Abstract
Genital herpes is caused by herpes simplex virus 1 (HSV-1) and HSV-2, and its incidence is constantly increasing in the human population. Regardless of the clinical manifestation, HSV-1 and HSV-2 infections are highly transmissible to sexual partners and enhance susceptibility to other sexually transmitted infections. An effective vaccine is not yet available. Here, HSV-1 glycoprotein B (gB1) was delivered by a feline immunodeficiency virus (FIV) vector and tested against HSV-1 and HSV-2 vaginal challenges in C57BL/6 mice. The gB1 vaccine elicited cross-neutralizing antibodies and cell-mediated responses that protected 100 and 75% animals from HSV-1- and HSV-2-associated severe disease, respectively. Two of the eight fully protected vaccinees underwent subclinical HSV-2 infection, as demonstrated by deep immunosuppression and other analyses. Finally, vaccination prevented death in 83% of the animals challenged with a HSV-2 dose that killed 78 and 100% naive and mock-vaccinated controls, respectively. Since this FIV vector can accommodate two or more HSV immunogens, this vaccine has ample potential for improvement and may become a candidate for the development of a truly effective vaccine against genital herpes.
INTRODUCTION
Genital herpes is a recurrent, life-long viral infection caused by herpes simplex virus 1 (HSV-1) and HSV-2. The incidence of this infection varies from country to country, but at least 50 million people in the United States (between 15 and 20% of the adult population) have genital HSV infection (10), with an increment of at least 30% in the last 25 years (22) and in spite of a possible reduction in recent years (66, 67). The incidence is also escalating worldwide, including low-income countries where the epidemiology has been investigated (41). No matter whether the infection is clinically manifested or asymptomatic, the virus replicates and is shed for relatively long periods of time in the genital mucosa epithelia and is eventually transmitted to sexual partners and offspring. Recognized vehicles of transmission are saliva, semen, cervical fluid, or vesicle fluid from active lesions (63). In general, primary and recurrent infections are so mild that go unnoticed but, depending on immune status, gender, and age, patients may develop clinical signs that require hospitalization in certain cases. Herpes lesions develop within 2 to 20 days after contact, can occur anywhere in the genital area, and are characterized by small blisters that may ulcerate and take up to 4 weeks to heal. The infected area is usually painful and may itch, burn or tingle, during the outbreak (13, 47). Women tend to have more severe disease and higher rates of complications associated with primary infection; HSV-2 seropositivity increases the risk of acquiring other sexually transmitted infections, including the human immunodeficiency virus (HIV) infection, and developing cervical cancer (1, 53). Antivirals are available but are only effective postexposure and are unable to avoid latency and reactivation of infections (11). Among pre-exposure measures, anti-HSV microbicides are an apt option but, except for an HIV reverse transcriptase-inhibitor also effective against HSV (2), those under testing have a narrow window of efficacy that strictly depends on time from exposure and infectious dose (65). Anti-HSV-1 and anti-HSV-2 vaccines would thus be the most effective pre-exposure means to contain virus dissemination.
Over the last 2 decades, numerous efforts have been made to develop effective vaccines against genital herpes. The strategies tested included genetically attenuated live virus, whole inactivated virion preparations, recombinant subunit vaccines, gene-delivery vehicles expressing structural or nonstructural HSV antigens, and DNA plasmid expressing HSV antigens (reviewed in references 17 and 38). Most of these approaches failed during animal study or in early phases of human trials, but a few have received the more consideration since they were able to reduce the rate of acquiring HSV-2 genital infection in HSV-seronegative women (45). These results offered hope that vaccination against HSV-2 is possible but also showed that improvement of current and development of novel concepts were clearly necessary (17). Notably, a recent vaccination trial using HSV-2 glycoprotein D and two adjuvants and including 8,323 young seronegative women was effective at preventing HSV-1 but not HSV-2 infection and/or disease (5).
Among novel strategies, delivery of the immunogens by lentiviral vectors proved particularly effective to vaccinate against infectious diseases and cancer (26, 31, 37, 48). Compared to vectors derived from other viruses, those derived from lentiviruses offer advantages such as low or null preexisting population immunity, ability to transport one or more heterologous genes (transgenes), delivery of the transgenes to replicating and quiescent cells (transduction), and prolonged transgene expression. Vectors derived from HIV-1 are among the most efficient, but potentially residual pathogenicity has hitherto restricted their clinical use (19). The feline immunodeficiency virus (FIV) may be a good alternative as a vector. FIV is a lentivirus in domestic cats and similar to HIV-1 in many respects, including genomic organization, but is unable to infect humans (20). Vectors derived from FIV have been successfully used to transduce primary cells from feline and nonfeline species (24, 52), including humans (60), and proved safe in vitro and in vivo (4, 8, 52).
Here, we used an FIV vector to deliver and trigger a specific immune response against the HSV-1 glycoprotein B (gB1) that, together with other glycoproteins, serves as viral receptor (7, 55, 57). The nonmuscle myosin heavy chain IIA (NMHC-IIA), a subunit of nonmuscle myosin IIA (NM-IIA), has been recently proposed as a gB1 cellular receptor (3). Since NM-IIA is ubiquitously expressed in various human tissues and cells, gB1-NMHC-IIA interaction may be responsible for the HSV-1 broad host range in vitro (62). Eliciting specific anti-gB1 immune responses may thus result in a significant reduction in viral infectivity. The gB1 recombinant FIV vector was tested in mice against vaginal challenges with fully virulent HSV-1 and HSV-2 isolates. Vaccination triggered gB1-specific humoral and cell-mediated immune responses that cross-protected animals from primary and recurrent infections with sublethal and lethal challenge doses.
MATERIALS AND METHODS
Cells.
Human epithelial 293T cells were grown in Dulbecco modified Eagle medium (DMEM) supplemented with 10% heat-inactivated fetal calf serum (FCS), penicillin (100 U/ml), streptomycin (10 μg/ml), and l-glutamine (2 mM) (all from Sigma-Aldrich, Milan, Italy) (complete DMEM). Murine fibroblast NIH 3T3 and simian epithelial Vero cells were grown in Eagle minimum essential medium supplemented as described above (complete EMEM).
Mice and challenge viruses.
Inbred C57BL/6 mice were purchased from Harlan Italy (Correzzana, Milan, Italy) and housed and bred in a biosafety level 3 animal facility approved for mouse detention and reproduction. Animals were maintained on a 12/12-h dark/light cycle and manipulated according to European Union and Italian guidelines and legislation. All manipulations were performed under deep anesthesia using 20 mg of 2,2,2-tribromoethanol (Sigma-Aldrich)/hg inoculated intraperitoneally. The project was approved by the University of Pisa Ethical Committee for Animal Research.
The neurovirulent strains LV (59) and MS (American Type Culture Collection, catalog no. VR-540) of HSV-1 and HSV-2, respectively, were used for challenge. These strains were expanded in semiconfluent T-175 flasks (BD Biosciences, Milan, Italy) of Vero cells. The flasks were inoculated with 1 PFU of HSV-1 or HSV-2/cell in complete EMEM, and after 6 h fed fresh EMEM, and cultured for further 2 to 3 days or until cell lysis. Culture fluids were then spun at 1,200 × g, and the pellets were freeze-thawed three times to release intracellular virions. After clarification at 600 × g for 5 min, the supernatants were pooled, 0.45-μm-pore-size filtered, and ultracentrifuged at 50,000 × g for 90 min to concentrate the virus. Pelleted virus was resuspended in saline, at 1/100 of the initial volume, and stored in small aliquots at −80°C until use. Plaque titration of the viral stocks was carried out on Vero cells. The cells were seeded (1.2 × 105/well) in 48-well plates and 1 day later inoculated with 10-fold dilutions (range, 10−3 to 10−10) of the viral stocks in triplicate. After 6 h, the medium was replaced with complete EMEM plus 1% methylcellulose (Sigma-Aldrich), and the plates were incubated for 2 to 3 days. The cells were then fixed with 10% formalin and stained with gentian violet, and viral plaques were counted under a light microscope. Titers are expressed as PFU/ml.
Since age and estrous cycle are known to influence the susceptibility to genital herpes (58), in vivo titration of the viral stocks was performed in mice at age 11 weeks, that is, at the age we had planned to perform the challenge in the vaccination experiments, and with their estrous cycles synchronized. Cycle synchronization was achieved with 2 mg of depot medroxyprogesterone acetate (Depo-Provera; Pfizer Italia, Latina, Italy) inoculated subcutaneously 5 days before infection. To facilitate virus absorption, the vaginas were preswabbed with a dry-tipped swab immediately prior to the instillation of 10-fold dilutions of the viral stocks. The animals were then examined daily for signs of infection. These usually showed up at day 5 or 6 with local erythema and ulcers and rapidly evolved into manifestations of nervous system involvement (arched backs, feeble movements, and paralysis of one or both hind limbs). The latter stage was usually irreversible, and death occurred within 1 to 2 days. Titrations were performed using five to eight animals/virus dilution. The 50% lethal dose (LD50) was calculated according to the Reed and Muench formula (46). Animals that survived despite paralysis or other irreversible lesions were euthanized by cervical dislocation under anesthesia.
Construction of vaccine vector and generation of vector particles.
gB1 gene was amplified by PCR from pTZ18U-gB1 (33) using the primers BglII-HSV1F (5′-ATAGATCTCTGTCCGCTGGTG-3′) and BglII-gBR (5′-GAAGATCTTCTCACAGGTCGTCCTCGTCGGCGTC-3′) and denaturation at 94°C for 2 min, followed by 25 cycles of 94°C for 30 s, 56°C for 30 s, and 72°C for 3 min, with a final extension at 72°C for 7 min. Amplification product was digested with BglII enzyme and cloned into the multiple cloning site of the LAW34 plasmid (43). The resulting pLAW-gB1 construct was sequenced for gB1 correct insertion and absence of mutations.
