Abstract
The general stress regulon of Bacillus subtilis comprises approximately 200 genes and is under the control of the alternative sigma factor σB. The activation of σB occurs in response to multiple physical stress stimuli as well as energy starvation conditions. The expression of the general stress proteins provides growing and stationary nonsporulating vegetative cells with nonspecific and broad stress resistance. A previous comprehensive phenotype screening analysis of 94 general stress gene mutants in response to severe growth-inhibiting stress stimuli, including ethanol, NaCl, heat, and cold, indicated that secondary oxidative stress may be a common component of severe physical stress. Here we tested the individual contributions of the same set of 94 mutants to the development of resistance against exposure to the superoxide-generating agent paraquat and hydrogen peroxide (H2O2). In fact, 62 mutants displayed significantly decreased survival rates in response to paraquat and/or H2O2 stress compared to the wild type at a confidence level of an α value of ≤0.01. Thus, we were able to assign 47 general stress genes to survival against superoxide, 6 genes to protection from H2O2 stress, and 9 genes to the survival against both. Furthermore, we show that a considerable overlap exists between the phenotype clusters previously assumed to be involved in oxidative stress management and the actual group of oxidative-stress-sensitive mutants. Our data provide information that many general stress proteins with still unknown functions are implicated in oxidative stress resistance and further support the notion that different severe physical stress stimuli elicit a common secondary oxidative stress.
INTRODUCTION
The genome of the Gram-positive model organism Bacillus subtilis comprises approximately 4,250 genes; about 1,300 of these genes encode proteins of still unknown functions (6). By the use of comparative transcriptome and proteome analyses of wild-type strains and mutant strains defective in regulatory proteins, it has become possible to define several stimulon, regulon, modulon, as well as operon structures in B. subtilis. These approaches also led to the characterization of the general stress regulon that is under the control of the alternative sigma factor σB and comprises approximately 200 genes (19, 35, 38, 40). The activation of σB occurs “generally” in response to various stress stimuli. These stimuli include physical stress, such as high and low temperatures, salt, ethanol, low pH, the nitrogen oxide (NO) donor sodium nitroprusside (SNP), and direct exposure to NO gas; irradiation with blue light; the addition of antibiotics such as bacitracin and vancomycin as well as starvation for glucose, phosphate, and oxygen; or treatment with compounds such as azide, mycophenolic acid, or carbonyl cyanide m-chlorophenylhydrazone (CCCP), which cause a deprivation of ATP and GTP levels (15, 39). It has been shown that the σB-dependent stress response is important for survival against severe ethanol, heat, salt, and alkaline shocks as well as repeated freezing-thawing cycles (14, 21, 44). Furthermore, it has been demonstrated that σB is necessary for growth as well as stationary-phase survival during prolonged periods of low temperatures (8, 21, 28). Thus, the characteristic function of the general stress proteins is to provide the cell with comprehensive cross-protective and preemptive stress resistance (14, 16, 21, 39, 44): (i) cross-protective, because it also confers resistance to oxidative (2, 12, 17) or alkaline (14) stresses that are not typical σB-inducing stimuli, and (ii) preemptive, because the protective functions of the general stress proteins also equip a nongrowing vegetative and nonsporulating cell against possible future stresses (17, 21, 44). The importance of the general stress response in cross-protection against oxidative stress was recognized about 15 years ago (3, 12–14, 16). Nevertheless, primary oxidative stress plays only a minor role in the activation of σB (35), and previous reports showed that oxidative stress induces only a subset of the σB regulon members (18, 32).
Although the overall physiological significance of the σB regulon for the stress adaptation and survival of B. subtilis is obvious, more than one-third of the general stress genes encode proteins with still unknown functions. The first attempt to comprehensively characterize the contribution of these general stress proteins with undefined functions to resistance against one or more specific stresses was made by Höper et al. (21). A phenotype screening analysis of 94 mutations of individual general stress genes was carried out. The mutants were exposed to heat (54°C), ethanol (10%), cold (4°C and 12°C), and hyperosmotic (10% NaCl) stresses to assign them to specific stress clusters on the basis of sensitive phenotypes. Notably, most of the mutants exhibited multiple stress management defects, indicating that different stress stimuli must cause a related damage to the cell that is counteracted by the same set of general stress gene products. In this context, many mutants with severe multiple-stress phenotypes could be associated with protection against oxidative damage. Thus, it was assumed that secondary oxidative stress may be a common component of multiple severe growth-inhibiting and σB-inducing stress stimuli (21).
The idea of secondary oxidative stress is corroborated by the identification of MgsR, a paralogue of the global regulator of the diamide stress response, Spx (41). The expression of MgsR is driven by σB and is necessary to achieve the full induction of a subregulon within the general stress response whose members can be linked to oxidative stress management (41). Recently, it was demonstrated that the regulator MgsR is activated by a redox switch beyond the primary decision of σB activation caused by the physical stress stimulus (A. Reder, D. Pöther, U. Gerth, and M. Hecker, unpublished data). Thus, the MgsR protein senses and integrates secondary oxidative stress signals caused by the imposition of ethanol stress.
