Abstract
Rationale
Skeletal muscle blood flow is coupled with the oxygenation state of hemoglobin in young adults, whereby the erythrocyte functions as an oxygen sensor and releases ATP during deoxygenation to evoke vasodilation. Whether this function is impaired in humans of advanced age is unknown.
Objective
To test the hypothesis that older adults demonstrate impaired muscle blood flow and lower intravascular ATP during conditions of erythrocyte deoxygenation.
Methods and Results
We show impaired forearm blood flow (FBF) responses during two conditions of erythrocyte deoxygenation (systemic hypoxia and graded handgrip exercise) with age, and this is due to reduced local vasodilation. In young adults, both hypoxia and exercise significantly increased venous [ATP] and ATP effluent (FBF × [ATP]) draining skeletal muscle. In contrast, hypoxia and exercise did not increase [ATP]v in older adults, and both [ATP]v and ATP effluent were substantially reduced compared with young despite similar levels of deoxygenation. Next, we demonstrate that this cannot be explained by augmented extracellular ATP hydrolysis in whole blood with age. Finally, we found that deoxygenation-mediated ATP release from isolated erythrocytes is essentially non-existent in older adults.
Conclusions
Skeletal muscle blood flow during conditions of erythrocyte deoxygenation is markedly reduced in aging humans, and reductions in plasma ATP and erythrocyte-mediated ATP release may be a novel mechanism underlying impaired vasodilation and oxygen delivery during hypoxemia with advancing age. Because aging is associated with elevated risk of ischemic cardiovascular disease and exercise intolerance, interventions targeting erythrocyte-mediated ATP release may offer therapeutic potential.
Keywords: Aging, Blood Flow, ATP, Erythrocyte, Hypoxia
Evidence indicates a close coupling between skeletal muscle blood flow and the oxygenation state of hemoglobin (Hb) in young healthy humans1, 2. In this respect, the erythrocyte may function as an oxygen sensor in addition to an oxygen carrier and facilitate local vasodilation in relation to the degree of unoccupied hemoglobin binding sites2–4. In addition to the production of nitric oxide via deoxyhemoglobin-mediated nitrite reduction4 and the possible formation of S-nitrosohemoglobin5 during red blood cell deoxygenation, ATP is also released from the red cell and likely contributes to local vasodilation observed during mismatches in oxygen supply and demand2, 4, 6, 7. Specifically, during physiological stimuli such as hypoxia and muscle contractions, ATP is believed to assist in the regulation of blood flow and oxygen delivery to meet the metabolic demands of the tissue via binding to purinergic (P2) receptors on the endothelium2, 7. Importantly and unique to ATP vasomotor signaling in humans, we and others have demonstrated that exogenous ATP administration evokes not only substantial vasodilation, but also blunts sympathetic vasoconstriction in a dose-dependent fashion similar to what occurs in contracting muscle8, 9. Moreover, because sympathoexcitation typically occurs during such conditions10, 11, intravascular ATP is thought to have a critical role in blood flow regulation in humans. Collectively, this schema provides a means of rapidly matching tissue oxygen demand with oxygen delivery via alterations in arteriolar resistance as sensed by the red blood cell oxygenation state.
While alterations in skeletal muscle vascular tone during conditions of Hb deoxygenation have been determined in young healthy humans, little information exists in aging humans; a population that demonstrates endothelial dysfunction as well as heightened sympathetic activity and is at greater risk for ischemic cardiovascular diseases and exercise intolerance12, 13. In this context, whether humans of advanced age have significantly impaired blood flow control during conditions of Hb deoxygenation is unclear. Further, whether this decline in local skeletal muscle vascular control and oxygen delivery with age is related to the loss of circulating vasoactive ATP and more specifically, the loss of ATP release from red blood cells during Hb deoxygenation is unknown. Therefore, we tested the hypothesis that local control of skeletal muscle blood flow is impaired in aging humans during systemic hypoxia as well as during muscle contractions (both conditions of erythrocyte deoxygenation), and that plasma ATP of blood draining skeletal muscle is lower in these conditions in older adults in vivo. Further, in an effort to gain mechanistic insight underlying the diminished circulating ATP with human aging, we tested the hypothesis that any age-associated impairment may be due in part to augmented ATP hydrolysis by blood components and impaired release of ATP from the red blood cell during Hb deoxygenation in vitro.
Methods
A detailed and expanded Methods section is available in the Online Data Supplement at http://circres.ahajournals.org
Subjects
With Institutional Review Board approval and after written informed consent, a total of 37 young and 25 older healthy adults participated in the present investigation. This study was approved by the Human Research Committee of Colorado State University and was performed according to the Declaration of Helsinki.
Experimental Protocols
Protocol 1: Forearm Plasma [ATP] during Intra-arterial ATP Infusion
Evidence in the lower limb indicates that ATP administered intra-arterially is undetectable in the venous circulation2, 8, however the upper limb is a much smaller mass and less distance is traveled for a single pass through the microcirculation. To establish that venous plasma [ATP] measures (vs arterial) are most closely reflective of effluent draining skeletal muscle, we determined whether: (a) plasma arterial [ATP] equates to plasma venous [ATP], and (b) ATP is rapidly catabolized in a single passage of the forearm microcirculation. To do so, we infused saline via brachial artery catheter for 3 minutes (control) followed by infusion of ATP (8.35 nmol/dL/min) for 3 minutes in young adults. Blood was sampled from both catheters at the end of saline and the deep venous catheter during ATP infusion and analyzed for plasma [ATP] via the luciferin-luciferase technique. Arterial [ATP] during infusion was calculated as: (8.35 nmol/dL/min × forearm volume)/forearm blood flow.
Protocol 2: Systemic Hypoxia and Age
An initial rest period of 3 min was given to establish baseline values while the subject breathed on the mouthpiece open to room air. As previously described by our laboratory14, the level of inspired O2 was then titrated to achieve an O2 saturation of ~80% as established by pulse oximetry on the earlobe, while simultaneously clamping ETCO2 at baseline values via self-regulating partial rebreathe system. This transition from baseline to the target level of hypoxia typically occurred in 2 min. The subject was then maintained at ~80% for 5 minutes to establish steady-state levels of SpO2, ETCO2, as well as forearm hemodynamics during hypoxia. Forearm blood flow (FBF; Doppler ultrasound14) and vascular conductance (FVC) were calculated and venous blood was sampled for blood gases as well as plasma [ATP] at the end of normoxia and the end of hypoxia.
Protocol 3: Graded Intensity Forearm Exercise and Age
After 3 minutes of baseline measurements, subjects performed dynamic rhythmic handgrip exercise in the supine position with the non-dominant arm for a total of 15 minutes. This 15 minute trial was graded in exercise intensity whereby 5 minute segments each of 5, 15, and 25% maximum voluntary contraction (MVC) workloads were completed. FBF and FVC were calculated and venous blood was sampled for blood gases as well as plasma [ATP] at the end of rest and each exercise intensity level.
Protocol 4: ATP Hydrolysis in Whole Blood from Exercising Forearm and Age
Given the observations of reduced extracellular ATP in the blood of older adults during exercise (see Results), we determined whether any observed age-associated differences in venous plasma [ATP] were due to an enhanced capacity to degrade extracellular ATP within the blood of older adults during exercise15, 16. To do so, subjects performed exercise as described above in Protocol 3 and blood was sampled at the end of rest and each exercise intensity level. Because data indicates that whole blood degrades ATP more rapidly than plasma, and that blood cells such as leukocytes are a primary source for nucleotidase activity, we determined the catabolism of 100 μM ATP in whole blood rather than plasma alone17. In this respect, intraluminal extracellular ATP is exposed to the mixed media of blood cells, and thus determination of ATP degradation in whole blood (vs plasma alone) is a more accurate representation of ATP breakdown in vivo.
Protocol 5: Erythrocyte ATP Release during Hb Deoxygenation and Age
Given the collective observations of reduced extracellular ATP in the blood of older adults during two conditions of Hb deoxygenation and that ATP is catabolized to a similar extent in young and older adults during exercise (see results), we aimed to determine whether the release of ATP from erythrocytes of aging humans during low O2 exposure was impaired. To do so, erythrocytes were diluted to 20% hematocrit and equilibrated with normoxic gas (16% O2/6%CO2) for 15 minutes in a rotating bulb tonometer. The gas mixture was then changed to 3% O2/6%CO2 mixture for 7 minutes followed by a secondary drop in O2 (1.5%/6%CO2) for 7 more minutes. Our goal was to reduce PO2 and FO2Hb to levels within the range observed in vivo. Red cells were sampled at the end of each gas equilibration period and ATP was measured as described in the Online Data Supplement and normalized to red cell count18, 19.
Data Acquisition and Analysis
Data was collected and stored on a computer at 250 Hz and analyzed off-line. Baseline FBF, HR, and MAP represent an average of the last minute of the normoxic or resting time period, and steady-state hyperemic values represent an average of the last 30 seconds of each condition9. To account for changes in FBF and its impact on [ATP] concentration measurements and to quantify the rate of total ATP draining the active muscle, ATP effluent was calculated as FBF * [ATP]/1000, similar to other methods of data quantification when blood flow is altered2, 20.
