Abstract
DNA damage response (DDR) is a sophisticated cellular network that detects and repairs DNA breaks. Viruses are known to activate the DDR and usurp certain DDR components to facilitate replication. Intriguingly, viruses also inhibit several DDR proteins, suggesting that this cellular network has both proviral and antiviral features, with the nature of the latter still poorly understood. In this study we show that irradiation of primary murine macrophages was associated with enhanced expression of several antiviral interferon (IFN)-stimulated genes (ISGs). ISG induction in irradiated macrophages was dependent on type I IFN signaling, a functional DNA damage sensor complex, and ataxia-telangiectasia mutated kinase. Furthermore, IFN regulatory factor 1 was also required for the optimal expression of antiviral ISGs in irradiated macrophages. Importantly, DDR-mediated activation of type I IFN signaling contributed to increased resistance to mouse gammaherpesvirus 68 replication, suggesting that the coordinate regulation of DDR and type I IFN signaling may have evolved as a component of the innate immune response to virus infections.
INTRODUCTION
DNA damage response (DDR) is a highly conserved cellular network that is critical for the detection and repair of double-stranded DNA breaks, arguably, the most catastrophic DNA lesions. DNA breaks are sensed by the MRN complex consisting of the Mre11, Nbs1, and Rad50 proteins (reviewed in reference 65). This complex is highly conserved in eukaryotes ranging from yeast to mammals, and mutations in any of the components of the MRN complex are associated with genomic instability and cancer. Two additional signaling events closely coincide with the sensing of the DNA break: phosphorylation of histone variant H2AX and recruitment and activation of ataxia-telangiectasia mutated (ATM). ATM is a serine/threonine kinase that, via phosphorylation of numerous substrates, mediates signaling downstream of the DNA break, facilitates DNA repair, and evokes a multitude of changes within the cellular environment. Serine 139 phosphorylated H2AX (γH2AX) is an established marker of irradiation-induced foci that are associated with double-stranded DNA breaks. Once phosphorylated by ATM, γH2AX is important for the recruitment of DNA repair factors, such as Rad51 and Brca1, to the DNA lesion (6, 10), enabling timely DNA repair.
Many viral infections are associated with increased levels of γH2AX and activated ATM in infected cells, suggesting that induction of DDR is an integral part of the infected cell environment (reviewed in references 28 and 29). Induction of DDR markers can be a virus-driven process, as evidenced by mouse gammaherpesvirus 68 (MHV68) that relies on a conserved viral protein kinase to induce DDR in infected primary macrophages (56). Viruses frequently usurp components of the DDR network to enhance viral DNA synthesis and gene expression (1, 3, 22, 24, 35, 63). Thus, on one hand, viruses exploit DDR to facilitate viral replication. On the other hand, aspects of DDR signaling are frequently blocked in virus infections. Several adenovirus proteins inactivate the MRN complex in infected cells (52), in spite of significant γH2AX induction later in infection (38). Furthermore, ICP0 of herpes simplex virus 1 (HSV-1) targets DDR-associated ubiquitin ligases for degradation (26, 27). Thus, DDR appears to play both proviral and antiviral roles in infected cells; however, the mechanism of the latter is still poorly understood.
Innate immune signaling, best represented by type I interferons (IFN), is a powerful antiviral host cell response. Virus infection is typically recognized by a variety of pattern recognition receptors that interact with components of the incoming virion or, alternatively, with biological entities unique to virus replication, i.e., double-stranded RNA (31, 32, 46). Engagement of pattern recognition receptor results in the activation of a number of signaling cascades that include the IFN regulatory factors (IRFs [20, 47]) that induce the transcription of several genes, most notably type I IFN. Interaction of secreted type I IFN with the ubiquitously expressed receptor triggers enhanced expression of hundreds of genes, some of which encode a known antiviral function. Intriguingly, gammaherpesvirus protein kinases encoded by MHV68 (orf36) and Epstein-Barr virus (BGLF4) induce γH2AX and inhibit type I IFN transcription and signaling in infected cells (16, 56, 59), suggesting that type I IFN and DDR signaling may be connected and that viruses further modify this connection in infected cells.
In the present study we show that induction of DNA damage led to the upregulation of several antiviral IFN-stimulated genes (ISGs) in primary macrophages, a cell type critically important for the innate immunity. Type I IFN signaling was required for irradiation-induced ISG transcription, indicating that activation of DDR was associated with production of type I IFN in primary macrophages. Furthermore, signaling downstream of type I IFN receptor was further regulated by a functional MRN complex in irradiated cells. Importantly, the irradiation-induced upregulation of antiviral ISGs was blocked in MHV68-infected macrophages and the viral kinase orf36 was partially responsible for this inhibition. These studies provide a mechanism that explains the antiviral nature of the DDR.
MATERIALS AND METHODS
Animals and primary cell cultures.
C57BL/6J (BL6), IRF-1-deficient mice (33), and ATM deficient mice (4) were obtained from Jackson Laboratories (Bar Harbor, ME). The Nbs1 hypomorph strain (ΔB/ΔB) was obtained from John Petrini (64). H2AX-deficient mice were obtained from Frederick Alt (7). IFNAR1-deficient mice were obtained from Mitchell Grayson (54). IRF-3- and IRF-7-deficient mice were obtained from Michael Diamond (15, 47). Mice were housed and bred in a specific-pathogen-free barrier facility in accordance with federal and institutional guidelines. All experimental manipulations of mice were approved by the Institutional Animal Care and Use Committee of the Medical College of Wisconsin. Bone marrow was harvested from mice at between 7 and 10 weeks of age. Primary bone marrow macrophages were generated as previously described (56).
Qualitative real-time PCR.
Total RNA was harvested, DNase treated, and reverse transcribed as described previously (57). cDNA was 8-fold serially diluted, and dilutions and corresponding −RT reactions were assessed, in triplicate, by real-time PCR using iCycler (Bio-Rad, Hercules, CA). Gene-specific cDNA was amplified using the primers listed in Table 1. The threshold cycle method, i.e., ΔCT, was used to quantify relative abundance of each cDNA, using corresponding GAPDH (glyceraldehyde-3-phosphate dehydrogenase) levels for normalization. The CT values for minus RT controls did not exceed background levels.
Table 1.
Primer sequences
| mRNA | Orientation | Primer sequence (5′–3′) |
|---|---|---|
| IRF-1 | Forward | ACA-CTA-AGA-GCA-AAA-CCA-AGA-G |
| Reverse | TTT-CCA-TAT-CCA-AGT-CCT-GA | |
| Viperin | Forward | CTT-CAA-CGT-GGA-CGA-AGA-CA |
| Reverse | GAC-GCT-CCA-AGA-ATG-TTT-CA | |
| GBP-1 | Forward | AGT-CTC-ACA-CAA-AGG-GCA-TCT-GGA |
| Reverse | TGT-CTT-TCA-GGC-CCT-CAG-TGT-CAA | |
| Mx1 | Forward | AGC-TAG-ACA-GAG-CAA-ACC-AAG-CCA |
| Reverse | TCC-CTG-AAG-CAG-ACA-CAG-CTG-AAA | |
| RNase L | Forward | AAG-CAG-ATA-GGT-GGC-TGT-CAC-TGT |
| Reverse | AAG-TGC-TGG-GAT-TAA-AGG-CGT-GTG | |
| IRF-3 | Forward | ACT-GCG-TCT-AGG-CTG-GTG-GTT-ATT |
| Reverse | TCT-GGA-CCT-GTC-TTG-TCC-TTG-CTT | |
| IRF-7 | Forward | AGA-AGC-AGC-TGC-ACT-ACA-CAG-AGA |
| Reverse | TAC-CTC-CCA-GTA-CAC-CTT-GCA-CTT | |
| IFN-β | Forward | ATA-AGC-AGC-TCC-AGC-TCC-AAG |
| Reverse | GTC-TCA-TTC-CAC-CCA-GTG-CTG | |
| IFN-γ | Forward | AGC-GGC-TGA-CTG-AAC-TCA-GAT-TGT |
| Reverse | ACT-GCT-TTC-TTT-CAG-GGA-CAG-CCT | |
| GAPDH | Forward | TGT-GAT-GGG-TGT-GAA-CCA-CGA-GAA |
| Reverse | GAG-CCC-TTC-CAC-AAT-GCC-AAA-GTT |
Western analysis.
Macrophages were collected into Laemmli buffer and analyzed as previously described (23). The antibodies used were anti-IRF-1 (1:2,000), anti-β actin (1:8,000), and anti-IRF-7 (1:2,000; Novus Biologicals, Littleton, CO), anti-IRF-3 (1:1,000; Santa Cruz Biotechnology, Santa Cruz, CA), anti-Y701 phospho Stat1 (1:3,000; Millipore, Billerica, MA), anti-total Stat1 (1:3,000; Santa Cruz Biotechnology), anti-viperin (1:2,000; Abcam, Cambridge, MA), anti-γH2AX (1:2;000; Millipore), anti-histone H3 (1:10,000; Millipore), and a secondary goat anti-mouse or anti-rabbit horseradish peroxidase-conjugated secondary antibody (1:25,000; Jackson Immunoresearch, Westgrove, PA).