Several studies further supported the idea that σB-inducing stress stimuli trigger secondary oxidative stress within the cell. Acid stress has been reported to induce the oxidative stress response in B. subtilis (46) and to cause hydroxyl radical (OH) and peroxynitrite (ONOO−) formation in Bacillus cereus (31). A radical-mediated mechanism for cell death caused by bactericidal antibiotics such as bacitracin was proposed previously (26), and the formation of superoxide and hydroxyl radicals was demonstrated to occur upon heat stress in B. subtilis (29) and B. cereus (30). Furthermore, ethanol, salt, and cold stresses have been shown to induce PerR-regulated genes in B. subtilis (20, 21, 24), and the respiratory chain of mitochondria was reported to be the origin of the extensive generation of reactive oxygen species (ROS) during ethanol metabolism in hepatocytes (1, 5).
Due to this increasing amount of data pointing at the significance of secondary oxidative stress management by the general stress proteins, we extended the previous analysis of Höper et al. (21) by monitoring the effect of the superoxide-generating agent paraquat as well as hydrogen peroxide (H2O2) stress on the survival of 94 mutants with singular defects in σB-dependent general stress genes. The results confirm that many general stress proteins are indeed involved in the oxidative stress resistance of B. subtilis. This functional genomics approach provides a valuable basis for more detailed biochemical investigations of the exact functions of general stress proteins to counteract secondary oxidative stress.
MATERIALS AND METHODS
Bacterial strains and culture conditions.
The two reference strains for this study were Bacillus subtilis wild-type strain 168 (10) and sigB mutant strain ML6 (22). Apart from two exceptions, the same set of 92 strains with mutations in single general stress genes described previously by Höper et al. was used (21). Mutants were constructed by the disruption of the reading frames of the general stress genes by the Campbell-type insertion of nonreplicative plasmids (pMutin1 to pMutin4) (25) or deletions internal to the structural gene ctc::spc (strain GF500) (42). The two pMUTIN mutant strains of spx (yjbD) (strain 2842) and mgsR (yqgZ) (strain 4773) that were used previously (21) have been replaced here by the ΔmgsR (BAR1) (41) and Δspx (spx::spc) (strain BAR8) (this study) deletion mutants. For the creation of the spx mutant, a modified two-step-fusion PCR protocol (45) was used to generate linear DNA fragments carrying a central spectinomycin resistance marker flanked by homologous sequences of the chromosomal up- and downstream regions of spx. The following primers were used: spx_up_for (GATGAAGGCAAACATCATATT) and spx_up_rev (TATTAATTTGTTCGTATGTATTCATTCATCTTCACTCCTCTAATTAGT) to generate the upstream fragment, spx_do_for (TAACAGATTAAAAAAATTATAATAGATCGTATCATCAAAAG) and spx_do_rev (TTATTCTCGGAACATTTATTGC) to generate the downstream fragment with B. subtilis wild-type chromosomal DNA as the template, as well as spec_for (ATGAATACATACGAACAAATTAATA) and spec_rev (TTATAATTTTTTTAATCTGTTATTT) for the spectinomycin resistance marker with plasmid pUS19 (7) as the template. The purification and fusion of the PCR products were carried out as described previously (41). For all experiments, a synthetic medium (43) was used. Growth was monitored by measuring the optical density at 500 nm (OD500). Fifty milliliters of prewarmed medium was inoculated with exponentially growing cells to obtain a starting OD500 of 0.06. Cultures were routinely grown in 500-ml Erlenmeyer flasks in a shaking water bath at 180 rpm at 37°C.
Exposure to stress and viability assays.
When the cultures reached an OD500 of 0.4, 18 ml was transferred into a prewarmed 100-ml flask, and the cells were stressed with 2% (vol/vol) ethanol to induce the general stress response for 20 min. After this preadaptation period, the cells were stressed with final concentrations of either 100 mM paraquat or 5 mM H2O2. Samples were taken prior to the imposition of oxidative stress (time zero [t0] control) and after 60, 120, 180, and 240 min for paraquat-stressed cells and after 30, 60, and 90 min for H2O2-stressed cells. Aliquots (100 μl) of the samples were diluted in a 0.9% (wt/vol) NaCl solution, appropriate dilutions of the cultures were plated onto LB agar and incubated overnight at 37°C, and the CFU were counted. All experiments were performed at least in triplicate, and at least two technical replicates from each dilution step were utilized to determine the number of CFU. The analysis of the survival experiment data was carried out as described previously by Höper et al. (21).
In silico analysis.
The amino acid sequences of the respective proteins were downloaded from the GenoList website (27) and were analyzed with the BLASTP protein-protein blast tool at the National Center for Biotechnology Information (NCBI) website (http://blast.ncbi.nlm.nih.gov/Blast.cgi).
RESULTS AND DISCUSSION
Impact of superoxide and peroxide stress on survival of B. subtilis wild-type and ΔsigB mutant cells.
