 1B). Initial exposure to NO (10 min) and then to H2O2 (15 min) resulted in approximately 3-fold less activation of the promoter than exposure to NO alone for 25 min, consistent with the abolishment/impairment of NO induction upon introduction of the oxidative stress.
We next performed a set of experiments using a lower concentration of NO and H2O2 (25 μM) that is within the range of physiological concentrations described to be produced by macrophages (8, 35, 42). Under these conditions, the activation of the promoter in cells sequentially and simultaneously exposed to both stresses was higher than that in nonexposed cells but considerably lower than that caused by NO alone (Fig. 1C).
Hence, we concluded that in cells exposed to both nitric oxide and hydrogen peroxide, the expression of FlRd is essentially impaired.
Effect of hydrogen peroxide on properties of NorR.
It is well established that the transcription of E. coli norR is not altered upon exposure to nitrosative stress (12). However, the lack of norV induction by NO in the presence of H2O2 led to the analysis of the effect of hydrogen peroxide on norR transcription levels. We observed that hydrogen peroxide did not cause changes in the level of norR mRNA (data not shown).
Given that the compromised expression of FlRd in cells treated with NO plus H2O2 could not be attributed to lowered NorR levels, we hypothesized that the ligation of NO to the mononuclear iron site located in the GAF domain of NorR, which triggers NorR activation of the norV promoter (6), was impaired in the presence of hydrogen peroxide, an issue that was studied by EPR spectroscopy.
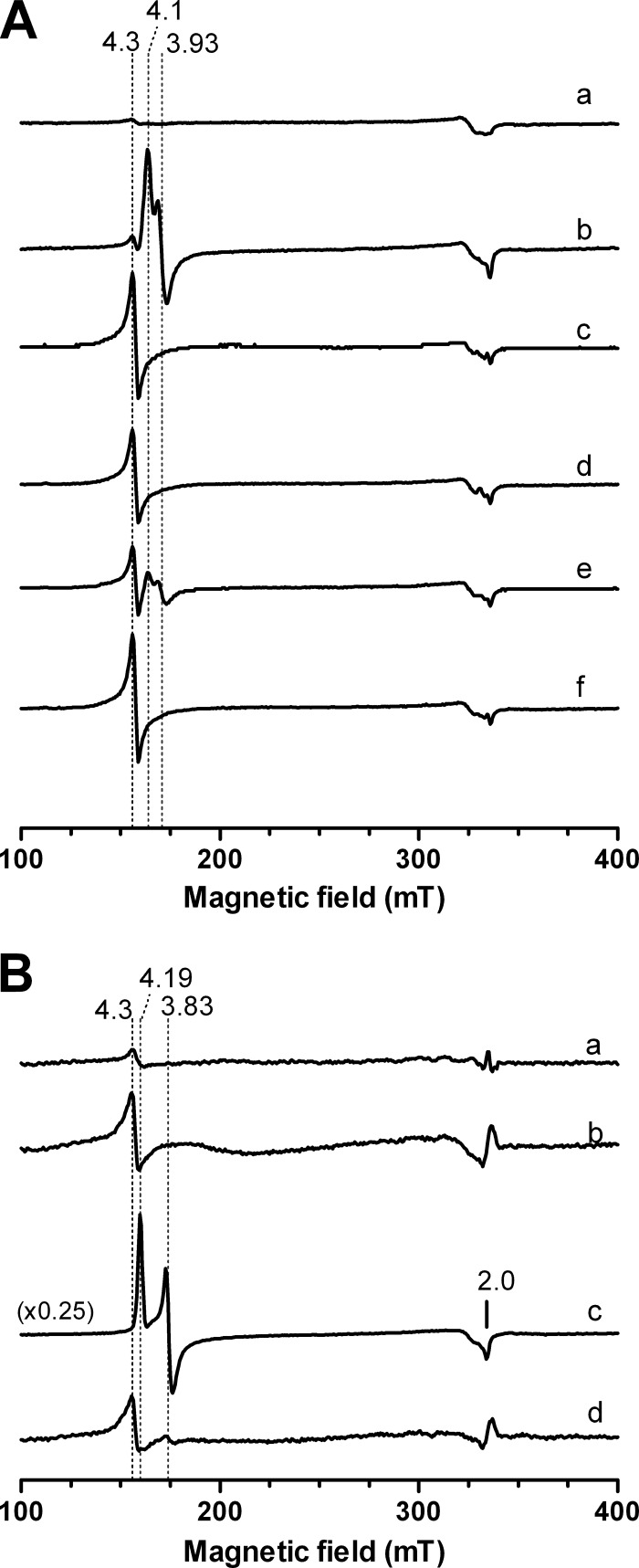
To this end, E. coli cells overexpressing the NorR protein were exposed to NO, H2O2, or a combination of the two, and the EPR spectra were recorded (Fig. 2A). The spectrum of cells treated with 150 μM NO displayed an intense signal, with g values of 4.1 and 3.93, attributed to a high-spin ferrous NO complex with a total spin (S) value of 3/2 and a rhombicity (E/D) of ∼0.03, as previously observed (6), and compatible with the formation of a mononitrosyl iron complex. Cells treated with 4 mM H2O2 exhibited a signal with a g value of 4.3, which is characteristic of high-spin (S = 5/2) ferric ions. The EPR spectrum of cells exposed simultaneously to NO and H2O2 revealed in the same region only the resonance due to ferric ions, with a g value of 4.3, i.e., the signal corresponding to the Fe-NO species was absent.
Fig 2.