Statistics
All values are reported as means ± S.E.M. Specific hypothesis testing during hypoxia or exercise studies across age was performed using two-way repeated measures ANOVA. In the case of a significant F value, Student-Newman-Keuls post hoc pairwise comparisons were made to identify significant differences. Comparison of the outcome variables at specific time points between conditions were made with unpaired t-tests, and the values within each condition with paired t-tests. SigmaPlot 11.0 (Systat Software, Inc., San Jose, CA, USA) was used for all analysis and statistical significance was set a priori at P<0.05.
Results
Subject Characteristics
The mean age difference between the young and older adults was 41 years (23±1 vs 64±1 yrs). Older adults had a greater BMI, % body fat, total and LDL-cholesterol, and triglycerides; however all measured variables were within the normal healthy range. All subject characteristics are noted in Supplemental Table-IA. Additional characteristics of those in Protocol 5 are presented in Supplemental Table-IB.
Protocol 1: Intra-arterial ATP Infusion in Young Adults
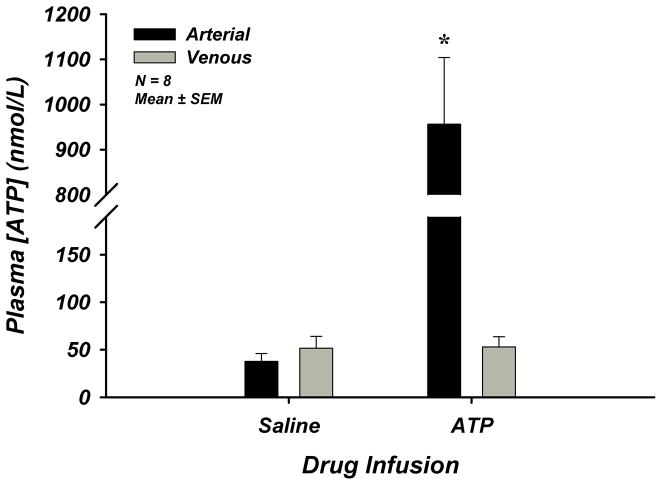
Exogenous ATP infusion in young adults (n=8) significantly increased arterial plasma [ATP] compared to saline control (38±8 vs 956±148 nmol/L), however venous plasma [ATP] was unchanged (52±13 vs 53±11 nmol/L) (Figure-1). Forearm and systemic hemodynamics are presented in Supplemental Table-II. These data demonstrate elevations in arterial plasma [ATP] without subsequent increases in venous plasma [ATP] supporting the concept that extracellular ATP is rapidly degraded once in the circulation2, 17, 21. Given these findings and the fact that the milieu (PO2, PCO2, pH) of venous blood is more closely representative of the microcirculatory environment and conducive to ATP release, we focused our investigation in aging humans to plasma [ATP] within venous blood draining skeletal muscle.
Figure 1. Lack of Increase in Venous Plasma [ATP] During Intra-arterial ATP Infusion.
ATP was infused intra-arterially through the brachial artery at a dose of 8.35 nmol/dL/min for 3 minutes in young adults. Calculated arterial plasma ATP was significantly elevated during drug infusion, while measured venous plasma ATP was unchanged. * P < 0.05 vs saline.
Protocol 2: Systemic Hypoxia and Age
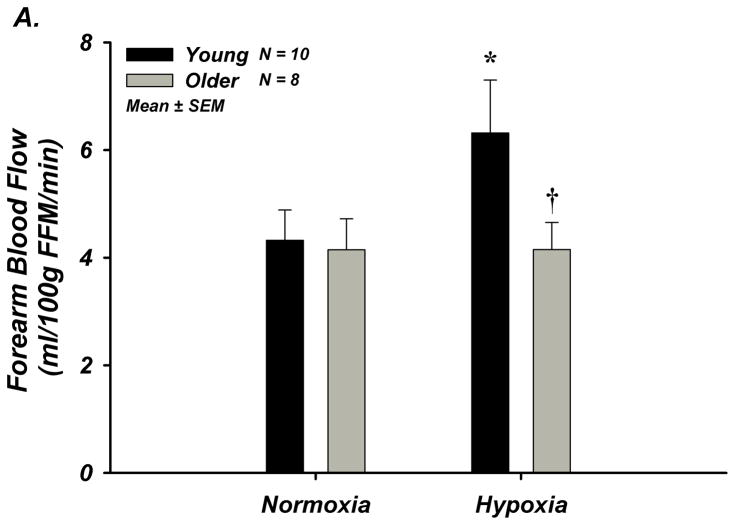
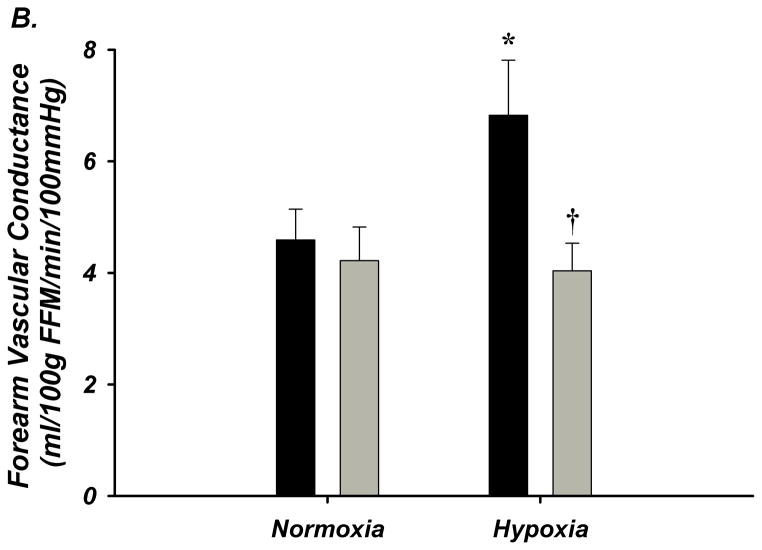
The degree of systemic hypoxia was not different between young (n=10) and older (n=8) adults (80.8±0.9 vs 81.5±0.9% SpO2) and ETCO2 was clamped at baseline (Supplemental Table-III). FBF and FVC significantly increased during systemic hypoxia from normoxia in young adults, but not in older adults (Figure-2A–B, Supplement Table-IIIB). MAP increased in older adults in response to hypoxia, and absolute MAP was greater than young adults (Supplemental Table-IIIB). Heart rate increased during systemic hypoxia in both groups, however the response was lower in older adults (Supplemental Table-IIIB).
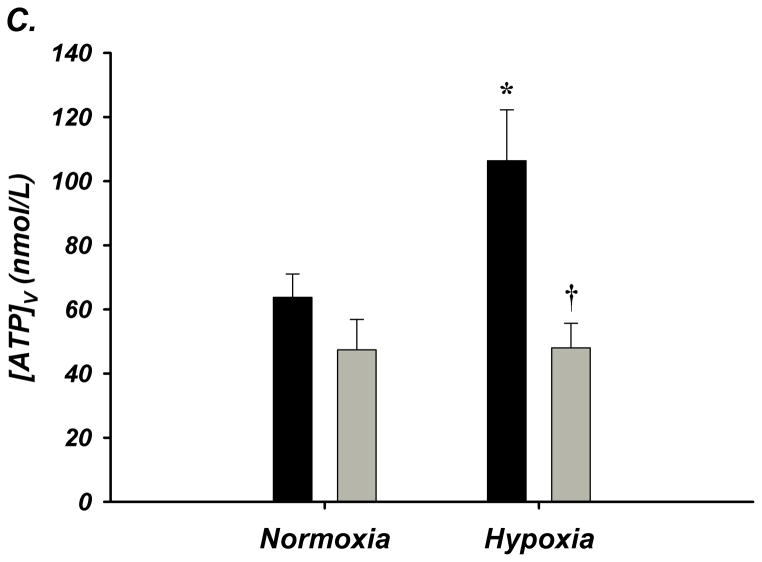
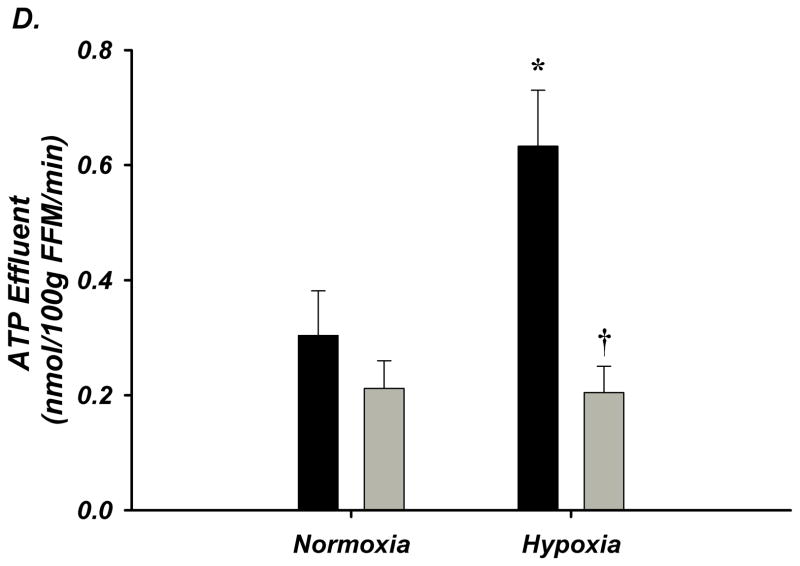
Figure 2. A–D. Forearm Hemodynamic Responses and Plasma ATP during Systemic Hypoxia with Age.