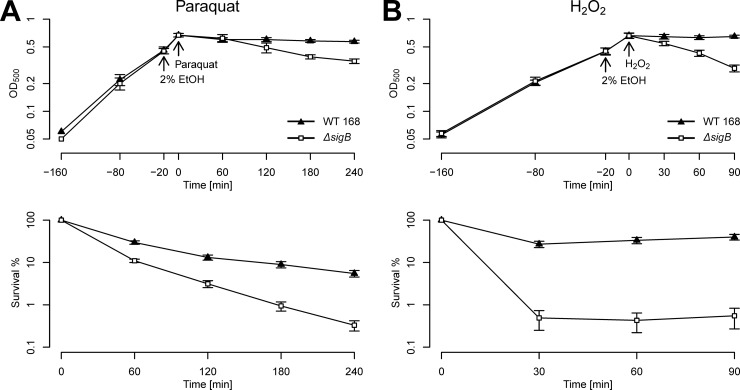
In the present study, we characterized the stress resistance profiles of 94 strains with mutations of individual general stress genes in response to superoxide and peroxide stresses. To be able to observe sensitive phenotypes for the individual mutants and to compare them with those of the B. subtilis wild-type strain and sigB mutant strain ML6, we used stress conditions that generated a maximum difference between the two reference strains without killing one of them completely. Hence, we introduced a preadaptation period by the imposition of 2% (vol/vol) ethanol stress 20 min prior to exposure to the detrimental oxidative stress. The preadaptation step equips the wild type with the protective functions of all general stress proteins and provides it with a maximal selective advantage (21). After preadaptation, cells were exposed to either 100 mM paraquat or 5 mM H2O2. Both stress conditions produced statistically significant differences in the survival rates between the wild-type and the sigB mutant strains (Fig. 1). The sigB mutant displayed a clear survival disadvantage compared to the wild-type strain upon either stress stimulus. Exposure to 100 mM paraquat reduced the viability of the wild type to approximately 10% after 240 min. The survival rates of the sigB mutant, however, decreased to a final level of 0.6% after 240 min (Fig. 1A). The imposition of 5 mM H2O2 did not severely affect the wild type but produced a strong and immediate decline in the survival rates of the sigB mutant to approximately 1% compared to wild-type levels throughout the time course (Fig. 1B). Thus, the results shown here again demonstrate that the general stress proteins provide B. subtilis with functions necessary for the efficient management of and survival against oxidative stress. Furthermore, the chosen stress levels lead to an appropriately wide range of selectivity between the wild type and the sigB mutant that allowed us to assess the effect of single mutations of general stress genes on survival against oxidative stress.
Fig 1.