EPR analysis of the influence of H2O2 on the binding of NO to the mononuclear iron center of NorR. (A) Whole-cell EPR analysis of E. coli overexpressing NorR. EPR spectra of E. coli BL21Gold(DE3) carrying pT7.7-NorR were recorded in the absence of stress (a), after 5 min of treatment with 150 μM NO (b) or 4 mM H2O2 (c), after sequential treatment with 150 μM NO (5 min) followed by 4 mM H2O2 (5 min) (d) or with 4 mM H2O2 (5 min) followed by 150 μM NO (5 min) (e), and after a simultaneous 5 min of treatment with 150 μM NO and 4 mM H2O2 (f). (B) EPR spectra of the purified NorR protein (75 μM), as isolated (a) or treated for 5 min with 150 μM H2O2 (b), 150 μM NO (c), or NO plus H2O2 (150 μM each) (d). With the exception of spectrum c in panel B, all spectra have the same intensity scale. EPR spectra were recorded at a 9.39-MHz microwave frequency, 2.4-mW microwave power, and 6 to 7 K.
Cells were also treated with NO and H2O2, but in a sequential mode. For cells treated first with NO (5 min) and then exposed to H2O2 (5 min), the EPR spectrum contained only one signal, with a g value of 4.3, suggesting that H2O2 destroys the already formed Fe-NO complex. The EPR spectrum of cells that were exposed first to H2O2 (5 min) and then to NO (5 min) exhibited an overlay of signals, with g values of 4.3, 4.1, and 3.93, that could be attributed to the superimposition of the resonances due to the high-spin ferric iron and Fe-NO species.
A similar study was conducted using purified recombinant E. coli NorR (Fig. 2B). NorR as isolated was essentially EPR silent, suggesting that it was purified in the ferrous form. Upon reaction with hydrogen peroxide, a resonance with a g value of 4.3 developed that was consistent with the oxidation of the mononuclear iron center. NorR incubation with NO resulted in the formation of a high-spin ferrous NO complex (Fig. 2B) with g values of 4.19, 3.83, and 2.0, which were slightly different from those of the protein in whole cells; upon treatment with H2O2 plus NO, the spectrum displayed a single signal with a g value of 4.3, as also observed in whole cells under the same conditions. Moreover, the intensity of this signal was similar to that of the H2O2-treated protein, indicating that formation of a ferric NO species did not occur. To confirm that lack of formation of the Fe-NO complex was due to iron oxidation, identical experiments were performed with NorR oxidized with potassium ferricyanide, which revealed that there was no binding of NO (data not shown).