Vector particles delivering gB1 (vLAW-gB1), green fluorescent protein (GFP; vLAW-GFP), or vector backbone alone (vLAW) were generated. These were produced by cotransfecting the respective plasmids with packaging pΔenv1, which provided Gag and Pol, and pRD114/TR or pVSV-G, which provided the envelope glycoprotein (Env). RD114/TR is a chimeric Env with the surface and transmembrane regions of the feline endogenous retrovirus RD114 fused with the cytoplasmic tail of an amphotropic murine leukemia virus (50). VSV-G is the glycoprotein G of vesicular stomatitis virus. Genomic maps of the pLAW, pΔenv1, and Env constructs are shown in Fig. 1A, while detailed descriptions are available elsewhere (43). Cotransfection was performed in 293T cells (3 × 106) using 35 μg of plasmid DNA and a 4:2:1 vector/pΔEnv1/Env ratio. DNA was diluted in 700 μl of 150 mM NaCl and added to a mixture of 600 μl of 150 mM NaCl and 100 μl of 10 μM polyethyleneimine (Sigma-Aldrich). After 15 min at room temperature, the DNA mixtures were added to the cells, and the medium was replaced with complete DMEM 6 h later. Vector supernatants were collected 48 to 72 h posttransfection, 0.45-μm-pore-size filtered, titrated, and stored in small aliquots at −80°C until use.
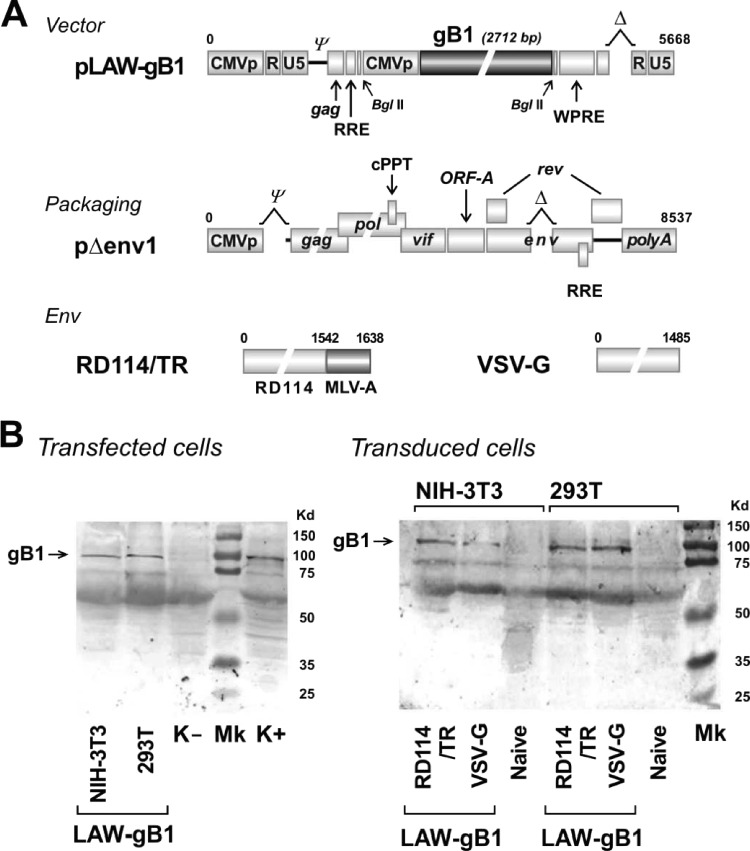
Fig 1.
Genomic maps of vector, packaging, and Env plasmids used to generate vLAW-gB1 and analysis of gB1 expression in vitro. (A) Schematic organization of plasmid vector pLAW-gB1, packaging pΔenv1, RD114/TR, a chimeric Env glycoprotein with surface and transmembrane regions of feline endogenous retrovirus RD114 and cytoplasmic tail of murine leukemia virus (50), and VSV-G, the Env glycoprotein G of vesicular stomatitis virus. pLAW-gB1 was produced by cloning full-length HSV-1 glycoprotein B (gB1) into pLAW34. A full description of pLAW34, pΔenv1, and Env constructs is available elsewhere (43). CMVp, CMV promoter; ψ, packaging site; RRE, Rev-responsive element; WPRE, woodchuck hepatitis posttranscriptional regulatory element. (B) WB analysis of gB1 expression as evaluated in transfected (left panel) or transduced (right panel) human 293T and murine NIH 3T3 cell lines. Transfection was performed with pLAW-gB1 alone. Transduction was carried out with vLAW-gB1 pseudotyped with either RD114/TR, or VSV-G. Arrow indicates gB1 protein band. K− and K+, uninfected and HSV-1-infected Vero cells, respectively. Naive, mock-transduced 293T or NIH 3T3 cells. Mk, high-range SDS-PAGE standard (Bio-Rad).
Cell transduction and titration of vector preparations.
Transduction was performed in 7 × 104 293T and 5 × 104 NIH 3T3 cells seeded the day before in 24-well plates. After 24 h, the medium was replaced with 1 ml of vector suspension diluted to contain ∼10 vector transduction units (TU) per cell. Vector titers were determined by transducing 7 × 104 293T cells with serial 10-fold dilutions of vLAW-GFP supernatants. At day 2 posttransduction, the cells were harvested, and GFP-positive cells were counted by flow cytometry with a FACScan (BD Biosciences). Dilutions were tested in triplicate, and titers were expressed in TU/ml. Equivalent vLAW-gB1 and vLAW TU/ml values were obtained by normalizing these and titrated vLAW-GFP supernatants for FIV p25 capsid protein and LAW RNA genome copies as described previously (43). On average, vector titers ranged between 2 × 106 and 5 × 107 TU/ml.
Vaccination, challenge, follow-up, and immunosuppression.
The immunizing protocols started when the mice were 5 weeks old. Groups of animals were inoculated with vLAW-gB1 (vaccinees) or vLAW (mock vaccinees) or left untreated (naive controls). Inoculations were performed intradermally in the hind footpad (50 μl of vector) and tail base (100 μl). These immunization routes were selected in the attempt to facilitate cell-mediated immune responses (23, 69). No local or systemic adverse reactions were observed. Elicited immune responses were analyzed in blood (collected by cardiac puncture), spleen, lymph nodes, and bone marrow of randomly selected vaccinated and mock-vaccinated animals. Tissues were immediately processed or stored at −80°C until use.
Challenge with HSV-1 or HSV-2 was performed via vagina following estrous cycle synchronization as described above. The challenge dose was 1 LD50 (roughly corresponding to 106 PFU) in all experiments except the last one, in which 10 LD50 (108 PFU) were used. All animals were monitored for about 3 weeks postchallenge (p.c.). Clinical signs of infection were graded according to a five-point scale: 0, no signs; 1, slight genital erythema and/or edema; 2, papules, ulcers and/or swelling; 3, fused ulcers, purulent genital lesions and/or hind limb paralysis; and 4, death (40).
Immunosuppression was achieved with an intraperitoneal bolus of 350 mg of cyclophosphamide (CY; Baxter, Rome, Italy)/kg. To check for immune cell depletion, mice were bled the following day retro-orbitally; circulating CD4+ and CD8+ T lymphocytes were labeled with fluorescein isothiocyanate (FITC)-conjugated monoclonal antibodies (eBioscience, San Diego, CA) and counted using flow cytometry as described previously (42).
gB1 expression.
Transfected and transduced 293T and NIH 3T3 cells were examined for gB1 expression by Western blotting (WB). The cells were lysed with WB lysis buffer (50 mM Tris [pH 7.5], 150 mM NaCl, 1% Triton, 1 mM EDTA, 0.1% protease inhibitor, 5 mg of MG123 proteasome inhibitor [Merck, Milan, Italy]/ml). Proteins were separated on a 10% sodium dodecyl sulfate (SDS)-polyacrylamide gel and transferred onto a nitrocellulose membrane (GE Healthcare, Milan, Italy). Membrane was blocked with 3% skim milk (Sigma-Aldrich) in phosphate-buffered saline (PBS)–1% Tween 20 and probed with anti-gB1 polyclonal antibody (9) diluted 1:4,000 in 1% skim milk and horseradish peroxidase (HRP)-conjugated anti-rabbit secondary antibody diluted 1:2,000 in 0.5% skim milk. Detection was carried out using an HRP substrate kit (Bio-Rad, Milan, Italy).
Humoral responses.
Elicited anti-gB1 antibody (gB1-Ab), total anti-HSV antibody, and neutralizing antibody (NAb) were examined by WB, enzyme-linked immunoassay (ELISA), and neutralization assay. WB was performed using HSV-1- or HSV-2-infected Vero cells as a substrate. Briefly, 106 cells were seeded the day before in a T-75 flask, infected with 10 PFU of HSV-1 or HSV-2/cell, and scraped off at 24 h postinfection. Cells were lysed with WB lysis buffer, aliquoted in 20-μl portions, and stored at −80°C. WB was carried out as described above except that protein lysate was separated on an 8% SDS–polyacrylamide gel, and murine sera and anti-mouse-HRP conjugates were used at dilutions of 1:100 and 1:1,000, respectively. ELISA was performed with murine sera diluted 1:100 and an Enzygnost anti-HSV/IgG test plate assay (Siemens, Milan, Italy), which contains antigens purified from chronically HSV-infected or uninfected simian kidney cells. Detection was carried out with biotinylated anti-mouse IgG antibody diluted 1:1,000 (eBioscience), extravidin-HRP diluted 1:5,000 (Sigma-Aldrich), and 3,3′,5,5′-tetramethylbenzidine-hydrogen peroxide as a substrate (Pierce, Rockford, IL). Plates were read at 490 nm. Anti-HSV-1 and -HSV-2 NAbs were assayed by plaque assay. Heat-inactivated sera were preadsorbed in Vero cells and diluted 1:10 to 1:80 in a 2-fold fashion in complete EMEM. The diluted samples were incubated with 100 PFU of HSV-1 or HSV-2 at 37°C for 1 h and then added to Vero cells (1.2 × 105/well) seeded the day before in 48-well plates. After 6 h at 37°C, the medium was replaced with 200 μl of complete EMEM, 5% FCS, and 3% methylcellulose, and the plates were further incubated for 48 to 72 h. The cells were stained, and lysis plaques were counted as for the in vitro HSV titration. The percent neutralization was calculated using the formula (1 − the number of infected cells in the presence of serum dilution/the number of cells incubated with virus alone) × 100. Each serum dilution was tested in triplicate.