Comparative analysis of the oxidative stress resistances of wild-type (WT) strain 168 and its isogenic sigB mutant strain ML6. The upper graphs indicate the growth curves, and the lower graphs indicate the survival rates. Both strains were cultivated in synthetic medium, and growth was monitored by measuring the OD500. At an OD500 of 0.4 (t of −20 min), the cells were treated with 2% ethanol (EtOH) (as indicated), followed by a preadaptation period of 20 min. After preadaptation (t0), the cells were stressed with 100 mM paraquat (A) or 5 mM H2O2 (B) (as indicated). Survival rates were determined by plating appropriate dilutions of control samples taken before oxidative stress treatment at t0 and after 60, 120, 180, and 240 min for the paraquat-stressed cells and after 30, 60, and 90 min for the H2O2-stressed cells. The values are arithmetic means and standard errors of the means.
Paraquat and H2O2 stress resistances of mutants defective in individual general stress genes.
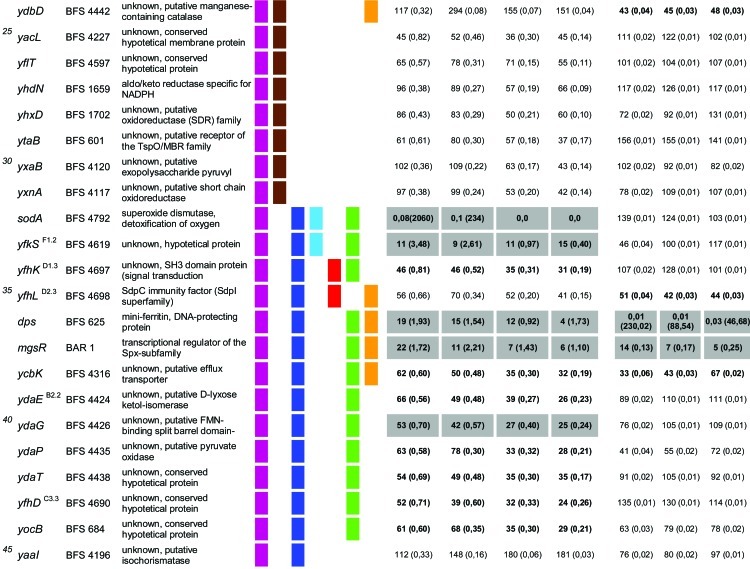
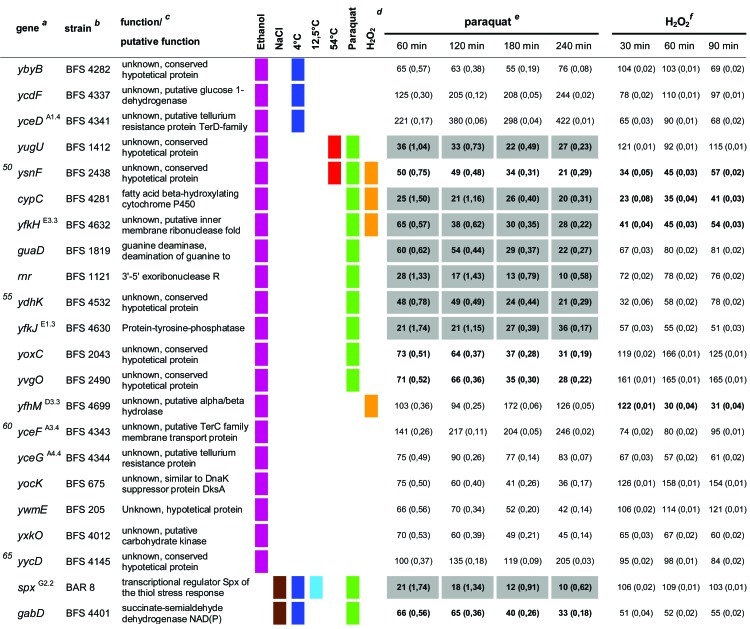
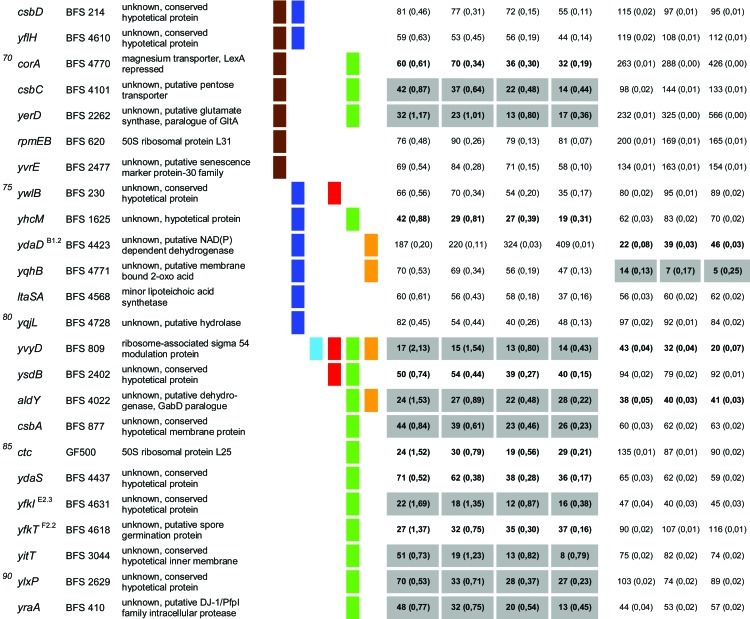
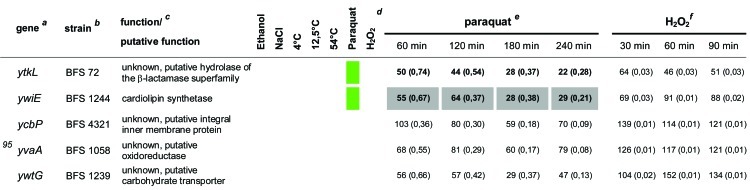
In total, 92 of the 94 mutants from the previous study (21) were used in our experimental setup. Only two mutant strains that were used previously, the spx (yjbD) (strain 2842) and mgsR (yqgZ) (strain 4773) mutants, were replaced here by the ΔmgsR (BAR1) and Δspx (BAR8) deletion mutants. Thus, the same 94 gene defects described previously by Höper et al. (21) were tested here for paraquat- and H2O2-sensitive phenotypes under the same conditions as those described above for the B. subtilis wild-type and ΔsigB reference strains. The survival rates of each mutant were determined, compared to those of the wild-type and sigB mutant strains, and tested for statistically significant differences (Table 1).
Table 1.
Quantitative evaluation of the survival rates of B. subtilis wild-type strain 168, the sigB mutant (ML6), and 94 individual general stress gene mutants in response to paraquat and H2O2 stressesa,b,c,d,e,f
The genes are ordered by sensitivity clusters according to reference 21. The genes of each cluster are in alphabetical order. The operon affiliation of the respective genes is indicated at the top right. The code A1.4, for example, means that this gene is the first of four genes of operon A.
Mutant strains of the respective genes used in this study.
Known or putative function of the respective gene products.
Summary of all sensitive phenotypes determined previously by Höper et al. (21) and in this study. The list was sorted according to clusters of sensitive phenotypes in the following order: ethanol (pink), NaCl (brown), 4°C (light blue), 12.5°C (dark blue), 54°C (red), paraquat (green), and H2O2 (orange).