Irradiation.
Bone marrow derived macrophages (day 7 of in vitro culture) were gamma irradiated with indicated doses using Gammacell 40 (MDS Nordion, Ottawa, Ontario, Canada). Mock-treated macrophages were treated identically to the irradiated cultures with the exception of the irradiation.
Viral stock preparation.
MHV68 wild-type control and orf36 mutant virus stocks (16, 56) were prepared and titered on NIH 3T12 cells as previously described (36).
Irradiation of infected macrophages.
Bone marrow-derived macrophages (day 7 of in vitro culture) were infected with wild-type, N36S mutant, or 36KN mutant viruses at a multiplicity of infection (MOI) of 10 PFU/cell for 1 h at 37°C and 5% CO2 to allow for adsorption and washed twice with phosphate-buffered saline prior to replenishment of the medium. Macrophages received a single dose of gamma irradiation (5 Gy) or mock treatment at 8 or 28 h postinfection, and the total RNA was harvested 8 h postirradiation.
Virus spread assays.
Bone marrow-derived macrophages were irradiated and immediately infected at 0.01 PFU per cell as described above. Duplicate cultures were collected at 72 and 96 h postinfection and freeze-thawed once, and the virus titer was determined using NIH 3T12 cells.
Statistical analysis.
A Student t test (GraphPad Prism; GraphPad, La Jolla, CA) was used to measure the statistical significance with an α-value of 0.05.
RESULTS
DNA damage stimulates the transcription of ISGs in primary macrophages.
To determine whether DDR induction was associated with antiviral signaling, primary bone marrow-derived macrophages were exposed to a single dose of gamma irradiation, and the levels of several ISG transcripts were assessed. Although hundreds of proteins are expressed in response to type I IFN (14), very few of them have been proven to mediate antiviral effects. Thus, for the present study, only IFN-induced genes encoding effectors with defined antiviral functions within at least two different viral systems were chosen (2, 25, 41, 60). To control for nonspecific activation, mock-treated macrophages were subjected to the same manipulations as irradiated cultures with the exception of gamma irradiation. Irradiation, but not mock treatment, stimulated the expression of GBP-1, viperin, and Mx1, with maximum levels observed 6 to 8 h postirradiation (Fig. 1A to C). Interestingly, gamma irradiation failed to enhance RNase L expression (Fig. 1D), suggesting that only a subset of ISGs was induced by DNA damage. Mx1 and viperin induction by irradiation occurred in a dose-dependent manner (Fig. 1E and F). However, even the highest dose of irradiation used (10 Gy) failed to enhance the expression of RNase L (Fig. 1G). Thus, the irradiation of primary macrophages was associated with the transcriptional induction of antiviral genes.
Fig 1.
Irradiation stimulates transcription of IFN-stimulated genes (ISGs) in primary macrophages. Bone marrow-derived BL6 macrophages were mock treated or irradiated with a single 5-Gy dose (A to D) or the indicated doses (E to G) of gamma irradiation. RNA was harvested at the indicated time points, and the levels of individual mRNAs measured by quantitative reverse transcription-PCR (qRT-PCR) with subsequent normalization to GAPDH. For each independent experiment, the relative levels of ISG transcripts were set at 1 for mock-irradiated samples collected at 1 h postirradiation. The data were pooled from three independent experiments (two replicates within each experiment) and are presented as means with the standard errors of the mean (SEM). *, P < 0.05 compared to mock 8-h treatment.
Type I IFN response is required for DNA damage-induced ISG transcription.
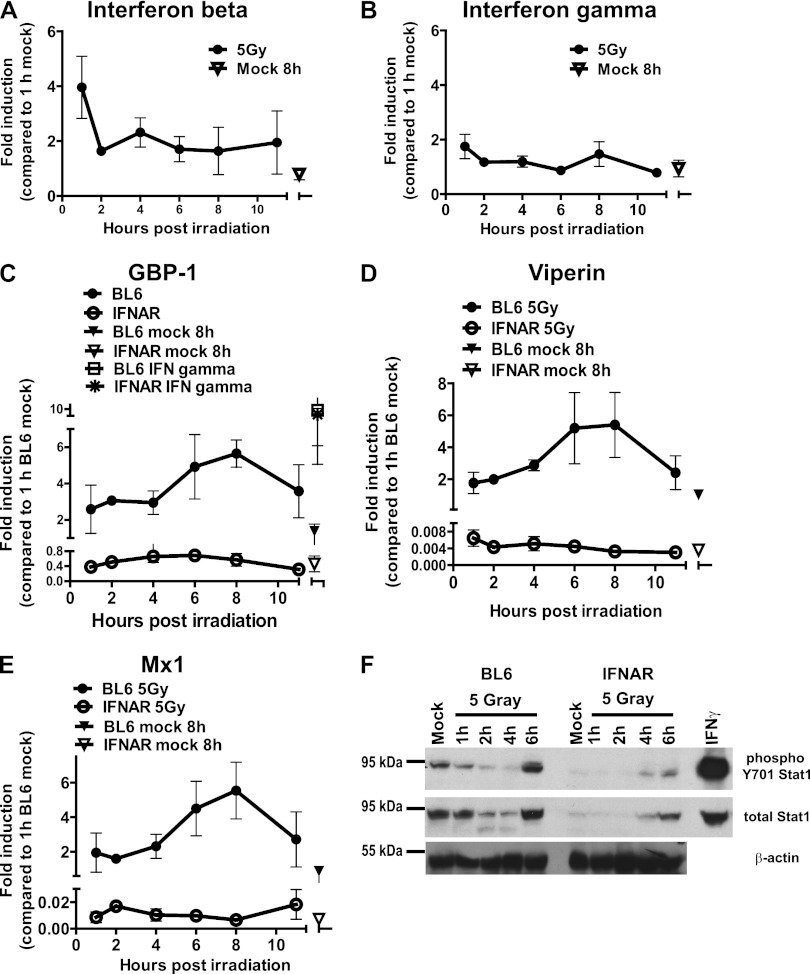
Because IFN signaling can induce ISG expression, the mRNA accumulation of type I and type II IFN transcripts was examined in irradiated macrophages. As early as 1 h postirradiation, there was a 4-fold increase in the accumulation of IFN-β mRNA (Fig. 2A). This response appeared to be selective for type I IFN, since irradiation failed to stimulate the accumulation of IFN-γ mRNA under all of the examined conditions (Fig. 2B).
Fig 2.
Type I IFN signaling is required for irradiation-induced ISG transcription. Bone marrow-derived BL6 or type I IFN receptor-deficient (IFNAR [C to F]) macrophages were treated as described in Fig. 1. Some macrophages were treated with 10 U of IFN-γ/ml for 2 h prior to measuring GBP-1 expression (C). (A to E) The levels of the indicated transcripts were measured by qRT-PCR. The relative levels of the indicated transcripts in wild-type mock-treated samples collected at 1 h postirradiation were set to 1 for each independent experiment. The data from three independent experiments (two replicates per experiment) were pooled and are presented as means with the SEM. (F) Protein levels of β-actin, total, and Y701-phosphorylated Stat1 were measured in BL6 or IFNAR macrophages subjected to the indicated treatments. The IFN-γ sample is a lysate of macrophages treated with 10 U of IFN-γ/ml for 2 h. The data are representative of two independent experiments.
At least 17 different cytokines, including IFN-β, constitute the type I IFN family (reviewed in reference 42). Importantly, the IFNAR1 subunit of the type I IFN receptor is required for signaling by all type I IFN family members. To determine whether type I IFN signaling was required for ISG induction by irradiation, ISG expression was examined in primary macrophages derived from wild-type (wt; BL6) or IFNAR1-deficient (IFNAR) mice (37). Although irradiation induced the accumulation of GBP-1, viperin, and Mx1 mRNA in wt cells, it failed to do so in IFNAR1-deficient irradiated macrophages (Fig. 2C to E). The absence of ISG induction in irradiated IFNAR1-deficient macrophages was not due to the inability of these cells to mediate IFN signaling, since the induction of GBP-1 transcript in response to IFN-γ treatment was similar in BL6 and IFNAR1-deficient macrophages. Thus, irradiation-stimulated ISG transcription relied on the ability of macrophages to respond to type I IFN.
