What are scientists, physicians, and the general public to make of the many null findings from randomized controlled trials (RCTs) of vitamin supplements? These trials are usually conducted on the basis of positive findings from prospective epidemiological studies and laboratory evidence of biological mechanisms. A common view is that the negative findings from the RCTs offer incontrovertible evidence that the nutrient is unrelated to disease and that the epidemiological studies are biased. An alternative explanation is that most RCTs of vitamin supplements are designed to test the hypothesis that supplementation, no matter the nutrient status, is protective. Vitamin treatment may not be effective in these trials because nutrient intake among the participants is already at optimum levels. To specify, examples are provided from the field of dementia and investigations of 3 dietary components: vitamin E, B vitamins, and docosahexaenoic acid.
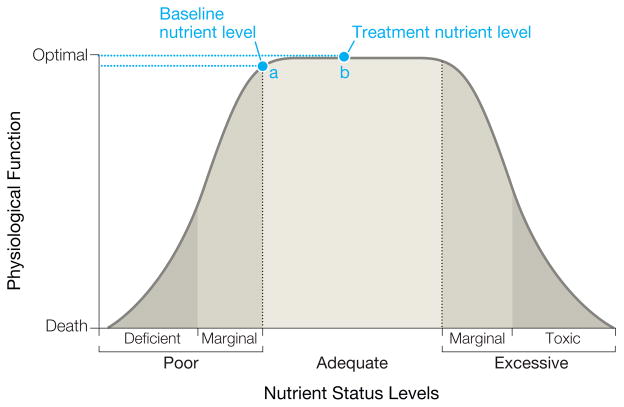
A basic principle of nutrition is that most nutrients have a nonlinear, inverted U-shaped association with optimum physiological function (Figure). Very low nutrient levels in diet or tissues result in poor function or even death. As the nutrient level increases, function also increases. Optimal functioning occurs over a fairly wide range of nutrient levels but at some point, higher levels become toxic and result in suboptimum function.
Figure. Relationship Between Level of Nutrient Status and Physiological Function.
For individuals who are already at a nutrient level for optimum functioning (point a), further vitamin supplementation (point b) would provide no additional benefit or treatment effect.
Consideration of nutrient level is critically important in the assessment of the epidemiological evidence. For example, among the first 3 prospective epidemiological studies1–3 reporting on the association of dietary antioxidants and the risk of Alzheimer disease, 2 studies found inverse associations with vitamin E from food sources1,2 and 1 found no association.3 Close examination of the reported intake levels of these study populations revealed that vitamin E intake was very low in the study with null findings, and the median intake level of the highest quartile of 4.7 mg per day was in the lowest categories of intake in the other 2 studies (lowest tertile <10.5 mg/d1 and quintile median of 4.2 mg/d2). Thus, a plausible explanation for the null association is that the range of vitamin intake in this study population was below the level of protective benefit to observe an association.
Nutrient levels are rarely considered in trial inclusion criteria. Further, trial volunteers are typically healthy behavior–seeking individuals and are unlikely to have low nutrient intake. More probably, intake levels are already at the level for optimal functioning (Figure, point a) and further supplementation provides no additional benefit (Figure, point b). Three RCTs examining the effect of vitamin E supplementation on cognition have been published and all have null results.4–6 Of note, none of these trials targeted individuals who had low dietary intake.
Post hoc analyses reported in 2 of the trials suggested that this could have accounted for the absence of effect. In the Women’s Health Study, among women whose vitamin E consumption at baseline was less than the median intake of 6.1 mg per day, vitamin E treatment of 435 mg every other day significantly slowed the rate of cognitive decline compared with placebo (rate difference=0.05), whereas there was no effect among women whose baseline intake was greater than 6.1 mg per day (rate difference of −0.01; P value for interaction=.04).4 In a similar post hoc analysis for the Women’s Antioxidant and Cardiovascular Study (WACS), there was no significant effect of intake of 402 mg of vitamin E on alternate days among women whose baseline intake levels were less than the median of 15 mg per day, currently the recommended dietary allowance (RDA).5
This raises the question of whether 15 mg per day is already within the range of optimum cognitive health and thus too high for observing a protective effect with additional supplementation. To further complicate interpretation of these data, trial participants were allowed to take multivitamin supplements containing nutrient levels that were as high as RDA levels; thus reported baseline levels were likely higher during the course of the trial.
The Women’s Antioxidant and Folic Acid Cardiovascular Study (WAFCS) also tested effects on cognition of B-vitamin supplementation, including folic acid (2.5 mg/d), vitamin B12 (1 mg/d), and vitamin B6 (50 mg/d).7 Overall, there was no effect of B-vitamin supplementation on cognitive decline but protective effects were observed among women who had dietary folate intakes of less than 279 mg per day (mean difference of 1.24 in cognitive change scores for B-vitamin treatment vs placebo compared with −0.04 among women with intakes ≥279 mg/d; P value for interaction=.002); vitamin B12 intakes less than the RDA of 2.4 μg per day (mean difference of 1.35 vs 0.10 in individuals with intakes ≥2.4 μg/d; P value for interaction=.05); and low intakes of at least 1 of the B vitamins (mean difference of 0.74 vs −0.10 in individuals with adequate intakes, P value for interaction=.01).