(A) Forearm blood flow (FBF), (B) forearm vasodilation (FVC), (C) plasma [ATP]V, and (D) ATP effluent significantly increased during systemic hypoxia from normoxia in young adults, but this was not observed in older adults. Older adults had significantly lower FBF, FVC, plasma [ATP]V, and ATP effluent during systemic hypoxia compared to young adults. * P < 0.05 vs normoxia; † P < 0.05 vs young.
Venous plasma [ATP] was not different at rest between young and older adults (64±8 nmol/L vs 47±10 nmol/L; Figure-2C). Young adults had significantly greater [ATP] during hypoxia compared to normoxia (106±16 nmol/L; Δ 43±19 nmol/L; Figure-2C). Remarkably, older adults had significantly lower [ATP] compared to young during hypoxia and did not significantly increase [ATP] above rest (48±8 nmol/L; Δ 1±9 nmol/L; Figure-2C). Similar to plasma [ATP], resting ATP effluent was not different with age. During hypoxia, ATP effluent increased in young adults (2.6±0.7 vs 5.3±1 nmol/min; Figure-2D), while there was no change in older adults (1.8±0.4 vs 1.7±0.3 nmol/min; Figure-2D). Respiratory variables and venous blood gases are presented in Supplemental Table-III.
Protocol 3: Graded Intensity Forearm Exercise and Age
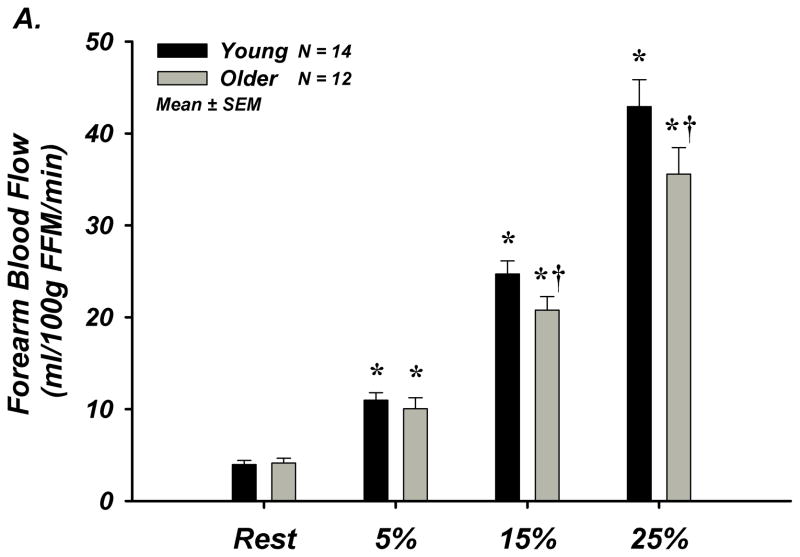
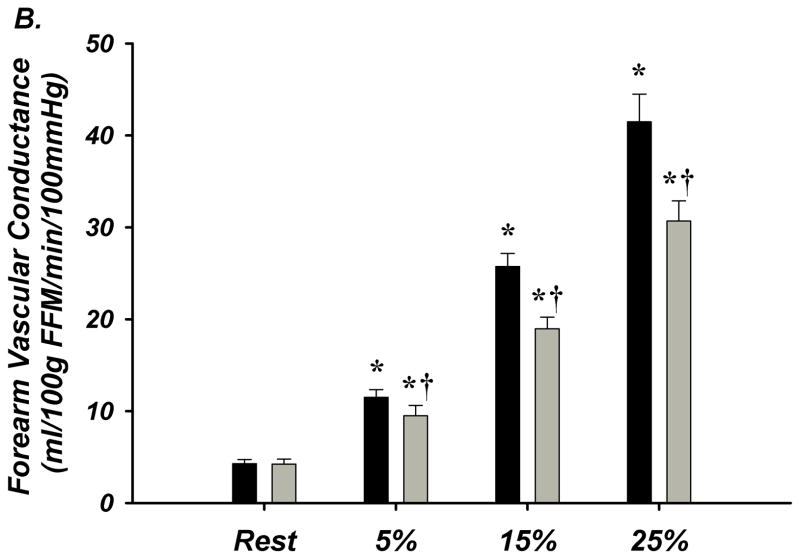
FBF and FVC increased in a graded fashion during progressive handgrip exercise. Older (n=12) compared to young (n=14) adults had significantly lower absolute FBF at 15 and 25% MVC exercise as well as significantly less forearm vasodilation (FVC) during all exercise workloads (Figure-3A–B). HR and MAP increased in both young and older adults in response to exercise, and absolute MAP was greater in older subjects (Supplemental Table-IVB).
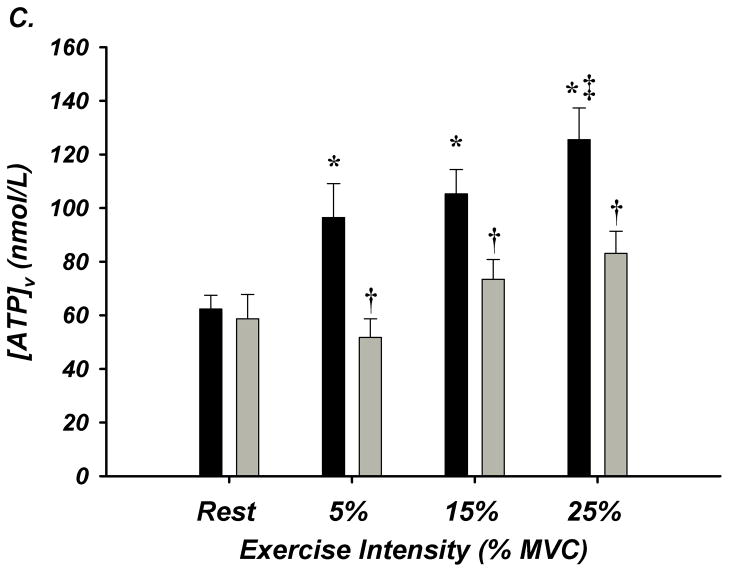
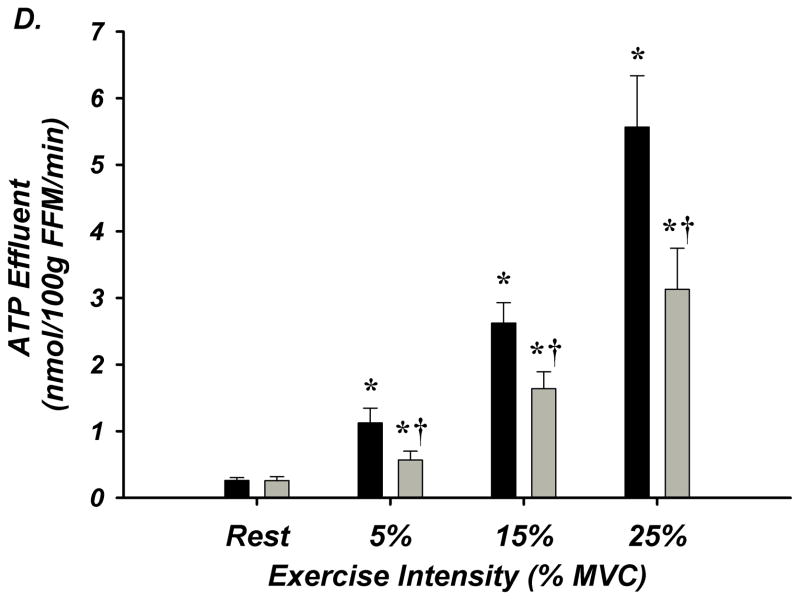
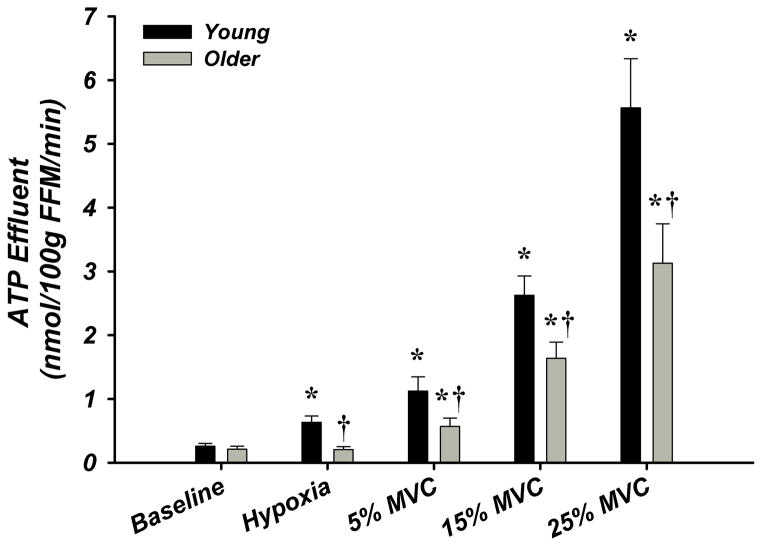
Figure 3. A–E. Forearm Hemodynamic Responses and Plasma ATP Measures during Graded Intensity Rhythmic Handgrip Exercise with Age.