IFN-β transcription was induced as early as 1 h postirradiation (Fig. 2A). However, peak ISG expression was not evident until 6 to 8 h postirradiation (Fig. 1). IFN stimulates the phosphorylation of Y701 of Stat1, resulting in Stat1 oligodimerization and subsequent transcription of ISGs (49). To determine whether DNA damage was associated with the activation of Stat1, Y701-phosphorylated and total Stat1 levels were measured by Western blot analysis in irradiated macrophages. Surprisingly, a significant decrease in both phosphorylated and total Stat1 levels was observed in BL6 macrophages between 1 and 4 h postirradiation (Fig. 2F). Importantly, Stat1 Y701 phosphorylation and total Stat1 levels increased between 4 and 6 h postirradiation, an observation consistent with the timing of ISG induction by irradiation. As expected, Stat1 levels (total and phosphorylated) were decreased in macrophages lacking type I IFN receptor compared to wt controls at 6 h postirradiation (Fig. 2F). Thus, Stat1 levels were dynamically regulated in irradiated cells, and the induction of antiviral ISGs by irradiation coincided with an increase in total and phosphorylated Stat1.
ISG induction by irradiation is dependent on the functional MRN complex and ATM expression.
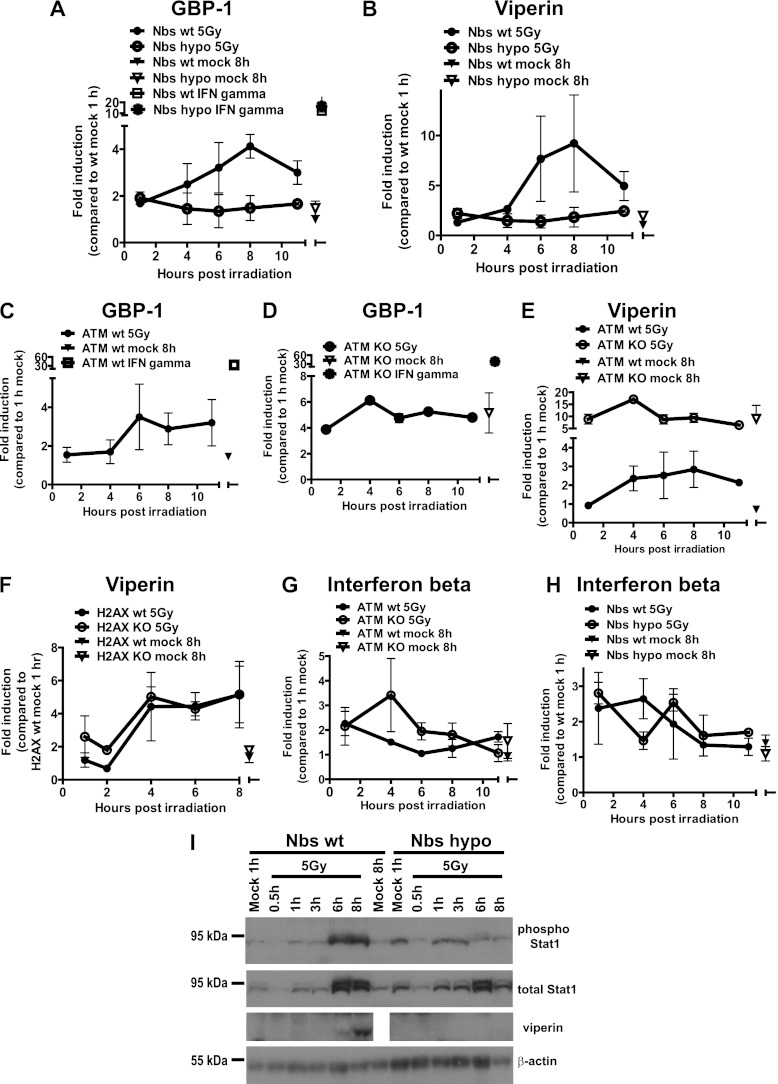
Having identified a requirement for type I IFN signaling in the induction of ISGs by irradiation, we examined the involvement of proximal DDR proteins in the regulation of ISG transcription. Macrophages were derived from ATM-deficient mice (4), mice homozygous for a hypomorphic Nbs1 allele (64), or wt littermates and subjected to mock or gamma irradiation. Irradiation failed to stimulate the accumulation of GBP-1, viperin, and Mx1 mRNA and viperin protein in macrophages lacking a fully functional MRN complex (Fig. 3A, B, and I; also, data not shown). However, the Nbs1 hypomorphic macrophages were responsive to IFN-γ, as evidenced by the enhanced expression of GBP-1 in IFN-γ-treated controls (Fig. 3A), suggesting that the absence of a functional MRN complex does not globally disrupt the IFN signaling network. Consistent with previous reports (50, 53), the steady-state levels of all three ISG transcripts were elevated in mock-treated ATM-deficient macrophages (Fig. 3C to E and data not shown). However, irradiation failed to further increase GBP-1, viperin, and Mx1 mRNA levels in ATM-deficient cells. The lack of ISG induction in irradiated ATM-deficient cells was not due to the altered responsiveness to IFN, since IFN-γ stimulated GBP-1 mRNA accumulation in ATM-deficient cells (Fig. 3D). Surprisingly, the induction of viperin mRNA by irradiation was normal in H2AX-deficient macrophages (Fig. 3F). Thus, ATM expression and a functional MRN complex, but not H2AX, were required for the induction of antiviral ISGs by irradiation.
Fig 3.
A functional MRN complex and ATM regulate irradiation-induced antiviral responses in primary macrophages. Nbs hypomorphic (A, B, H, and I), ATM-deficient (C to E and G), and H2AX-deficient (F) primary macrophages or macrophages derived from wt littermates were treated as described for Fig. 1 and 2, and the indicated mRNA transcripts (A to H) or proteins (I) were measured as described in Fig. 2. In panels A to H, the data were pooled from three independent experiments (two replicates per experiment) and are presented as means with the SEM. In panel I, the data are representative of two independent experiments.
The role of ATM and the MRN complex in the induction of type I IFN was examined following the irradiation of macrophages isolated from mice deficient in these targets. There were no defects in irradiation-stimulated IFN-β expression by macrophages deficient in ATM or lacking a functional MRN complex (Fig. 3G and H), suggesting that ATM and MRN may modify signaling downstream of type I IFN receptor, such as Stat1 phosphorylation, in irradiated cells. Similar to BL6 macrophages, Nbs wt cells responded to irradiation by upregulating levels of total and phosphorylated Stat1 between 3 and 6 h postirradiation (Fig. 3I). Importantly, in spite of the increase in total Stat1 by 6 h posttreatment, irradiation failed to enhance the phosphorylation of Stat1 above basal levels in Nbs1 hypomorphic macrophages, suggesting that signaling downstream of type I IFN receptor relied on a functional MRN complex in irradiated macrophages.
IRF-1 expression contributes to the induction of antiviral ISGs in irradiated macrophages.
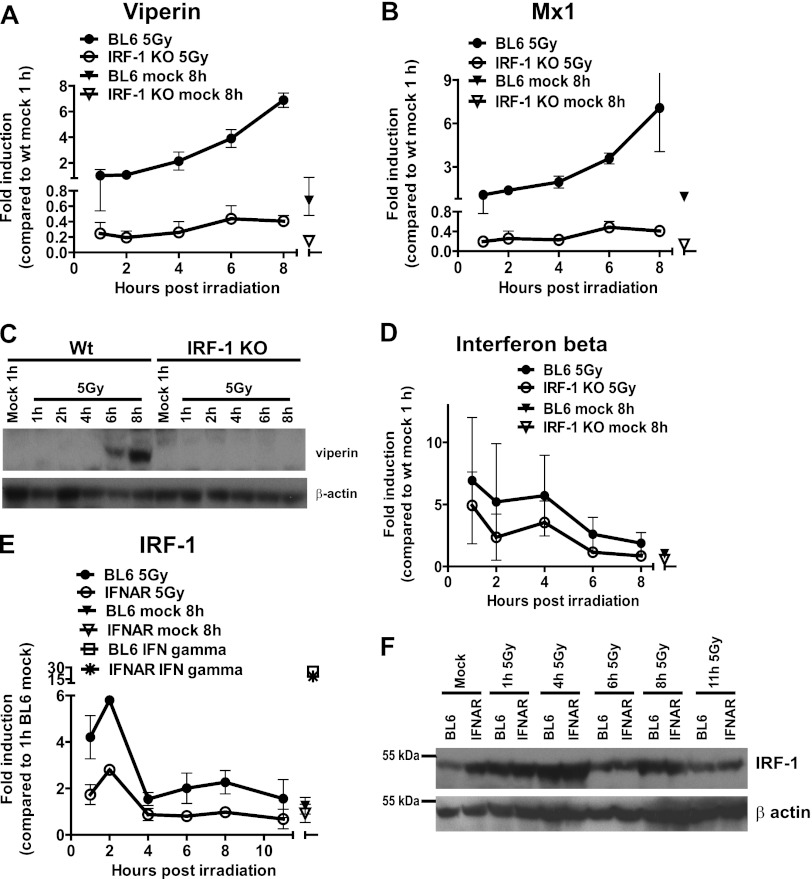
IFN regulatory factor 1 (IRF-1) is a transcriptional activator of the IFN-β promoter (44) and a tumor suppressor that mediates irradiation-induced apoptosis and cell cycle arrest (55). To determine the contribution of IRF-1 to the upregulation of ISGs in response to irradiation, the levels of antiviral ISG mRNA were measured in wild-type BL6 or IRF-1-deficient macrophages. Irradiation-stimulated viperin and Mx1 mRNA accumulation was attenuated in IRF-1-deficient macrophages (IRF-1 KO) compared to wt controls (Fig. 4A and B), and the viperin protein levels remained undetectable in IRF-1-deficient irradiated macrophages (Fig. 4C), suggesting that IRF-1 expression was required for the optimal induction of antiviral ISGs in irradiated macrophages.
