Abstract
Vaccinia virus has a broad range of infectivity in many cell lines and animals. Although it is known that the vaccinia mature virus binds to cell surface glycosaminoglycans and extracellular matrix proteins, whether additional cellular receptors are required for virus entry remains unclear. Our previous studies showed that the vaccinia mature virus enters through lipid rafts, suggesting the involvement of raft-associated cellular proteins. Here we demonstrate that one lipid raft-associated protein, integrin β1, is important for vaccinia mature virus entry into HeLa cells. Vaccinia virus associates with integrin β1 in lipid rafts on the cell surface, and the knockdown of integrin β1 in HeLa cells reduces vaccinia mature virus entry. Additionally, vaccinia mature virus infection is reduced in a mouse cell line, GD25, that is deficient in integrin β1 expression. Vaccinia mature virus infection triggers the activation of phosphatidylinositol 3-kinase (PI3K)/Akt signaling, and the treatment of cells with inhibitors to block P13K activation reduces virus entry in an integrin β1-dependent manner, suggesting that integrin β1-mediates PI3K/Akt activation induced by vaccinia virus and that this signaling pathway is essential for virus endocytosis. The inhibition of integrin β1-mediated cell adhesion results in a reduction of vaccinia virus entry and the disruption of focal adhesion and PI3K/Akt activation. In summary, our results show that the binding of vaccinia mature virus to cells mimics the outside-in activation process of integrin functions to facilitate vaccinia virus entry into HeLa cells.
INTRODUCTION
Vaccinia virus is the prototype of the orthopoxvirus genus of the family Poxviridae. It has a broad host range, producing multiple infectious forms of virus particles, e.g., mature virus (MV), wrapped virus (WV), and extracellular virus (EV) (14). Vaccinia MV particles are produced in large quantities in infected cells and contain ∼80 viral proteins in viral particles (10, 51, 69). The large number of viral proteins in the MV composition contributes to its complex viral entry processes. First, vaccinia MV binds to multiple cell surface components, such as glycosaminoglycans (11, 29, 42), extracellular matrix laminin (9), and sulfatides (49). Furthermore, vaccinia MV contains an entry fusion complex (EFC) of 12 viral envelope proteins that are evolutionarily conserved and essential for membrane fusion (6, 7, 33, 47, 52, 56, 68). Additionally, different strains of vaccinia MV harboring mutations in the viral envelope proteins A25 and A26 enter cells differently (8). Finally, vaccinia MV entry pathways also vary among different cell lines (66), and multiple kinases, such as extracellular signal-regulated kinase (ERK), protein kinase A (PKA), protein kinase C (PKC), and PAK1, have been shown to be activated upon virus entry (18, 43, 45). However, the cellular receptor mediating vaccinia MV entry and signal transduction remains unknown. Our previous work showed that cell-bound MV particles were clustered at the plasma membrane lipid rafts prior to virus entry and that the interruption of lipid raft integrity with m-β-cyclodextran significantly reduced vaccinia MV entry into HeLa cells (12). Since lipid rafts on the plasma membrane are known to act as platforms for receptor clustering, endocytosis, and signal transduction, we hypothesized that cellular proteins within plasma membrane lipid rafts mediate vaccinia MV entry into HeLa cells. Therefore, we isolated detergent-resistant domains from HeLa cells upon vaccinia MV infections and extracted proteins for quantitative proteomic analyses (55). Here, we investigated one of the raft-associated proteins, integrin β1 (ITGβ1), for its role in vaccinia MV entry.
Integrins are a large family of cell surface receptors composed of 18 different α and 8 different β subunits (35). Integrin β1 is known to associate with multiple α subunits, including α1-11 and αV, and is widely distributed in virtually all mammalian cell types (35, 38, 39, 60). Through interactions with the extracellular matrices, integrin β1 regulates multiple intracellular kinase activation pathways. Our results demonstrate that integrin β1 mediates MV entry in both mouse embryonic fibroblast (MEF) and HeLa cells and that vaccinia virus-induced phosphatidylinositol 3-kinase (PI3K)/Akt requires integrin β1.
(R. Izmailyan conducted this research in partial fulfillment of the requirements for a Ph.D. from Academia Sinica, Taipei, Taiwan, Republic of China.)
MATERIALS AND METHODS
Cells and viruses.
Two mouse cell lines, GD25 and GD25β1A, were kindly provided by Reinhard Fässler (Max Planck Institute of Biochemistry, Germany). The GD25 cell line was derived from integrin β1 knockout (KO) embryonic stem cells (20). The stably transformed cell line GD25β1A resulted from the electroporation of GD25 cells with wild-type human integrin β1 cDNA (48). HeLa, BSC40, GD25, and GD25β1A cells were cultured in Dulbecco's modified Eagle medium (DMEM) supplemented with 10% fetal bovine serum (FBS) and 2% penicillin-streptomycin (Gibco) in a 5% CO2 incubator at 37°C. The Western Reserve strain of vaccinia MV (WR-VV) was purified through 25 to 40% sucrose gradients as previously described (32, 37). A recombinant WR-VV was also used in this study (8). It was constructed previously to express a dual gene cassette inserted into the thymidine kinase (tk) locus containing the luciferase (luc) gene driven by a viral early promoter and the lacZ gene driven by a viral late promoter (8).
Antibodies and reagents.
Anti-integrin β1 monoclonal antibodies (MAbs) Ts2/16 and 12G10 were purchased from Santa Cruz Biotechnology and Abcam, respectively, and 9EG7 and Mab13, rat MAbs, were acquired from BD Pharmingen. Anti-transferrin receptor (TfR) antibody (CD71) was obtained from AbD Serotec. Anti-paxillin antibody was purchased from BD Transduction Laboratories. Alexa Fluor 647-phalloidin was purchased from Invitrogen. Anti-phospho-Akt (Ser473) and anti-Akt antibodies were purchased from Cell Signaling Technology. Anti-phos-pho-focal adhesion kinase (FAK) (pY397) antibody was purchased from Invitrogen. Anti-FAK antibody was purchased from BD Biosciences. Anti-cyclophilin B (CypB) antibody was obtained from Santa Cruz Biotechnology. Anti-β-actin antibody was purchased from Sigma-Aldrich. Anti-A4 and anti-vaccinia MV (anti-VV) rabbit antibodies were previously described (30). Mouse MAb clone 2D5 against the vaccinia virus L1 protein was obtained from Y. Ichihashi (31). Bafilomycin A1 (BFLA), cycloheximide (CHX), and blebbistatin (Bleb) were purchased from Sigma-Aldrich. The PI3K inhibitor (LY294002) and Akt inhibitor (Akt IV) were purchased from Calbiochem. Laminin-1 (LN), fibronectin (FN), and poly-l-lysine (PLL) were purchased from Sigma-Aldrich. The CypB small interfering RNA (siRNA) duplex and the integrin β1 siRNA duplex (AAUGUAACCAACCGUAGCAUU) were purchased from Dharmacon Inc.
Biological network analysis.
Cellular proteins identified in lipid rafts isolated from HeLa cells (55) were subjected to subcellular localization analyses with NCBI Gene Ontology. The “integrin β1 (ITGβ1) signaling network” contains cellular proteins that are known to physically interact with integrin β1 and was constructed by using ARIADNE Pathway Studio 7.0 software, which uses automated text-mining engines to extract information (Ariadne Genomics). Plasma membrane proteins identified in lipid rafts were compared with those in the integrin β1 signaling network and were displayed in a graphical network by using the open-source software Cytoscape (57).
Virus entry assays.
Several cell-based biological assays were used to quantify vaccinia MV entry into host cells based on previously established methods (8, 24, 61–63). MV particles bound to cells were quantified by vaccinia MV virion binding assays at 4°C for 60 min with anti-L1 antibody (2D5) (63). Viral core numbers present in the cytoplasm after membrane fusion were quantified by viral core-uncoating assays using an antibody against A4 (62). Luciferase assays driven by a viral early promoter were performed with cell lysates harvested at 2 h postinfection (p.i.), as described previously (8, 61). Acid bypass treatment, which forced cell-bound MV to fuse with the plasma membrane, was performed as previously described (24). In brief, HeLa cells were pretreated with 25 nM bafilomycin A1 or 50 μM PI3K inhibitor at 37°C for 30 min, cooled at 4°C for 20 min, and subsequently infected with vaccinia MV at a multiplicity of infection (MOI) of 20 PFU per cell for 1 h. After washing, the infected cells were treated with neutral (pH 7.2) or acidic (pH 5) buffer for 5 min, incubated in growth medium, and fixed at 2 h p.i. These infected cells were permeabilized and stained with anti-core A4 antibody for confocal microscopy analyses as described previously (62).
Plaque formation on GD25β1A and GD25 cells.
Freshly confluent cells were infected with WR-VV (approximately 300 PFU per well in a 6-well plate) at 37°C for 1 h; washed and cultured in growth medium containing 1% agarose; fixed at 2 days p.i.; and stained with X-Gal (5-bromo-4-chloro-3-indolyl-β-d-galactopyranoside) as previously described (4). Alternatively, cells were pretreated with dimethyl sulfoxide (DMSO) or LY294002 (25 or 50 μM) in serum-free DMEM prior to infection, and the inhibitors remained in cultures after infection until cell fixation and X-Gal staining as described above.
Integrin β1 siRNA.
HeLa cells were either mock transfected (si-control) or transfected with siRNA duplexes (20 nM) targeting either cyclophilin B (si-CypB) or integrin β1 (si-ITGβ1) using the Lipofectamine 2000 reagent (Invitrogen) as described previously (30).