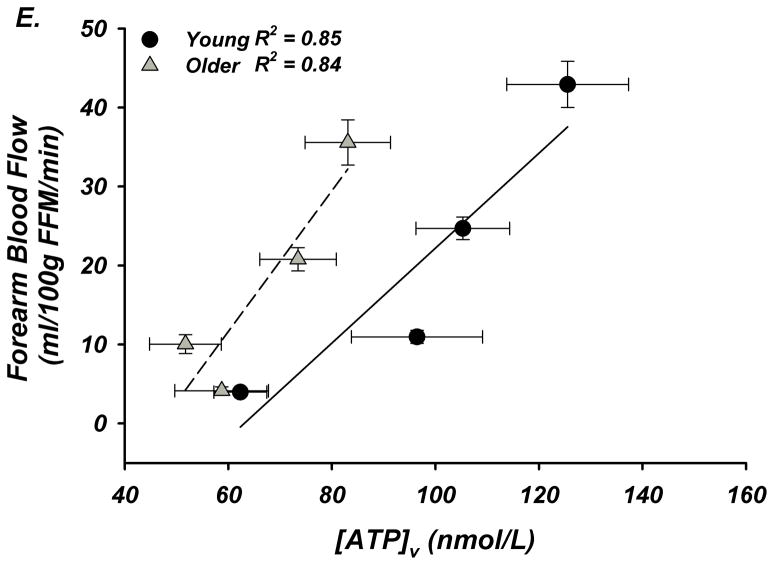
(A) Forearm blood flow (FBF) and (B) forearm vasodilation (FVC) increased as result of exercise despite age. However, older adults had lower FBF at 15 and 25% MVC, and FVC was lower at all intensities. (C) Plasma [ATP]v was elevated during exercise from rest in young adults but not older adults, and aged humans had significantly lower values at all exercise intensities. (D) ATP effluent was increased from rest during all intensities in young and older adults, yet a markedly blunted response was observed with age. (E) Association between venous plasma [ATP] and FBF at rest and during exercise. * P < 0.05 vs rest; † P < 0.05 vs young; ‡ P<0.05 vs 5% and 15% MVC within young.
Venous plasma [ATP] was not different at rest between young and older adults (62±5 nmol/L vs 59±9 nmol/L; Figure-3C). Young adults had significantly greater [ATP] during all exercise intensities compared to rest conditions (5% = 96±13, 15% = 105±9, 25% = 126±12 nmol/L; Figure-3C). Older adults did not significantly increase [ATP] above rest and had significantly lower [ATP] compared to young at all exercise intensities (5% = 52±7, 15% = 73±7, 25% = 83 ± 8 nmol/L; Figure-3C). ATP effluent was not different with age at rest (young = 2.4±0.4 vs older = 2.0±0.3 nmol/min; Figure-3D). Although both young and older adults had greater ATP effluent during exercise compared to rest, ATP effluent was significantly attenuated ~50–60% with age (Figure-3D, Supplemental Figure-I). Venous blood gases are presented in Supplement Table-V.
We measured arterial plasma [ATP] sampled from the brachial artery during exercise in 5 young adults who were instrumented with arterial catheters for other studies in the laboratory. Arterial [ATP] was 34 ± 9 nmol/L at rest and 34 ± 6 nmol/L during 5% MVC exercise. During 15 and 25% MVC exercise, arterial [ATP] was 44 ± 10 and 50 ± 4 nmol/L (P = 0.2 vs rest), respectively. Importantly, arterial [ATP] at rest or during any exercise intensity was always lower than the average resting venous [ATP] (~60 nmol/L; Supplemental Figure-I), suggesting that arterial plasma [ATP] detectable at the brachial artery does not increase in this model of exercise and further, is not explanatory for elevations in venous plasma [ATP] resultant from exercise nor the age-group differences found in this Protocol.
Protocol 4: ATP Hydrolysis in Whole Blood from Exercising Forearm and Age
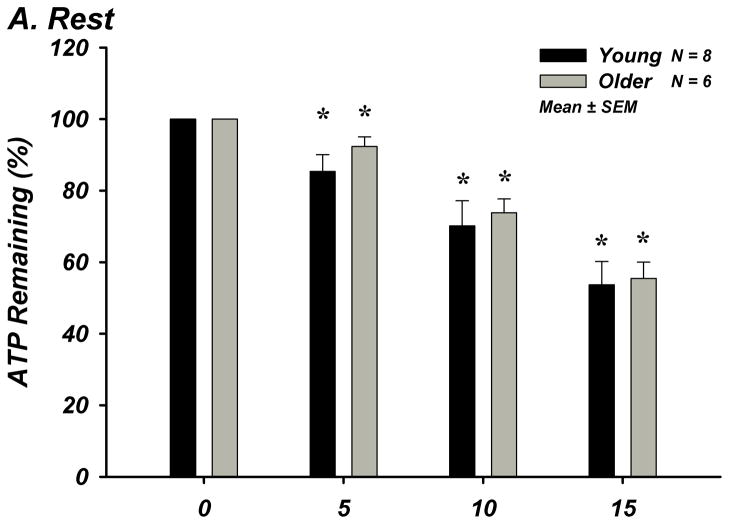
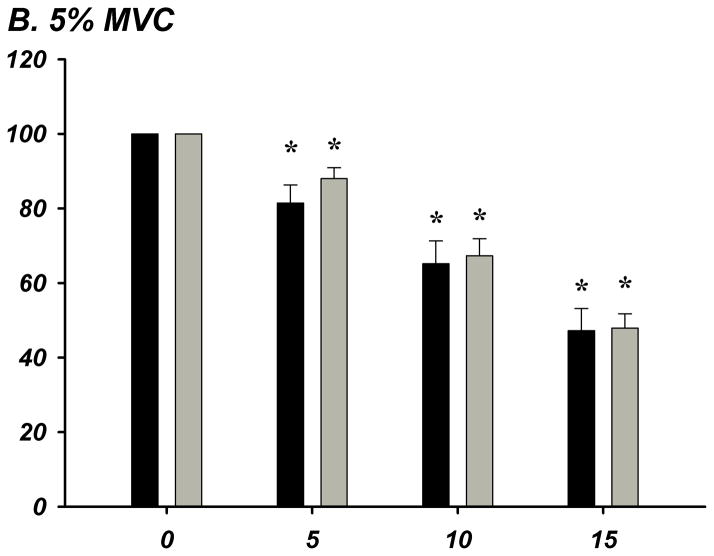
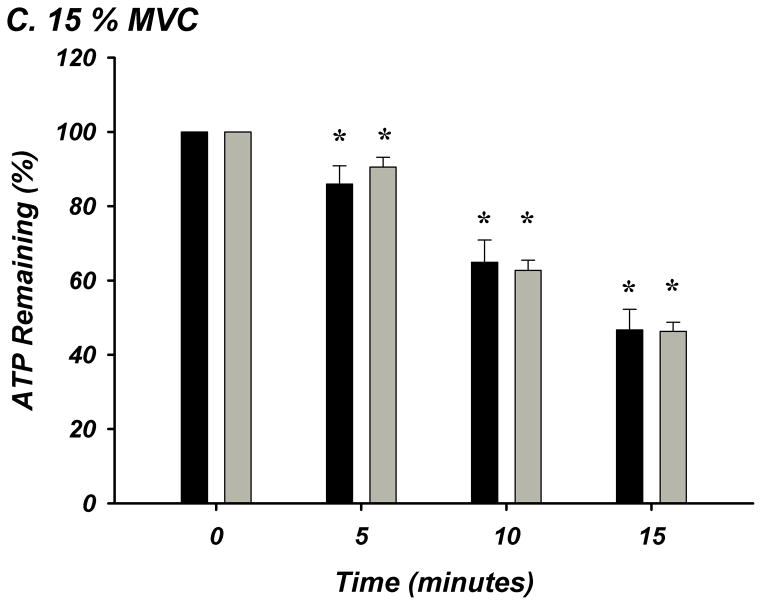
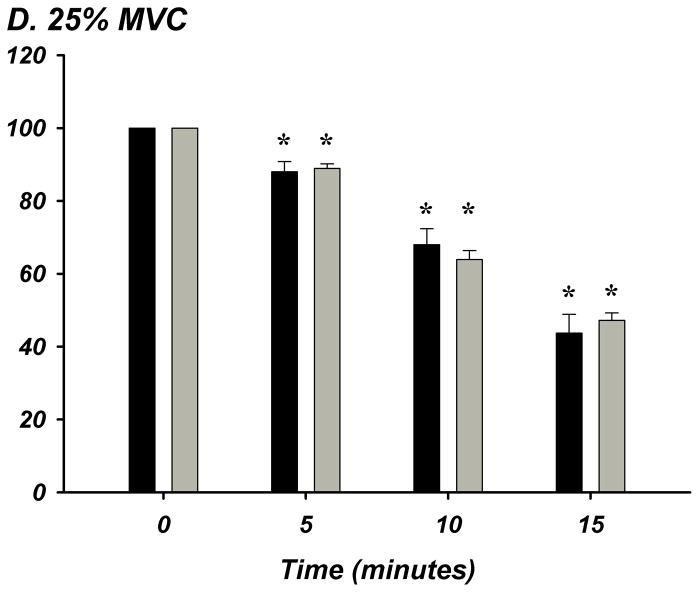
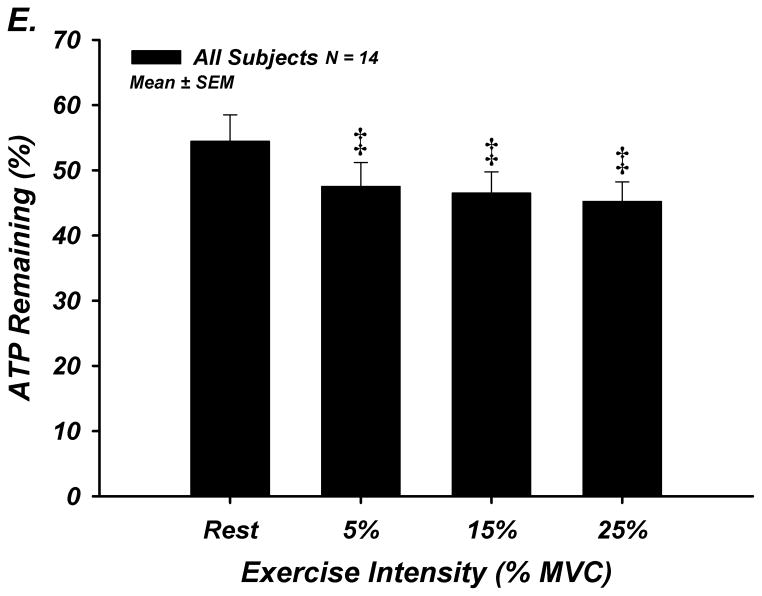
The % of ATP remaining of 100 μM ATP in whole blood was significantly lower from the previous time point of measurement indicating rapid and progressive ATP degradation over time within whole blood in young (n=8) and older (n=8) adults (P<0.05; Figure-4A–E). There were no age-group differences in ATP hydrolysis at any exercise intensity or timepoint. At minute 15, the % of ATP remaining was lower at all exercise intensities compared to rest for all subjects (P<0.05), indicating that acute exercise augments ATP catabolism in whole blood.