Fig 4.
IRF-1 contributes to induction of antiviral ISGs in irradiated macrophages. (A, B, and D) BL6 and IRF-1-deficient (IRF-1 KO) bone marrow-derived macrophages were treated, and the levels of the indicated transcripts were measured as described for Fig. 2. (C) The viperin and β-actin protein levels were measured by Western analysis. The IRF-1 mRNA (E) and protein levels (F) were measured by qRT-PCR and Western analysis, respectively, in irradiated BL6 or IFNAR1-deficient macrophages. IRF-1 mRNA was also assessed in BL6 or IFNAR1-deficient macrophages treated with 10 U of IFN-γ/ml for 120 min (E). In panels A, B, D, and E, the data were pooled from three independent experiments (two replicates per experiment) and are presented as means with the SEM. In panels C and F, the data are representative of two independent experiments.
To determine whether the attenuation in ISG expression observed in IRF-1-deficient macrophages was due to inadequate IFN induction, the accumulation of IFN-β mRNA was measured in wt and IRF-1-deficient cultures following irradiation. The absence of IRF-1 did not modify irradiation-stimulated IFN-β expression (Fig. 4D), suggesting that IRF-1 acts downstream of type I IFN receptor to mediate the induction of antiviral ISGs in irradiated macrophages.
Irradiation, in an ATM-dependent manner, induces the expression and stabilization of IRF-1 in fibroblasts (40). Furthermore, transcription, but not protein stabilization, of IRF-1 is also induced by type I IFN (40). Therefore, the role of type I IFN in IRF-1 induction was evaluated in macrophages isolated from wt (BL6) or IFNAR1-deficient (IFNAR) mice. Irradiation induced the biphasic accumulation of IRF-1 mRNA in BL6 macrophages, with an initial peak of early accumulation 1 h postirradiation and a second, more modest mRNA induction at 6 to 8 h postirradiation (Fig. 4E). IRF-1 mRNA accumulation was only observed during the first 2 h postirradiation in IFNAR1-deficient macrophages, since the second phase of IRF-1 transcription was absent (Fig. 4E). In contrast to mRNA accumulation, irradiation stimulated IRF-1 protein accumulation to similar levels in BL6 and IFNAR1 macrophages, as determined by Western blot analysis (Fig. 4F), suggesting that the lack of irradiation-mediated ISG induction in IFNAR1-deficient macrophages (Fig. 2) was not due to the inability of these cells to stimulate IRF-1 expression in response to irradiation.
IRF-7 and IRF-3 expression is not required for the induction of ISGs in irradiated macrophages.
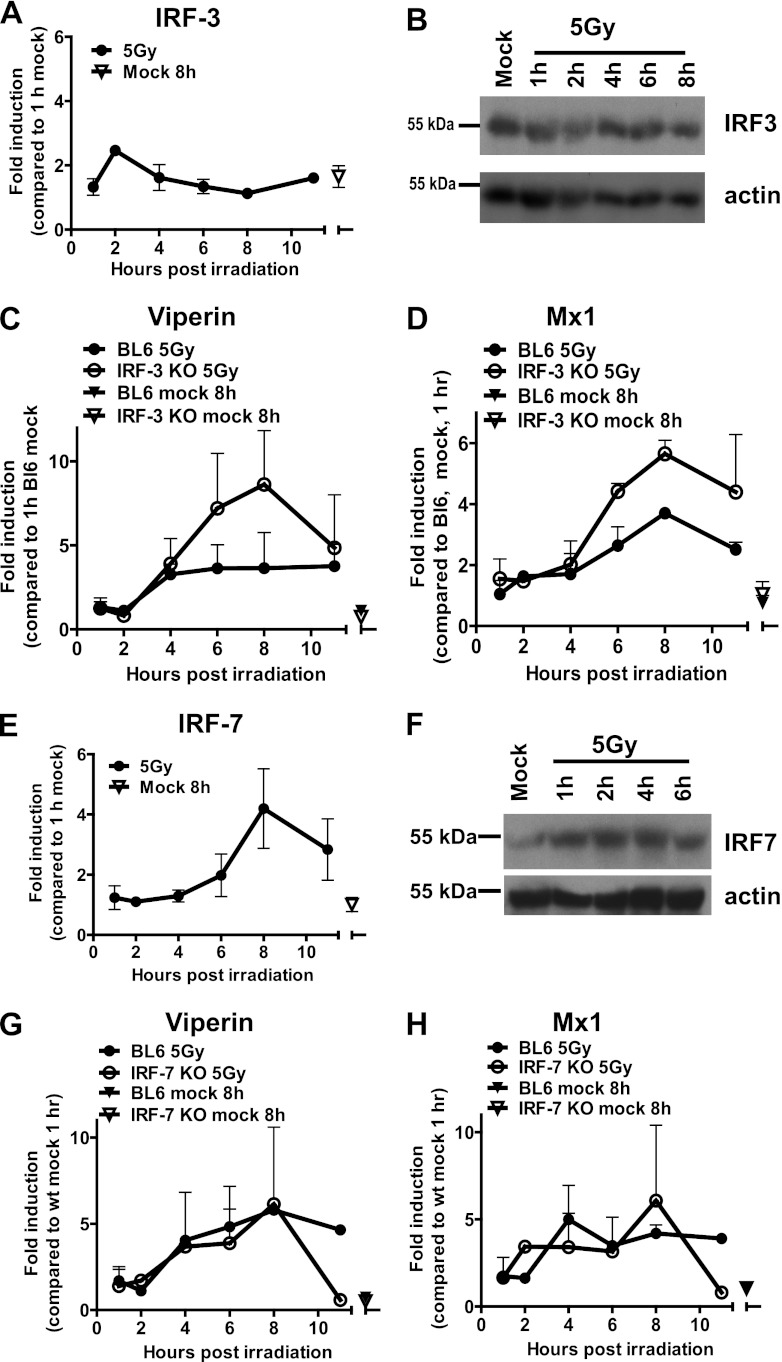
IRF-1, IRF-3, and IRF-7 are central to induction of IFN-α and IFN-β expression in virus-infected cells (44, 61, 66). Irradiation induces the phosphorylation and nuclear translocation of latent IRF-3 in HeLa cells (18); however, it is not clear whether IRF-3 induces IFN-β and ISG transcription in irradiated cells. IRF-3 transcription and protein levels were similar in mock-treated and irradiated macrophages at all of the time points examined (Fig. 5A and B). Furthermore, there was no reduction in the levels of viperin and Mx1 mRNA that accumulate in irradiated macrophages derived from IRF-3-deficient mice (Fig. 5C and D) (47) compared to the levels that accumulated in macrophages isolated from wt control mice. These findings indicate that IRF-3 was not necessary for the enhanced expression of antiviral ISGs in irradiated macrophages.
Fig 5.
IRF-7and IRF-3 expression is not required for the induction of ISGs in irradiated macrophages. (A and E) IRF-3 and IRF-7 mRNA levels were measured in BL6 macrophages treated as described for Fig. 1. (B and F) IRF-3 and IRF-7 protein levels were measured in mock-treated or irradiated BL6 macrophages at the indicated times postirradiation. Viperin and Mx1 mRNA levels were measured by qRT-PCR in BL6 and IRF-3 (C and D)- or IRF-7 (G and H)-deficient macrophages subjected to mock or gamma irradiation treatments. The relative levels of the indicated transcripts in wt mock-treated samples collected at 1 h postirradiation were set to 1 for each independent experiment. Transcript data were pooled from two to three independent experiments (two replicates per experiment) and are presented as means with the SEM. The results for the Western analyses are representative of three to four independent experiments.