Cell-mediated immune response.
T lymphocytes secreting gamma interferon (IFN-γ) upon recognition of a specific gB1 T-cell epitope (gB1-γTL) were measured by intracellular staining. Single-cell suspensions from spleen and iliac lymph nodes were prepared by mechanical disruption. Bone marrow-derived cells were obtained by flushing out cells from femurs. Cells were washed twice with RPMI 1640 medium (Sigma-Aldrich) supplemented as described above, counted, and placed (3 × 106) in FACScan tubes (Sarstedt, Verona, Italy). Samples were stimulated with 1 μg of gB1 T-lymphocyte epitope (498SSIEFARL505) (25)/ml or scrambled peptide ASFLRSEI (both peptides were synthesized by Espikem, Florence, Italy). Nonspecific stimulation with 5 ng of phorbol myristate acetate/ml and 2 μg of ionomycin/ml (both from Sigma-Aldrich) served as a positive control. Brefeldin A (5 μg/ml; Sigma-Aldrich) was added 1 h after stimulation; 5 h later, the cells were washed once with fluorescence-activated cell sorting (FACS) buffer (0.2% bovine serum albumin and 0.1% sodium azide in PBS [pH 7.4]) and fixed with FACS Fix (FACS buffer and 1% formaldehyde). After further washes, the cells were incubated with 0.1% saponin (Sigma-Aldrich) and labeled with 50 μg of FITC-conjugated anti-mouse CD8 and phycoerythrin-conjugated anti-mouse-IFN-γ antibody (eBioscience)/ml. Labeled cells were counted by flow cytometry, and the data were analyzed using the CellQuest version 2 software (BD Biosciences).
HSV-2 DNA genome in genital swabs and tissues.
Vaginal swabs were soaked in 600 μl of PBS at room temperature for 15 min, and cervical epithelial cells were pelleted by centrifugation. HSV DNA was extracted with a QIAamp DNA minikit as recommended by the manufacturer (Qiagen, Milan, Italy). Sacral nerves and genital ganglia were protease digested, and DNA was extracted as described above. Molecular analysis was carried out by using an HSV-2-specific nested PCR as described previously (16, 21). The first and second PCR primer pairs were 6AF (5′-TCAGCCCATCCTCCTTCGGCAGTA-3′)/6BR (5′-GATCTGGTACTCGAATGTCTCCG-3′) and 6CF (5′-AGACGTGCGGGTCGTACACG-3′)/6DR (5′-CGCGCGGTCCCAGATCGGCA-3′), respectively. The amplification profile (denaturation at 94°C for 2 min; five cycles of 94°C for 1 min, 56°C for 1 min, and 72°C for 1 min; 40 cycles of 94°C for 45 s, 56°C for 30 s, and 72°C for 1 min; final extension at 72°C for 15 min) was the same for both PCRs, except that the second amplification profile was decreased to 30 cycles. Amplicons were examined on a 1% agarose gel.
Statistics.
Statistical analyses were performed using SPSS 15.0 software (SPSS, Inc., Chicago, IL). The Fisher exact test was applied to evaluate heterogeneity of contingency tables. P values of ≤0.05 were considered statistically significant. One-way analysis of variance (ANOVA) was applied to evaluate differences among means of different groups. Tukey's multiple-comparison test was used to determine which means were significantly different.
RESULTS
Vector LAW-gB1 construction and in vitro characterization.
Vector LAW-gB1 delivers gB1 cloned into the LAW34 backbone. LAW34 is a self-inactivating vector derived from the FIV strain Petaluma. It contains the minimal FIV residues necessary for viral RNA encapsidation into progeny particles, importation into the nucleus, and integration in the host cell genome. To comply with today's safety and efficiency standards (26), LAW34 has both long terminal repeats inactivated by an internal deletion in U3, thus minimizing the risk of insertional mutagenesis. Other salient features of LAW34 include the presence of a cytomegalovirus (CMV) promoter and a woodchuck hepatitis virus posttranscriptional regulatory element, which allowed us to drive the expression of the gene of interest and to stabilize its mRNA, respectively (43). Maps of the LAW-gB1, of the packaging pΔenv1, and of the Env constructs that were used to generate pseudotyped particles (see below) are shown in Fig. 1A.
Analysis of gB1 expression in vitro was preliminarily evaluated in pLAW-gB1 transfected 293T and NIH 3T3 cells. Cells were examined at day 2 posttransfection by WB with a gB1 polyclonal antibody. As shown in Fig. 1B, the WB profile was indistinguishable from that of a protein lysate of HSV-1-infected Vero cells that were run in parallel as a positive control. This result demonstrated that gB1 was encoded in full and correctly processed in both 293T and NIH 3T3 cells.
In previous work we demonstrated that it is possible, to some extent, to retarget vLAW34 by changing Env. For instance, using the chimeric RD114/TR, i.e., the surface and transmembrane protein of the feline endogenous retrovirus RD114 fused with the cytoplasmic tail of the murine leukemia virus (50), led to a high efficiency of transduction for ex vivo immature murine bone marrow-derived dendritic cells (DCs). In contrast, ex vivo murine T lymphocytes were better transduced by VSV-G-pseudotyped particles (43). We therefore considered it to be of interest to compare the properties of LAW-gB1 pseudotyped with either RD114/TR (vLAW-gB1/RD) or VSV-G (vLAW-gB1/VSV). 293T cells were equally transduced by the two vectors (ca. 80%). In contrast, NIH 3T3 cells were more efficiently transduced with vLAW-gB1/RD (65%) than with vLAW-gB1/VSV (30% [data not shown]). As a consequence, in transduced NIH 3T3 cells the gB1 expression level was similarly affected (Fig. 1B). In either case, however, gB1 was produced full length and for at least 2 weeks posttransduction, suggesting that LAW-gB1 DNA integrated into the cell genome (Fig. 1B and data not shown).
Vaccination experiments using HSV-1 as a challenge.
In a first pilot experiment not reported in detail for brevity, groups of eight mice were immunized with 105 or 106 TU of vLAW-gB1/RD- or vLAW-gB1/VSV-inoculated twice in the footpads 1 week apart. Similar groups of mice were given 106 TU of vLAW/RD or vLAW/VSV and served as mock-vaccinated controls. One week after each inoculation, two animals per group were sacrificed and examined for gB1-Ab and gB1-γTL in the blood. As expected, the mock-vaccinated animals showed no detectable anti-gB1 immunity at any time, and the same was true for those given 105 TU of the immunogens. In contrast, the animals immunized with 106 TU of vLAW-gB1/RD or vLAW-gB1/VSV consistently showed both gB1-Ab and gB1-γTL. Interestingly, whereas gB1-Ab levels were uniformly low regardless of the immunogen used, those of gB1-γTL were highest in mice given vLAW-gB1/RD, suggesting that this immunogen might have been more effective at triggering cell-mediated immunity. Three weeks after the last immunizing dose, four animals per group and six naive animals were challenged intravaginally with 1 LD50 of HSV-1 as described in Materials and Methods. All naive and mock-vaccinated mice became obviously sick starting from day 6 p.c. The animals injected with 105 TU were also totally unprotected. On the other hand, two of four mice given 106 TU of vLAW-gB1/RD and one of four mice given 106 TU of vLAW-gB1/VSV showed no clinical signs. Furthermore, the animals in both of these two groups that became sick uniformly developed milder clinical manifestations than did the controls. It was thus tentatively concluded that both vLAW-gB1/RD and vLAW-gB1/VSV were promising immunogens, provided that they were administered at doses of at least 106 TU.
In the subsequent experiment, groups of 14 mice were treated as described above except that, in an attempt to potentiate the extent of protection, they also received a third dose of immunogens and mock immunogens (again, 106 TU) administered at the base of the tail 2 weeks after the second footpad inoculation (Fig. 2). Anti-gB1 immunity was monitored in three mice/group sacrificed 1 week after the first and second immunizations and at challenge (i.e., 3 weeks after the third inoculation). Similar to what was observed in the previous experiment, vLAW-gB1/RD- and vLAW-gB1/VSV-vaccinated mice had comparably low gB1-Ab levels after the first two immunizations; however, at the time of challenge, animals given vLAW-gB1/VSV showed a substantial increase in antibody titers, whereas the ones in the vLAW-gB1/RD group did not (Fig. 3A). The development of gB1-γTL also differed depending on the immunogen used: in the mice vaccinated with vLAW-gB1/RD, gB1-γTL developed very promptly, peaked after the second dose, and by the time of challenge had substantially decreased. In contrast, in animals vaccinated with vLAW-gB1/VSV the gB1-γTL developed more slowly, so that the peak was observed only at the time of challenge. Thus, by this time, the two groups had equivalent numbers of gB1-γTL in the blood. As expected, mock-vaccinated mice were constantly negative for both gB1-Ab and gB1-γTL (Fig. 3A).
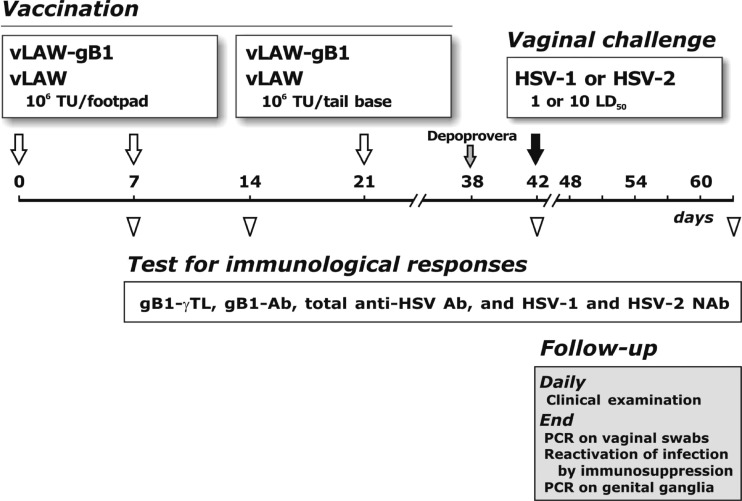
Fig 2.