Figure 4. A–E. Hydrolysis of ATP in Whole Blood Acquired During Graded Rhythmic Handgrip Exercise.
Exogenous ATP (100 μM) was progressively catabolized in human whole blood samples over 15 minutes at all exercise intensities. No difference with age at rest or across exercise intensities was observed when % ATP remaining was determined (A) Rest, (B) 5% MVC, (C) 15% MVC, (D) 25% MVC. (E) ATP hydrolysis (after 15 minutes) was significantly greater than rest during exercise for all subjects, but was not dependent on intensity. * P <0.05 vs prior time point. ‡ P<0.05 vs rest.
Protocol 5: Erythrocyte ATP Release during Hb Deoxygenation and Age
Erythrocytes from young (n=8) and older (n=8) adults were similarly deoxygenated when exposed to 3% O2 and 1.5% O2 compared to 16% O2 normoxic gas (Supplemental Table-V). PO2, PCO2, and pH were not different between age-groups during normoxic and hypoxic gas exposure.
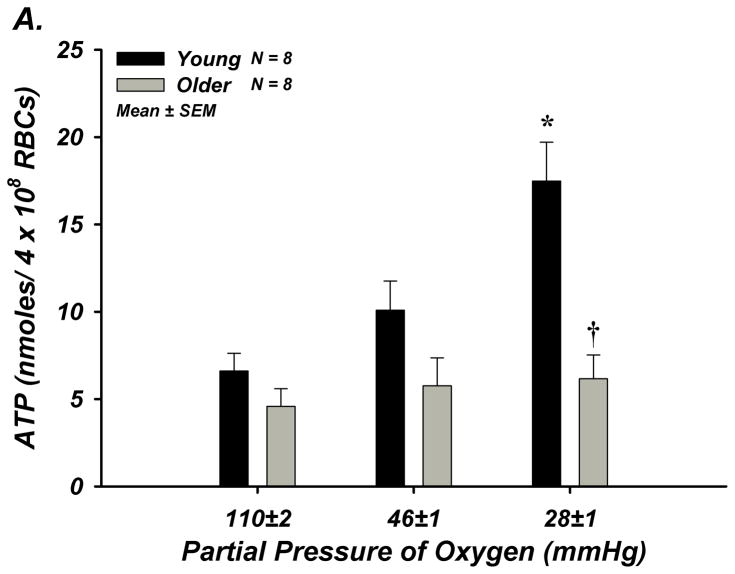
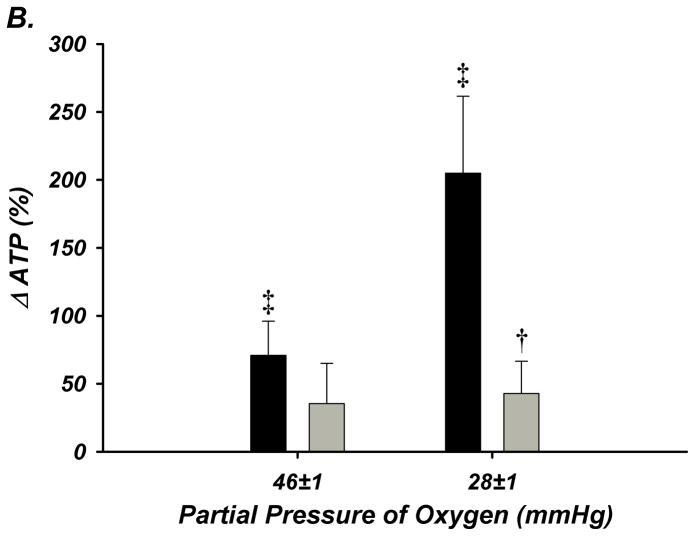
Absolute ATP release from red cells of young adults tended to increase during the first level of hypoxia (P = 0.087) and was significantly elevated during the second level of hypoxia (Figure-5A). The relative (%) change in ATP from baseline was graded with the level of deoxygenation. In contrast, red blood cells of older adults did not release ATP at either level of deoxygenation, and a clear age-associated impairment was observed during the second level of hypoxia (205±57 vs 43±24%; Figure-5B).
Figure 5. Deoxygenation-Induced ATP Release from Isolated Erythrocytes of Young and Older Adult Humans.
(A) ATP release tended to increase from red blood cells of young adults during 3% O2 (P =0.087; PO2 = 46±1 mmHg), and significantly increased during 1.5% O2 (PO2 = 28±1 mmHg), but no increase occurred from red cells of older adults. (B) The percent increase in ATP release was significant from red blood cells of young adults during both levels of hypoxia, however this was not observed from red cells of older adults. * P < 0.05 vs normoxia (16% O2; PO2 = 110±2 mmHg); † P < 0.05 vs young; ‡ P < 0.05 vs zero.
Discussion
To the best of our knowledge, this is the first study to directly investigate alterations in extracellular intravascular ATP in aging humans and the control of blood flow and vascular tone at rest or during Hb deoxygenating stimuli such as systemic hypoxia and exercise. There are several new findings from the present series of in vivo and in vitro experiments. First, despite an augmented perfusion pressure in older adults, skeletal muscle blood flow during conditions of erythrocyte deoxygenation (systemic hypoxia and exercise) is markedly reduced in aging humans due to impaired local vasodilation. Second, while venous plasma [ATP] increases during systemic hypoxia and during dynamic forearm exercise in young adults, healthy older humans do not increase [ATP] and have significantly lower [ATP] than young adults under these conditions. Third, the rate of ATP effluent from skeletal muscle increases during systemic hypoxia and graded intensity forearm exercise in young adults, whereas no increase in ATP effluent is observed during hypoxia in older adults and the increase is substantially blunted during exercise (~50–65% vs young; Figure-6). Importantly, the reduced [ATP] and ATP effluent in older adults occurs concomitantly with less vasodilation. Fourth, although extracellular ATP is rapidly degraded within the human circulation and this catabolism is enhanced by exercise, aging does not appear to augment ATP hydrolysis in whole blood at rest or during exercise. Finally, release of ATP from erythrocytes of older adults in response to deoxygenation is markedly impaired and these data are the first evidence of such impairment in aging humans. Collectively, reductions in erythrocyte-mediated ATP release and intravascular ATP may be a novel mechanism by which skeletal muscle blood flow and oxygen delivery is impaired during hypoxemia with advancing age in humans.
Figure 6. Summary of ATP Effluent Across Conditions in Young and Older Adults.
ATP effluent was calculated for each experimental condition based on [ATP]v and forearm blood flow. ATP effluent for baseline is the combined average of baseline values prior to hypoxia and exercise trials and was not different with age. ATP effluent in young adults (black) was significantly increased during hypoxia (~80% O2 saturations) alone and during exercise. Older adults did not increase ATP effluent during hypoxia but did during exercise. An age-associated reduction in ATP effluent was observed for all experimental conditions of hypoxia alone and exercise. (A). * P < 0.05 vs rest; † P < 0.05 vs young. MVC = maximum voluntary contraction
Aging, Blood Flow, and Intravascular ATP during Conditions of Hb Deoxygenation (in vivo studies)
Evidence indicates a close coupling between skeletal muscle blood flow and the oxygenation state of Hb1, and deoxygenation-mediated ATP release from red blood cells is believed to have a role in this coupling by evoking local vasodilation via binding to P2 receptors on the endothelium. Intravascular ATP evokes a robust endothelium-dependent vasodilation and also can blunt sympathetically-mediated vasoconstriction in young healthy humans, both which will serve to improve blood flow and oxygen delivery to hypoxic or metabolically-active tissue. Because aging is classically associated with endothelial dysfunction22 and older adults present with an impaired ability to modulate sympathetic vasoconstriction in contracting muscle (“functional sympatholysis”)23, we recently directly tested whether these unique properties of ATP were impaired with age. In contrast to our hypotheses, older adults demonstrated preserved ATP-mediated vasodilation and the ability to blunt sympathetic α-adrenergic vasoconstriction23, 24. These collective observations suggest that if ATP is involved in the impairment of vascular control during mismatches in oxygen supply and demand in aging humans, older adults are likely to have diminished intravascular ATP during conditions of Hb deoxygenation.