Altogether, it was concluded that the presence of H2O2 inhibits the binding of NO to the iron center of NorR due to oxidation of the mononuclear iron center impairing the formation of the nitrosylated ferrous site.
The ATPase activity of NorR is induced by conformational changes triggered by the formation of the mononitrosyl iron complex (6). Since our results suggest that hydrogen peroxide prevents binding of NO, the ATPase activity of NorR under these stress conditions was examined (Table 2). The basal level of the ATPase activity of NorR remained essentially unchanged when the protein was exposed to H2O2; in contrast, when it was treated with NO, the activity increased approximately 7-fold. However, in the presence of both NO and H2O2, no enhancement of the activity was seen, thus confirming that hydrogen peroxide hinders the NO-dependent ATPase activity of NorR.
Table 2.
ATPase activity of NorR upon treatment with NO and H2O2a
| Presence of NO | Presence of H2O2 | ATPase activity (μmol ATP/min-mg protein) |
|---|---|---|
| − | − | 0.4 ± 0.1 |
| + | 0.4 ± 0.1 | |
| + | − | 2.9 ± 0.4 |
| + | 1.1 ± 0.1 |
NorR was treated for 5 min with 100 μM H2O2, 20 μM NO, or a mixture of the two. The results represent the averages ± SE for three independent samples.
Role of FlRd in protection of E. coli within macrophages.
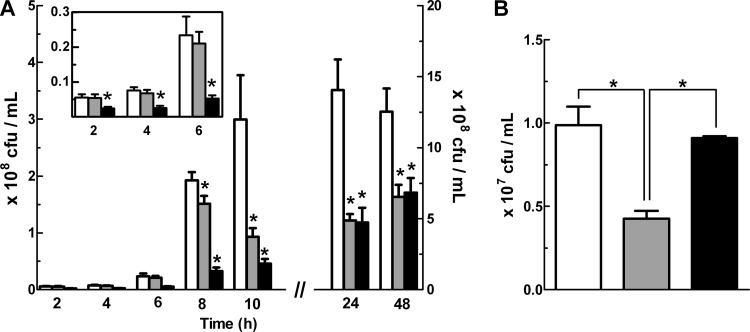
The contribution of FlRd to the survival of E. coli was investigated in macrophages and compared with that of the other soluble NO detoxifier, Hmp. For this purpose, macrophages were infected with wild-type E. coli and with strains mutated in the norV and hmp genes. The results showed that for up to 6 h of macrophage infection, ΔnorV cell counts had no significant difference compared to wild-type cell counts, while the survival of Δhmp cells was lower (Fig. 3A). However, for longer incubation times, survival of the ΔnorV mutant strain within macrophages decreased and became similar to that of the Δhmp strain (Fig. 3A), indicating that FlRd enhances the long-term survival of E. coli within macrophages.
Fig 3.
Survival of E. coli wild-type and norV mutant strains upon macrophage infection. (A) J774A.1 murine macrophages were infected with E. coli K-12 wild-type (white), ΔnorV (gray), and Δhmp (black) strains. Viable counts of E. coli cells were determined at the indicated times. Data from 24 h and 48 h of infection are depicted with the right y axis, and data from early times are expanded in the inset. (B) Intracellular survival of the E. coli wild type carrying the empty vector pFLAG (white) or the Δhmp ΔnorV strain carrying pFLAG (gray) or pFLAG-norV, which expresses norV from a NorR-independent promoter (black), after 6 h of infection of macrophages. Values are means ± SE for at least 6 independent experiments. *, P < 0.05 (one-way analysis of variance and the Bonferroni multiple-comparison test).