Genotoxic agents induce the phosphorylation and nuclear translocation of IRF-7 in HeLa cells (19); however, the effect of these changes on the transcription of IRF-7-dependent genes is unknown. IRF-7 transcription was increased in irradiated macrophages between 6 and 8 h postirradiation, a finding consistent with the timing of ISG induction (Fig. 5E). Furthermore, the protein levels of IRF-7 were increased in response to irradiation, and this increase was evident prior to the transcriptional induction (Fig. 5F), suggesting that, similar to IRF-1, irradiation may increase IRF-7 protein stability. To determine whether IRF-7 was involved in the expression of ISGs in irradiated macrophages, ISG induction was measured in mock-treated or irradiated macrophages derived from wt or IRF-7-deficient (15) mice. Irradiation stimulated the accumulation of viperin and Mx1 mRNA to similar levels in macrophages isolated from wt and IRF-7-deficient mice at all of the time points examined (Fig. 5G and H). Thus, IRF-3 and IRF-7 expression was dispensable for irradiation-induced antiviral ISG expression.
Irradiation-mediated upregulation of antiviral ISG expression is blocked in MHV68-infected macrophages.
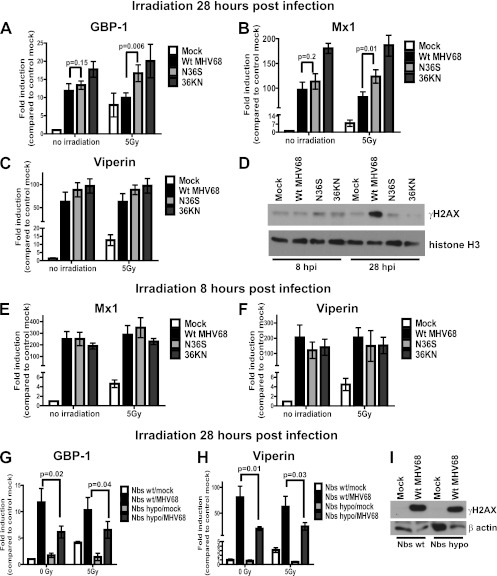
The results presented in Fig. 1 to 5 provide evidence for an unexpected connection between DDR and the type I IFN antiviral response in macrophages that includes the irradiation-induced expression of antiviral ISGs. One prediction from these results is that the connection between DDR and the type I IFN antiviral responses would be severed in virus-infected cells, especially in cases when virus infection is associated with induction of DDR. MHV68 system offers a unique opportunity to test such prediction, since this virus encodes a conserved protein kinase orf36 that induces DDR and suppresses IFN-β transcription (16, 56). Interestingly, unlike orf36-mediated DDR induction, the ability of orf36 to suppress IFN-β transcription appears to be independent of the orf36 enzymatic activity (16). Thus, the use of the two MHV68 orf36 mutants allows us to discriminate between the lack of DDR induction and no suppression of IFN-β transcription (N36S mutant, no orf36 expression) versus the lack of DDR induction and the control of IFN-β transcription (36KN mutant, enzymatically inactive orf36 expressed). To determine whether DDR induction was uncoupled from the induction of antiviral ISGs in MHV68-infected cells and whether the orf36 kinase played a role in this uncoupling, primary macrophages were mock infected, infected with wt MHV68, or infected with either of the two orf36 mutant viruses and then subjected to irradiation. The induction of ISGs in irradiated cells was measured at 8 h postirradiation, which is consistent with the highest levels of ISG induction in uninfected macrophages (Fig. 1 to 5).
Initially, ISG induction in response to irradiation was measured in macrophages that were mock treated or irradiated at 28 h postinfection, followed by the assessment of ISG mRNA levels 8 h after irradiation (36 h postinfection). At 28 h postinfection under high-MOI conditions (MOI = 10), viral DNA synthesis, the expression of all classes of viral genes, and orf36-dependent induction of γH2AX are evident in wt MHV68-infected macrophages (Fig. 6D) (36, 56). Baseline mRNA levels of Gbp-1, Mx1, and viperin were significantly elevated in infected cells compared to mock-infected controls, with similar levels of Gbp-1 and Mx1 observed in wt MHV68- or N36S-infected cells (Fig. 6A to C). Because N36S-infected macrophages are expected to have higher IFN-β transcription, these results failed to determine the degree to which orf36-induced DDR and IFN-β expression contribute to the transcriptional induction of ISGs in infected macrophages. In order to resolve this issue, ISG expression was measured in macrophages infected with the 36KN virus mutant. Surprisingly, ISG expression was not attenuated in macrophages infected with the 36KN mutant compared to wt MHV68 infections (Fig. 6A to C), suggesting that orf36-induced DDR is unlikely to contribute to the baseline induction of ISGs in infected macrophages at 36 h postinfection.
Fig 6.
Irradiation-mediated upregulation of antiviral ISG expression is blocked in MHV68-infected macrophages. BL6 macrophages were mock infected or infected with wt MHV68, a kinase-deficient virus mutant (N36S), or virus mutant expressing an enzymatically inactive orf36 (36KN) at 10 PFU/cell. The cells were irradiated at 28 (A to C) or 8 (E and F) h postinfection, RNA was harvested at 8 h postirradiation, and the expression of the ISGs was determined by qRT-PCR. The relative levels of the indicated transcripts in mock-infected mock-irradiated cells were set to 1 for each independent experiment. Transcript data were pooled from four to six independent experiments (two replicates per experiment) and are presented as means with the SEM. (D) The protein levels of γH2AX and histone H3 were measured by Western analysis in BL6 macrophages infected as indicated for 8 or 28 h. (G and H) Nbs1 wt or hypomorphic macrophages were mock infected or infected with wt MHV68 and irradiated at 28 h postinfection, the RNA was harvested at 8 h postirradiation, and the expression of Mx1 and viperin was assessed by qRT-PCR as for panel A. (I) The protein levels of γH2AX and β-actin were measured by Western analysis at 28 h postinfection.
As expected, irradiation of mock-infected macrophages at 28 h postinfection was associated with accumulation of all three ISG mRNA at 8 h posttreatment (Fig. 6A to C). Irradiation failed to increase ISG transcription in macrophages infected with wt MHV68 (Fig. 6A to C), suggesting that the connection between DDR and type I IFN signaling was uncoupled by MHV68. Importantly, while baseline levels of GBP-1 and Mx1 were similar in wt MHV68- and N36S-infected cells, the mRNA levels of these two ISGs were elevated in irradiated macrophages infected with the N36S virus compared to irradiated macrophages infected with wt virus (Fig. 6A and B). The difference in the levels of Mx1 and Gbp1 between irradiated and mock-treated cells failed to reach statistical significance for wt MHV68- or N36S-infected cultures, suggesting that multiple mechanisms, in addition to orf36, controlled irradiation-induced ISG expression in MHV68-infected macrophages. Consistent with this observation, orf36 expression did not differentially regulate viperin mRNA levels in irradiated macrophages (Fig. 6C). Furthermore, the levels of all three ISGs remained equally elevated in 36KN-infected macrophages upon irradiation. Thus, irradiation-induced ISG expression was blocked in MHV68-infected macrophages irradiated at 28 h postinfection.
To determine whether the ability to block irradiation-induced ISG upregulation was maintained during the early stages of MHV68 infection, macrophages were infected with wt or orf36 mutant viruses and subjected to irradiation at 8 h postinfection, prior to the orf36-mediated γH2AX induction (Fig. 6D), with subsequent measurement of ISG mRNA levels at 8 h posttreatment (16 h postinfection).
The baseline levels of Mx1 and viperin induction in wt MHV68-infected macrophages were higher at 16 h postinfection compared to 36 h postinfection (Fig. 6B and E and Fig. 6C and F, respectively), suggesting that MHV68 exercises a better control of baseline antiviral ISG expression later in infection. Furthermore, irradiation failed to further induce ISG transcription in wt MHV68-infected macrophages irradiated at 8 h postinfection (Fig. 6E and F), suggesting that the uncoupling of the DDR and type I IFN response was not specific to late stages of infection but instead occurred throughout the replication cycle of MHV68. Alternatively, high levels of ISG expression observed at 8 h postinfection could have blunted further induction of ISGs in response to irradiation. Unlike that observed at 28 h postinfection, orf36 expression had no role in the control of Mx1 mRNA induction in irradiated infected macrophages (Fig. 6B and E), providing further support to the conclusion that MHV68 uses multiple mechanisms to uncouple the connection between DDR and type I IFN signaling.