Timeline and details of vaccination, challenge, and immunological and follow-up analyses. Empty arrows indicate inoculations of 106 TU/dose of vaccine vLAW-gB1 and mock-vaccine vLAW. Empty triangles indicate the time at which randomly selected animals were sacrificed and examined for the indicated immunological parameters. At 3 weeks after the last vaccine inoculation, the animals were challenged via vagina with 1 or 10 LD50s of HSV-1 or HSV-2. Challenge, indicated by solid arrow, was preceded by the inoculation of depot medroxyprogesterone acetate (Depoprovera, gray arrow) to synchronize the estrous cycle. Animals were monitored postchallenge for clinical signs of infection for about 3 weeks and, at termination, examined as detailed in the gray box.
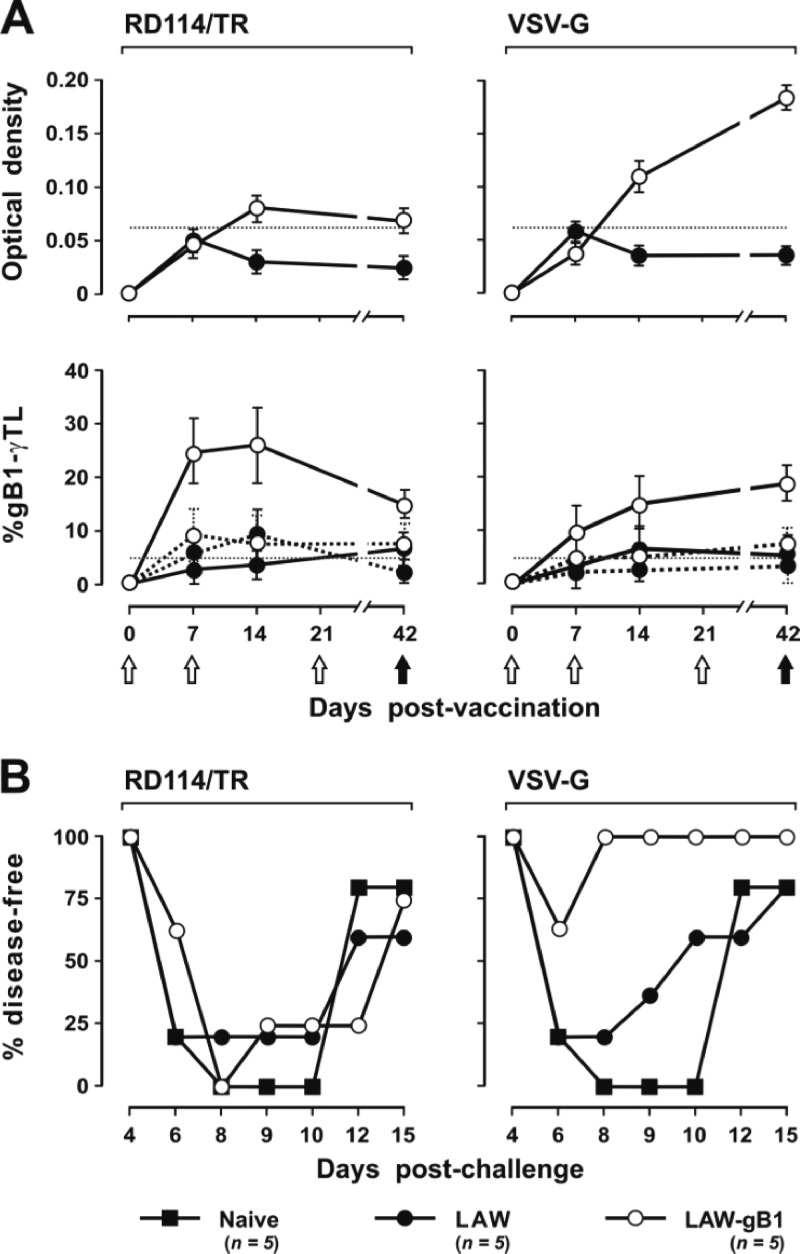
Fig 3.
Analysis of elicited immunity and outcome of HSV-1 challenge in animals vaccinated (LAW-gB1) or mock-vaccinated (LAW) with the respective vector pseudotyped with RD114/TR or VSV-G. (A) The top panels show the levels of anti-gB1 antibodies (gB1-Ab), measured with a commercial anti-HSV antibodies ELISA kit; the lower panels show the percentages of IFN-γ-secreting CD8+ T lymphocytes recognizing the gB1 T-lymphocyte epitope 498SSIEFARL505 (gB1-γTL; continuous line) or scrambled peptide ASFLRSEI (dotted line) were determined by intracellular staining of spleen cells stimulated in vitro. IFN-γ-secreting CD4+ T lymphocytes were below cutoff or undetectable. Empty and solid circles indicate LAW-gB1 and LAW animals, respectively. Whiskers indicate the standard deviation. Dotted lines indicate the cutoff, as calculated according to the manufacturer's instructions (gB1-Ab) or experimentally (gB1-γTL). White and black arrows indicate inoculation of above immunogens and challenge with HSV-1, respectively. (B) Percentages of naive, LAW, and LAW-gB1 animals that remained disease-free after challenge with 1 LD50 HSV-1. n, Number of animals/group.
Finally, the remaining five animals per group and an equal number of naive mice were challenged with HSV-1 exactly as described above. At day 6 p.c., all naive mice and four of the five mock-vaccinated mice were overtly sick, with clinical signs that scored 2 or more by days 8 to 10. Four of these animals (one naive and three mock vaccinees) died between days 10 and 12, whereas in the others the infection subsided so that all appeared fully recovered at the termination of the experiment, i.e., at day 15 p.c. In the vLAW-gB1/RD vaccinees the outcome of challenge was indistinguishable from the controls: all animals developed severe signs of disease, and 1 died at day 10 p.c. In contrast, in the vLAW-gB1/VSV vaccinated group, three of five animals remained completely disease-free throughout the observation period, and the other 2 only showed some redness around the vagina that disappeared within 2 days (Fig. 3B). These results were a clear indication that vLAW-gB1/VSV, possibly due to its wider spectrum of cells transduced, was a much better protective immunogen than was vLAW-gB1/RD. Thus, the subsequent experiments were conducted with vLAW-gB1/VSV alone.
Vaccination experiments using HSV-2 as a challenge.
In the first of the experiments using HSV-2 as a challenge, the immunogen was vLAW-gB1/VSV, the mock immunogen vLAW/VSV, the experimental plan the same depicted by Fig. 2, and the intravaginal challenge was conducted with 1 LD50 of HSV-2. Of the 30 mice that were given the immunogen and the 25 that received the mock immunogen, 14 and 13, respectively, were used to monitor the anti-gB1 immune response at the three times indicated in Fig. 2 (three to five animals per group per time point). These analyses showed that, while the mock vaccinees had no detectable responses, the vaccinees had responded exactly as observed with the same inocula in the previous experiment, with regard to both the kinetics and the levels of gB1-γTL and gB1-Ab generated (data not shown).