Survival rates of the mutants in response to 100 mM paraquat are expressed relative to those of the wild type and sigB mutant strain ML6 for each time point tested. The wild-type values were defined as 100% for direct comparison with the mutant strains; thus, the survival rates of the mutants reflect percent survival rates based on the wild-type level. The comparison with the sigB mutant (values in parentheses) represents the fold difference compared to the single mutant strains. Thus, values lower than 1 indicate that the mutant strain was less sensitive than the sigB mutant, and values higher than 1 indicate that the mutant was more sensitive than the sigB mutant. The values for direct comparisons of the wild type and the sigB mutant are given in the first and second rows. Boldface type indicates significant differences with a confidence level of an α value of ≤0.01. Values that passed an even more stringent confidence level of an α value of ≤0.001 are shaded in light gray.
Survival rates of the mutants in response to 5 mM H2O2 are expressed relative to those of the wild type and sigB mutant strain ML6 for each time point tested (see above).
A total of 56 mutants exhibited a significant (α ≤ 0.01) paraquat-sensitive phenotype, and 15 strains were sensitive to H2O2 treatment compared to the wild type (Table 1). Nine of the 15 H2O2-sensitive strains were also paraquat sensitive. These data demonstrate that a large fraction of the general stress gene products is either directly or indirectly involved in oxidative stress management. With regard to this, Höper et al. (21) previously reported a broad overlap of the ethanol-sensitive as well as the salt-sensitive and cold-sensitive (4°C) groups (summarized in Table 1). It was proposed that these three stress stimuli or a combination of these stresses could result in the generation of reactive oxygen species representing a secondary overall or common stress. Notably, 41 of the paraquat- and/or H2O2-sensitive mutants are indeed ethanol sensitive. Furthermore, 13 of 17 strains sensitive to ethanol and cold (4°C) stresses as well as 13 of 22 ethanol- and salt-sensitive mutants were also sensitive to the oxidative stress treatment. This represents a remarkable overlap between the ethanol, salt, and cold stress phenotype clusters that were previously assumed to be involved in secondary oxidative stress management with the identified group of paraquat- and H2O2-sensitive mutants (Table 1).
Moreover, it is noteworthy that gene mutations known to be directly involved in oxidative stress resistance mechanisms belong to these phenotype clusters: the dps gene encodes a miniferritin and a paralogue of the DNA-binding and -protecting protein MrgA (3, 11). MrgA is part of the PerR regulon and is strongly induced by sublethal levels of H2O2 (9, 11). The Dps protein was shown previously to be required for the increased H2O2 resistance of glucose-starved stationary B. subtilis cells (3), and the study by Höper et al. (21) revealed that this mutant was also highly sensitive to ethanol and cold (4°C) stresses. In addition to this, we demonstrate here that the dps mutant also exhibits a severe sensitivity to paraquat treatment (Table 1, and see Fig. S1A in the supplemental material). The stronger survival disadvantage of the dps mutant in response to H2O2 treatment than of the sigB mutant may be related to the fact that the dps gene is also constitutively expressed from a second upstream promoter (3). Furthermore, mutations of spx and mgsR, encoding two redox-sensitive regulatory proteins, were also part of this study. The Δspx strain, lacking the global regulator of the diamide stress response, Spx (33, 34), was reported previously to be salt and cold (4°C) sensitive (21) and was identified here to be paraquat sensitive as well (Table 1, and see Fig. S1B in the supplemental material). σB induces the spx gene as part of the bicistronic yjbC-spx operon (4, 37). The yjbC mutant was also identified to be ethanol and salt sensitive in the previous study and in addition was proven here to be paraquat sensitive (Table 1, and see Fig. S1C in the supplemental material). Furthermore, our experimental setup also included 4 genes, (i) ytkL, (ii) gabD, (iii) sodA, and (iv) yraA, that belong to both the σB-dependent general stress regulon (35) and the Spx-regulated diamide/thiol stress regulon (34).
For the ytkL mutant, no sensitive phenotype could be described previously (21), but here it could be assigned to the paraquat-sensitive group (Table 1).
The gabD mutant exhibited a salt- and cold (4°C)-sensitive phenotype in the previous study and is shown here to be paraquat sensitive (Table 1, and see Fig. S1D in the supplemental material). The gabD gene encodes an NADP+-dependent succinate-semialdehyde dehydrogenase. Thus, GabD may be necessary for the generation of reduction equivalents (NADPH) under conditions of oxidative stress. Notably, an aldY mutant strain encoding a paralogue of gabD was also identified here to be sensitive to paraquat and H2O2 treatment (Table 1).
The sodA mutant strain was shown to be ethanol and cold (4°C and 12°C) sensitive previously and displayed a strong paraquat-sensitive phenotype in this study (Table 1, and see Fig. S1E in the supplemental material). Although the latter observation was not surprising due to the fact that sodA encodes the superoxide dismutase SodA, which is directly involved in the detoxification of oxygen radicals and necessary for oxidative stress resistance (23), this is another good example of the correlation between known enzyme functions, stress phenotypes, and regulon clusters observed in this study. Nevertheless, due to the fact that the mutation of sodA produced a stronger negative effect on the survival rate under paraquat stress conditions than the sigB mutant, it is necessary to emphasize that sodA expression is under the control of multiple systems, only one of which is σB.