The findings from Protocol 2 in the present investigation are the first to demonstrate an age-related impairment in the local vasodilation and muscle blood flow response to systemic isocapnic hypoxia in humans (SPO2 ~80%). Specifically, while local vascular conductance increases in young adults during systemic hypoxia, this is essentially non-existent in older adults (Figure-2B). In line with our central hypothesis, plasma [ATP] and ATP effluent increases during systemic hypoxia in young, however we observed no significant change in either indices of vasoactive ATP in aged humans (Figure-2C–D). This absence of local vasodilation in conjunction with a lack of net ATP release in vivo in older adults is consistent with prior in vitro studies demonstrating that red blood cells are crucial to observe hypoxic vasodilation4, 5, 7, 18, and within the present study, implicate the loss of vasoactive ATP as a potential mechanism.
The findings from Protocol 3 are consistent with many studies in older adults demonstrating that local vascular conductance (vasodilation) is impaired and thus muscle blood flow during exercise is reduced despite an augmented perfusion with age22, 25. However, whether age-associated reductions in exercise hyperemia and oxygen delivery are associated with lower levels of intravascular ATP has never been determined. In young subjects, mild contractions (5% MVC) evoked a significant increase in plasma [ATP] which was maintained during 15% MVC and increased further during 25% MVC exercise. In contrast, despite similar levels at rest, plasma [ATP] did not increase above baseline values in older subjects during any exercise intensity and these concentrations were significantly lower compared with the young. Somewhat similarly, ATP effluent increased progressively with exercise intensity in both young and older adults, however ATP effluent was significantly lower in older adults at every exercise intensity (~50–65%). As in Protocol 2, we clearly show that older adults have significantly lower muscle blood flow during exercise which occurs concomitantly with less plasma [ATP] and ATP effluent. Although the mechanism underlying these impairments in hyperemia and oxygen delivery with age may be somewhat dependent on the stimulus, our collective data imply that blunted vasodilation with age during two physiological conditions of erythrocyte deoxygenation may be due, in part, to reductions in intravascular ATP. In turn, this could lead to reductions in P2 receptor stimulation and reduced endothelium-dependent signaling for direct vasodilation and/or the modulation of sympathetic vasoconstriction and thus impaired control of peripheral vascular tone in aged humans10, 22, 23.
Potential Mechanisms for Attenuated Plasma [ATP] with Age during Conditions of Hb Deoxygenation (in vitro studies)
Evidence indicates that ATP catabolism to downstream metabolites occurs rapidly within whole blood17, and that this process is enhanced during exercise15 and in humans that demonstrate endothelial dysfunction16. We recently observed under resting conditions that vasodilation evoked via exogenous ATP infusions does not occur via P1-receptors in both young and older adults, providing indirect evidence that nucleotidase activity is not augmented with age at rest24. However, whether ATP catabolism is augmented during exercise with human aging (independent of other co-morbidities) and could in part explain the reduced ATP levels we observed in circulation of older adults during exercise is unknown. Therefore, we directly determined ATP degradation in whole blood at rest and during exercise. We found no evidence of augmented ATP breakdown in blood of older vs young adults sampled at rest or during exercise. Nevertheless, our data support the concept that ATP hydrolysis is greater during conditions of exercise (Figure-4E)15. It should be noted here that an [ATP] greater than that observed in our samples was used for the ATP degradation assay similar to previous reports16, 17, therefore true in vivo catabolism rates cannot be extrapolated from our findings. However, in light of our observations, we speculate that the reduced intravascular ATP during hypoxia and exercise in aging humans may result from either augmented breakdown via endothelial cell bound nucleotidases (which is not accounted for in our catabolism studies) or a loss of ATP production and/or efflux of extracellular ATP into circulation.
Studies in isolated red blood cells have clearly demonstrated that Hb deoxygenation evokes ATP release that is graded with the level of deoxygenation3. Therefore, in an effort to reveal a possible mechanism underlying our in vivo observations, we determined whether ATP release from erythrocytes of healthy older adult humans was impaired compared to red cells of healthy young adults. By study design, we focused on deoxygenating erythrocytes to FO2Hb levels similar to our in vivo measures. In support of this hypothesis, we observed that ATP release from red blood cells of young adults increased by 71% from baseline normoxia (FO2Hb = ~95%) during first level of low oxygen exposure (FO2Hb = ~71%) and by 205% during the second level (FO2Hb = ~43%). In contrast, red cells from older adults did not significantly release ATP during either low oxygen condition despite similar total intracellular [ATP] and a clear age-impairment was present (Figure-5). Although it is currently unclear why this impairment exists, these data are consistent with observations from red cells of diabetic patients that demonstrate blunted ATP release and subsequent vasodilation compared to red cells of healthy adults18. Interestingly, deoxygenation-induced ATP release is significantly impaired from red cells of poor deformability26, and reduced erythrocyte deformability occurs with advanced donor age27. Therefore, it is plausible to speculate that our observed ‘erythrocyte dysfunction’ may be secondary to red cell stiffening with advancing age. Nonetheless, it is apparent that red blood cells from humans of advanced age fail to release ATP during Hb deoxygenation, and this is likely one mechanism underlying the age-related reduction in plasma [ATP] and vasodilation during hypoxemia with age. Although not directly established in our in vivo studies, data obtained in vitro clearly demonstrate that blunted ATP release from red cells is associated with impaired vasomotor responses, and importantly that vasodilation can be restored when ATP release from red cells is rescued18.
Integrative Perspectives
We utilized several in vivo and in vitro experimental approaches to gain an understanding of whether intravascular ATP is impaired in aging humans during physiological conditions of Hb deoxygenation and, if so, what potential mechanisms are involved. The concept that the red blood cell can serve as an oxygen carrier and sensor is certainly intriguing, however this leads to complex physiology and associated measures in vivo particularly during conditions of high blood flow within contracting skeletal muscle. One important consideration under these experimental conditions relates to the measurement of plasma [ATP] as solely representing the available circulating vasoactive ATP. In our experiments, there is a clear increase in [ATP] in young adults during 5% MVC exercise which did not increase significantly above this level until 25% MVC (Figure-3A). Because total muscle blood flow (and hence blood volume) increased up to 10-fold during exercise, this means that a significant release of ATP release into circulation must occur otherwise [ATP] would decrease via dilution effect by the large volume of blood. This latter point has recently been demonstrated experimentally during high flow non-exercise conditions (acetylcholine infusions) in humans28. Thus, although plasma [ATP] may represent one measure of intravascular ATP, we believe that quantification of ATP effluent ([ATP] × flow) may be important as well to describe the total circulating rate of vasoactive ATP available to evoke vasodilation during a given stimulus2. Accordingly, we have summarized ATP effluent across all in vivo conditions in the present study and demonstrate that although this is normal under resting conditions in older adults, ATP effluent during systemic hypoxia is abolished and during forearm exercise is substantially reduced with age (Figure-6).
A second point relates to the influence of elevated blood flow due to vasodilation and the number of red blood cells exposed to the low oxygen environments of systemic hypoxia and exercise, as total released ATP is dependent on the number of red cells19, 29. By means of the Fahraeus and Phase Separation effects, vasodilation increases red cell supply and microvascular hematocrit7, 30. This is important in that during exercise, PO2 and Hb oxygenation within skeletal muscle significantly decrease at the onset of low intensity exercise and remain relatively constant as exercise intensity increases2, 31 (see Supplemental Table-IVA). However, given the elevation in total muscle blood flow, redistribution of blood to the active muscle fibers, and elevations in microvascular hematocrit, the total number of red cells exposed to the low oxygen environment is significantly elevated providing a greater stimulus for net ATP release in vivo. Further, the integrative physiological conditions of contracting muscle not only results in Hb deoxygenation, but also evokes changes in pH, CO2, as well as mechanical stresses all of which can induce extracellular ATP release from erythrocytes into circulation2, 6, 7, 32. Thus, within skeletal muscle, a simple correlation between Hb deoxygenation and [ATP] in vivo does not exist nor should it be requisite to exist to support this overall hypothesized regulation of tissue blood flow and oxygen delivery by the red cells. A final consideration regarding the complexity of this physiology is the ability of endothelial cell bound ectonucleotidases21 as well as other blood constituents to rapidly degrade ATP in vivo, the latter of which have been shown to be upregulated during exercise15 (Figure-4E). This very tight regulation between red cell delivery, ATP release, and ATP degradation may aid in explaining why venous plasma [ATP] are not substantially greater as exercise intensity and blood flow is increased even in young adults.