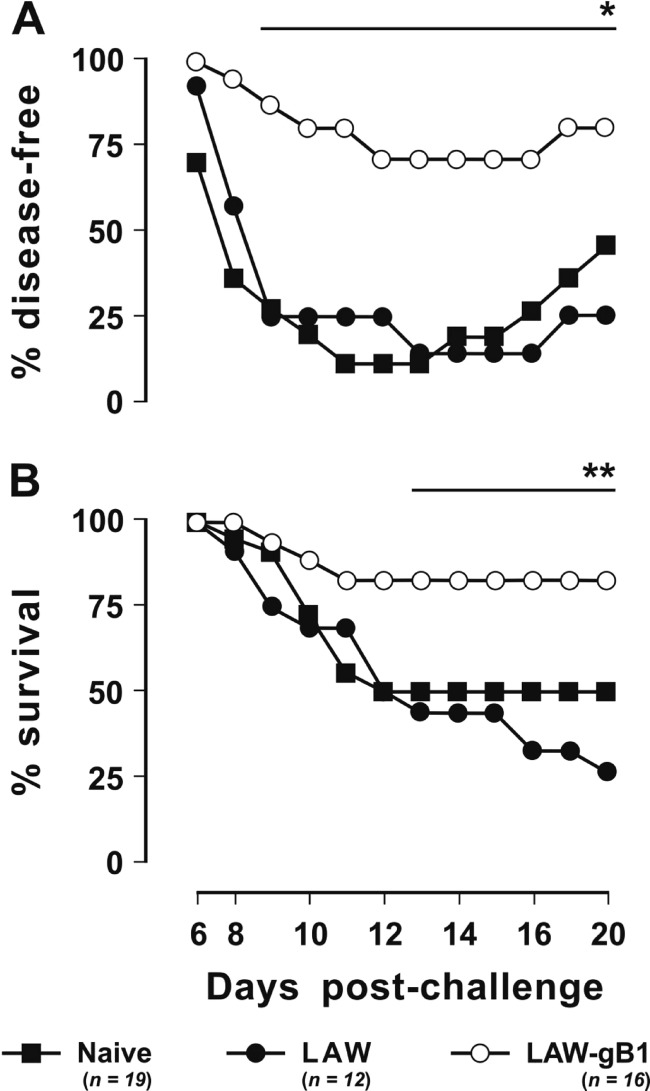
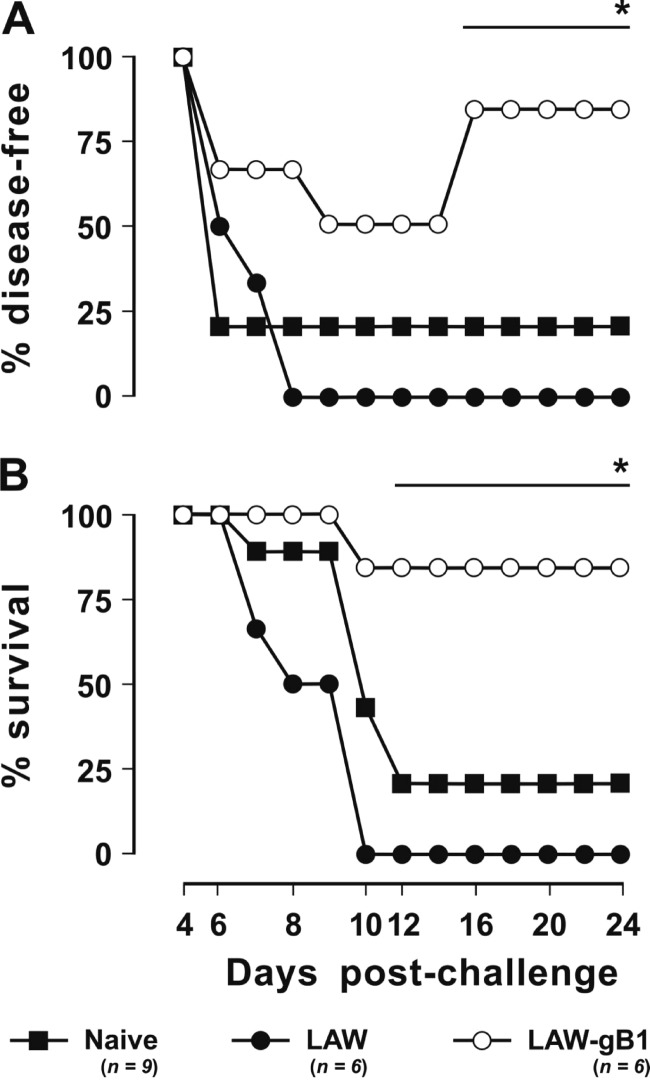
The HSV-2 used for challenge was known to be especially virulent. Thus, after the challenge of the 12 mock vaccinees and of the 19 naive mice that were used as unmanipulated controls, only 1 (8%) and 2 (11%), respectively, remained free of signs of infection (Fig. 4). All of the others became clearly sick, with clinical pictures that in most cases had a clear tendency to worsen with time; as a result, when the experiment was terminated at 20 days p.c., of the 17 naive mice and 11 mock vaccinees that had developed clinical signs, only 8 (47%) and 2 (18%), respectively, had apparently recovered, while the others had either succumbed or were still clearly diseased. In sharp contrast, the outcome of challenge was much less severe in the vaccinees: of the 16 animals in the group, half resisted the challenge completely, 5 (31%) developed very mild infection (clinical score < 2), which promptly subsided, and only 3 (19%) developed severe clinical manifestations that led to their euthanasia (Fig. 4 and Table 1). Statistical analysis showed that the proportion of animals which had developed lesions of any severity postchallenge was significantly smaller in the vaccinated group than in the naive plus the mock-vaccinated group (P = 0.003) and that the same was true, at a much higher level of significance, for the proportion of animals with lesions scoring 2 to 4 (P < 0.0001).
Fig 4.
Outcome of challenge performed with 1 LD50 of HSV-2. Animals were vaccinated (LAW-gB1) or mock vaccinated (LAW) with vector particles pseudotyped with VSV-G. (A) Percentages of animals that remained disease-free throughout the observation period. (B) Percentages of surviving animals. The number of dead animals includes those that succumbed to the infection or were euthanized following paralysis or other irreversible lesions. n, Number of animals/group. One and two asterisks indicates significant differences relative to vaccinated animals at P ≤ 0.03 and P ≤ 0.003, respectively.
Table 1.
Clinical status of animals challenged with 1 or 10 LD50s of HSV-2, as measured at the end of follow-upa
| Group | No. (%) of animals challenged with 1 LD50 |
No. (%) of animals challenged with 10 LD50 |
||||||
|---|---|---|---|---|---|---|---|---|
| Total | Sick or dead | Recovering | Healthy | Total | Sick or dead | Recovering | Healthy | |
| Naive | 19 | 11 (58.0) | 6 (31.5) | 2 (10.5) | 9 | 7 (77.8) | 2 (22.2) | 0 (0.0) |
| Mock vaccinated | 12 | 9 (75.0) | 2 (16.7) | 1 (8.3) | 6 | 6 (100) | 0 (0.0) | 0 (0.0) |
| Vaccinated | 16 | 3 (18.7)* | 5 (31.3) | 8 (50.0)† | 6 | 1 (16.7)‡ | 4 (66.6) | 1 (16.7) |
“Recovering” refers to animals that developed transient infection and were in complete or partial remission at the end of follow-up. “Healthy” refers to animals that showed no signs of disease during the observation period.
, Statistically different from naive and mock-vaccinated animals at P = 0.027 and P = 0.004, respectively;
, statistically different from naive and mock-vaccinated animals at P = 0.015 and P = 0.027, respectively;
, statistically different from naive and mock-vaccinated animals at P = 0.024 and P = 0.004, respectively.
In the next experiment, mice were immunized exactly as described above, but the HSV-2 dose was 10 LD50. In the 15 mice used as controls (9 naive and 6 mock vaccinated), this challenge produced a very severe clinical picture: compared to the 1-LD50 challenge, the viral lesions became evident 3 to 4 days earlier, had a more evident onset, and progressed more rapidly to score 3 to 4 (large ulcers and/or paralysis) and death (Fig. 5). Thus, by the end of the experiment at day 24, only two naive mice (13% of total controls) were still alive and had apparently resolved the infection. All of the others had succumbed between days 10 and 12. Relative to the dramatic picture observed in the controls, the six mice that had been vaccinated with vLAW-gB1/VSV exhibited a much less dramatic postchallenge outcome. Although one animal died at day 10 p.c., three showed transient lesions that scored 2 or above, one had modest redness around the vagina that lasted 1 day, and one showed no lesions at all (Fig. 5). At the end of the experiment, the difference in the proportion of vaccinated and control animals with lesions scoring 2 to 4 was statistically significant at P = 0.003.
Fig 5.
Outcome of challenge performed with 10 LD50s of HSV-2. Animals were vaccinated (LAW-gB1) or mock vaccinated (LAW) with vector particles pseudotyped with VSV-G. (A) Percentages of animals that remained disease-free throughout the observation period. (B) Percentages of surviving animals. The numbers of dead animals were calculated as in Fig. 4. n, Number of animals/group. An asterisk indicates a significant difference relative to vaccinated animals at P ≤ 0.003.
vLAW-gB1/VSV protects both against HSV-2 disease and infection.
The data presented above indicated that immunization with vLAW-gB1/VSV reduced the clinical consequences of an HSV-2 challenge; however, these findings were not informative as to whether vaccination did so just by increasing the likelihood that the infection remained subclinical or also by preventing infection acquisition. In an attempt to shed light on this aspect, the eight vaccinees that in the above experiment had remained completely disease-free after challenge with 1 LD50 of HSV-2 were extensively investigated for the presence of traces of ongoing or past infection. First, vaginal swabs collected at the end of follow-up from these mice were examined for the presence of HSV-2 genomes by nested PCR. Five vaccinees that in the same experiment had developed transient lesions and five naive mice that in the same experiment had become overtly sick were used as positive controls. However, the PCR assay was positive only in three naive controls that still presented with extensive genital lesions at the time of sampling (Table 2), thus showing that the approach was not sufficiently sensitive.
Table 2.
Results of tests carried out to examine whether eight vaccinated animals, which showed no signs of disease after HSV-2 challenge, developed subclinical infection
| Disease status (no. of animals)a | No. of animals displaying a virological or immunological marker |
|||
|---|---|---|---|---|
| HSV-2 genome in vaginab | Anti-HSV antibodyc | Reactivation of HSV-2 infectiond | HSV-2 genome in nervous tissuese | |
| Sick (5) | 3 | 5 | 5/3 | 5 |
| Transiently infected (5) | 0 | 5 | 3/1 | 5 |
| Healthy (8) | 0 | 2 | 2/1ef | 2f |
That is, animals that either were still sick, were transiently infected and fully recovered, or never showed signs of disease by the end of follow-up.
That is, the number of HSV-2 nested PCR-positive animals. Analysis was performed in cervical epithelial cells collected at the end of follow-up.
That is, the number of animals positive for anti-HSV antibody as determined by WB analysis of sera collected at day 30 p.c. The sera of HSV-2-infected or gB1-vaccinated animals were used as positive or negative controls, respectively. Six healthy animals were positive for gB1 antibody only.
That is, the number of animals diseased/number of animals dead after HSV-2 reactivation induced by deep immunosuppression by CY inoculation at day 80 p.c.
That is, the number of HSV-2 nested PCR-positive animals. Analysis was performed in sciatic nerve and cervical ganglia collected at day 21 post-CY inoculation.
Significantly different from sick naive controls at P = 0.013.
Sera then collected at days 30 p.c. from the eight protected animals were examined by WB for the presence, gB1-Ab aside, of antibodies recognizing other HSV proteins that might therefore indicate that an infection had taken place. Sera from naive and vLAW-gB1/VSV-vaccinated (and therefore positive for gB1-Ab only) mice served as negative controls, while positive controls were sera from symptomatic naive and vaccinated mice. As expected, all of the latter sera showed WB profiles with multiple bands typical of a full-blown HSV-2 infection. In contrast, six of the vaccine protected animal sera showed the gB1-Ab band alone, and only two had an extended, albeit weak WB reactivity (Table 2 and data not shown). This suggested that in some animals the vaccine had prevented not only the clinical emergence of the infection but also the infection itself.