Macrophages lacking a functional MRN complex (Nbs1 hypomorphic) failed to induce ISG expression in response to irradiation (Fig. 3A, B, and I). To determine the contribution of a functional MRN complex to induction of ISG expression in MHV68-infected macrophages, macrophages isolated from Nbs1 hypomorphic or wt littermates were mock infected or infected with wt MHV68, irradiated at 28 h postinfection, with subsequent assessment of the ISG mRNA levels. As expected, the baseline Mx1 and viperin levels were elevated in MHV68-infected Nbs1 wt macrophages compared to mock-infected controls (Fig. 6G and H). Furthermore, irradiation failed to induce ISG transcription in Nbs1 hypomorphic mock-infected macrophages or in macrophages of either genotype infected with MHV68. Interestingly, the baseline levels of Mx1 and viperin mRNA were lower in MHV68-infected Nbs1 hypomorphic macrophages compared to Nbs1 wt MHV68-infected controls (Fig. 6G and H), in spite of similar γH2AX levels observed under both conditions at 28 h postinfection (Fig. 6I). Thus, a functional MRN complex contributed to the baseline expression of antiviral ISGs in MHV68-infected macrophages independent of MHV68-induced γH2AX.
Irradiation confers an antiviral effect that attenuates the replication of MHV68 in primary macrophages.
Another prediction based on the integration of DDR and type I IFN signaling is that irradiation could confer an antiviral state that would limit virus replication. To test this prediction, primary macrophages were irradiated or mock treated and subsequently infected with MHV68 at a low MOI. The ability of MHV68 to spread throughout the macrophage cultures was assessed by measuring the viral yields at 72 and 96 h postinfection. MHV68 spread to uninfected cells was attenuated in irradiated cultures, as evidenced by decreased virus titers at 72 and 96 h postinfection (Fig. 7A). This attenuation in viral spread occurred in spite of the ability of MHV68 to suppress irradiation-induced ISG transcription (Fig. 6). Type I IFN contributed to the resistance of irradiated macrophages to MHV68 infection, since differences in MHV68 titers between irradiated and control IFNAR1-deficient macrophages were less than those observed in BL6 macrophages and did not reach statistical significance (Fig. 7A). Importantly, although not statistically significant, there was a trend toward a decrease in MHV68 titers in irradiated IFNAR1-deficient macrophages, suggesting that additional signaling pathways activated by irradiation may contribute to the antiviral state of irradiated macrophages. It was difficult to determine whether irradiation restricted the replication of orf36 MHV68 mutant viruses (Fig. 7B) due to the attenuated replication of these virus mutants in primary macrophages (56). Decreased MHV68 replication in irradiated macrophages was not due to increased irradiation-induced cell death, since the macrophages are resistant to irradiation-induced apoptosis (30), and irradiated IFNAR1-deficient macrophages could support adequate MHV68 replication (Fig. 7B). Thus, irradiation increased the resistance of primary macrophages to MHV68 infection, and this resistance was in part mediated by type I IFN.
Fig 7.
Irradiation confers an antiviral state that attenuates MHV68 replication in primary macrophages. BL6 (A and B) and IFNAR (A) macrophages were irradiated and immediately infected with wt MHV68 (A and B) and N36S or 36KN virus mutants (B) at 0.01 PFU/cell. The virus yield was measured at 72 and 96 h postinfection. The data were pooled from four independent experiments and are shown as means with the SEM. Within each experiment the wt MHV68 titer measured in a single replicate of control BL6 macrophages at 72 h postinfection was set to 1, with subsequent normalization of all data obtained within the same experiment. (C) Working model. An unknown sensor leads to transcription of type I IFN following induction of double-stranded DNA breaks in primary macrophages. Subsequently, type I IFN initiates antiviral signaling that is further modified by select DDR components, such as the MRN complex and ATM. In addition, type I IFN signaling and DDR-induced IRF-1 cooperate to upregulate the transcription of antiviral ISGs in irradiated cells.
DISCUSSION
In this study we show that specific induction of DNA damage by gamma irradiation was associated with activation of type I IFN signaling that was required for enhanced antiviral gene expression. Type I IFN signaling in irradiated cells was modified by select DDR proteins, including the DNA damage sensor complex and ATM, a central DDR regulatory kinase. Furthermore, IRF-1, but not IRF-3 and IRF-7, was required for the optimal expression of antiviral ISGs examined here, further differentiating innate immune signaling in irradiated cells from the classical signaling induced by pattern recognition receptors. Importantly, the type I IFN responses induced by DDR were actively blocked in the context of MHV68 infection and conferred an antiviral state that attenuated viral replication. Thus, induction of type I IFN signaling in the context of DNA damage, as shown here, extends the DDR signaling landscape to include innate immune responses.
In spite of many previous publications investigating gene expression changes in response to irradiation, the direct connection between DDR and type I IFN has not been appreciated, since a majority of gene expression studies were conducted in transformed cell lines with altered DDR and IFN responses and/or were limited to well-established DDR effects, such as apoptosis and cell cycle regulation. Several studies have examined changes in gene expression in response to irradiation in vivo, with the most recent study reporting differential gene expression associated with immune activation (11). However, it still remains to be determined whether immune activation in vivo is simply a response to tissue damage induced by irradiation. Irradiation was also shown to stimulate the expression of ligands for NKG2D, an activating NK receptor; however, the involvement of immune signaling pathways in NKG2D ligand expression has not been defined (12). In a recent study published during the preparation of this manuscript, the Reich group had shown induction of IFN-α and -λ, but not IFN-β, in primary human monocytes treated with etoposide (8). The differences in the form of type I IFN induction observed between our study and the work of Brzostek-Racine et al., are likely to stem from different experimental approaches (immediate versus delayed responses and a single dose of gamma irradiation versus 24 h of etoposide treatment) and different sources of tissue (human versus mouse).
Based on the studies described here, we propose the following working model of the cross talk between DDR and type I IFN responses (Fig. 7C). DNA damage activates transcription of type I IFN. In irradiated primary macrophages, type I IFN signaling downstream of IFN receptor intersects with select DDR components, such as the MRN complex and ATM, and these interactions contribute to regulation of Stat1 phosphorylation. In addition, type I IFN signaling and IRF-1 cooperate to induce the transcription of antiviral ISGs in irradiated cells. One prediction of this model is that viruses uncouple DDR induction from type I IFN activation, a prediction corroborated by the results of our study (Fig. 6). While gamma irradiation is unlikely to be present during viral infection in vivo, low levels of DNA damage may be induced in both infected and bystander cells via the generation of reactive oxygen species and other oxidative moieties released in the context of inflammation. Thus, induction of type I IFN by DDR may reflect an innate immune host defense system that has evolved to confer a protective, antiviral state in vivo that attenuates viral spread prior to the generation of adaptive immune responses. To support this hypothesis, MHV68 spread was attenuated in irradiated macrophage cultures, and this attenuation was in part mediated by type I IFN signaling (Fig. 7A).
The plethora of virus interactions with host DDR has been recently appraised in a number of excellent reviews (28, 62). Importantly, several viruses directly induce DDR and subsequently usurp DDR components for efficient viral replication. MHV68-encoded viral kinase initiates DDR in infected macrophages by mediating serine 139 phosphorylation of H2AX, including at the core promoter of gene50, an immediate-early gene encoding a key viral regulator of the lytic replication cycle (35, 56). H2AX and ATM are subsequently usurped by MHV68 to facilitate viral gene expression and replication (35, 56). Furthermore, several herpesvirus kinases target Tip60, an important activator of ATM, to stimulate viral gene expression and replication (24). Active induction of DDR is not limited to the herpesvirus family, since human papillomavirus E1- and simian virus 40 large T antigen-induced DDR is commandeered for viral replication (13, 34, 45, 48). Viruses that actively induce DDR are most likely to use several mechanisms to uncouple the connection between DDR and type I IFN signaling. Indeed, the induction of ISGs by irradiation was blocked in MHV68-infected macrophages, likely by using multiple mechanisms, including the expression of orf36 viral kinase (Fig. 7A and B). Intriguingly, our findings may also explain why certain DDR components are inhibited in virus-infected cells. The MRN complex is targeted for degradation or is relocalized to viral replication compartments in adenovirus and Epstein-Barr virus infection, respectively. However, downstream DDR events, such as γH2AX, are induced in infected cells (21, 38, 52). It is tempting to speculate that these viruses target the MRN complex to attenuate its role in activation of ISG transcription. Indeed, the transcription of GBP-1 and viperin was attenuated in MHV68-infected Nbs1 hypomorphic macrophages (Fig. 6G and H). Other DDR components inhibited in the course of viral infection may also participate in the DDR-immune signaling cross talk. Restoring the DDR type I IFN cross talk in infected cells may constitute a potent antiviral therapy approach, especially for viruses that actively induce DDR.
In addition to its antiviral nature, the induction of type I IFN signaling in the context of DDR may also participate in cancer development and treatment. DDR is activated in a majority of premalignant lesions (5), and the induction of type I IFN signaling in this context may contribute to the DDR tumor-suppressive effects. Type I IFN has potent antitumor activity, can facilitate the recruitment of immune cells, and is already in wide use as an adjuvant therapy for melanoma, renal cell carcinoma, and lymphoma (58). Type I IFN is also induced in a mouse model of local radiotherapy, and this induction facilitates the recruitment and activation of immune cells (9). Thus, harnessing the interaction between a tumor suppressor and immune signaling pathway could improve cancer therapy and prevention approaches.