Confocal immunofluorescence microscopy. (i) Copatching experiments.
The experiments for the copatching of integrin β1 and vaccinia MV were performed as previously described (30, 58). In brief, HeLa cells seeded onto glass coverslips were infected with MV at an MOI of 50 PFU per cell for 1 h at 4°C, washed, and transferred to 12°C, where the cells were incubated with anti-VV antibody (1:500) and anti-integrin β1 MAb (12G10) (1:1,000) for 1 h. Tetramethylrhodamine-conjugated goat anti-rabbit IgG (1:1,000) and fluorescein isothiocyanate (FITC)-conjugated goat anti-mouse IgG (1:1,000) were subsequently added for another 1 h of incubation prior to cell fixation for confocal microscopy. Cells were mounted in Vectashield medium (Vector Laboratories, Burlingame, CA), and images were collected with an LSM510 Meta confocal laser scanning microscope (Carl Zeiss) using a 63× objective lens.
(ii) Surface staining of integrin β1 with virus in GD25β1A cells.
GD25β1A cells were seeded (1.2 × 105 cells) onto glass coverslips in 12-well plates. The next day, cells were cooled at 4°C for 20 min and subsequently infected with vaccinia MV at an MOI of 60 PFU per cell for 1 h, washed 3 times in phosphate-buffered saline (PBS), fixed, and incubated with primary anti-integrin β1 MAb (12G10) (1:1,000) and anti-VV rabbit antibody (1:500) for 1 h. Tetramethylrhodamine-conjugated goat anti-rabbit IgG (1:1,000) and FITC-conjugated goat anti-mouse IgG (1:1,000) were subsequently added for 30 min, and cells were analyzed by confocal microscopy.
Outside-in integrin signaling activation assay.
Outside-in integrin signaling activation assays were performed as follows. (i) Glass coverslips in 24-well plates were coated with FN (10 μg/ml), LN (20 μg/ml), or PLL (100 μg/ml) in PBS at 4°C. After 24 h, the dishes were blocked with 1% bovine serum albumin (BSA) in PBS at 37°C for 1 h. Serum-starved HeLa cells were seeded at a density of 8 × 104 cells into each well in serum-free DMEM; incubated at 37°C for 20, 30, 45, and 90 min; and lysed in cold lysis buffer (10 mM Tris [pH 7.4], 150 mM NaCl, 0.5% NP-40, 1% sodium deoxycholate, 0.1% SDS, 1% Triton X-100, 1 mM EGTA, and 1 mM EDTA with 1× protease inhibitor cocktail [tablets purchased from Roche Applied Science]). Samples were then prepared for immunoblot analyses with anti-phospho-Akt (1:1,000), anti-phospho-FAK (1:1,000), anti-Akt (1:1,000), and anti-FAK (1:1,000) antibodies. Alternatively, cells were fixed after 90 min of plating, permeabilized, and stained with anti-paxillin antibody (1:1,000). (ii) HeLa cells seeded for 90 min, as described above, were subsequently infected with vaccinia MV at an MOI of 5 PFU per cell at 37°C for 1.5 h and harvested for luciferase assays. (iii) HeLa cells were seeded, as described in above, for 15 min at 37°C. DMSO or blebbistatin (25 and 50 μM) was added to cells, and the cells were incubated for another 45 min. The cells either were fixed, permeabilized, and stained with anti-paxillin antibody (1:1,000) or were infected with vaccinia MV at an MOI of 5 PFU per cell for 1.5 h and harvested for luciferase assays.
Flow cytometry.
Cells were detached from the dishes with 20 mM EDTA, washed, and stained with anti-integrin β1 MAb (9EG7) (1:1,000) in PBS containing 150 mM NaCl and 1% BSA for 1 h at 4°C. Samples were then washed 3 times, followed by incubation with FITC-conjugated goat anti-rat secondary antibody for 1 h at 4°C. Following a final wash, the cells were analyzed by fluorescence-activated cell sorting (FACS).
Vaccinia MV infections activated Akt phosphorylation.
HeLa cells (7.5 × 104 cells/well) or GD25β1A cells (1 × 105 cells/well) were cultured in 12-well plates for 24 h. Cells were serum starved at 37°C for 2 h (for HeLa cells) or 18 h (for GD25β1A cells) and then stimulated with medium containing 20% FBS or purified vaccinia MV (at MOIs of 40 PFU per cell for HeLa cells and 60 PFU per cell for GD25β1A cells) at 37°C for various times. Cell lysates were prepared with cold lysis buffer (10 mM Tris [pH 7.4], 150 mM NaCl, 0.5% NP-40, 1% sodium deoxycholate, 0.1% SDS, 1% Triton X-100, 1 mM EGTA, and 1 mM EDTA with 1× protease inhibitor cocktail [tablets purchased from Roche Applied Science]) for immunoblot analysis.
Statistical analysis.
Statistical analyses were performed by using Student's t test with Prism software (GraphPad).
RESULTS
Integrin β1 associates with vaccinia MV on HeLa cells.
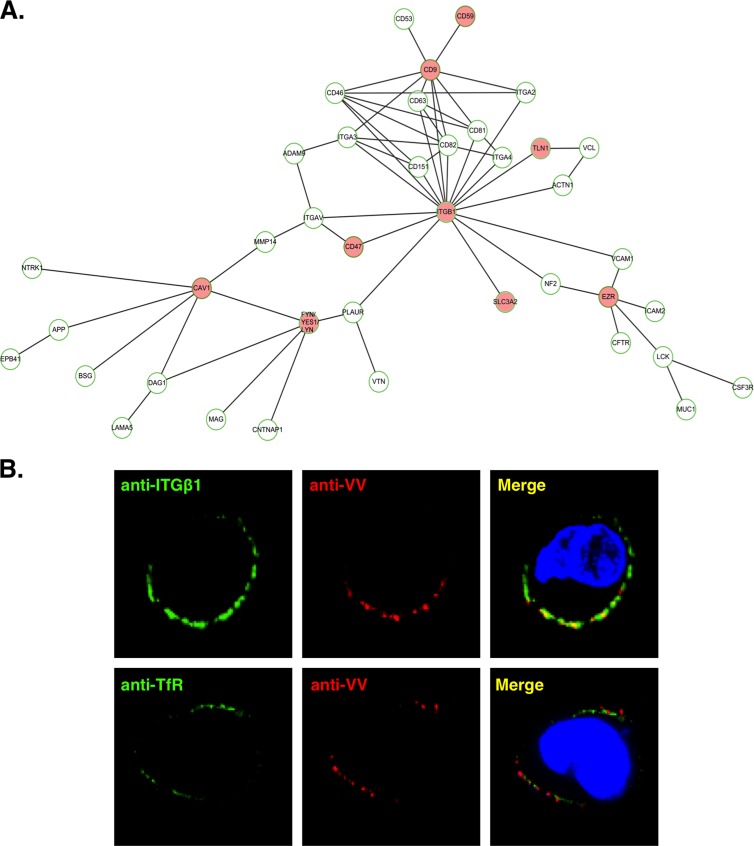
In order to identify cellular proteins within lipid raft microdomains, we previously performed stable isotope labeling for quantitative proteomic analyses and identified 570 cellular proteins. Those proteins with altered levels after vaccinia virus infection constitute about 3% of the total candidates and were described elsewhere previously (55). To complement the above-mentioned study, here we analyzed the remaining 97% of the “constitutive” raft-associated proteins whose quantifications fell within the range of a ratio of 1.0 ± 0.5, i.e., a ratio that was considered not significantly changed by virus infection. These proteins were analyzed through biological network analyses in order to identify specific signaling complexes resident in the rafts. As described in Materials and Methods, biological network analyses (Fig. 1A) revealed the presence of integrin β1 and its associated proteins, such as CD9, CD47, CD59, CD98, talin, ezrin, and Fyn/yes/lyn, suggesting a possibility that integrin β1-mediated biological signaling may participate in vaccinia MV entry. We thus investigated whether integrin β1 associates with vaccinia MV on the surface of infected HeLa cells. HeLa cells were infected with the vaccinia MV WR strain at an MOI of 50 PFU per cell for 60 min at 4°C and then washed and transferred to a temperature of 12°C. Anti-integrin β1 (ITGβ1) and anti-vaccinia MV (VV) antibodies were subsequently added to cells for copatching (Fig. 1B), as previously described (30, 58). An anti-transferrin receptor (TfR) antibody was also included as a negative control. The results show that vaccinia MV on HeLa cells copatched with cell surface integrin β1 but not with the transferrin receptor on HeLa cells (Fig. 1B), suggesting a role of integrin β1 in vaccinia MV entry.
Fig 1.
Integrin β1 was identified in lipid rafts and is associated with vaccinia MV on HeLa cells. (A) The hierarchical ITGβ1 network, constructed by ARIADNE Pathway Studio 7.0 software (see Materials and Methods), shows integrin β1 and its direct and indirect interacting membrane proteins. The nodes are labeled with gene symbols, and the red nodes represent the cellular proteins identified in the lipid raft fractions upon vaccinia MV infections (55). ITGβ1, integrin β1; TLN1, talin-1; EZR, ezrin; CAV1, caveolin-1; SLC3A2, CD98. (B) Vaccinia MV colocalizes with ITGβ1 at the surface of HeLa cells. HeLa cells were infected with vaccinia MV at an MOI of 50 PFU per cell for 1 h at 4°C, washed, and transferred to 12°C, where the cells were incubated with rabbit anti-VV antibody (1:500) (red) and anti-integrin β1 MAb (12G10) (1:1,000) (green) for 1 h for copatching as previously described (30). Alternatively, anti-VV antibody was incubated with anti-transferrin receptor (TfR) (green) MAb as a control. Tetramethylrhodamine-conjugated goat anti-rabbit IgG (1:1,000) and FITC-conjugated goat anti-mouse IgG (1:1,000) were subsequently added, and cells were fixed for confocal microscopy. DNA was visualized by 4′-6-diamidino-2-phenylindole (DAPI) staining (blue).