The lack of differences in the survival of the wild type and the ΔnorV mutant upon incubation in macrophages observed within the first 6 h of macrophage infection may be due, in light of our earlier findings that norV induction in response to NO is compromised when H2O2 is present, to the NorR inhibition caused by the macrophage oxidative burst. However, if regulation by NorR was lifted and flavorubredoxin was expressed, it should be able to detoxify nitric oxide and afford protection to E. coli. To test this hypothesis, we designed a complementation experiment in which macrophages were incubated for 6 h with a strain expressing the norV gene from a NorR-independent, IPTG-controlled plasmid. To avoid scavenging of NO by Hmp, it was necessary to use the double ΔnorV Δhmp mutant strain, whose phenotype within macrophages at this early time is due to the absence of hmp. Under these conditions, the behavior of the E. coli wild-type strain was rescued (Fig. 3B), showing that when it is expressed independently of its own promoter, i.e., not regulated by NorR, FlRd confers protection to E. coli against macrophages.
DISCUSSION
In this study, we present evidence that the combination of NO and H2O2 impairs induction of FlRd, the flavodiiron NO reductase of E. coli. Cell growth was done under anaerobic conditions to avoid the interference of oxygen and the possible formation of peroxynitrite that is generated under aerated environments when superoxide, derived from molecular oxygen, reacts with NO.
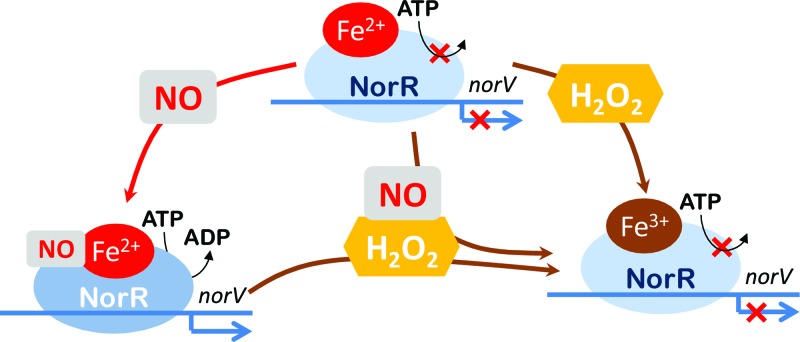
It was previously shown that the Fe(II)-GAF domain of NorR inhibits its ATPase activity and that binding of NO to the iron center is required to stimulate the ATPase activity (6). Our EPR studies suggest that upon incubation of NorR with NO plus H2O2, the oxidation of the iron center blocks NO ligation. Consequently, the ATPase activity of NorR is not triggered. Hence, it might be concluded that NorR needs to be in the reduced state in order to bind NO and to promote the transcription of norV, i.e., the iron oxidation state influences the activation of NorR (Fig. 4).
Fig 4.
Schematic representation of FlRd expression under stress conditions. The ferrous mononitrosyl iron complex formed in NorR upon exposure to NO promotes transcription of the norV gene. In the presence of hydrogen peroxide, the iron center is oxidized and no longer able to bind NO, resulting in the lack of norV expression.
We observed that hmp accounts for successful E. coli infection in macrophages, demonstrating the importance of the protein for bacterial stress resistance, which is in agreement with previous reports (37, 38). Moreover, we provide evidence that Hmp protects E. coli at all stages of macrophage infection. In contrast, the contribution of norV to survival of E. coli within macrophages exhibited a time-dependent profile, as protection occurred only for incubations in mammalian cells of longer than 8 h. Therefore, previous failures to demonstrate a role for FlRd in protection against macrophage killing were most probably due to the macrophage infection times studied, which ranged from 15 to 120 min (31). Interestingly, a study of the gene expression profile of Salmonella enterica following macrophage infection indicated that induction of norV transcription is higher at times corresponding to the generation of the NO burst (7). An apparent oscillation in norV mRNA levels was also detected in E. coli exposed to acidified nitrite and grown under aerobic conditions while no variation was observed under anaerobic conditions, also suggesting that oxygen is required for the oscillatory expression pattern of norV (24).