Because of the novel nature of the DDR-type I IFN signaling cross talk, many questions remain to be addressed in future studies, including the mechanism of type I IFN induction by DDR. The Reich group reported an attenuated IFN-λ and -α transcription in IRF-7-deficient mouse embryonic fibroblasts in response to long-term etoposide treatment (8). In contrast, we show that the induction of viperin and Mx1 is not attenuated in IRF-7-deficient irradiated macrophages (Fig. 5G and H), suggesting that several IRF factors may collaborate to induce type I IFN-dependent transcriptional responses in irradiated cells. It is also possible that DNA fragments generated by irradiation are recognized by novel DNA sensor proteins with subsequent activation of type I IFN transcription. Recently, IFN-γ-inducible protein 16 (IFI16) was proposed to be a sensor of Kaposi's sarcoma-associated herpesvirus genome in infected nuclei (17). It is tempting to speculate that IFI16 or a similar nuclear sensor may be involved in sensing large fragments of DNA or DDR-associated changes in chromatin structure (67) to mediate the induction of type I IFN transcription. These sensors are likely to be distinct from the classical sensors of double-stranded DNA breaks, since in our studies IFN-β was induced in cells deficient in ATM or a functional MRN complex (Fig. 3F and G).
We observed an immediate decrease in Stat1 levels and phosphorylation in response to irradiation, with subsequent upregulation of Stat1 phosphoprotein and total protein levels in irradiated cells. This observation was unexpected since Stat1 is a long-lived protein (51). The decrease in Stat1 protein levels and phosphorylation following irradiation likely contribute to the delayed induction of ISGs in irradiated macrophages (6 to 8 h postirradiation) in spite of increased IFN-β transcription evident as early as 1 h postirradiation (Fig. 2A). An important question to be resolved in future studies is the mechanism by which Stat1 levels and phosphorylation are regulated in irradiated cells and the physiological relevance of such regulation.
It is also likely that other immune signaling pathways are activated by DNA damage. The immune signaling landscape evoked by DNA damage may be further modified by cell type and differentiation stage, since DDR signaling differs in actively proliferating and terminally differentiated cells (39) and type II IFN signaling evokes differential gene expression in macrophages and mouse embryonic fibroblasts (43). An unbiased identification of the immune signaling landscape in the context of virus-induced and physiological DDR is an important future undertaking.
ACKNOWLEDGMENTS
We thank Bryon Johnson for his advice and suggestions throughout this study. We are grateful to John Petrini, Fred Alt, Mitchell Grayson, and Mike Diamond for their generous gifts of mouse strains.
This study was funded by the Concern Foundation, the Medical College of Wisconsin Cancer Center, and Advancing Healthier Wisconsin (V.L.T.) and by National Institute of Allergy and Infectious Diseases grant AI44458 (J.A.C.).
Footnotes
Published ahead of print 11 April 2012
REFERENCES
- 1. Adeyemi RO, Landry S, Davis ME, Weitzman MD, Pintel DJ. 2010. Parvovirus minute virus of mice induces a DNA damage response that facilitates viral replication. PLoS Pathog. 6:e1001141 doi:10.1371/journal.ppat.1001141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Anderson SL, Carton JM, Lou J, Xing L, Rubin BY. 1999. Interferon-induced guanylate binding protein-1 (GBP-1) mediates an antiviral effect against vesicular stomatitis virus and encephalomyocarditis virus. Virol. 256:8–14 [DOI] [PubMed] [Google Scholar]
- 3. Bailey SG, et al. 2009. Functional interaction between Epstein-Barr virus replication protein Zta and host DNA damage response protein 53BP1. J. Virol. 83:11116–11122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Barlow C, et al. 1996. ATM-deficient mice: a paradigm of ataxia telangiectasia. Cell 86:159–171 [DOI] [PubMed] [Google Scholar]
- 5. Bartkova J, et al. 2005. DNA damage response as a candidate anti-cancer barrier in early human tumorigenesis. Nature 434:864–870 [DOI] [PubMed] [Google Scholar]
- 6. Bassing CH, et al. 2002. Increased ionizing radiation sensitivity and genomic instability in the absence of histone H2AX. Proc. Natl. Acad. Sci. U. S. A. 99:8173–8178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Bassing CH, et al. 2003. Histone H2AX: a dosage-dependent suppressor of oncogenic translocations and tumors. Cell 114:359–370 [DOI] [PubMed] [Google Scholar]
- 8. Brzostek-Racine S, Gordon C, Van Scoy S, Reich NC. 2011. The DNA damage response induces IFN. J. Immunol. 187:5336–5345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Burnette BC, et al. 2011. The efficacy of radiotherapy relies upon induction of type I interferon-dependent innate and adaptive immunity. Cancer Res. 71:2488–2496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Celeste A, et al. 2002. Genomic instability in mice lacking histone H2AX. Science 296:922–927 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Chang CT, et al. 2011. Comprehensive assessment of host responses to ionizing radiation by nuclear factor-κB bioluminescence imaging-guided transcriptomic analysis. PLoS One 6:e23682 doi:10.1371/journal.pone.0023682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Gasser S, Orsulic S, Brown EJ, Raulet DH. 2005. The DNA damage pathway regulates innate immune system ligands of the NKG2D receptor. Nature 436:1186–1190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Hein J, et al. 2009. Simian virus 40 large T antigen disrupts genome integrity and activates a DNA damage response via Bub1 binding. J. Virol. 83:117–127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Hertzog P, Forster S, Samarajiwa S. 2011. Systems biology of interferon responses. J. Interferon Cytokine Res. 31:5–11 [DOI] [PubMed] [Google Scholar]
- 15. Honda K, et al. 2005. IRF-7 is the master regulator of type-I interferon-dependent immune responses. Nature 434:772–777 [DOI] [PubMed] [Google Scholar]
- 16. Hwang S, et al. 2009. Conserved herpesviral kinase promotes viral persistence by inhibiting the IRF-3-mediated type I interferon response. Cell Host. Microbe 5:166–178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kerur N, et al. 2011. IFI16 acts as a nuclear pathogen sensor to induce the inflammasome in response to Kaposi sarcoma-associated herpesvirus infection. Cell Host. Microbe 9:363–375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kim T, et al. 1999. Activation of interferon regulatory factor 3 in response to DNA-damaging agents. J. Biol. Chem. 274:30686–30689 [DOI] [PubMed] [Google Scholar]
- 19. Kim TK, Kim T, Kim TY, Lee WG, Yim J. 2000. Chemotherapeutic DNA-damaging drugs activate interferon regulatory factor-7 by the mitogen-activated protein kinase kinase-4-cJun NH2-terminal kinase pathway. Cancer Res. 60:1153–1156 [PubMed] [Google Scholar]
- 20. Kimura T, et al. 1994. Involvement of the IRF-1 transcription factor in antiviral responses to interferons. Science 264:1921–1924 [DOI] [PubMed] [Google Scholar]
- 21. Kudoh A, et al. 2005. Epstein-Barr virus lytic replication elicits ATM checkpoint signal transduction while providing an S-phase-like cellular environment. J. Biol. Chem. 280:8156–8163 [DOI] [PubMed] [Google Scholar]
- 22. Kudoh A, et al. 2009. Homologous recombinational repair factors are recruited and loaded onto the viral DNA genome in Epstein-Barr virus replication compartments. J. Virol. 83:6641–6651 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Lenschow DJ, et al. 2005. Identification of interferon-stimulated gene 15 as an antiviral molecule during Sindbis virus infection in vivo. J. Virol. 79:13974–13983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Li R, et al. 2011. Conserved herpesvirus kinases target the DNA damage response pathway and TIP60 histone acetyltransferase to promote virus replication. Cell Host Microbe 10:390–400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Li XL, Blackford JA, Hassel BA. 1998. RNase L mediates the antiviral effect of interferon through a selective reduction in viral RNA during encephalomyocarditis virus infection. J. Virol. 72:2752–2759 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Lilley CE, Chaurushiya MS, Boutell C, Everett RD, Weitzman MD. 2011. The intrinsic antiviral defense to incoming HSV-1 genomes includes specific DNA repair proteins and is counteracted by the viral protein ICP0. PLoS Pathog. 7:e1002084 doi:10.1371/journal.ppat.1002084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Lilley CE, et al. 2010. A viral E3 ligase targets RNF8 and RNF168 to control histone ubiquitination and DNA damage responses. EMBO J. 29:943–955 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Lilley CE, Chaurushiya MS, Weitzman MD. 2009. Chromatin at the intersection of viral infection and DNA damage. Biochim. Biophys. Acta. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Lilley CE, Schwartz RA, Weitzman MD. 2007. Using or abusing: viruses and the cellular DNA damage response. Trends Microbiol. 15:119–126 [DOI] [PubMed] [Google Scholar]