Vaccinia virus entry is reduced upon integrin β1 knockdown in HeLa cells.
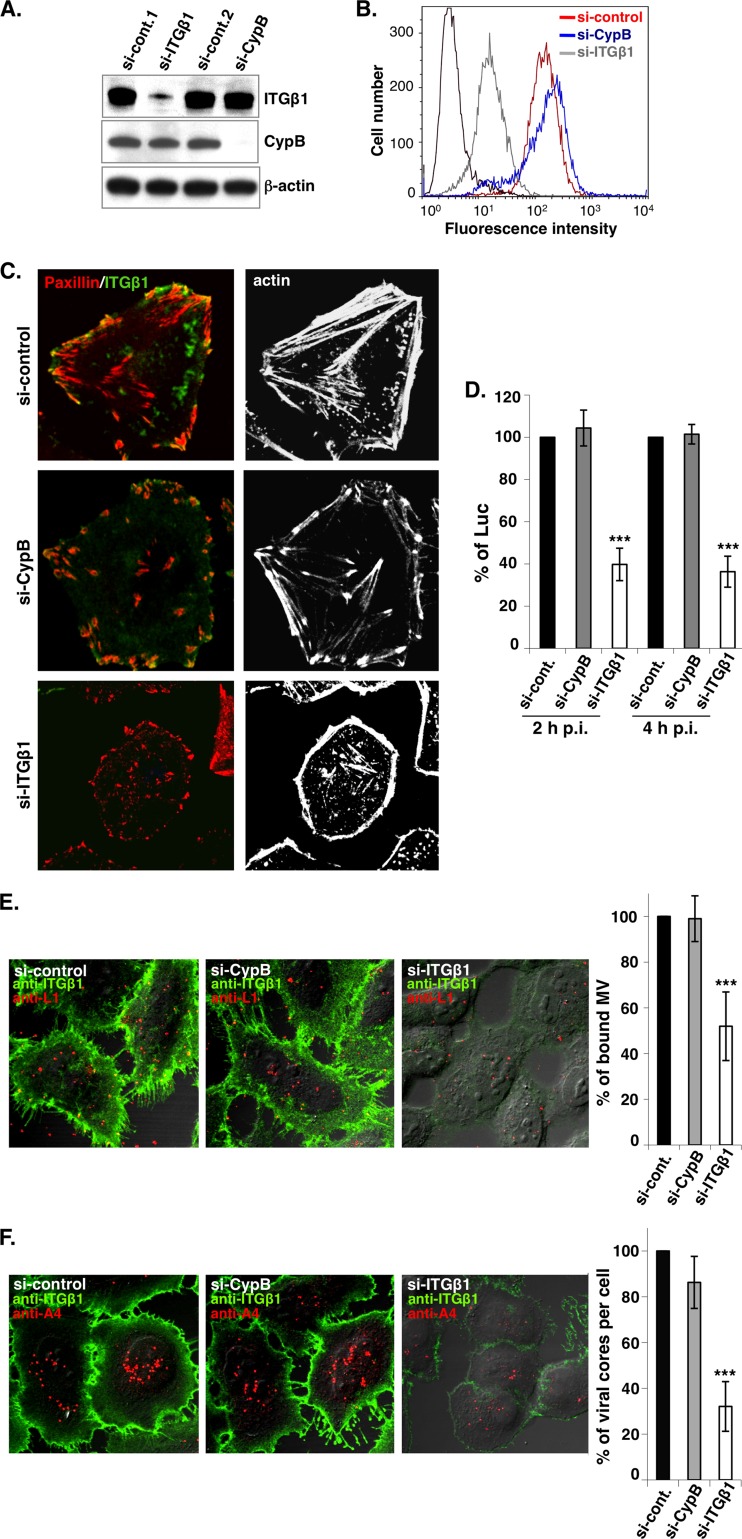
To test whether integrin β1 mediates vaccinia MV entry into HeLa cells, control siRNA (si-control) or siRNA targeting integrin β1 (si-ITGβ1) or cyclophilin B (si-CypB) was transfected into HeLa cells, followed by harvesting for immunoblot analyses (Fig. 2A) and FACS analyses (Fig. 2B). The si-ITGβ1 and si-CypB constructs specifically knocked down (KD) the total amounts of integrin β1 and cyclophilin B proteins, respectively (Fig. 2A). In addition, integrin β1 expression on the cell surface was effectively reduced in si-ITGβ1 KD HeLa cells but not in si-control or si-CypB KD cells (Fig. 2B). si-ITGβ1 also affected integrin β1-mediated cell adhesion, resulting in alterations in cell morphology and a disorganization of focal adhesions, as determined by actin and paxillin staining in si-ITGβ1 KD HeLa cells (Fig. 2C), confirming the specificity of si-ITGβ1. We then infected these HeLa KD cells with vaccinia MV at an MOI of 5 PFU per cell and harvested the cells at 2 or 4 h p.i. for viral early luciferase activity assays. The results showed that the rates of vaccinia virus infection of si-ITGβ1 KD HeLa cells were reduced to 40% (at 2 h p.i.) and 38% (at 4 h p.i.) of the infection rates seen for the si-control and si-CypB KD HeLa cells (Fig. 2D), suggesting that the reduction was not due to delayed kinetics. Finally, we performed vaccinia MV binding assays and virus core-uncoating assays as previously described (62, 63). The results showed that the rate of MV attachment was reduced to 52% in si-ITGβ1 KD HeLa cells (Fig. 2E) and that the rate of MV penetration was reduced to 32% (Fig. 2F), indicating that integrin β1 is important for vaccinia virus entry at both the attachment and penetration steps.
Fig 2.
Vaccinia virus entry is reduced in si-ITGβ1 KD HeLa cells. (A) Immunoblots of lysates prepared from mock-KD (si-Cont.1 and 2), si-ITGβ1 KD, and si-CypB KD HeLa cells using anti-ITGβ1 (Mab13), anti-CypB, or anti-β-actin antibodies. (B) Cell surface expression of ITGβ1 determined by flow cytometry. The KD HeLa cells described above (A) were stained with anti-ITGβ1 MAb (9EG7), followed by FITC-conjugated goat anti-rat secondary antibody, and analyzed by FACS. si-control is shown in red, si-CypB is shown in blue, and si-ITGβ1 is shown in gray. The background staining (shown in black) represents the si-control cells that were stained with the secondary antibody only. (C) Immunofluorescence analysis of ITGβ1, paxillin, and actin proteins in KD HeLa cells. The KD HeLa cells described above (A) were fixed and stained with anti-ITGβ1 MAb (Mab13), followed by FITC-conjugated goat anti-rat secondary antibody (green). These cells were subsequently permeabilized and stained with Alexa Fluor 647-phalloidin (white) and anti-paxillin antibodies, followed by tetramethylrhodamine-conjugated goat anti-mouse IgG (red). (D) Viral early luciferase assays. The KD HeLa cells described above (A) were infected with VV-WR at an MOI of 5 PFU per cell and harvested at 2 and 4 h p.i. for luciferase assays as described in Materials and Methods. The luciferase activity in the si-control cells was defined as 100%. The bars represent the standard deviations from three independent experiments. (E) Immunofluorescence analyses of vaccinia virus binding assay. The KD HeLa cells described above (A) were infected with purified vaccinia MV particles at an MOI of 20 PFU per cell for 60 min at 4°C, fixed, and stained with anti-ITGβ1 Mab13 (green) and anti-L1 MAb 2D5 (red). The cell-bound virions were quantified as described previously (63). (F) Immunofluorescence analyses of vaccinia virus core-uncoating assays. The KD HeLa cells described above (A) were infected as described above, cultured in the presence of cycloheximide (10 μg/ml) for an additional 2 h at 37°C, fixed, and stained with anti-ITGβ1 MAb Mab13 as described above. These cells were subsequently permeabilized and stained with anti-A4 antibody, and internalized viral cores were quantified as described previously (62). Particle numbers counted in the si-control HeLa cells were used as 100%. The bars represent the standard deviations from five independent experiments. Statistical analyses in panels D to F were performed by using Student's t test in Prism software (GraphPad). The P value is shown (***, P < 0.0001).
Vaccinia virus entry is reduced in mouse cells lacking integrin β1 expression.