Experimental Considerations
First, there are multiple sources of ATP including sympathetic nerve terminals as well as skeletal muscle. However, recent studies have demonstrated that ATP does not cross the endothelium2, 28, 33, and thus intravascular [ATP] from these sources is unlikely. Further, older adults demonstrate greater levels of sympathetic activity both at rest and during conditions of deoxygenation10, 11 and the exercise intensity and forearm muscle mass was similar between groups; thus these cannot explain the reductions in intravascular ATP with age.
Second, to definitively determine the contribution of intravascular ATP to vasomotor control during systemic hypoxia and exercise, the use of a specific P2Y receptor antagonist would be ideal. Unfortunately, agents used in animal preparations in vivo are significantly flawed34 and no substances are currently approved for human use. Regardless, the loss of ATP release from red cells has been repeatedly linked with impaired vascular function and lends credibility to our in vivo and in vitro observations4, 5, 7, 18, 35, 36. Independent from vascular control, ATP as a signaling molecule has a wide range of effects on cardiovascular function. Profoundly, attenuated intravascular ATP is associated with a pro-thrombotic environment, leukocyte adhesion, platelet activation, enhanced ischemia-reperfusion injury, and hyper-inflammation37. Therefore, the present observations of diminished intravascular ATP in humans of advanced age have clear implications and therapeutic potential for many cardiovascular complications in addition to oxygen delivery during hypoxemia.
Third, in the present in vivo aging experiments we did not sample for arterial plasma [ATP]. However, studies in young subjects demonstrated that arterial infusion of ATP is not detectable in the venous blood of the same limb (Figure-1) and that arterial [ATP] is lower than venous values and does not increase during handgrip exercise (Supplemental Figure-I). Finally, a wealth of data exists demonstrating no significant elevation in arterial plasma [ATP] during systemic hypoxia, or in response to exercise (e.g.38). Thus, these data are consistent with our observations that arterial plasma [ATP] is not explanatory of venous plasma [ATP] nor the observed impairments with age.
Fourth, blood flow regulation to contracting muscle is complex and involves many factors in addition to intravascular ATP such as K+ efflux from myocytes, mechanical factors, as well as a variety of endothelial and metabolically-derived substances39. In this context, we show a robust association between plasma ATP and forearm blood flow (Figure-3E) that is left-shifted in older adults and likely depicts a compensation of lowered plasma [ATP] by other vasodilatory signals, rather than enhanced ATP receptor responsiveness23, 24. Thus, despite clear impairments in intravascular ATP and hyperemic responses during exercise in older subjects, blood flow still increases during exercise and this would be expected given the redundant control of blood flow to active muscle.
Conclusions
In young healthy humans, oxygen delivery is effectively matched to oxygen demand during hypoxia and exercise via increases in skeletal muscle blood flow; however, older adults demonstrate an altered control of the vasculature (impaired vasodilation and functional sympatholysis) that may predispose this population to increased risk for ischemia related cardiovascular disease and exercise intolerance12, 13. The collective findings from the present investigation indicate that skeletal muscle blood flow during conditions of erythrocyte deoxygenation is markedly reduced in aging humans due to impaired local vasodilation associated with reductions in plasma ATP. Further, we suggest that loss of erythrocyte-mediated ATP release may be a novel mechanism underlying impaired oxygen delivery during hypoxemia with advancing age, and that rectification of this dysfunction could prove beneficial for older healthy and diseased humans.
Supplementary Material
NOVELTY AND SIGNFICANCE.
What is Known?
Human aging is a predominant risk factor for ischemia-related cardiovascular events.
Blood flow in the muscle is closely coupled to the oxygenation state of hemoglobin in young healthy humans.
Intravascular ATP in the extracellular space has potent vasoactive properties and is released from erythrocytes.
What New Information Does This Article Contribute?
Skeletal muscle blood flow is markedly reduced in humans of advanced age during conditions of erythrocyte deoxygenation due to impaired local vasodilation.
Unlike young adults, intravascular plasma [ATP] does not increase during conditions of erythrocyte deoxygenation in older adults.
While ATP hydrolysis by blood is augmented during exercise, aging does not alter this capacity.
Red blood cells from humans of advanced age are significantly impaired in their ability to release ATP during hemoglobin deoxygenation.
Aging is associated with endothelial dysfunction as well as heightened sympathetic activity and it increases the risk for ischemic cardiovascular diseases and exercise intolerance. Nevertheless, it is unclear whether a decline in local skeletal muscle vascular control and oxygen delivery with age is related to the loss of circulating vasoactive ATP and more specifically, the loss of ATP release from red blood cells during Hb deoxygenation Here, we present evidence supporting the notion that skeletal muscle blood flow, local vasodilation, intravascular plasma ATP, and erythrocyte-mediated ATP release during conditions of erythrocyte deoxygenation are markedly impaired in aged humans. This ‘erythrocyte dysfunction’ may be a novel mechanism underlying impaired vasodilation and oxygen delivery during hypoxemia with advancing age. Because aging is associated with elevated risk for ischemic cardiovascular disease as well as exercise intolerance, and ATP has notable effects on vascular function, interventions to improve erythrocyte-mediated ATP release may of therapeutic value.
Acknowledgments
We thank Jennifer Richards and Leora Garcia for their assistance, as well as the volunteers for their participation.
SOURCES OF FUNDING
This research was supported by National Institutes of Health awards HL095573 and HL102720 (F.A.D).
Non-standard Abbreviations
- ATP
adenosine triphosphate
- BMI
body mass index
- ctO2
oxygen content
- ctCO2
carbon dioxide content
- DEXA
dual-energy X-ray absorptiometry
- ETCO2
end-tidal carbon dioxide
- FBF
forearm blood flow
- FO2Hb
fraction of oxyhemoglobin
- FVC
forearm vascular conductance
- HCT
hematocrit
- HDL
high-density lipoprotein
- Hb
hemoglobin
- HbA1C
glycated hemoglobin
- LDL
low-density lipoprotein
- MAP
mean arterial pressure
- MBV
mean blood velocity
- MVC
maximal voluntary contraction
- PO2
partial pressure of oxygen
- PCO2
partial pressure of carbon dioxide
- RBC
red blood cell
- RLU
relative light units
- SpO2
oxygen saturation-pulse oximetry
- tHb
total hemoglobin
Footnotes
DISCLOSURES
None.
References
- 1.Gonzalez-Alonso J, Richardson RS, Saltin B. Exercising skeletal muscle blood flow in humans responds to reduction in arterial oxyhaemoglobin, but not to altered free oxygen. J Physiol. 2001;530:331–341. doi: 10.1111/j.1469-7793.2001.0331l.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gonzalez-Alonso J, Olsen DB, Saltin B. Erythrocyte and the regulation of human skeletal muscle blood flow and oxygen delivery: Role of circulating atp. Circ Res. 2002;91:1046–1055. doi: 10.1161/01.res.0000044939.73286.e2. [DOI] [PubMed] [Google Scholar]
- 3.Jagger JE, Bateman RM, Ellsworth ML, Ellis CG. Role of erythrocyte in regulating local o2 delivery mediated by hemoglobin oxygenation. American Journal of Physiology - Heart & Circulatory Physiology. 2001;280:H2833–2839. doi: 10.1152/ajpheart.2001.280.6.H2833. [DOI] [PubMed] [Google Scholar]
- 4.Crawford JH, Isbell TS, Huang Z, Shiva S, Chacko BK, Schechter AN, Darley-Usmar VM, Kerby JD, Lang JD, Jr, Kraus D, Ho C, Gladwin MT, Patel RP. Hypoxia, red blood cells, and nitrite regulate no-dependent hypoxic vasodilation. Blood. 2006;107:566–574. doi: 10.1182/blood-2005-07-2668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Diesen DL, Hess DT, Stamler JS. Hypoxic vasodilation by red blood cells: Evidence for an s-nitrosothiol-based signal. Circ Res. 2008;103:545–553. doi: 10.1161/CIRCRESAHA.108.176867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang L, Olivecrona G, Gotberg M, Olsson ML, Winzell MS, Erlinge D. Adp acting on p2y13 receptors is a negative feedback pathway for atp release from human red blood cells. Circ Res. 2005;96:189–196. doi: 10.1161/01.RES.0000153670.07559.E4. [DOI] [PubMed] [Google Scholar]