The yraA mutant strain was one of 14 mutants without a defined sensitive phenotype in the previous study (21). Here we show that the yraA mutant is sensitive to paraquat stress (Table 1, and see Fig. S1F in the supplemental material). Besides positive regulation by σB and Spx, the yraA gene was also reported previously to be specifically upregulated by AdhR as part of the adhA-yraA operon in response to aldehyde stress (36). In this context, it was proposed that yraA, encoding a putative DJ-1/Pf1 family protease, may be involved in the degradation or repair of oxidatively damaged proteins (36). The yfkM gene, encoding a paralogue of YraA, is also a σB regulon member and was tested here. Interestingly, the yfkM mutant also displayed an ethanol-sensitive, salt-sensitive (21), and paraquat-sensitive phenotype (Table 1, and see Fig. S1G in the supplemental material), further strengthening the hypothesis that these two putative proteases are specifically needed under conditions of oxidative stress.
As mentioned above, the ΔmgsR mutant strain as well as 11 strains with mutations in MgsR target genes were also included in this screening. It is known from a previous study that MgsR is a paralogue of Spx that controls a subregulon within the framework of the general stress response (41). Recently, MgsR was shown to be activated by a redox switch in response to ethanol stress, integrating secondary oxidative stress signals into a σB- and MgsR-mediated regulatory cascade (Reder et al., unpublished). Due to these observations, it has been assumed that the products of the MgsR target genes are needed for secondary oxidative stress management (41; Reder et al., unpublished). With regard to this, we show here that the mgsR mutant strain is sensitive not only to ethanol and cold stresses (21) but also to both paraquat and H2O2 stresses (Table 1, and see Fig. S1H in the supplemental material). Furthermore, our experimental setup included 11 strains with mutations of known MgsR target genes. Of this group, only three mutations, yhxD, yxaB, and yflH (Table 1) did not have oxidative stress management defects. The remaining 8 strains, with mutations of yjgB, yjgC, yjgD, ydbD, ydaE, ydaG, ysnF, and ydaD, were all sensitive to paraquat and/or H2O2 stress (Table 1). For some of these gene products, it is possible to predict a putative function that would also be in line with their determined phenotypes. For example, ydaD encodes a putative NADP+-dependent dehydrogenase. YjgC represents a putative formate dehydrogenase that may also be involved in the generation of NADH or NADPH. The ydaG gene encodes a potential flavin mononucleotide (FMN)-binding protein that may participate in redox processes. Finally, ydbD most likely encodes a manganese-dependent catalase that may be involved in the detoxification of H2O2. Notably, the catalase katX mutant and the putative catalase ydbD mutant are the only two strains that exhibited identical sensitivity patterns for all tested stimuli (Table 1, and see Fig. S1I and S1J in the supplemental material).
Conclusion.
Because an increasing number of reports pointed to an involvement of B. subtilis general stress proteins in oxidative stress management, this follow-up study assessed the effects of individual mutations of a large set of σB-dependent general stress genes on resistance against severe paraquat (superoxide) and H2O2 stresses. We demonstrate here that a substantial number of general stress genes are indeed involved in the development of oxidative stress resistance, which is very likely a common “secondary” component of multiple “primary” σB-inducing physical stress stimuli. Together with the results of the previous study by Höper et al. (21), all but 3 strains, the ycbP, ywtG, and yvaA mutants, could be associated with at least one or more stress resistance defects. Although it is obvious that some of the general stress proteins exert functions that are related primarily to relieving oxidative stress, the contributions of others may be more general, with a secondary oxidative stress component among them.
Because most of the general stress proteins are crucial for the survival of B. subtilis but are still of undefined functions, these functional genomics data are of great importance. One example where the results of Höper et al. (21) provided the basis for a detailed analysis and guided experimental design was the characterization of the regulator MgsR and the identification of its subregulon within the σB response (41; Reder et al., unpublished). Therefore, we believe that the information gained by detailed phenotypic screening analyses is a first and important step for directed assays to determine the exact biochemical functions of uncharacterized proteins of B. subtilis involved in stress management.
Supplementary Material
ACKNOWLEDGMENTS
We are deeply grateful to Anita Harang for her excellent technical assistance. We thank Holger Kock for his critical comments on the manuscript. We thank M. Schmalisch and all BFA consortium members for sharing their mutant strains with us.
This work was supported by the BMBF (FK2:0313978A) and the DFG (HE 1887/7-4 and HE 1887/8-1).
All authors discussed the results and implications and commented on the manuscript at all stages. We declare no competing financial interests.
Footnotes
Published ahead of print 11 May 2012
Supplemental material for this article may be found at http://jb.asm.org/.