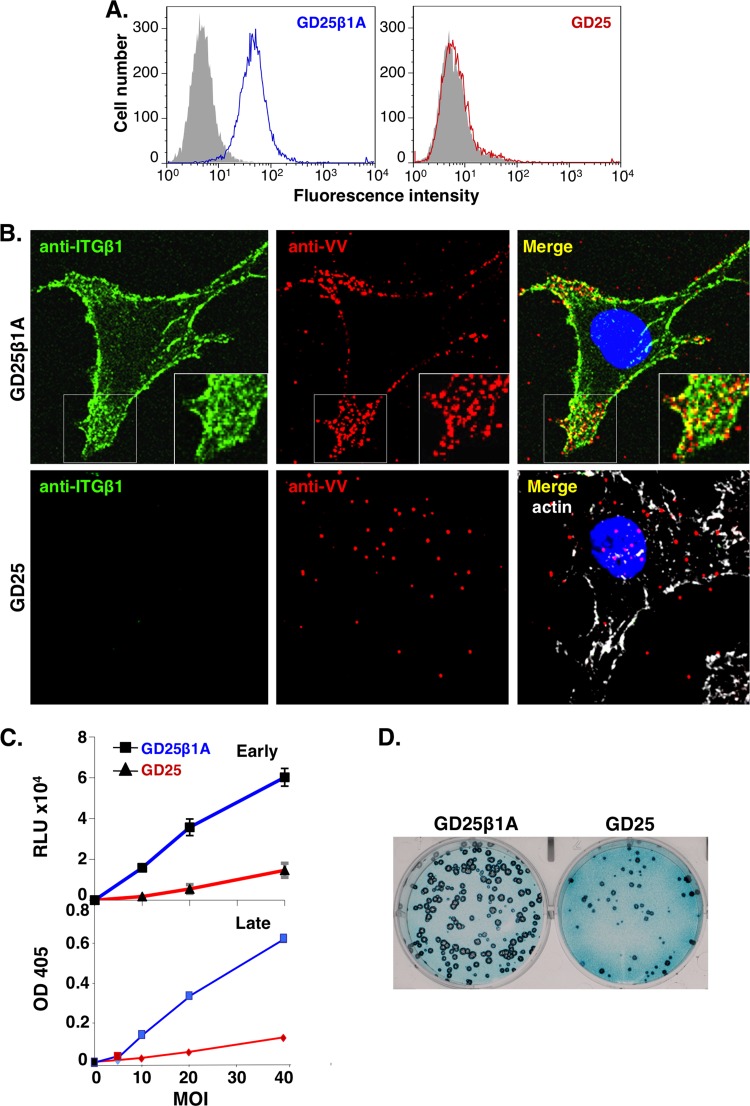
In addition to HeLa cells, we obtained a mouse cell line, GD25β1A, derived from an integrin β1 KO cell line, GD25 (20), which expresses only human integrin β1 (48). Indeed, integrin β1 was detected on the surface of GD25β1A cells but not on the surface of GD25 cells by FACS analyses (Fig. 3A). GD25β1A and GD25 cells were infected with vaccinia MV at an MOI of 60 PFU per cell at 4°C for 60 min, washed, and fixed for immunofluorescence analyses. Abundant MV particles bound to GD25β1A cells and colocalized with surface integrin β1 concentrated at cellular protrusions, whereas fewer MV particles (∼34%) bound to GD25 cells (Fig. 3B). In addition, both GD25β1A and GD25 cells were infected with vaccinia WR-VV at different MOIs from 10 to 40 PFU per cell and were harvested to measure the early luciferase activity at 2 h p.i. or the late β-galactosidase (β-Gal) activity at 8 h p.i. (Fig. 3C). Data from both enzymatic activity assays and the core-uncoating assay (data not shown) revealed that vaccinia MV entry into GD25 cells was less efficient than entry into GD25β1A cells. We also performed plaque assays on GD25β1A and GD25 cells and stained the plaques with X-Gal. Consistently, vaccinia MV produced fewer plaques (∼30%) in GD25 cells than in GD25β1A cells (Fig. 3D). Interestingly, plaques formed in GD25 cells also appeared smaller than those formed in GD25β1A cells, implying a role of integrin β1 in vaccinia virus spreading among cells, although we did not pursue it further. Taken together, the results show that cell surface integrin β1 mediates vaccinia MV infections.
Fig 3.
Vaccinia virus entry is reduced in mouse cells lacking integrin β1 expression. (A) Flow cytometry analyses of cell surface expression of ITGβ1. GD25β1A (blue) and GD25 (red) cells were stained with anti-ITGβ1 MAb (9EG7), followed by FITC-conjugated goat anti-rat secondary antibody, and analyzed by FACS. The background staining (in gray) represents cell staining with the secondary antibody only. (B) Confocal immunofluorescence of vaccinia MV particles colocalized with integrin β1 in GD25β1A and GD25 cells. GD25β1A and GD25 cells were infected with purified vaccinia MV particles at an MOI of 60 PFU per cell at 4°C for 1 h, washed, and stained with anti-ITGβ1 (12G10) (green) and anti-VV (red) antibodies, followed by FITC-conjugated goat anti-mouse IgG and tetramethylrhodamine-conjugated goat anti-rabbit IgG. GD25 cells were negative for ITGβ1 staining and were subsequently permeabilized and stained with Alexa Fluor 647-phalloidin (white) to mark the cell body. (C) GD25β1A and GD25 cells were infected with WR-VV at MOIs of 10, 20, and 40 PFU per cell and harvested at 2 h p.i. for luciferase assays (Early) and 8 h p.i. for β-Gal activity assays (Late). RLU, relative light units; OD 405, optical density at 405 nm. (D) Vaccinia MV formed more plaques in GD25β1A cells than in GD25 cells. Both cell types were infected with ∼300 PFU of WR-VV, fixed, and stained for X-Gal at 2 days p.i. to visualize the blue plaques.
Vaccinia MV binds to integrin β1 to induce PI3K/Akt activation, which leads to virus endocytosis in HeLa cells.
Integrin β1 was shown previously to mediate multiple inside-out and outside-in signaling pathways that play crucial roles in cell-to-cell and cell-to-matrix communications (28, 38, 46). The clustering of integrin at the cell membrane leads to kinase activation, which is followed by alterations to the cytoskeleton and receptor endocytosis. Since it was shown previously that PI3K and Akt are critical regulators downstream of integrin β1 on GD25β1A cells (15, 48, 65), we wanted to test whether vaccinia MV binding to integrin β1 activates PI3K/Akt signaling in GD25β1A cells and whether such kinase activation is critical for vaccinia MV entry.
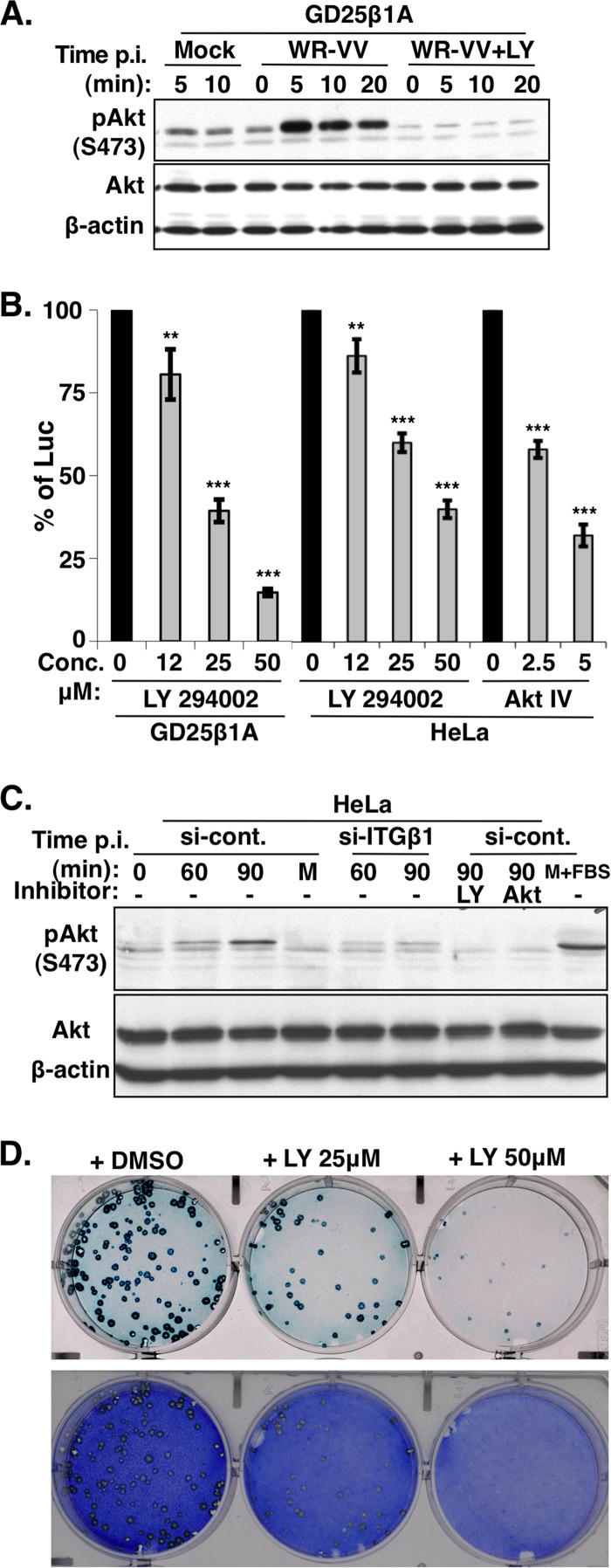
GD25β1A cells were serum starved and subsequently infected with vaccinia MV at an MOI of 60 PFU per cell at 37°C for 5, 10, and 20 min and then harvested for immunoblot analyses with anti-phospho-Akt (Ser473) antibody. As shown in Fig. 4A, vaccinia MV stimulated the robust phosphorylation of Akt in GD25β1A cells as early as 5 min after the addition of virus, compared to that in medium alone. The pretreatment of GD25β1A cells with the PI3K inhibitor LY294002 completely abolished MV-induced Akt phosphorylation, showing that vaccinia MV infection triggered the activation of Akt through PI3K. To determine whether PI3K/Akt activation was required for vaccinia virus entry, we pretreated both GD25β1A and HeLa cells with inhibitors that blocked PI3K (LY294002) and Akt (Akt IV) activities, subsequently infected the cells with vaccinia MV, and measured viral early promoter luciferase activity at 2 h p.i. As shown in Fig. 4B, PI3K as well as Akt inhibitors readily reduced vaccinia MV infections in both GD25β1A and HeLa cells in a dose-dependent manner, showing that PI3K/Akt signaling is crucial for vaccinia MV entry into both cell lines. It is worth noting that GD25 cells were not suited to kinase signaling studies because it was previously shown that this cell line contains altered kinase regulation that compensates for the loss of integrin β1 and that multiple tyrosine kinases are activated without integrin β1 (3). Thus, in order to demonstrate that MV-induced PI3K activation requires integrin β1, we infected si-control and si-ITGβ1 KD HeLa cells with vaccinia MV and harvested the cells at 0, 60, and 90 min for phospho-Akt immunoblot analyses. When these cells were infected with vaccinia MV, the phosphorylation of Akt was also induced in si-control HeLa cells although with slower kinetics than in GD25β1A cells (Fig. 4C). Most importantly, the phosphorylation of Akt was significantly reduced in si-ITGβ1 KD HeLa cells. Finally, GD25β1A cells were pretreated with 25 and 50 μM LY294002 and infected with vaccinia MV for plaque formation assays, which showed a dosage-dependent reduction of plaque numbers with LY294002 and not the DMSO control (Fig. 4D), consistent with the data from the luciferase assays (Fig. 4B). Taken together, these results demonstrate that vaccinia MV-induced PI3K/Akt activation is mediated through integrin β1 and that such an activation is required for vaccinia virus entry and plaque formation.