The expression of NADPH oxidase and nitric oxide synthase is induced in macrophages upon phagocytosis of bacteria, and the subsequent production of superoxide and nitric oxide is used to suppress bacterial growth (25, 34). Studies on the production of chemical species by macrophages during the first hours after bacterial invasion revealed that H2O2 resulting from spontaneous dismutation of superoxide via SODs is the most abundant species (15, 28). The ROS level is considered to abate within 6 to 10 h, with increasingly abundant generation of nitrosative species (42). In spite of the toxicity of the species generated, macrophages do not provide complete protection against infection partially because several bacterial proteins have the ability to detoxify reactive oxygen and nitrogen species. Moreover, some bacteria contain more than one enzyme that apparently detoxifies the same chemical species. This is the case for E. coli, which has at least two soluble NO-scavenging enzymes. So far, the need for two systems has remained largely unclear. The present data reveal, for the first time (to the best of our knowledge), that FlRd has a time-differentiated action within macrophages. The blockage of norV transcription that is expected to occur during the first stage of macrophage infection due to the predominance of hydrogen peroxide decreases the ability of bacteria to scavenge the NO produced by the macrophages. Consequently, the remaining NO will stimulate the expression of Hmp, as its gene is regulated by iron-sulfur-containing transcription factors, such as NsrR, that lift their repression and trigger Hmp's NO activity only upon binding to NO. Over time, macrophages decrease the oxidative burst while NO production increases, which will allow binding of NO to NorR and subsequent triggering of norV expression.
It is recognized that NO at low concentrations has a protective role for bacteria, as it activates transcription factors, such as SoxRS, OxyR, and Fur, whose regulons encode antioxidant enzymes (17). Bacillus subtilis and Staphylococcus aureus express an NO synthase enzyme that is proposed to play a critical role in adaptation to oxidative stress (18, 33). While a gene encoding NO synthase is apparently absent in E. coli, the existence of two NO-detoxifying systems may go beyond the need for functional redundancy to represent an alternative way of tuning the intracellular NO concentration. Moreover, the two systems endow bacteria with the ability to profit from the fact that mammalian Nox and iNOS activities peak at different times after phagocytosis and provide a metabolic flexibility that helps to protect E. coli from the variety of environmental conditions experienced during the course of macrophage infection. Indeed, survival within macrophages is an important mechanism of infection of pathogens (23, 40).
Flavodiiron-like proteins are widespread in nature, including in several commensal and pathogenic Gram-negative and Gram-positive bacteria that colonize the human orogastric tract (32, 43). These include members of the genera Bacillus, Bacteroides, Clostridium, Fusobacterium, Ruminococcus, Porphyromonas, Prevotella, Vibrio, Salmonella, Shigella, and Yersinia and uropathogenic, enteropathogenic, and enterohemorrhagic E. coli strains. Consistent with the significant in vitro activity of this enzyme, in this work we succeeded in demonstrating the contribution of FlRd to microbial survival against iNOS-mediated host immune defenses.
ACKNOWLEDGMENTS
We are grateful to Robert Poole, University of Sheffield, United Kingdom, for providing the antibody against E. coli Hmp.
This work was funded by project grants PEst-OE/EQB/LA0004/2011, PTDC/BIA-PRO/098224/2008 (L.M.S.), and PTDC/QUI-BIOQ/111080/2009 (M.T.) from Fundação para a Ciência e Tecnologia (FCT). J.M.B. and M.C.J. are recipients of FCT grants SFRH/BD/41209/2007 and SFRH/BPD/43172/2008, respectively.