To address this aspect more in depth, at 80 days p.c. the eight vaccine-protected mice, as well as some vaccinated and naive animals of the same experiment that had developed and then resolved signs of HSV-2 disease postchallenge, were given a bolus of cyclophosphamide (CY). This treatment depleted by ca. 90% the circulating lymphocytes of the mice within 1 day and left them strongly leukopenic for more than 2 weeks (data not shown), creating conditions favorable for the reactivation of a latent HSV-2 infection. In the five naive animals that had resolved the disease, reactivation was indeed immediate: all again developed HSV-2 lesions starting 2 days post-CY and 3 died by day 11. Infection also reactivated in three of five of the vaccinees that had transiently exhibited viral lesions postchallenge. In these mice, however, the clinical relapses occurred later (days 4 to 7 post-CY) and were generally more benign compared to the relapses occurred in the former animals. One of these animals died at day 15 post-CY, but the death was deemed unrelated to the HSV-2 since the clinical score never exceeded 2 and the conditions had been stable for almost 1 week. Of the eight vaccine-protected mice, the same six animals that showed the gB1-Ab band alone remained disease-free throughout the observation period of 3 weeks, one developed a mild genital erythema that appeared at day 4 and lasted 1 week, and one remained healthy until day 9 post-CY but then developed severe and rapidly progressive signs of HSV-2 disease which led to its euthanasia at day 15 post-CY. Statistical analysis showed that reactivation was significantly less frequent in the vaccine-protected animals than in the naive animals at P = 0.013. Finally, at the end of the experiment, all surviving animals were sacrificed, and their sciatic nerves and cervical ganglia PCR were examined for the presence of the HSV-2 genome. All animals that had undergone clinical reactivation tested positive, and the same was true for two vaccinees that had been transiently sick postchallenge but had not reactivated the infection post-CY. In contrast, the six vaccine-protected animals that were gB1-Ab positive only and had remained disease free even post-CY tested negative. The difference between the rate of PCR positivity in the vaccine-protected and naive animals was also statistically significant (Table 2).
Dissection of the immune responses elicited by vLAW-gB1/VSV.
In the present study, 15 mice per group were either vaccinated with vLAW-gB1/VSV or mock vaccinated with vLAW/VSV and examined for gB1-Ab, HSV-1 and HSV-2 NAb, and gB1-γTL in the blood, spleen, bone marrow, and iliac lymph nodes according to the standard schedule depicted by Fig. 2. The mock vaccinees were constantly negative in all of the assays, while examination of the vaccines revealed some interesting findings. The kinetics and level of gB1-Ab and gB1-γTL were very similar to those illustrated by Fig. 3 and are therefore not further described. The NAbs in the sera were detectable from the second sampling (1 week after first immunizing dose) onward and were inhibitory for both HSV-1 and HSV-2, albeit with different efficacies; typically, sera diluted 1:10 reduced plaque formation by HSV-1 and HSV-2 by 70 and 60%, respectively, and, although higher dilutions were increasingly less effective, marginal neutralizing activity was observed up to a dilution of 1:80 (Fig. 6A and data not shown). Similarly to what is depicted in Fig. 3 and despite the fact that the third immunizing dose had led to a substantial increment of gB1-Ab, the neutralizing activity of sera underwent only a marginal increase. Indeed, the combined ANOVA-Tukey's test found that only the difference between means at 7 and 14 days postvaccination and at 7 and 42 days postvaccination were statistically significant at P ≤ 0.005. Comparison of the means at 14 and 42 days postvaccination did not reach statistical significance.
Fig 6.
Analysis of anti-HSV immune responses in vaccinated (LAW-gB1) and mock-vaccinated (LAW) animals. The timeline of the vaccination schedule and testing for elicited immune responses is as described in Fig. 2. (A) Percentages of anti-HSV-1 (circles)- and anti-HSV-2 (triangles)-neutralizing activity in sera collected at the indicated times and tested at 1:10 (solid symbols) and 1:20 (empty symbols) dilution. The percent neutralization refers to the number of infected cells incubated with the virus alone. Serum samples from naive animals diluted 1:10 showed a neutralization activity of ≤10%. An asterisk indicates a statistically significant increment at P ≤ 0.005. (B) Percentages of gB1-γTL from spleen (circles), iliac lymph nodes (triangles), or bone marrow (squares) as measured by intracellular staining after stimulation with gB1 T epitope (solid symbols) or scrambled peptide (empty symbols). Analysis was performed in animals euthanized at the indicated times. The dotted line indicates the cutoff. The solid line indicates the average value. IFN-γ-secreting CD4+ T lymphocytes were below cutoff or undetectable. **, Statistically significant increment at P < 0.001.
In the vaccinees, gB1-γTL were present in all of the lymphoid tissues examined, but their development followed different kinetics depending on the organ. In the spleen, these cells were already rather abundant (ca. 15%) after the first immunization and then remained essentially stable throughout the experiment. The bone marrow after the first immunization also had elevated proportions of these effectors, which further increased after the second dose of immunogen to return to the previous levels at the time of challenge. In contrast, the iliac lymph nodes contained very few if any gB1-γTL after the first dose but at later times exhibited the largest values that were observed in the study (ca. 30%). The combined ANOVA-Tukey's test showed no statistical significance between means of spleen and bone marrow tissues at any time tested. In contrast, in iliac lymph nodes the difference between means at 7 and 14 days postvaccination and at 7 and 42 days postvaccination reached statistical significance at P < 0.001 (Fig. 6B). Again, there was no statistical significance between means at 14 and 42 days postvaccination.
DISCUSSION
Genital infections caused by HSV-1 and HSV-2 are exceedingly common, spread at an unrelenting pace, and increase host susceptibility to other sexually transmitted infections (10, 41, 67). Despite many efforts in vaccine development and several clinical tests (5, 14, 27), no truly effective measures to prevent infection or disease are currently available (17, 38).
To address this issue, we focused on gB1. This HSV-1 envelope glycoprotein has several qualifying features as a vaccinal immunogen. (i) Together with other glycoproteins (7, 55, 57), gB1 mediates HSV-1 entry into susceptible cells. Its cellular receptor is the nonmuscle myosin heavy chain IIA (NMHC-IIA), a subunit of the nonmuscle myosin IIA (NM-IIA) (3). Because of its essential role in cell adhesion, cell migration, and tissue architecture (62), NM-IIA is expressed in various tissues and cell types in vivo and may thus favor the broad spectrum of HSV-1 infection (7). Specific immune responses blocking gB1-NMHC-IIA interplay could therefore greatly reduce viral infectivity. (ii) gB1 induces strong humoral and cell-mediated immune responses (17, 35). (iii) It contains “protective” cytotoxic-T-lymphocyte (CTL) epitopes that are preferentially recognized by asymptomatic subjects, are important to prevent recurrent diseases, and maintain HSV-1 in a latent state (12, 45). (iv) Finally, gB1 is highly conserved within and between HSV-1 and HSV-2 serotypes. The interserotype homology is 85% at amino acid level and, with the exception of the N and C-terminal regions, the secondary structure is also highly similar (6). As a consequence, HSV-1 and HSV-2 gB share several B- and T-lymphocyte epitopes that induce cross-reactive NAb and CTL responses in both natural and experimental infections (35, 51) and have allowed the development of cross-neutralizing antibodies (29).
Thus, vaccinations with HSV-1 and HSV-2 gB have been attempted in many ways by using the whole gene either as a protein or as a DNA coding sequence (9, 14), selected immunodominant T-lymphocyte epitope(s) as peptides or DNA sequences (39, 44), and various delivery systems and immunization routes (28, 36). Finally, gB was tested either alone or combined with other HSV immunogens (18, 34, 64). In general, these vaccines, whether tested in animal models or clinical studies, prevented HSV-1 disease but were poorly protective against lethal doses of HSV-1 and were clearly inadequate against HSV-2. The studies also showed that mucosal immunity is important to prevent or curb the course of infection (17, 27, 30, 38).
The novelty of our study is the delivery of gB with a lentiviral vector that, to the best of our knowledge, has never been used as a vaccine vector against HSV. Lentiviral vectors are well suited to stimulate immunity. Compared to other vectors and most adjuvants, lentiviruses also transduce nondividing cells, including dendritic cells (DCs) and macrophages (26), thus setting the basis for an efficient stimulation of the immune system. Here, we used LAW34, an FIV vector complying with today's safety standards (19) and tested in various animal species (cats, mice, pigs, and rats). A concern with any lentiviral vector is the potential consequence of the integration of their genomes into the host's DNA. To overcome this possible risk, the use in vaccine development of integrase-deficient lentiviral vectors has been proposed with promising results (15, 37). This may represent an interesting avenue for future studies. Possibly because the long terminal repeat of LAW34 are inactivated, in more than 3 years of follow-up studies lasting a few weeks to several months and dozens of animals inoculated, neither short- nor long-term noxious effects were observed. In one of these studies, porcine cardiac stem cells were transduced ex vivo, implanted in rats with myocardial infarction, and monitored for 3 months by nuclear magnetic resonance imaging. The cells survived, differentiated, and expressed the transduced reporter gene throughout the observation period, a clear indication that LAW34 does not harm or affect physiological functions of implanted cells and the delivered gene is expressed for protracted periods of time (8). The use of LAW34 for vaccination was prompted by an earlier study in which we demonstrated that this vector transduced DCs very efficiently when pseudotyped with RD/114 (43). Since DCs play a key role in building up the adaptive host defense against an invading pathogen (54), we expected this vector makeup to be better than conventional pseudotyping with VSV-G. Surprisingly, the results demonstrated otherwise. Although vLAW-gB1/RD induced prompter immune responses, these declined faster compared to vLAW-gB1/VSV so that both vaccine groups had equivalent anti-gB1 immune responses at the time of challenge. Even more disappointingly, vLAW-gB1/RD afforded no protection against HSV-1 challenge. In contrast, the vLAW-gB1/VSV vaccinees were fully protected from disease, paralleling the results obtained from most HSV vaccines hitherto developed (17, 38).