Fig 4.
PI3K/Akt activation induced by WR-VV is required for virus entry. (A) WR-VV induced phosphorylation of Akt in GD25β1A cells. GD25β1A cells were serum starved, pretreated with DMSO or the PI3K inhibitor (LY294002), and either mock infected or infected with medium containing purified MV particles (WR-VV) at an MOI of 60 PFU per cell at 37°C. Cells were harvested at 0, 5, 10, and 20 min after the addition of virus for immunoblot analyses with anti-phospho-Akt (pAkt) (S473), anti-Akt, and anti-β-actin antibodies. (B) Viral early luciferase activity in GD25β1A and HeLa cells was blocked by PI3K/Akt inhibitors. Cells were pretreated with DMSO; the PI3K inhibitor (LY294002) at a concentration of 12, 25, or 50 μM; or the Akt inhibitor (Akt IV) at a concentration of 2.5 or 5 μM and subsequently infected with WR-VV at MOIs of 10 PFU (for GD25β1A cells) and 5 PFU (for HeLa cells) per cell and harvested at 2 h p.i. for luciferase assays as described above. The luciferase activity present in the DMSO-treated samples was used as 100%. The bars represent the standard deviations from three independent experiments. Statistical analyses were performed by using Student's t test in Prism software (GraphPad). P values are shown (**, P < 0.001; ***, P < 0.0001). (C) WR-VV-induced phosphorylation of Akt requires integrin β1 in HeLa cells. The si-control and si-ITGβ1 KD HeLa cells, as described in the legend of Fig. 2A, were serum starved, pretreated with or without inhibitors of PI3K (LY294002) or Akt (Akt IV), and subsequently infected with purified MV particles (WR-VV) at an MOI of 40 PFU per cell at 37°C. Cells were harvested at 0, 60, and 90 min after the addition of virus for immunoblot analyses with anti-phospho-Akt (pAkt) (S473), anti-Akt, and anti-β-actin antibodies. The si-control HeLa cells stimulated with medium containing 20% fetal bovine serum (M+FBS) for 30 min were used as a control. (D) Vaccinia virus plaque formation was reduced with the PI3K inhibitor LY294002 in GD25β1A cells. GD25β1A cells were pretreated with DMSO or the inhibitor LY294002 at concentrations of 25 and 50 μM for 1 h at 37°C and subsequently infected with WR-VV (∼300 PFU/well), cultured in medium containing inhibitors, fixed at 2 days p.i., and stained with X-Gal to visualize the blue plaques. After photography, cells were subsequently stained with crystal violet to reveal the monolayer of cells.
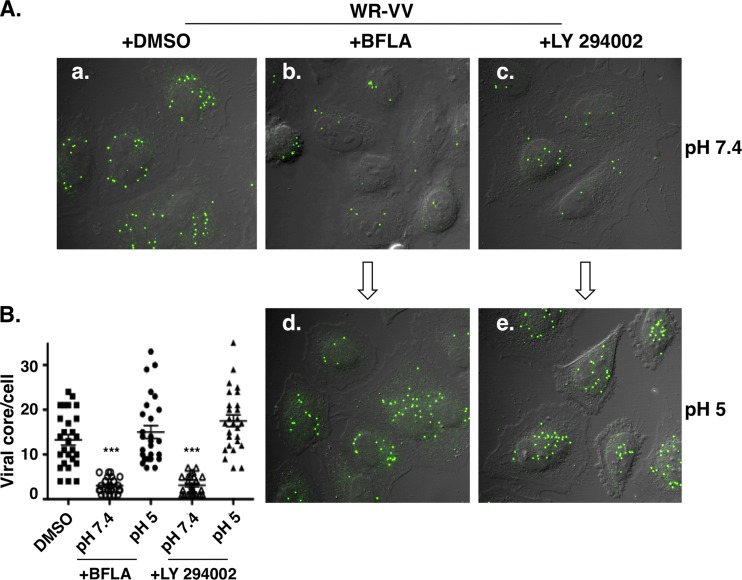
To determine whether the PI3K inhibitor specifically blocks viral endocytic entry, we performed acid bypass experiments in which the cell-bound viruses were briefly treated with acidic buffer to force viral entry through plasma membrane fusion, as described previously (24). HeLa cells were pretreated with DMSO, bafilomycin A (a drug known to inhibit endosomal acidification), or the PI3K inhibitor LY294002 and infected with vaccinia MV at 4°C for 1 h. The unbound virions were washed, and the infected cells were briefly exposed to either neutral (pH 7.4) or acidic (pH 5) buffer for 5 min at 37°C. The cultures were then maintained for another 2 h in growth medium prior to fixation for viral core-uncoating assays as previously described (8). As shown in Fig. 5A, control HeLa cells treated with DMSO (Fig. 5Aa) were successfully infected, and abundant viral cores were detected in the cytoplasm. BFLA pretreatment significantly reduced viral core numbers in cells, confirming virus entry through a low-pH-dependent endocytic process (Fig. 5Ab). The pretreatment of HeLa cells with LY294002 also reduced numbers of viral cores in cells, suggesting that PI3K/Akt is important for vaccinia virus uncoating (Fig. 5Ac). Most importantly, the exposure of these cells to a low-pH buffer rendered MV entry through plasma membrane fusion resistant to inhibition by BFLA (Fig. 5Ad) and LY294002 (Fig. 5Ae). Quantifications of viral uncoated cores in each sample are shown in Fig. 5B. Taken together, these results demonstrate that PI3K/Akt activation induced by vaccinia MV is important for virus endocytosis in HeLa cells.
Fig 5.
PI3K/Akt activation is required for vaccinia MV endocytosis. (A) Immunofluorescence analyses of virus-uncoating assays with HeLa cells. HeLa cells were pretreated with DMSO (a), 25 nM BFLA (b and d), or 50 μM PI3K inhibitor (LY294002) (c and e) and subsequently infected with purified MV particles at an MOI of 20 PFU per cell for 60 min at 4°C. Cells were then treated with neutral (pH 7.4) (a to c) or acidic (pH 5) (d and e) buffer for 5 min, washed, cultured at 37°C in medium containing 10 μg/ml cycloheximide for an additional 2 h, and analyzed by virus core-uncoating assays using anti-A4 antibody, as described in the text. (B) Quantification of viral core numbers per cell from each group (30 cells/group) described above (A). Statistical analyses were performed by using Student's t test in Prism software (GraphPad). The P value is shown (***, P < 0.0001).
Outside-in activation of integrin β1 facilitates vaccinia MV entry.