- 30. Lin HS, Hsu S. 1989. Biochemical mechanisms underlying the development of radioresistance by cultured peritoneal exudate macrophages. Radiat. Res. 117:70–78 [PubMed] [Google Scholar]
- 31. Lund J, Sato A, Akira S, Medzhitov R, Iwasaki A. 2003. Toll-like receptor 9-mediated recognition of Herpes simplex virus-2 by plasmacytoid dendritic cells. J. Exp. Med. 198:513–520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Lund JM, et al. 2004. Recognition of single-stranded RNA viruses by Toll-like receptor 7. Proc. Natl. Acad. Sci. U. S. A. 101:5598–5603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Matsuyama T, et al. 1993. Targeted disruption of IRF-1 or IRF-2 results in abnormal type I IFN gene induction and aberrant lymphocyte development. Cell 75:83–97 [PubMed] [Google Scholar]
- 34. Moody CA, Laimins LA. 2009. Human papillomaviruses activate the ATM DNA damage pathway for viral genome amplification upon differentiation. PLoS Pathog. 5:e1000605 doi:10.1371/journal.ppat.1000605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Mounce BC, et al. 2011. Gammaherpesvirus gene expression and DNA synthesis are facilitated by viral protein kinase and histone variant H2AX. Virol. 420:73–81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Mounce BC, Tsan FC, Kohler S, Cirillo LA, Tarakanova VL. 2011. Dynamic association of gammaherpesvirus DNA with core histone during de novo lytic infection of primary cells. Virol. 421:167–172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Muller U, et al. 1994. Functional role of type I and type II interferons in antiviral defense. Science 264:1918–1921 [DOI] [PubMed] [Google Scholar]
- 38. Nichols GJ, Schaack J, Ornelles DA. 2009. Widespread phosphorylation of histone H2AX by species C adenovirus infection requires viral DNA replication. J. Virol. 83:5987–5998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Nouspikel T. 2007. DNA repair in differentiated cells: some new answers to old questions. Neuroscience 145:1213–1221 [DOI] [PubMed] [Google Scholar]
- 40. Pamment J, Ramsay E, Kelleher M, Dornan D, Ball KL. 2002. Regulation of the IRF-1 tumour modifier during the response to genotoxic stress involves an ATM-dependent signaling pathway. Oncogene 21:7776–7785 [DOI] [PubMed] [Google Scholar]
- 41. Pavlovic J, Zurcher T, Haller O, Staeheli P. 1990. Resistance to influenza virus and vesicular stomatitis virus conferred by expression of human MxA protein. J. Virol. 64:3370–3375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Platanias LC. 2005. Mechanisms of type-I- and type-II-interferon-mediated signaling. Nat. Rev. Immunol. 5:375–386 [DOI] [PubMed] [Google Scholar]
- 43. Presti RM, Popkin DL, Connick M, Paetzold S, Virgin HW. 2001. Novel cell type-specific antiviral mechanism of interferon gamma action in macrophages. J. Exp. Med. 193:483–496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Reis LF, Harada H, Wolchok JD, Taniguchi T, Vilcek J. 1992. Critical role of a common transcription factor, IRF-1, in the regulation of IFN-β and IFN-inducible genes. EMBO J. 11:185–193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Sakakibara N, Mitra R, McBride AA. 2011. The papillomavirus E1 helicase activates a cellular DNA damage response in viral replication foci. J. Virol. 85:8981–8995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Samanta M, Iwakiri D, Kanda T, Imaizumi T, Takada K. 2006. EB virus-encoded RNAs are recognized by RIG-I and activate signaling to induce type I IFN. EMBO J. 25:4207–4214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Sato M, et al. 2000. Distinct and essential roles of transcription factors IRF-3 and IRF-7 in response to viruses for IFN-alpha/beta gene induction. Immunity 13:539–548 [DOI] [PubMed] [Google Scholar]
- 48. Shi Y, Dodson GE, Shaikh S, Rundell K, Tibbetts RS. 2005. Ataxia-telangiectasia-mutated (ATM) is a T-antigen kinase that controls SV40 viral replication in vivo. J. Biol. Chem. 280:40195–40200 [DOI] [PubMed] [Google Scholar]
- 49. Shuai K, Stark GR, Kerr IM, Darnell JE., Jr 1993. A single phosphotyrosine residue of Stat91 required for gene activation by interferon-gamma. Science 261:1744–1746 [DOI] [PubMed] [Google Scholar]
- 50. Siddoo-Atwal C, Haas AL, Rosin MP. 1996. Elevation of interferon beta-inducible proteins in ataxia telangiectasia cells. Cancer Res. 56:443–447 [PubMed] [Google Scholar]
- 51. Siewert E, Muller-Esterl W, Starr R, Heinrich PC, Schaper F. 1999. Different protein turnover of interleukin-6-type cytokine signaling components. Eur. J. Biochem. 265:251–257 [DOI] [PubMed] [Google Scholar]
- 52. Stracker TH, Carson CT, Weitzman MD. 2002. Adenovirus oncoproteins inactivate the Mre11-Rad50-NBS1 DNA repair complex. Nature 418:348–352 [DOI] [PubMed] [Google Scholar]
- 53. Sugihara T, Murano H, Nakamura M, Ichinohe K, Tanaka K. 2011. Activation of interferon-stimulated genes by gamma-ray irradiation independently of the ataxia telangiectasia mutated-p53 pathway. Mol. Cancer Res. 9:476–484 [DOI] [PubMed] [Google Scholar]
- 54. Sun S, Zhang X, Tough DF, Sprent J. 1998. Type I interferon-mediated stimulation of T cells by CpG DNA. J. Exp. Med. 188:2335–2342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Tanaka N, et al. 1996. Cooperation of the tumour suppressors IRF-1 and p53 in response to DNA damage. Nature 382:816–818 [DOI] [PubMed] [Google Scholar]
- 56. Tarakanova VL, et al. 2007. Gamma-herpesvirus kinase actively initiates a DNA damage response by inducing phosphorylation of H2AX to foster viral replication. Cell Host Microbe 1:275–286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Tarakanova VL, Molleston JM, Goodwin M, Virgin HW., IV 2010. MHV68 complement regulatory protein facilitates MHV68 replication in primary macrophages in a complement-independent manner. Virology 405:50–61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Wang BX, Rahbar R, Fish EN. 2011. Interferon: current status and future prospects in cancer therapy. J. Interferon Cytokine Res. 31:545–552 [DOI] [PubMed] [Google Scholar]
- 59. Wang JT, et al. 2009. Epstein-Barr virus BGLF4 kinase suppresses the interferon regulatory factor 3 signaling pathway. J. Virol. 83:1856–1869 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Wang X, Hinson ER, Cresswell P. 2007. The interferon-inducible protein viperin inhibits influenza virus release by perturbing lipid rafts. Cell Host Microbe 2:96–105 [DOI] [PubMed] [Google Scholar]
- 61. Wathelet MG, et al. 1998. Virus infection induces the assembly of coordinately activated transcription factors on the IFN-β enhancer in vivo. Mol. Cell 1:507–518 [DOI] [PubMed] [Google Scholar]
- 62. Weitzman MD, Lilley CE, Chaurushiya MS. 2010. Genomes in conflict: maintaining genome integrity during virus infection. Annu. Rev. Microbiol. 64:61–81 [DOI] [PubMed] [Google Scholar]
- 63. Wilkinson DE, Weller SK. 2004. Recruitment of cellular recombination and repair proteins to sites of herpes simplex virus type 1 DNA replication is dependent on the composition of viral proteins within prereplicative sites and correlates with the induction of the DNA damage response. J. Virol. 78:4783–4796 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Williams BR, et al. 2002. A murine model of Nijmegen breakage syndrome. Curr. Biol. 12:648–653 [DOI] [PubMed] [Google Scholar]
- 65. Williams GJ, Lees-Miller SP, Tainer JA. 2010. Mre11-Rad50-Nbs1 conformations and the control of sensing, signaling, and effector responses at DNA double-strand breaks. DNA Repair (Amst.) 9:1299–1306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Yoneyama M, et al. 1998. Direct triggering of the type I interferon system by virus infection: activation of a transcription factor complex containing IRF-3 and CBP/p300. EMBO J. 17:1087–1095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Ziv Y, et al. 2006. Chromatin relaxation in response to DNA double-strand breaks is modulated by a novel ATM- and KAP-1-dependent pathway. Nat. Cell Biol. 8:870–876 [DOI] [PubMed] [Google Scholar]