REFERENCES
- 1. Albano E. 2006. Alcohol, oxidative stress and free radical damage. Proc. Nutr. Soc. 65:278–290 [DOI] [PubMed] [Google Scholar]
- 2. Antelmann H, Engelmann S, Schmid R, Hecker M. 1996. General and oxidative stress responses in Bacillus subtilis: cloning, expression, and mutation of the alkyl hydroperoxide reductase operon. J. Bacteriol. 178:6571–6578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Antelmann H, et al. 1997. Expression of a stress- and starvation-induced dps/pexB-homologous gene is controlled by the alternative sigma factor sigmaB in Bacillus subtilis. J. Bacteriol. 179:7251–7256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Antelmann H, Scharf C, Hecker M. 2000. Phosphate starvation-inducible proteins of Bacillus subtilis: proteomics and transcriptional analysis. J. Bacteriol. 182:4478–4490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Bailey SM, Pietsch EC, Cunningham CC. 1999. Ethanol stimulates the production of reactive oxygen species at mitochondrial complexes I and III. Free Radic. Biol. Med. 27:891–900 [DOI] [PubMed] [Google Scholar]
- 6. Barbe V, et al. 2009. From a consortium sequence to a unified sequence: the Bacillus subtilis 168 reference genome a decade later. Microbiology 155:1758–1775 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Benson AK, Haldenwang WG. 1993. Regulation of sigma B levels and activity in Bacillus subtilis. J. Bacteriol. 175:2347–2356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Brigulla M, et al. 2003. Chill induction of the SigB-dependent general stress response in Bacillus subtilis and its contribution to low-temperature adaptation. J. Bacteriol. 185:4305–4314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Bsat N, Herbig A, Casillas-Martinez L, Setlow P, Helmann JD. 1998. Bacillus subtilis contains multiple Fur homologues: identification of the iron uptake (Fur) and peroxide regulon (PerR) repressors. Mol. Microbiol. 29:189–198 [DOI] [PubMed] [Google Scholar]
- 10. Burkholder PR, Giles NH. 1947. Induced biochemical mutations in Bacillus subtilis. Am. J. Bot. 34:345–348 [PubMed] [Google Scholar]
- 11. Chen L, Helmann JD. 1995. Bacillus subtilis MrgA is a Dps (PexB) homologue: evidence for metalloregulation of an oxidative-stress gene. Mol. Microbiol. 18:295–300 [DOI] [PubMed] [Google Scholar]
- 12. Engelmann S, Hecker M. 1996. Impaired oxidative stress resistance of Bacillus subtilis sigB mutants and the role of katA and katE. FEMS Microbiol. Lett. 145:63–69 [DOI] [PubMed] [Google Scholar]
- 13. Engelmann S, Lindner C, Hecker M. 1995. Cloning, nucleotide sequence, and regulation of katE encoding a sigma B-dependent catalase in Bacillus subtilis. J. Bacteriol. 177:5598–5605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Gaidenko TA, Price CW. 1998. General stress transcription factor σB and sporulation transcription factor σH each contribute to survival of Bacillus subtilis under extreme growth conditions. J. Bacteriol. 180:3730–3733 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Hecker M, Pané-Farré J, Völker U. 2007. SigB-dependent general stress response in Bacillus subtilis and related gram-positive bacteria. Annu. Rev. Microbiol. 61:215–236 [DOI] [PubMed] [Google Scholar]
- 16. Hecker M, Völker U. 2001. General stress response of Bacillus subtilis and other bacteria. Adv. Microb. Physiol. 44:35–91 [DOI] [PubMed] [Google Scholar]
- 17. Hecker M, Völker U. 1998. Non-specific, general and multiple stress resistance of growth-restricted Bacillus subtilis cells by the expression of the σB regulon. Mol. Microbiol. 29:1129–1136 [DOI] [PubMed] [Google Scholar]
- 18. Helmann JD, et al. 2003. The global transcriptional response of Bacillus subtilis to peroxide stress is coordinated by three transcription factors. J. Bacteriol. 185:243–253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Helmann JD, et al. 2001. Global transcriptional response of Bacillus subtilis to heat shock. J. Bacteriol. 183:7318–7328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Höper D, Bernhardt J, Hecker M. 2006. Salt stress adaptation of Bacillus subtilis: a physiological proteomics approach. Proteomics 6:1550–1562 [DOI] [PubMed] [Google Scholar]
- 21. Höper D, Völker U, Hecker M. 2005. Comprehensive characterization of the contribution of individual SigB-dependent general stress genes to stress resistance of Bacillus subtilis. J. Bacteriol. 187:2810–2826 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Igo M, et al. 1987. Genetic studies of a secondary RNA polymerase sigma factor in Bacillus subtilis. J. Bacteriol. 169:3464–3469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Inaoka T, Matsumura Y, Tsuchido T. 1999. SodA and manganese are essential for resistance to oxidative stress in growing and sporulating cells of Bacillus subtilis. J. Bacteriol. 181:1939–1943 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Kaan T, Homuth G, Mäder U, Bandow J, Schweder T. 2002. Genome-wide transcriptional profiling of the Bacillus subtilis cold-shock response. Microbiology 148:3441–3455 [DOI] [PubMed] [Google Scholar]