Footnotes
Published ahead of print 4 May 2012
REFERENCES
- 1. Beckman JS, Wink DA, Crow JP. 1996. Nitric oxide and peroxynitrite, p 61–70 In Feelisch M, Stamler JS. (ed), Methods in nitric oxide research. John Wiley & Sons Ltd., Chichester, United Kingdom [Google Scholar]
- 2. Bowman LA, McLean S, Poole RK, Fukuto JM. 2011. The diversity of microbial responses to nitric oxide and agents of nitrosative stress close cousins but not identical twins. Adv. Microb. Physiol. 59:135–219 [DOI] [PubMed] [Google Scholar]
- 3. Constantinidou C, et al. 2006. A reassessment of the FNR regulon and transcriptomic analysis of the effects of nitrate, nitrite, NarXL, and NarQP as Escherichia coli K12 adapts from aerobic to anaerobic growth. J. Biol. Chem. 281:4802–4815 [DOI] [PubMed] [Google Scholar]
- 4. da Costa PN, Teixeira M, Saraiva LM. 2003. Regulation of the flavorubredoxin nitric oxide reductase gene in Escherichia coli: nitrate repression, nitrite induction, and possible post-transcription control. FEMS Microbiol. Lett. 218:385–393 [DOI] [PubMed] [Google Scholar]
- 5. D'Autreaux B, Tucker N, Spiro S, Dixon R. 2008. Characterization of the nitric oxide-reactive transcriptional activator NorR. Methods Enzymol. 437:235–251 [DOI] [PubMed] [Google Scholar]
- 6. D'Autreaux B, Tucker NP, Dixon R, Spiro S. 2005. A non-haem iron centre in the transcription factor NorR senses nitric oxide. Nature 437:769–772 [DOI] [PubMed] [Google Scholar]
- 7. Eriksson S, Lucchini S, Thompson A, Rhen M, Hinton JC. 2003. Unravelling the biology of macrophage infection by gene expression profiling of intracellular Salmonella enterica. Mol. Microbiol. 47:103–118 [DOI] [PubMed] [Google Scholar]
- 8. Fang FC. 2011. Antimicrobial actions of reactive oxygen species. mBio 2:e00141–11 doi: 10.1128/mBio.00141-11.2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Filenko N, et al. 2007. The NsrR regulon of Escherichia coli K-12 includes genes encoding the hybrid cluster protein and the periplasmic, respiratory nitrite reductase. J. Bacteriol. 189:4410–4417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Fischer DS, Price DC. 1964. A simple serum iron method using the new sensitive chromogen tripyridyl-S-triazine. Clin. Chem. 10:21–31 [PubMed] [Google Scholar]
- 11. Gardner AM, Gardner PR. 2002. Flavohemoglobin detoxifies nitric oxide in aerobic, but not anaerobic, Escherichia coli. Evidence for a novel inducible anaerobic nitric oxide-scavenging activity. J. Biol. Chem. 277:8166–8171 [DOI] [PubMed] [Google Scholar]
- 12. Gardner AM, Gessner CR, Gardner PR. 2003. Regulation of the nitric oxide reduction operon (norRVW) in Escherichia coli. Role of NorR and sigma54 in the nitric oxide stress response. J. Biol. Chem. 278:10081–10086 [DOI] [PubMed] [Google Scholar]
- 13. Gardner AM, Helmick RA, Gardner PR. 2002. Flavorubredoxin, an inducible catalyst for nitric oxide reduction and detoxification in Escherichia coli. J. Biol. Chem. 277:8172–8177 [DOI] [PubMed] [Google Scholar]
- 14. Gardner PR. 2005. Nitric oxide dioxygenase function and mechanism of flavohemoglobin, hemoglobin, myoglobin and their associated reductases. J. Inorg. Biochem. 99:247–266 [DOI] [PubMed] [Google Scholar]
- 15. Giorgio M, Trinei M, Migliaccio E, Pelicci PG. 2007. Hydrogen peroxide: a metabolic by-product or a common mediator of ageing signals? Nat. Rev. Mol. Cell. Biol. 8:722–728 [DOI] [PubMed] [Google Scholar]