As in previous vaccine studies (17, 27), HSV-2 was more pathogenic and difficult to contain by immunological measures compared to HSV-1. At 1 LD50, the HSV-2 isolate yielded symptomatic infection in all naive and mock-vaccinated animals and killed more than 60% of the animals. In most of these, death was preceded by paralysis starting from hind limbs and rapidly extending to the back, suggesting a pronounced neurovirulence of the isolate. Vaccination, which was performed only with vLAW-gB1/VSV, spared 75 and 80% of the animals from severe infection and death, respectively. We then wanted to ascertain whether the 8 of 16 vaccinees, which showed no signs of disease, not even transient redness at the site of challenge, were fully protected or underwent subclinical infection. This was investigated through several approaches. Searching for the viral genome in vaginal brushes by real-time or nested PCR proved to be not sufficiently sensitive. HSV recurrence induction by CY treatment and PCR analysis of nervous tissues proved to be a more effective way to determine infection. These methods allowed us to identify the virus in all controls that were sick following challenge but fully recovered before CY treatment. As frequently observed in patients (51), severity of recurrences were proportional to postchallenge clinical onset; in animals where primary infection was severely debilitating and prolonged, HSV-2 reactivated sooner and caused comparable or worse disease. In contrast, of the animals that had had mild primary infection, two had no clinical relapse and yet were determined to be HSV positive by PCR in nervous system tissues, and three had a modest and transient clinical episode. Among the eight fully protected animals, as judged by the absence of clinical signs postchallenge, only two were, in fact, infected, whereas there was no trace of the virus in the remaining vaccinees. In all, vLAW-gB1/VSV was able to protect from HSV-2 infection and severe disease, as defined by clinical score 2 or higher, 38 and 80% animals, respectively, thus outperforming most previously developed vaccines based on the sole gB (14, 27). Protection efficacy was further addressed against a challenge dose 10 times higher than in the above tests and that killed nearly all of the naive and mock-vaccinated controls. Although the number of animals used in this experiment was low, vaccination spared 50 and 83% animals from severe infection and death, respectively.
The type and extent of elicited immunity was investigated by measuring gB1-Ab and virus NAb in serum, gB1-γTL in bone marrow and spleen, and, given their importance in prevention and immune control of viral latency and reactivation of genital herpes infection (68), iliac lymph nodes. These parameters were monitored in vaccinated and mock-vaccinated animals 1 week after first and second immunizations and at challenge, i.e., 3 weeks after the third immunization. Although we detected anti-gB1 IgA in vaginal washes and serum, we were not able to clearly discern them from IgG. Given the undisputed importance of IgA against mucosal infections in the genital tract (49), this is a limitation of this work. Interestingly, and in contrast to our expectations for immunization with lentiviral vectors, which are usually advantageous to Th1 responses (26), vaccination elicited a prompt, potent, and stable NAb response that was able to neutralize ca. 50% HSV-1 and HSV-2. Although there are limited studies where NAb and cross-neutralization activity are measured, our results were in line with vaccination experiments carried out with DNA vaccines encoding a gB1-secreting form (9) or combined with Th2-stimulating compounds (28) or multi-epitope peptides (64). In our study, NAb became detectable after the second immunization. Similar behavior was observed for gB1-γTL in iliac lymph nodes as opposed to the bone marrow and spleen, where gB1-γTL was already measurable after the first immunization and gradually increased thereafter. Despite the different kinetics, the percentages of gB1-γTL at challenge were high and at comparable levels in the three body sites and were similar to those observed in previous studies addressing the role of cell-mediated immune response against vaginal and systemic HSV-2 infections (56, 65, 68).
Taken together, these results demonstrate that vLAW-gB1/VSV favorably compares to most HSV reference vaccines. Immunization was accomplished with three inoculations of vLAW-gB1/VSV (106 TU/dose), an amount easy to produce and scale up, and by using a single immunogen. A larger dose might have been more effective but would have been difficult to scale up for human use. Since LAW34 can stably deliver two or more genes for up to 7 kb of heterologous DNA (61), this vaccine has ample room for improvement. Indeed, we are already testing in vitro LAW34 delivering gB and gD, another HSV receptor extensively used for vaccination (17, 38, 65), and gB and ICP27, a multifunctional early regulatory protein eliciting strong and protective cell-mediated immune responses (32). With a truly protective vaccine years away (5), LAW34, either administered alone or combined with other delivery systems, may become a structural component of an effective vaccine against the genital herpes. Further studies in this and other animal models are clearly worthwhile to confirm the protective efficacy of LAW34 and investigate in greater depth the immunological bases of this protection.
ACKNOWLEDGMENT
This study was supported by Ministero dell'Istruzione, dell'Università e della Ricerca, grant PRIN 2007.
Footnotes
Published ahead of print 4 April 2012
REFERENCES
- 1. Abu-Raddad LJ, et al. 2008. Genital herpes has played a more important role than any other sexually transmitted infection in driving HIV prevalence in Africa. PLoS One 3:e2230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Andrei G, et al. 2011. Topical tenofovir, a microbicide effective against HIV, inhibits herpes simplex virus-2 replication. Cell Host Microbe 10:379–389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Arii J, et al. 2010. Non-muscle myosin IIA is a functional entry receptor for herpes simplex virus-1. Nature 467:859–862 [DOI] [PubMed] [Google Scholar]
- 4. Barraza RA, et al. 2009. Prolonged transgene expression with lentiviral vectors in the aqueous humor outflow pathway of nonhuman primates. Hum. Gene Ther. 20:191–200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Belshe RB, et al. 2012. Efficacy results of a trial of a herpes simplex vaccine. N. Engl. J. Med. 366:34–43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Bzik DJ, Debroy C, Fox BA, Pederson NE, Person S. 1986. The nucleotide sequence of the gB glycoprotein gene of HSV-2 and comparison with the corresponding gene of HSV-1. Virology 155:322–333 [DOI] [PubMed] [Google Scholar]
- 7. Campadelli-Fiume G, et al. 2007. The multipartite system that mediates entry of herpes simplex virus into the cell. Rev. Med. Virol. 17:313–326 [DOI] [PubMed] [Google Scholar]
- 8. Campan M, et al. 2011. Ferritin as a reporter gene for in vivo tracking of stem cells by 1.5-T cardiac MRI in a rat model of myocardial infarction. Am. J. Physiol. Heart Circ. Physiol. 300:H2238–H2250 [DOI] [PubMed] [Google Scholar]
- 9. Caselli E, et al. 2001. Mice genetic immunization with plasmid DNA encoding a secreted form of HSV-1 gB induces a protective immune response against herpes simplex virus type 1 infection. Intervirology 44:1–7 [DOI] [PubMed] [Google Scholar]
- 10. Centers for Disease Control and Prevention 2012. HIV, STD and TB prevention. Centers for Disease Control and Prevention, Atlanta, GA: http://www.cdc.gov/std/Herpes/STDFact-Herpes.htm [Google Scholar]
- 11. Cernik C, Gallina K, Brodell RT. 2008. The treatment of herpes simplex infections: an evidence-based review. Arch. Intern. Med. 168:1137–1144 [DOI] [PubMed] [Google Scholar]
- 12. Chentoufi AA, et al. 2008. Asymptomatic human CD4+ cytotoxic T-cell epitopes identified from herpes simplex virus glycoprotein B. J. Virol. 82:11792–11802 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Corey L, Handsfield H. 2000. Genital herpes and public health: addressing a global problem. JAMA 283:791–794 [DOI] [PubMed] [Google Scholar]
- 14. Corey L, et al. 1999. Recombinant glycoprotein vaccine for the prevention of genital HSV-2 infection: two randomized controlled trials. JAMA 282:331–340 [DOI] [PubMed] [Google Scholar]
- 15. Coutant F, Frenkiel MDP, Charneau P. 2008. Protective antiviral immunity conferred by a nonintegrative lentiviral vector-based vaccine. PLoS One 3:e3973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Coyle P, Desai A, Wyatt D, McCaughey C, O'Neill H. 1999. A comparison of virus isolation, indirect immunofluorescence and nested multiplex polymerase chain reaction for the diagnosis of primary and recurrent herpes simplex type 1 and type 2 infections. J. Virol. Methods 83:75–82 [DOI] [PubMed] [Google Scholar]
- 17. Dasgupta G, Chentoufi AA, Nesburn AB, Wechsler SL, BenMohamed L. 2009. New concepts in herpes simplex virus vaccine development: notes from the battlefield. Expert Rev. Vaccines 8:1023–1035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Domingo C, et al. 2003. Immunological properties of a DNA plasmid encoding a chimeric protein of herpes simplex virus type 2 glycoprotein B and glycoprotein D. Vaccine 21:3565–3574 [DOI] [PubMed] [Google Scholar]
- 19. Dropulic B. 2011. Lentiviral vectors: their molecular design, safety, and use in laboratory and preclinical research. Hum. Gene Ther. 22:649–657 [DOI] [PubMed] [Google Scholar]
- 20. Elder JH, Lin YC, Fink E, Grant CK. 2010. Feline immunodeficiency virus (FIV) as a model for study of lentivirus infections: parallels with HIV. Curr. HIV Res. 8:73–80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Farley N, et al. 2010. Recurrent vaginal shedding of herpes simplex type 2 virus in the mouse and effects of antiviral therapy. Antivir. Res. 86:188–195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Fleming D, McQuillan G, Johnson R. 1997. Herpes simplex virus type 2 in the United States, 1976–1994. N. Engl. J. Med. 337:1105. [DOI] [PubMed] [Google Scholar]