- 25. Kobayashi K, et al. 2003. Essential Bacillus subtilis genes. Proc. Natl. Acad. Sci. U. S. A. 100:4678–4683 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Kohanski MA, Dwyer DJ, Collins JJ. 2010. How antibiotics kill bacteria: from targets to networks. Nat. Rev. Microbiol. 8:423–435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Lechat P, Hummel L, Rousseau S, Moszer I. 2008. GenoList: an integrated environment for comparative analysis of microbial genomes. Nucleic Acids Res. 36:D469–D474 doi: 10.1093/nar/gkm1042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Méndez MB, Orsaria LM, Philippe V, Pedrido ME, Grau RR. 2004. Novel roles of the master transcription factors Spo0A and sigma(B) for survival and sporulation of Bacillus subtilis at low growth temperature. J. Bacteriol. 186:989–1000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Mols M, Abee T. 2011. Primary and secondary oxidative stress in Bacillus. Environ. Microbiol. 13:1387–1394 [DOI] [PubMed] [Google Scholar]
- 30. Mols M, Pier I, Zwietering MH, Abee T. 2009. The impact of oxygen availability on stress survival and radical formation of Bacillus cereus. Int. J. Food Microbiol. 135:303–311 [DOI] [PubMed] [Google Scholar]
- 31. Mols M, van Kranenburg R, van Melis CC, Moezelaar R, Abee T. 2010. Analysis of acid-stressed Bacillus cereus reveals a major oxidative response and inactivation-associated radical formation. Environ. Microbiol. 12:873–885 [DOI] [PubMed] [Google Scholar]
- 32. Mostertz J, Scharf C, Hecker M, Homuth G. 2004. Transcriptome and proteome analysis of Bacillus subtilis gene expression in response to superoxide and peroxide stress. Microbiology 150:497–512 [DOI] [PubMed] [Google Scholar]
- 33. Nakano S, Erwin KN, Ralle M, Zuber P. 2005. Redox-sensitive transcriptional control by a thiol/disulphide switch in the global regulator, Spx. Mol. Microbiol. 55:498–510 [DOI] [PubMed] [Google Scholar]
- 34. Nakano S, Küster-Schöck E, Grossman AD, Zuber P. 2003. Spx-dependent global transcriptional control is induced by thiol-specific oxidative stress in Bacillus subtilis. Proc. Natl. Acad. Sci. U. S. A. 100:13603–13608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Nannapaneni P, et al. 2012. Defining the structure of the general stress regulon of Bacillus subtilis using targeted microarray analysis and random forest classification. Microbiology 158:696–707 [DOI] [PubMed] [Google Scholar]
- 36. Nguyen TT, et al. 2009. Genome-wide responses to carbonyl electrophiles in Bacillus subtilis: control of the thiol-dependent formaldehyde dehydrogenase AdhA and cysteine proteinase YraA by the MerR-family regulator YraB (AdhR). Mol. Microbiol. 71:876–894 [DOI] [PubMed] [Google Scholar]
- 37. Petersohn A, et al. 1999. Identification of σB-dependent genes in Bacillus subtilis using a promoter consensus-directed search and oligonucleotide hybridization. J. Bacteriol. 181:5718–5724 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Petersohn A, et al. 2001. Global analysis of the general stress response of Bacillus subtilis. J. Bacteriol. 183:5617–5631 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Price CW. 2000. Protective function and regulation of general stress response in Bacillus subtilis and related Gram-positive bacteria, p 179–197 In Storz G, Hengge-Aronis R. (ed), Bacterial stress responses. ASM Press, Washington, DC [Google Scholar]
- 40. Price CW, et al. 2001. Genome-wide analysis of the general stress response in Bacillus subtilis. Mol. Microbiol. 41:757–774 [DOI] [PubMed] [Google Scholar]
- 41. Reder A, et al. 2008. The Spx paralogue MgsR (YqgZ) controls a subregulon within the general stress response of Bacillus subtilis. Mol. Microbiol. 69:1104–1120 [DOI] [PubMed] [Google Scholar]
- 42. Schmalisch M, Langbein I, Stülke J. 2002. The general stress protein Ctc of Bacillus subtilis is a ribosomal protein. J. Mol. Microbiol. Biotechnol. 4:495–501 [PubMed] [Google Scholar]
- 43. Stülke J, Hanschke R, Hecker M. 1993. Temporal activation of β-glucanase synthesis in Bacillus subtilis is mediated by the Gtp pool. J. Gen. Microbiol. 139:2041–2045 [DOI] [PubMed] [Google Scholar]
- 44. Völker U, Maul B, Hecker M. 1999. Expression of the σB-dependent general stress regulon confers multiple stress resistance in Bacillus subtilis. J. Bacteriol. 181:3942–3948 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Wach A. 1996. PCR-synthesis of marker cassettes with long flanking homology regions for gene disruptions in S. cerevisiae. Yeast 12:259–265 [DOI] [PubMed] [Google Scholar]
- 46. Wilks JC, et al. 2009. Acid and base stress and transcriptomic responses in Bacillus subtilis. Appl. Environ. Microbiol. 75:981–990 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.