- 16. Gomes CM, et al. 2002. A novel type of nitric-oxide reductase. Escherichia coli flavorubredoxin. J. Biol. Chem. 277:25273–25276 [DOI] [PubMed] [Google Scholar]
- 17. Gusarov I, Nudler E. 2005. NO-mediated cytoprotection: instant adaptation to oxidative stress in bacteria. Proc. Natl. Acad. Sci. U. S. A. 102:13855–13860 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Hong IS, et al. 2003. Purification and characterization of nitric oxide synthase from Staphylococcus aureus. FEMS Microbiol. Lett. 222:177–182 [DOI] [PubMed] [Google Scholar]
- 19. Imlay JA. 2008. Cellular defenses against superoxide and hydrogen peroxide. Annu. Rev. Biochem. 77:755–776 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Justino MC, Goncalves VM, Saraiva LM. 2005. Binding of NorR to three DNA sites is essential for promoter activation of the flavorubredoxin gene, the nitric oxide reductase of Escherichia coli. Biochem. Biophys. Res. Commun. 328:540–544 [DOI] [PubMed] [Google Scholar]
- 21. Justino MC, Vicente JB, Teixeira M, Saraiva LM. 2005. New genes implicated in the protection of anaerobically grown Escherichia coli against nitric oxide. J. Biol. Chem. 280:2636–2643 [DOI] [PubMed] [Google Scholar]
- 22. Kurtz DM., Jr 2006. Avoiding high-valent iron intermediates: superoxide reductase and rubrerythrin. J. Inorg. Biochem. 100:679–693 [DOI] [PubMed] [Google Scholar]
- 23. Linehan SA, Holden DW. 2003. The interplay between Salmonella typhimurium and its macrophage host—what can it teach us about innate immunity? Immunol. Lett. 85:183–192 [DOI] [PubMed] [Google Scholar]
- 24. Mukhopadhyay P, Zheng M, Bedzyk LA, LaRossa RA, Storz G. 2004. Prominent roles of the NorR and Fur regulators in the Escherichia coli transcriptional response to reactive nitrogen species. Proc. Natl. Acad. Sci. U. S. A. 101:745–750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Nathan C, Shiloh MU. 2000. Reactive oxygen and nitrogen intermediates in the relationship between mammalian hosts and microbial pathogens. Proc. Natl. Acad. Sci. U. S. A. 97:8841–8848 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Nobre LS, et al. 2010. Binding of azole antibiotics to Staphylococcus aureus flavohemoglobin increases intracellular oxidative stress. J. Bacteriol. 192:1527–1533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Norby JG. 1988. Coupled assay of Na+,K+-ATPase activity. Methods Enzymol. 156:116–119 [DOI] [PubMed] [Google Scholar]
- 28. Paulsen CE, Carroll KS. 2010. Orchestrating redox signaling networks through regulatory cysteine switches. ACS Chem. Biol. 5:47–62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Pinto AF, Rodrigues JV, Teixeira M. 2010. Reductive elimination of superoxide: structure and mechanism of superoxide reductases. Biochim. Biophys. Acta 1804:285–297 [DOI] [PubMed] [Google Scholar]
- 30. Poole RK. 2005. Nitric oxide and nitrosative stress tolerance in bacteria. Biochem. Soc. Trans. 33:176–180 [DOI] [PubMed] [Google Scholar]
- 31. Pullan ST, et al. 2007. Nitric oxide in chemostat-cultured Escherichia coli is sensed by Fnr and other global regulators: unaltered methionine biosynthesis indicates lack of S nitrosation. J. Bacteriol. 189:1845–1855 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Saraiva LM, Vicente JB, Teixeira M. 2004. The role of the flavodiiron proteins in microbial nitric oxide detoxification. Adv. Microb. Physiol. 49:77–129 [DOI] [PubMed] [Google Scholar]