- 23. Fukushima A, Yamaguchi T, Ishida W, Fukata K, Ozaki A, Ueno H. 2005. The immunization protocol determines whether endogenous interferon-gamma suppresses the infiltration of eosinophils into the conjunctiva. Immunol. Lett. 100:189–194 [DOI] [PubMed] [Google Scholar]
- 24. Grinshpun A, et al. 2010. Neonatal gene therapy of glycogen storage disease type Ia using a feline immunodeficiency virus-based vector. Mol. Ther. 18:1592–1598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Hanke T, Graham F, Rosenthal K, Johnson D. 1991. Identification of an immunodominant cytotoxic T-lymphocyte recognition site in glycoprotein B of herpes simplex virus by using recombinant adenovirus vectors and synthetic peptides. J. Virol. 65:1177–1186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Hu B, Tai A, Wang P. 2011. Immunization delivered by lentiviral vectors for cancer and infectious diseases. Immunol. Rev. 239:45–61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Johnston C, Koelle DM, Wald A. 2011. HSV-2: in pursuit of a vaccine. J. Clin. Invest. 121:4600–4609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Kim SB, et al. 2009. Modulation of protective immunity against herpes simplex virus via mucosal genetic cotransfer of DNA vaccine with beta2-adrenergic agonist. Exp. Mol. Med. 41:812–823 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Krawczyk A, et al. 2011. Impact of valency of a glycoprotein B-specific monoclonal antibody on neutralization of herpes simplex virus. J. Virol. 85:1793–1803 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Lee AJ, Ashkar AA. 2012. Herpes simplex virus-2 in the genital mucosa: insights into the mucosal host response and vaccine development. Curr. Opin. Infect. Dis. 25:92–99 [DOI] [PubMed] [Google Scholar]
- 31. Lopes L, Dewannieux MT, Takayuki Y, Collins MK. 2011. A lentiviral vector pseudotype suitable for vaccine development. J. Gene Med. 13:181–187 [DOI] [PubMed] [Google Scholar]
- 32. Manickan E, et al. 1995. Vaccination with recombinant vaccinia viruses expressing ICP27 induces protective immunity against herpes simplex virus through CD4+ Th1+ T cells. J. Virol. 69:4711–4716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Manservigi R, et al. 1988. Constitutive expression in human cells of herpes simplex virus type 1 glycoprotein B gene cloned in an episomal eukaryotic vector. Virology 167:284–288 [DOI] [PubMed] [Google Scholar]
- 34. McClements WL, Armstrong ME, Keys RD, Liu MA. 1996. Immunization with DNA vaccines encoding glycoprotein D or glycoprotein B, alone or in combination, induces protective immunity in animal models of herpes simplex virus-2 disease. Proc. Natl. Acad. Sci. U. S. A. 93:11414–11420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Mueller SN, et al. 2003. The early expression of glycoprotein B from herpes simplex virus can be detected by antigen-specific CD8+ T cells. J. Virol. 77:2445–2451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Muller WJ, et al. 2008. Recombinant Listeria monocytogenes expressing an immunodominant peptide fails to protect after intravaginal challenge with herpes simplex virus-2. Arch. Virol. 153:1165–1169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Negri D, et al. 2011. Integrase-defective lentiviral-vector-based vaccine: a new vector for induction of T cell immunity. Expert Opin. Biol. Ther. 11:739–750 [DOI] [PubMed] [Google Scholar]
- 38. Nikolic DS, Piguet V. 2010. Vaccines and microbicides preventing HIV-1, HSV-2, and HPV mucosal transmission. J. Invest. Dermatol. 130:352–361 [DOI] [PubMed] [Google Scholar]
- 39. Orr MT, Orgun NN, Wilson CB, Way SS. 2007. Cutting edge: recombinant Listeria monocytogenes expressing a single immune-dominant peptide confers protective immunity to herpes simplex virus-1 infection. J. Immunol. 178:4731–4735 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Palliser D, et al. 2006. An siRNA-based microbicide protects mice from lethal herpes simplex virus 2 infection. Nature 439:89–94 [DOI] [PubMed] [Google Scholar]
- 41. Paz-Bailey G, Ramaswamy M, Hawkes SJ, Geretti AM. 2007. Herpes simplex virus type 2: epidemiology and management options in developing countries. Sex. Transm. Infect. 83:16–22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Pistello M, et al. 2006. AIDS vaccination studies with an ex vivo feline immunodeficiency virus model: analysis of the accessory ORF-A protein and DNA as protective immunogens. J. Virol. 80:8856–8868 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Pistello M, et al. 2007. Streamlined design of a self-inactivating feline immunodeficiency virus vector for transducing ex vivo dendritic cells and T lymphocytes. Genet. Vaccines Ther. 5:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Pouriayevali MH, Bamdad T, Parsania M, Sari RD. 2011. Full-length antigen priming enhances the CTL epitope-based DNA vaccine efficacy. Cell. Immunol. 268:4–8 [DOI] [PubMed] [Google Scholar]
- 45. Ramachandran S, Davoli KA, Yee MB, Hendricks RL, Kinchington PR. 2010. Delaying the expression of herpes simplex virus type 1 glycoprotein B (gB) to a true late gene alters neurovirulence and inhibits the gB-CD8+ T-cell response in the trigeminal ganglion. J. Virol. 84:8811–8820 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Reed L, Muench H. 1938. A simple method for estimating fifty percent end points. Am. J. Hyg. 1938:493–497 [Google Scholar]
- 47. Roizman B, Knipe DM, Whitley R. 2007. Herpes simplex viruses, p 2501–2602 In Knipe DM, et al. (ed), Fields virology, 5th ed Lippincott/The Williams & Wilkins Co, Philadelphia, PA [Google Scholar]
- 48. Rollier CS, Reyes-Sandoval A, Cottingham MG, Ewer K, Hill AV. 2011. Viral vectors as vaccine platforms: deployment in sight. Curr. Opin. Immunol. 23:377–382 [DOI] [PubMed] [Google Scholar]
- 49. Russell MW, Mestecky J. 2002. Humoral immune responses to microbial infections in the genital tract. Microbes Infect. 4:667–677 [DOI] [PubMed] [Google Scholar]
- 50. Sandrin V, et al. 2002. Lentiviral vectors pseudotyped with a modified RD114 envelope glycoprotein show increased stability in sera and augmented transduction of primary lymphocytes and CD34+ cells derived from human and nonhuman primates. Blood 100:823–832 [DOI] [PubMed] [Google Scholar]
- 51. Schiffer JT, et al. 2010. Mucosal host immune response predicts the severity and duration of herpes simplex virus-2 genital tract shedding episodes. Proc. Natl. Acad. Sci. U. S. A. 107:18973–18978 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Sinn PL, Arias AC, Brogden KA, McCray PB., Jr 2008. Lentivirus vector can be readministered to nasal epithelia without blocking immune responses. J. Virol. 82:10684–10692 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Smith JS, et al. 2002. Herpes simplex virus-2 as a human papillomavirus cofactor in the etiology of invasive cervical cancer. J. Natl. Cancer Inst. 94:1604–1613 [DOI] [PubMed] [Google Scholar]
- 54. Steinman RM. 2012. Decisions about dendritic cells: past, present, and future. Annu. Rev. Immunol. 30:1–22 [DOI] [PubMed] [Google Scholar]
- 55. Suenaga T, et al. 2010. Myelin-associated glycoprotein mediates membrane fusion and entry of neurotropic herpesviruses. Proc. Natl. Acad. Sci. U. S. A. 107:866–871 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Tang VA, Rosenthal KL. 2010. Intravaginal infection with herpes simplex virus type-2 (HSV-2) generates a functional effector memory T cell population that persists in the murine genital tract. J. Reprod. Immunol. 87:39–44 [DOI] [PubMed] [Google Scholar]
- 57. Taylor JM, et al. 2007. Alternative entry receptors for herpes simplex virus and their roles in disease. Cell Host Microbe 2:19–28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Teepe AG, Allen LB, Wordinger RJ, Harris EF. 1990. Effect of the estrous cycle on susceptibility of female mice to intravaginal inoculation of herpes simplex virus type 2 (HSV-2). Antivir. Res. 14:227–235 [DOI] [PubMed] [Google Scholar]
- 59. Tognon M, et al. 1985. Analysis of HSV isolated from patients with unilateral and bilateral herpetic keratitis. Int. Ophthalmol. 8:13–18 [DOI] [PubMed] [Google Scholar]
- 60. Valori CF, Ning K, Wyles M, Azzouz M. 2008. Development and applications of non-HIV-based lentiviral vectors in neurological disorders. Curr. Gene Ther. 8:406–418 [DOI] [PubMed] [Google Scholar]
- 61. Vannucci L, et al. 2010. Feline immunodeficiency virus vector as a tool for preventative strategies against human breast cancer. Vet. Immunol. Immunopathol. 134:132–137 [DOI] [PubMed] [Google Scholar]
- 62. Vicente-Manzanares M, Ma X, Adelstein RS, Horwitz AR. 2009. Non-muscle myosin II takes centre stage in cell adhesion and migration. Nat. Rev. Mol. Cell. Biol. 10:778–790 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Wald A. 2004. Herpes simplex virus type 2 transmission: risk factors and virus shedding. Herpes 11:130A–137A [PubMed] [Google Scholar]
- 64. Wang X, et al. 2011. Design and evaluation of a multi-epitope assembly peptide (MEAP) against herpes simplex virus type 2 infection in BALB/c mice. Virol. J. 8:232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Wilson SS, Fakioglu E, Herold BC. 2009. Novel approaches in fighting herpes simplex virus infections. Expert Rev. Anti-Infect. Ther. 7:559–568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Xu F, et al. 2010. Seroprevalence of herpes simplex virus type 2 among persons aged 14–49 years in United States, 2005–2008. MMWR Morb. Mortal. Wkly. Rep. 59:456–459 [PubMed] [Google Scholar]
- 67. Xu F, et al. 2006. Trends in herpes simplex virus type 1 and type 2 seroprevalence in the United States. JAMA 296:964–973 [DOI] [PubMed] [Google Scholar]
- 68. Zhu J, et al. 2007. Virus-specific CD8+ T cells accumulate near sensory nerve endings in genital skin during subclinical HSV-2 reactivation. J. Exp. Med. 204:595–603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Zhou X, Berg L, Motal UM, Jondal M. 1992. In vivo primary induction of virus-specific CTL by immunization with 9-mer synthetic peptides. J. Immunol. Methods 153:193–200 [DOI] [PubMed] [Google Scholar]