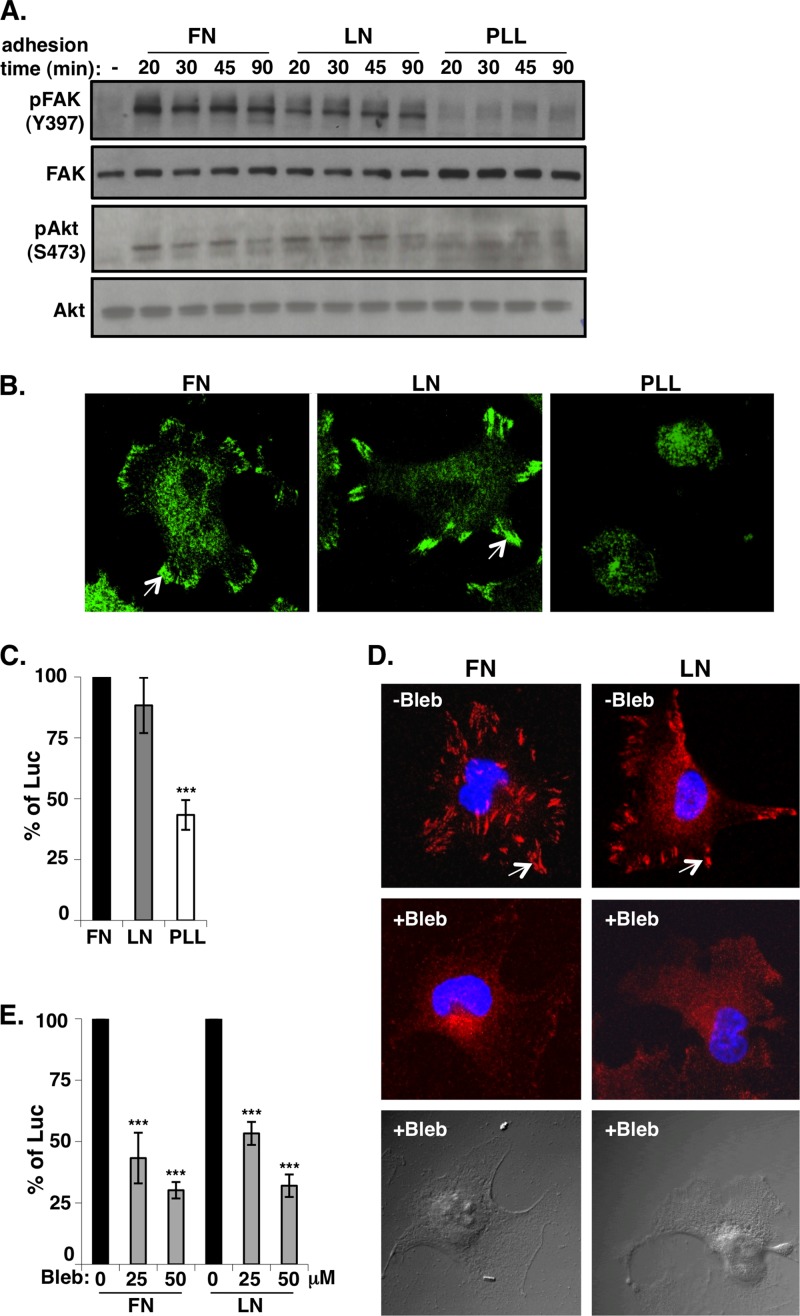
The fact that the integrin network is essential for cell viability, migration, and growth suggests that vaccinia MV exploits the integrin/PI3K/Akt signaling pathway to modulate cellular environments preferable for viral entry and growth. We thus performed outside-in activation experiments to turn on the integrin-dependent signaling pathway in HeLa cells. HeLa cells were plated onto dishes precoated with the extracellular matrix proteins fibronectin (FN) and laminin (LN) for only a short time so that few extracellular matrix proteins were secreted from cells to induce integrin-mediated cell adhesion. As a negative control, HeLa cells were plated onto dishes precoated with poly-l-lysine (PLL), which mediates cell attachment through electrostatic interactions and is independent of integrins (53, 54). Cells were allowed to adhere and spread for 20, 30, 45, and 90 min before they were harvested for immunoblot analysis. As shown in Fig. 6A, the integrin-mediated phosphorylation of FAK and Akt was detected as early as 20 min after cell plating onto FN and LN but not onto PLL, showing a specific activation of integrin/PI3K/Akt through cell-matrix interactions. As expected, immunofluorescence staining with anti-paxillin antibody revealed the formation of focal adhesions in cells plated onto FN and LN but not onto PLL (Fig. 6B). When these cells were infected with vaccinia MV, the early luciferase activity level was higher in HeLa cells plated onto FN and LN than in those plated onto PLL (Fig. 6C). Altogether, these results demonstrate that the outside-in activation of integrin β1-mediated PI3K signaling is important for vaccinia MV entry into HeLa cells.
Fig 6.
Outside-in integrin activation enhances vaccinia MV entry. (A) Cell adhesion to the extracellular matrix activates FAK and Akt phosphorylation. Serum-starved HeLa cells in suspension (−) were seeded onto fibronectin (FN), laminin-1 (LN), and poly-l-lysine (PLL) at 37°C and harvested at 20, 30, 45, and 90 min for immunoblot analysis with anti-phospho-FAK (pFAK) (Y397), anti-phospho-Akt (pAkt) (S473), anti-FAK, and anti-Akt antibodies. (B) Immunofluorescence staining of paxillin in HeLa cells seeded onto a different extracellular matrix. HeLa cells were seeded onto FN, LN, and PLL, as described above, for 90 min; fixed; permeabilized; and stained with anti-paxillin antibody, followed by FITC-conjugated goat anti-mouse IgG. (C) Viral early luciferase assays. HeLa cells were seeded as described above (B), infected with WR-VV at an MOI of 5 PFU per cell, and harvested at 1.5 h p.i. for luciferase assays as described in Materials and Methods. The luciferase activity in HeLa cells seeded onto FN was defined as 100%. The bars represent the standard deviations from three independent experiments. (D) Immunofluorescence staining of paxillin in HeLa cells treated with blebbistatin. HeLa cells were seeded onto FN and LN as described above (A) and incubated with 50 μM blebbistatin (+Bleb) or DMSO (−Bleb) (see Materials and Methods) for 60 min. Cells were fixed and permeabilized, and the focal adhesions were stained with anti-paxillin antibody (red). Cell nuclei were stained with DAPI (blue). In panels B and D, white arrows identify areas of focal adhesion. (E) HeLa cells seeded as described above were treated with DMSO or blebbistatin (25 and 50 μM) and subsequently infected with WR-VV at an MOI of 5 PFU per cell and harvested at 1.5 h p.i. for luciferase assays. The luciferase activity in DMSO-treated HeLa cells was defined as 100%. The bars represent the standard deviations from three independent experiments. Statistical analyses in panels B, C, and E were performed by using Student's t test in Prism software (GraphPad). The P value is shown (***, P < 0.0001).
Integrin adhesome formation induced by outside-in activation was shown previously to be disrupted upon blebbistatin treatment (53). When the above-described outside-in activation procedures were performed with HeLa cells in the presence of 25 and 50 μM blebbistatin, the formation of focal adhesions was completely dispersed (Fig. 6D). These drug-treated HeLa cells were subsequently infected with vaccinia MV, and a dosage-dependent reduction of viral entry was observed, compared with the mock-treated cells (Fig. 6E). These results suggested that the recruitment of cellular proteins to form integrin adhesomes, which was blocked by blebbistatin treatment, is important for vaccinia MV entry.
DISCUSSION
In this study, we demonstrated the role of the cellular protein integrin β1 in vaccinia MV entry by several experimental criteria. First, we showed that integrin β1 and vaccinia MV colocalized on plasma membrane lipid rafts and that the knockdown of integrin β1 in HeLa cells and the knockout of integrin β1 in mouse cells reduced vaccinia MV attachment. Second, we showed that the requirement of integrin β1 is not limited to virus attachment, since integrin β1 also mediated the activation of PI3K/Akt for subsequent MV endocytosis. We further showed that adhesome formation activated by the interaction of the extracellular matrix and integrin is also critical for vaccinia MV entry. Taken together, these data demonstrated that cell surface integrin β1 in lipid rafts serves as a signaling platform which activates downstream kinases and cellular adhesome formation, both of which are important for vaccinia MV entry.
Vaccinia virus infection is known to activate multiple kinases, including ERK (18), PKC, and PAK1 (43, 45). However, the virus-induced upstream signal that leads to kinase activation remains unknown. Our study shows that the binding of vaccinia MV to integrin β1 activated the PI3K/Akt kinase pathway. We also found that the KD of integrin β1 led to a reduction of ERK activity in HeLa cells and that the addition of the ERK inhibitor PD98059 interfered with vaccinia MV entry (R. Izmailyan, unpublished results). Although we did not investigate PAK1 activation in this study, interestingly, PAK1 was previously shown to be activated by integrin β1 (50). Taking these findings together, we conclude that vaccinia MV interacts with integrin β1 to activate a downstream kinase network to induce viral endocytosis (Fig. 7). At this point, we have not been able to identify any viral envelope protein specifically associated with integrin β1 in coimmunoprecipitation experiments. It could be that vaccinia MV interacts with multiple cellular receptor proteins during virus entry and that each receptor interaction is transient and dynamically regulated (see below).
Fig 7.
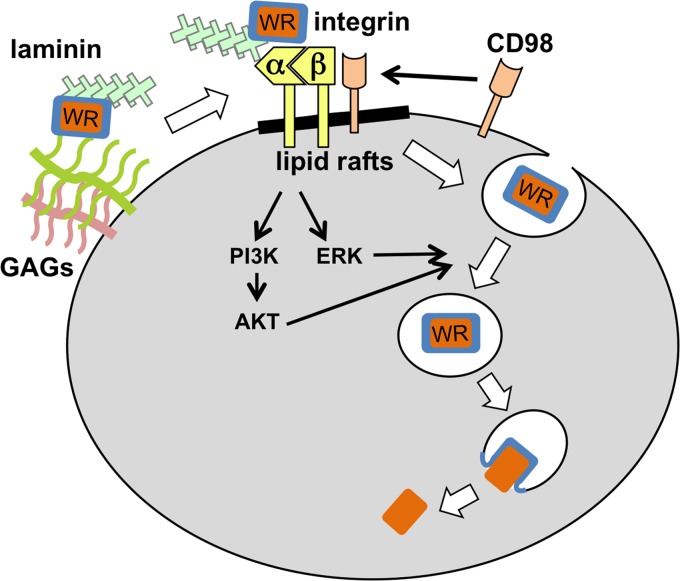
Current model of integrin β1 in vaccinia MV entry. The vaccinia MV WR strain binds to cell surface glycosaminoglycans (GAGs) and laminin, which induce further interactions between MV and cellular surface receptor integrin in lipid rafts. The subsequent recruitment of the cellular membrane protein CD98 and the activation of downstream kinases such as PI3K/Akt and ERK lead to the endocytosis of vaccinia MV.