The Folic Acid Cognitive Intervention Trial (FACIT) is a rare example of a vitamin supplement trial that targeted individuals who were found to have insufficient folate nutriture based on a stringent set of biochemical criteria.8 Participants were randomized to receive 800 μg per day of folic acid or placebo for 3 years. During this period, folate levels among treated individuals increased from a median 12 nmol/L to 76 nmol/L and homocysteine concentrations decreased from a median 13.0 μmol/L to 10.1 μmol/L. The folic acid–treated participants had significantly slower rates of decline in cognitive function on multiple tests compared with the placebo group.
Published and ongoing RCTs of the effect of docosahexaenoic acid, an omega-3 fatty acid in fish oil, on cognitive decline and dementia have the same design flaw. The published trials report null findings. The exclusion criteria for these trials at best omitted persons who consumed more than 3 fish meals per week.9,10 This is far above the level of just 1 fish meal per week observed to be inversely associated with dementia in the majority of prospective epidemiological studies. The epidemiological literature strongly supports exclusion of individuals from supplement trials who consume fish more than occasionally (eg, rarely or never).
Clinical trials are both costly and important for substantiation of nutrient effects on health. The public health may be better served by initially conducting trials in individuals with insufficient nutriture and, if effective, further testing the effectiveness in those with adequate nutrient levels.
Footnotes
Conflict of Interest Disclosures: Both authors have completed and submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest and none were reported.
References
- 1.Engelhart MJ, Geerlings MI, Ruitenberg A, et al. Dietary intake of antioxidants and risk of Alzheimer disease. JAMA. 2002;287(24):3223–3229. doi: 10.1001/jama.287.24.3223. [DOI] [PubMed] [Google Scholar]
- 2.Morris MC, Evans DA, Bienias JL, et al. Dietary intake of antioxidant nutrients and the risk of incident Alzheimer disease in a biracial community study. JAMA. 2002;287(24):3230–3237. doi: 10.1001/jama.287.24.3230. [DOI] [PubMed] [Google Scholar]
- 3.Luchsinger JA, Tang MX, Shea S, Mayeux R. Antioxidant vitamin intake and risk of Alzheimer disease. Arch Neurol. 2003;60(2):203–208. doi: 10.1001/archneur.60.2.203. [DOI] [PubMed] [Google Scholar]
- 4.Kang JH, Cook N, Manson J, Buring JE, Grodstein F. A randomized trial of vitamin E supplementation and cognitive function in women. Arch Intern Med. 2006;166(22):2462–2468. doi: 10.1001/archinte.166.22.2462. [DOI] [PubMed] [Google Scholar]
- 5.Kang JH, Cook NR, Manson JE, Buring JE, Albert CM, Grodstein F. Vitamin E, vitamin C, beta carotene, and cognitive function among women with or at risk of cardiovascular disease: the Women’s Antioxidant and Cardiovascular Study. Circulation. 2009;119(21):2772–2780. doi: 10.1161/CIRCULATIONAHA.108.816900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Petersen RC, Thomas RG, Grundman M, et al. Alzheimer’s Disease Cooperative Study Group. Vitamin E and donepezil for the treatment of mild cognitive impairment. N Engl J Med. 2005;352(23):2379–2388. doi: 10.1056/NEJMoa050151. [DOI] [PubMed] [Google Scholar]
- 7.Kang JH, Cook N, Manson J, Buring JE, Albert CM, Grodstein F. A trial of B vitamins and cognitive function among women at high risk of cardiovascular disease. Am J Clin Nutr. 2008;88(6):1602–1610. doi: 10.3945/ajcn.2008.26404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Durga J, van Boxtel MP, Schouten EG, et al. Effect of 3-year folic acid supplementation on cognitive function in older adults in the FACIT trial: a randomised, double blind, controlled trial. Lancet. 2007;369(9557):208–216. doi: 10.1016/S0140-6736(07)60109-3. [DOI] [PubMed] [Google Scholar]
- 9.Quinn JF, Raman R, Thomas RG, et al. Docosahexaenoic acid supplementation and cognitive decline in Alzheimer disease: a randomized trial. JAMA. 2010;304(17):1903–1911. doi: 10.1001/jama.2010.1510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.van de Rest O, Geleijnse JM, Kok FJ, et al. Effect of fish oil on cognitive performance in older subjects: a randomized, controlled trial. Neurology. 2008;71(6):430–438. doi: 10.1212/01.wnl.0000324268.45138.86. [DOI] [PubMed] [Google Scholar]