- 7.Ellsworth ML, Ellis CG, Goldman D, Stephenson AH, Dietrich HH, Sprague RS. Erythrocytes: Oxygen sensors and modulators of vascular tone. Physiology (Bethesda) 2009;24:107–116. doi: 10.1152/physiol.00038.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rosenmeier JB, Hansen J, Gonzalez-Alonso J. Circulating atp-induced vasodilatation overrides sympathetic vasoconstrictor activity in human skeletal muscle. Journal of Physiology. 2004;558:351–365. doi: 10.1113/jphysiol.2004.063107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kirby BS, Voyles WF, Carlson RE, Dinenno FA. Graded sympatholytic effect of exogenous atp on postjunctional alpha-adrenergic vasoconstriction in the human forearm: Implications for vascular control in contracting muscle. J Physiol. 2008;586:4305–4316. doi: 10.1113/jphysiol.2008.154252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Taylor JA, Hand GA, Johnson DG, Seals DR. Augmented forearm vasoconstriction during dynamic exercise in healthy older men. Circulation. 1992;86:1789–1799. doi: 10.1161/01.cir.86.6.1789. [DOI] [PubMed] [Google Scholar]
- 11.Davy KP, Jones PP, Seals DR. Influence of age on the sympathetic neural adjustments to alterations in systemic oxygen levels in humans. Am J Physiol Regul Integr Comp Physiol. 1997;273:R690–R695. doi: 10.1152/ajpregu.1997.273.2.R690. [DOI] [PubMed] [Google Scholar]
- 12.Luscher TF, Tanner FC, Tschudi MR, Noll G. Endothelial dysfunction in coronary artery disease. Annu Rev Med. 1993;44:395–418. doi: 10.1146/annurev.me.44.020193.002143. [DOI] [PubMed] [Google Scholar]
- 13.Fleg JL, Morrell CH, Bos AG, Brant LJ, Talbot LA, Wright JG, Lakatta EG. Accelerated longitudinal decline of aerobic capacity in healthy older adults. Circulation. 2005;112:674–682. doi: 10.1161/CIRCULATIONAHA.105.545459. [DOI] [PubMed] [Google Scholar]
- 14.Crecelius AR, Kirby BS, Voyles WF, Dinenno FA. Augmented skeletal muscle hyperaemia during hypoxic exercise in humans is blunted by combined inhibition of nitric oxide and vasodilating prostaglandins. J Physiol. 2011;589:3671–3683. doi: 10.1113/jphysiol.2011.209486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yegutkin GG, Samburski SS, Mortensen SP, Jalkanen S, Gonzalez-Alonso J. Intravascular adp and soluble nucleotidases contribute to acute prothrombotic state during vigorous exercise in humans. J Physiol. 2007;579:553–564. doi: 10.1113/jphysiol.2006.119453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Duarte MM, Loro VL, Rocha JB, Leal DB, Bem AF, Dorneles A, Morsch VM, Schetinger MR. Enzymes that hydrolyze adenine nucleotides of patients with hypercholesterolemia and inflammatory processes. FEBS J. 2007;274:2707–2714. doi: 10.1111/j.1742-4658.2007.05805.x. [DOI] [PubMed] [Google Scholar]
- 17.Coade SB, Pearson JD. Metabolism of adenine nucleotides in human blood. Circ Res. 1989;65:531–537. doi: 10.1161/01.res.65.3.531. [DOI] [PubMed] [Google Scholar]
- 18.Sprague RS, Bowles EA, Achilleus D, Stephenson AH, Ellis CG, Ellsworth ML. A selective phosphodiesterase 3 inhibitor rescues low po2-induced atp release from erythrocytes of humans with type 2 diabetes: Implication for vascular control. Am J Physiol Heart Circ Physiol. 2011;301:H2466–2472. doi: 10.1152/ajpheart.00729.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sridharan M, Adderley SP, Bowles EA, Egan TM, Stephenson AH, Ellsworth ML, Sprague RS. Pannexin 1 is the conduit for low oxygen tension-induced atp release from human erythrocytes. Am J Physiol Heart Circ Physiol. 2010;299:H1146–1152. doi: 10.1152/ajpheart.00301.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Giannarelli C, Virdis A, De Negri F, Magagna A, Duranti E, Salvetti A, Taddei S. Effect of sulfaphenazole on tissue plasminogen activator release in normotensive subjects and hypertensive patients. Circulation. 2009;119:1625–1633. doi: 10.1161/CIRCULATIONAHA.108.782482. [DOI] [PubMed] [Google Scholar]
- 21.Kohler D, Eckle T, Faigle M, Grenz A, Mittelbronn M, Laucher S, Hart ML, Robson SC, Muller CE, Eltzschig HK. Cd39/ectonucleoside triphosphate diphosphohydrolase 1 provides myocardial protection during cardiac ischemia/reperfusion injury. Circulation. 2007;116:1784–1794. doi: 10.1161/CIRCULATIONAHA.107.690180. [DOI] [PubMed] [Google Scholar]
- 22.Kirby BS, Voyles WF, Simpson CB, Carlson RE, Schrage WG, Dinenno FA. Endothelium-dependent vasodilatation and exercise hyperaemia in ageing humans: Impact of acute ascorbic acid administration. J Physiol. 2009;587:1989–2003. doi: 10.1113/jphysiol.2008.167320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kirby BS, Crecelius AR, Voyles WF, Dinenno FA. Modulation of postjunctional -adrenergic vasoconstriction during exercise and exogenous atp infusions in ageing humans. J Physiol. 2011;589:2641–2653. doi: 10.1113/jphysiol.2010.204081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kirby BS, Crecelius AR, Voyles WF, Dinenno FA. Vasodilatory responsiveness to adenosine triphosphate in ageing humans. J Physiol. 2010;588:4017–4027. doi: 10.1113/jphysiol.2010.197814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Poole JG, Lawrenson L, Kim J, Brown C, Richardson RS. Vascular and metabolic response to cycle exercise in sedentary humans: Effect of age. Am J Physiol Heart Circ Physiol. 2003;284:H1251–1259. doi: 10.1152/ajpheart.00790.2002. [DOI] [PubMed] [Google Scholar]
- 26.Sridharan M, Sprague RS, Adderley SP, Bowles EA, Ellsworth ML, Stephenson AH. Diamide decreases deformability of rabbit erythrocytes and attenuates low oxygen tension-induced atp release. Exp Biol Med (Maywood) 2010;235:1142–1148. doi: 10.1258/ebm.2010.010118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gelmini G, Coiro V, Ferretti P, Baroni MC, Delsignore R. Evaluation of whole blood filterability with increasing age in healthy men and women. Haematologica. 1989;74:15–18. [PubMed] [Google Scholar]
- 28.Mortensen SP, Thaning P, Nyberg M, Saltin B, Hellsten Y. Local release of atp into the arterial inflow and venous drainage of human skeletal muscle: Insight from atp determination with the intravascular microdialysis technique. J Physiol. 2011;589:1847–1857. doi: 10.1113/jphysiol.2010.203034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Edwards J, Sprung R, Sprague R, Spence D. Chemiluminescence detection of atp release from red blood cells upon passage through microbore tubing. Analyst. 2001;126:1257–1260. doi: 10.1039/b100519g. [DOI] [PubMed] [Google Scholar]
- 30.Pries AR, Secomb TW, Gaehtgens P, Gross JF. Blood flow in microvascular networks. Experiments and simulation. Circ Res. 1990;67:826–834. doi: 10.1161/01.res.67.4.826. [DOI] [PubMed] [Google Scholar]
- 31.Boushel R, Langberg H, Gemmer C, Olesen J, Crameri R, Scheede C, Sander M, Kjaer M. Combined inhibition of nitric oxide and prostaglandins reduces human skeletal muscle blood flow during exercise. J Physiol. 2002;543:691–698. doi: 10.1113/jphysiol.2002.021477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wan J, Ristenpart WD, Stone HA. Dynamics of shear-induced atp release from red blood cells. Proc Natl Acad Sci U S A. 2008;105:16432–16437. doi: 10.1073/pnas.0805779105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mortensen SP, Gonzalez-Alonso J, Nielsen JJ, Saltin B, Hellsten Y. Muscle interstitial atp and norepinephrine concentrations in the human leg during exercise and atp infusion. J Appl Physiol. 2009;107:1757–1762. doi: 10.1152/japplphysiol.00638.2009. [DOI] [PubMed] [Google Scholar]
- 34.Skinner MR, Marshall JM. Studies on the roles of atp, adenosine and nitric oxide in mediating muscle vasodilatation induced in the rat by acute systemic hypoxia. J Physiol. 1996;495 (Pt 2):553–560. doi: 10.1113/jphysiol.1996.sp021615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sprague RS, Goldman D, Bowles EA, Achilleus D, Stephenson AH, Ellis CG, Ellsworth ML. Divergent effects of low-o(2) tension and iloprost on atp release from erythrocytes of humans with type 2 diabetes: Implications for o(2) supply to skeletal muscle. Am J Physiol Heart Circ Physiol. 2010;299:H566–573. doi: 10.1152/ajpheart.00430.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhu H, Zennadi R, Xu BX, Eu JP, Torok JA, Telen MJ, McMahon TJ. Impaired adenosine-5′-triphosphate release from red blood cells promotes their adhesion to endothelial cells: A mechanism of hypoxemia after transfusion. Crit Care Med. 2011;39:2478–2486. doi: 10.1097/CCM.0b013e318225754f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Erlinge D, Burnstock G. P2 receptors in cardiovascular regulation and disease. Purinergic Signal. 2008;4:1–20. doi: 10.1007/s11302-007-9078-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dufour SP, Patel RP, Brandon A, Teng X, Pearson J, Barker H, Ali L, Yuen AH, Smolenski RT, Gonzalez-Alonso J. Erythrocyte-dependent regulation of human skeletal muscle blood flow: Role of varied oxyhemoglobin and exercise on nitrite, s-nitrosohemoglobin, and atp. Am J Physiol Heart Circ Physiol. 2010;299:H1936–1946. doi: 10.1152/ajpheart.00389.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Clifford PS, Hellsten Y. Vasodilatory mechanisms in contracting skeletal muscle. J Appl Physiol. 2004;97:393–403. doi: 10.1152/japplphysiol.00179.2004. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.