Although our study focused primarily on integrin β1, the significance of the involvement of integrin in vaccinia virus entry goes beyond this molecule. Blebbistatin, an inhibitor of myosin II light-chain ATPase (41), was recently shown to interrupt integrin adhesome formation (53), and in our experiments, blebbistatin-treated HeLa cells lost the outside-in activation of integrin and reduced vaccinia virus endocytosis. This finding implies that other components of the integrin adhesome may be involved in vaccinia virus endocytosis. Indeed, a recent study conducted in our laboratory by Schroeder et al. (55) demonstrated that CD98, a type II membrane protein found in lipid rafts (55) and a component of the integrin adhesome (53), is required for vaccinia MV endocytosis (55). The KD of CD98 does not affect vaccinia MV binding to cells but reduces virus endocytosis and core uncoating (55), suggesting that it participates in vaccinia virus entry at a step subsequent to integrin β1. It is worth noting that CD98 was reported previously to promote integrin-dependent signaling, leading to the activation of FAK, PI3K, Akt, and Rac (22, 23, 70). Currently, our model proposes that vaccinia MV binds to integrin β1, recruits CD98 in rafts, activates kinases, and induces cytoskeleton rearrangements to trigger MV endocytosis in cells (Fig. 7). Finally, we noticed that vaccinia MV formed smaller plaques on GD25 cells than those on GD25β1A cells. The KD of integrin β1 in HeLa cells also reduced MV and EV production in a low-MOI infection, implying that vaccinia virus spreading among cells may also require integrin β1 (data not shown). Further studies are needed to explore this possibility.
Integrins are involved in the entry of many viruses (2, 5, 19, 21, 26, 34, 36, 44, 59, 64) and bacteria (16, 27, 40). The pathogen-host interaction may promote the clustering of integrins and focal adhesion formation but subvert the downstream signaling network to modify membrane traffic and cytoskeletal dynamics to meet their own needs (1, 17). Several unique properties of integrins may explain why they are so frequently targeted for bacterial and viral invasions. Integrins are ubiquitously expressed on virtually all cells. They regulate both outside-in and inside-out signaling to maintain important cell functions related to cell adhesion, migration, and survival. Furthermore, integrins act as global regulators of endocytosis, affecting the intracellular trafficking of growth factor receptors and endosome localization in cells (67). A genomewide screen of endocytosis regulators identified adhesion-mediated molecules that support cross talk between cell adhesion regulation and endocytic activity (13). The fate of endocytosed vaccinia MV is not well understood. Recently, it was shown that integrin internalization is mediated through macropinocytosis. Internalized integrins were found to transit through early endosomes to recycling endosomes and then back to the cell surface to form new adhesions (25). Currently, we are investigating whether integrin β1 regulates the intracellular trafficking of vaccinia virions through the same route prior to virus-cell membrane fusion. More studies will be needed in the future to dissect the structure-function relationship of integrins in vaccinia virus entry.
ACKNOWLEDGMENTS
We thank Reinhard Fässler (Max Planck Institute of Biochemistry, Germany) for providing GD25β1A and GD25 cells and Jyrki Heino for suggestions and comments on the manuscript. We also thank Sue-Ping Lee for assistance with confocal microscopy.
This work was supported by grants from Academia Sinica and the National Science Council of the Republic of China (NSC-100-2320-B-001-006).
Footnotes
Published ahead of print 11 April 2012
REFERENCES
- 1. Agerer F, et al. 2005. Cellular invasion by Staphylococcus aureus reveals a functional link between focal adhesion kinase and cortactin in integrin-mediated internalisation. J. Cell Sci. 118:2189–2200 [DOI] [PubMed] [Google Scholar]
- 2. Akula SM, Pramod NP, Wang FZ, Chandran B. 2002. Integrin alpha 3 beta 1 (CD 49c/29) is a cellular receptor for Kaposi's sarcoma-associated herpesvirus (KSHV/HHV-8) entry into the target cells. Cell 108:407–419 [DOI] [PubMed] [Google Scholar]
- 3. Armulik A, Svineng G, Wennerberg K, Fassler R, Johansson S. 2000. Expression of integrin subunit beta 1B in integrin beta 1-deficient GD25 cells does not interfere with alpha V beta 3 functions. Exp. Cell Res. 254:55–63 [DOI] [PubMed] [Google Scholar]
- 4. Bair CH, Chung CS, Vasilevskaya IA, Chang W. 1996. Isolation and characterization of a Chinese hamster ovary mutant cell line with altered sensitivity to vaccinia virus killing. J. Virol. 70:4655–4666 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Bergelson JM, Shepley MP, Chan BMC, Hemler ME, Finberg RW. 1992. Identification of the integrin Vla-2 as a receptor for echovirus-1. Science 255:1718–1720 [DOI] [PubMed] [Google Scholar]
- 6. Bisht H, Weisberg AS, Moss B. 2008. Vaccinia virus L1 protein is required for cell entry and membrane fusion. J. Virol. 82:8687–8694 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Brown E, Senkevich TG, Moss B. 2006. Vaccinia virus F9 virion membrane protein is required for entry but not virus assembly, in contrast to the related L1 protein. J. Virol. 80:9455–9464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Chang SJ, Chang YX, Izmailyan R, Tang YL, Chang W. 2010. Vaccinia virus A25 and A26 proteins are fusion suppressors for mature virions and determine strain-specific virus entry pathways into HeLa, CHO-K1, and L cells. J. Virol. 84:8422–8432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Chiu WL, Lin CL, Yang MH, Tzou DL, Chang W. 2007. Vaccinia virus 4c (A26L) protein on intracellular mature virus binds to the extracellular cellular matrix laminin. J. Virol. 81:2149–2157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Chung CS, et al. 2006. Vaccinia virus proteome: identification of proteins in vaccinia virus intracellular mature virion particles. J. Virol. 80:2127–2140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Chung CS, Hsiao JC, Chang YS, Chang W. 1998. A27L protein mediates vaccinia virus interaction with cell surface heparan sulfate. J. Virol. 72:1577–1585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Chung CS, Huang CY, Chang W. 2005. Vaccinia virus penetration requires cholesterol and results in specific viral envelope proteins associated with lipid rafts. J. Virol. 79:1623–1634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Collinet C, et al. 2010. Systems survey of endocytosis by multiparametric image analysis. Nature 464:243–249 [DOI] [PubMed] [Google Scholar]
- 14. Condit RC, Moussatche N, Traktman P. 2006. In a nutshell: structure and assembly of the vaccinia virion. Adv. Virus Res. 66:31–124 [DOI] [PubMed] [Google Scholar]
- 15. Cordes N, Seidler J, Durzok R, Geinitz H, Brakebusch C. 2006. Beta 1-integrin-mediated signaling essentially contributes to cell survival after radiation-induced genotoxic injury. Oncogene 25:1378–1390 [DOI] [PubMed] [Google Scholar]
- 16. Cue D, Dombek PE, Lam H, Cleary PP. 1998. Streptococcus pyogenes serotype M1 encodes multiple pathways for entry into human epithelial cells. Infect. Immun. 66:4593–4601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. de Curtis I. 2011. Host-pathogen interactions: cheating the host by making new connections. Curr. Biol. 21:R192–R194 [DOI] [PubMed] [Google Scholar]
- 18. de Magalhães JC, et al. 2001. A mitogenic signal triggered at an early stage of vaccinia virus infection—implication of MEK/ERK and protein kinase in a virus multiplication. J. Biol. Chem. 276:38353–38360 [DOI] [PubMed] [Google Scholar]
- 19. Dorner M, et al. 2010. Beta(1) integrin expression increases susceptibility of memory B cells to Epstein-Barr virus infection. J. Virol. 84:6667–6677 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Fassler R, et al. 1995. Lack of b1 integrin gene in embryonic stem cells affects morphology, adhesion, and migration but not integration into the inner cell mass of blastocysts. J. Cell Biol. 128:979–988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Feire AL, Koss H, Compton T. 2004. Cellular integrins function as entry receptors for human cytomegalovirus via a highly conserved disintegrin-like domain. Proc. Natl. Acad. Sci. U. S. A. 101:15470–15475 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Fenczik CA, Sethi T, Ramos JW, Hughes PE, Ginsberg MH. 1997. Complementation of dominant suppression implicates CD98 in integrin activation. Nature 390:81–85 [DOI] [PubMed] [Google Scholar]
- 23. Feral CC, et al. 2005. CD98hc (SLC3A2) mediates integrin signaling. Proc. Natl. Acad. Sci. U. S. A. 102:355–360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Gong S, Lai CF, Esteban M. 1990. Vaccinia virus induces cell-fusion at acid pH and this activity is mediated by the N-terminus of the 14-kDa virus envelope protein. Virology 178:81–91 [DOI] [PubMed] [Google Scholar]