- 33. Shatalin K, et al. 2008. Bacillus anthracis-derived nitric oxide is essential for pathogen virulence and survival in macrophages. Proc. Natl. Acad. Sci. U. S. A. 105:1009–1013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Shiloh MU, et al. 1999. Phenotype of mice and macrophages deficient in both phagocyte oxidase and inducible nitric oxide synthase. Immunity 10:29–38 [DOI] [PubMed] [Google Scholar]
- 35. Slauch JM. 2011. How does the oxidative burst of macrophages kill bacteria? Still an open question. Mol. Microbiol. 80:580–583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Smith PK, et al. 1985. Measurement of protein using bicinchoninic acid. Anal. Biochem. 150:76–85 [DOI] [PubMed] [Google Scholar]
- 37. Stevanin TM, Poole RK, Demoncheaux EA, Read RC. 2002. Flavohemoglobin Hmp protects Salmonella enterica serovar Typhimurium from nitric oxide-related killing by human macrophages. Infect. Immun. 70:4399–4405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Stevanin TM, Read RC, Poole RK. 2007. The hmp gene encoding the NO-inducible flavohaemoglobin in Escherichia coli confers a protective advantage in resisting killing within macrophages, but not in vitro: links with swarming motility. Gene 398:62–68 [DOI] [PubMed] [Google Scholar]
- 39. Stewart V, MacGregor CH. 1982. Nitrate reductase in Escherichia coli K-12: involvement of chlC, chlE, and chlG loci. J. Bacteriol. 151:788–799 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Svensson L, et al. 2010. Role of flavohemoglobin in combating nitrosative stress in uropathogenic Escherichia coli—implications for urinary tract infection. Microb. Pathog. 49:59–66 [DOI] [PubMed] [Google Scholar]
- 41. van Wonderen JH, Burlat B, Richardson DJ, Cheesman MR, Butt JN. 2008. The nitric oxide reductase activity of cytochrome c nitrite reductase from Escherichia coli. J. Biol. Chem. 283:9587–9594 [DOI] [PubMed] [Google Scholar]
- 42. Vazquez-Torres A, Jones-Carson J, Mastroeni P, Ischiropoulos H, Fang FC. 2000. Antimicrobial actions of the NADPH phagocyte oxidase and inducible nitric oxide synthase in experimental salmonellosis. I. Effects on microbial killing by activated peritoneal macrophages in vitro. J. Exp. Med. 192:227–236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Vicente JB, Justino MC, Goncalves VL, Saraiva LM, Teixeira M. 2008. Biochemical, spectroscopic, and thermodynamic properties of flavodiiron proteins. Methods Enzymol. 437:21–45 [DOI] [PubMed] [Google Scholar]
- 44. Vine CE, Cole JA. 2011. Nitrosative stress in Escherichia coli: reduction of nitric oxide. Biochem. Soc. Trans. 39:213–215 [DOI] [PubMed] [Google Scholar]
- 45. Vine CE, Cole JA. 2011. Unresolved sources, sinks, and pathways for the recovery of enteric bacteria from nitrosative stress. FEMS Microbiol. Lett. 325:99–107 [DOI] [PubMed] [Google Scholar]
- 46. Wasser IM, de Vries S, Moenne-Loccoz P, Schroder I, Karlin KD. 2002. Nitric oxide in biological denitrification: Fe/Cu metalloenzyme and metal complex NO(x) redox chemistry. Chem. Rev. 102:1201–1234 [DOI] [PubMed] [Google Scholar]
- 47. Wasserfallen A, Ragettli S, Jouanneau Y, Leisinger T. 1998. A family of flavoproteins in the domains Archaea and Bacteria. Eur. J. Biochem. 254:325–332 [DOI] [PubMed] [Google Scholar]