- 25. Gu ZZ, Noss EH, Hsu VW, Brenner MB. 2011. Integrins traffic rapidly via circular dorsal ruffles and macropinocytosis during stimulated cell migration. J. Cell Biol. 193:61–70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Guerrero CA, et al. 2000. Integrin alpha(v)beta(3) mediates rotavirus cell entry. Proc. Natl. Acad. Sci. U. S. A. 97:14644–14649 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Gustavsson A, et al. 2002. Role of the beta1-integrin cytoplasmic tail in mediating invasin-promoted internalization of Yersinia. J. Cell Sci. 115:2669–2678 [DOI] [PubMed] [Google Scholar]
- 28. Harburger DS, Calderwood DA. 2009. Integrin signalling at a glance. J. Cell Sci. 122:159–163 (Erratum, 122:1472.) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Hsiao JC, Chung CS, Chang W. 1999. Vaccinia virus envelope D8L protein binds to cell surface chondroitin sulfate and mediates the adsorption of intracellular mature virions to cells. J. Virol. 73:8750–8761 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Huang CY, et al. 2008. A novel cellular protein, VPEF, facilitates vaccinia virus penetration into HeLa cells through fluid phase endocytosis. J. Virol. 82:7988–7999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Ichihashi Y, Oie M. 1996. Neutralizing epitope on penetration protein of vaccinia virus. Virology 220:491–494 [DOI] [PubMed] [Google Scholar]
- 32. Izmailyan R, Chang W. 2008. Vaccinia virus WR53.5/F14.5 protein is a new component of intracellular mature virus and is important for calcium-independent cell adhesion and vaccinia virus virulence in mice. J. Virol. 82:10079–10087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Izmailyan RA, Huang CY, Mohammad S, Isaacs SN, Chang W. 2006. The envelope G3L protein is essential for entry of vaccinia virus into host cells. J. Virol. 80:8402–8410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Jackson T, Mould AP, Sheppard D, King AMQ. 2002. Integrin alpha v beta 1 is a receptor for foot-and-mouth disease virus. J. Virol. 76:935–941 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Johnson MS, Lu N, Denessiouk K, Heino J, Gullberg D. 2009. Integrins during evolution: evolutionary trees and model organisms. Biochim. Biophys. Acta 1788:779–789 [DOI] [PubMed] [Google Scholar]
- 36. Jokinen J, et al. 2010. Molecular mechanism of alpha 2 beta 1 integrin interaction with human echovirus 1. EMBO J. 29:196–208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Joklik WK. 1962. The purification of four strains of poxvirus. Virology 18:9–18 [DOI] [PubMed] [Google Scholar]
- 38. Legate KR, Montanez E, Kudlacek O, Fassler R. 2006. ILK, PINCH and parvin: the tIPP of integrin signalling. Nat. Rev. Mol. Cell Biol. 7:20–31 [DOI] [PubMed] [Google Scholar]
- 39. Lenter M, et al. 1993. A monoclonal antibody against an activation epitope on mouse integrin chain-beta(1) blocks adhesion of lymphocytes to the endothelial integrin-alpha(6)beta(1). Proc. Natl. Acad. Sci. U. S. A. 90:9051–9055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Leong JM, Fournier RS, Isberg RR. 1990. Identification of the integrin binding domain of the Yersinia pseudotuberculosis invasin protein. EMBO J. 9:1979–1989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Limouze J, Straight AF, Mitchison T, Sellers JE. 2004. Specificity of blebbistatin, an inhibitor of myosin II. J. Muscle Res. Cell Motil. 25:337–341 [DOI] [PubMed] [Google Scholar]
- 42. Lin CL, Chung CS, Heine HG, Chang W. 2000. Vaccinia virus envelope H3L protein binds to cell surface heparan sulfate and is important for intracellular mature virion morphogenesis and virus infection in vitro and in vivo. J. Virol. 74:3353–3365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Locker JK, et al. 2000. Entry of the two infectious forms of vaccinia virus at the plasma membrane is signaling-dependent for the IMV but not the EEV. Mol. Biol. Cell 11:2497–2511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Maginnis MS, et al. 2006. Beta1 integrin mediates internalization of mammalian reovirus. J. Virol. 80:2760–2770 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Mercer J, Helenius A. 2008. Vaccinia virus uses macropinocytosis and apoptotic mimicry to enter host cells. Science 320:531–535 [DOI] [PubMed] [Google Scholar]
- 46. Morgan MR, Humphries MJ, Bass MD. 2007. Synergistic control of cell adhesion by integrins and syndecans. Nat. Rev. Mol. Cell Biol. 8:957–969 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Nichols RJ, Stanitsa E, Unger B, Traktman P. 2008. The vaccinia virus gene I2L encodes a membrane protein with an essential role in virion entry. J. Virol. 82:10247–10261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Pankov R, et al. 2003. Specific beta(1) integrin site selectively regulates Akt/protein kinase B signaling via local activation of protein phosphatase 2A. J. Biol. Chem. 278:18671–18681 [DOI] [PubMed] [Google Scholar]
- 49. Perino J, et al. 2011. Role of sulfatide in vaccinia virus infection. Biol. Cell 103:319–331 [DOI] [PubMed] [Google Scholar]
- 50. Price LS, Leng J, Schwartz MA, Bokoch GM. 1998. Activation of Rac and Cdc42 by integrins mediates cell spreading. Mol. Biol. Cell 9:1863–1871 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Resch W, Hixson KK, Moore RJ, Lipton MS, Moss B. 2007. Protein composition of the vaccinia virus mature virion. Virology 358:233–247 [DOI] [PubMed] [Google Scholar]
- 52. Satheshkumar PS, Moss B. 2009. Characterization of a newly identified 35-amino-acid component of the vaccinia virus entry/fusion complex conserved in all chordopoxviruses. J. Virol. 83:12822–12832 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Schiller HB, Friedel CC, Boulegue C, Fassler R. 2011. Quantitative proteomics of the integrin adhesome show a myosin II-dependent recruitment of LIM domain proteins. EMBO Rep. 12:259–266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Schottelndreier H, Mayr GW, Guse H. 1999. β1-integrins mediate Ca2+-signalling and T cell spreading via divergent pathways. Cell. Signal. 11:611–619 [DOI] [PubMed] [Google Scholar]
- 55. Schroeder N, Chung CS, Chen CH, Liao CL, Chang W. 2012. The lipid raft-associated protein CD98 is required for vaccinia virus endocytosis. J. Virol. 86:4868–4882 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Senkevich TG, Ojeda S, Townsley A, Nelson GE, Moss B. 2005. Poxvirus multiprotein entry-fusion complex. Proc. Natl. Acad. Sci. U. S. A. 102:18572–18577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Shannon P, et al. 2003. Cytoscape: a software environment for integrated models of biomolecular interaction networks. Genome Res. 13:2498–2504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Spiegel S, Kassis S, Wilchek M, Fishman PH. 1984. Direct visualization of redistribution and capping of fluorescent gangliosides on lymphocytes. J. Cell Biol. 99:1575–1581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Stewart PL, Nemerow GR. 2007. Cell integrins: commonly used receptors for diverse viral pathogens. Trends Microbiol. 15:500–507 [DOI] [PubMed] [Google Scholar]
- 60. Tarone G, et al. 1993. Expression of beta-1 integrin complexes on the surface of unfertilized mouse oocyte. Development 117:1369–1375 [DOI] [PubMed] [Google Scholar]
- 61. Townsley AC, Weisberg AS, Wagenaar TR, Moss B. 2006. Vaccinia virus entry into cells via a low-pH-dependent endosomal pathway. J. Virol. 80:8899–8908 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Vanderplasschen A, Hollinshead M, Smith GL. 1998. Intracellular and extracellular vaccinia virions enter cells by different mechanisms. J. Gen. Virol. 79(Pt 4):877–887 [DOI] [PubMed] [Google Scholar]
- 63. Vanderplasschen A, Smith GL. 1997. A novel virus binding assay using confocal microscopy: demonstration that the intracellular and extracellular vaccinia virions bind to different cellular receptors. J. Virol. 71:4032–4041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Veettil MV, et al. 2008. Kaposi's sarcoma-associated herpesvirus forms a multimolecular complex of integrins (alphaVbeta5, alphaVbeta3, and alpha3beta1) and CD98-xCT during infection of human dermal microvascular endothelial cells, and CD98-xCT is essential for the postentry stage of infection. J. Virol. 82:12126–12144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Velling T, Nilsson S, Stefansson A, Johansson S. 2004. Beta1-integrins induce phosphorylation of Akt on serine 473 independently of focal adhesion kinase and Src family kinases. EMBO Rep. 5:901–905 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Whitbeck JC, Foo CH, Ponce de Leon M, Eisenberg RJ, Cohen GH. 2009. Vaccinia virus exhibits cell-type-dependent entry characteristics. Virology 385:383–391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Wickstrom SA, Fassler R. 2011. Regulation of membrane traffic by integrin signaling. Trends Cell Biol. 21:266–273 [DOI] [PubMed] [Google Scholar]
- 68. Wolfe CL, Ojeda S, Moss B. 2012. Transcriptional repression and RNA silencing act synergistically to demonstrate the function of the eleventh component of the vaccinia virus entry-fusion complex. J. Virol. 86:293–301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Yoder JD, et al. 2006. Pox proteomics: mass spectrometry analysis and identification of vaccinia virion proteins. Virol. J. 3:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Zent R, et al. 2000. Class- and splice variant-specific association of CD98 with integrin beta cytoplasmic domains. J. Biol. Chem. 275:5059–5064 [DOI] [PubMed] [Google Scholar]