Abstract
Hepatocyte growth factor (HGF)-stimulated mitogenesis, motogenesis and morphogenesis in various cell types begins with activation of the Met receptor tyrosine kinase and the recruitment of intracellular adaptors and kinase substrates. The adapter protein Gab1 is a critical effector and substrate of activated Met, mediating morphogenesis, among other activities, in epithelial cells. To define its role downstream of Met in hematopoietic cells, Gab1 was expressed in the HGF-responsive, Gab1-negative murine myeloid cell line 32D. Interestingly, the adhesion and motility of Gab1-expressing cells were significantly greater than parental cells, independent of growth factor treatment. Downstream of activated Met, Gab1 expression was specifically associated with rapid Shp-2 recruitment and activation, increased mitogenic potency, suppression of GATA-1 expression and concomitant upregulation of GATA-2 transcription. In addition to enhanced proliferation, continuous culture of Gab1-expressing 32D cells in HGF resulted in cell attachment, filopodia extension and phenotypic changes suggestive of monocytic differentiation. Our results suggest that in myeloid cells, Gab1 is likely to enhance HGF mitogenicity by coupling Met to Shp-2 and GATA-2 expression, thereby potentially contributing to normal myeloid differentiation as well as oncogenic transformation.
Keywords: HGF, Met, Gab1, Shp-2, myeloid cells
INTRODUCTION
Hepatocyte growth factor (HGF) is a multifunctional cytokine that stimulates growth, survival, motility, and morphogenesis in a wide variety of target cell types. HGF signaling via its cell surface receptor, Met, is fundamentally important in development, homeostasis and tissue regeneration, as well as in tumorigenesis. The HGF/Met pathway is also important in hematopoiesis. HGF and Met are expressed in sites of primordial hematopoiesis, as well as in the adult hematopoietic environment. HGF produced by bone marrow stromal cells is essential for the differentiation and self-renewal of hematopoietic stem cells, and promotes the proliferation, differentiation and survival of hematopoietic precursor cells induced by other hematopoietic growth factors, such as interleukin (IL)-3 and stem cell factor (SCF) (Galimi et al., 1994; Weimar et al., 1998). Moreover, HGF regulates dendritic cell (Ovali et al., 2000) and antigen-specific B cell differentiation (Taher et al., 1999; Van Der Voort et al., 1997). HGF is also an important regulator of thrombopoiesis, and HGF transgenic mice overexpress thrombopoietin and display thrombocytosis with megakaryocytosis (Kosone et al., 2007).
Upon ligand binding, Met undergoes autophosphorylation on tyrosine residues, two of which (Y1349 and Y1356) form a binding site for several intracellular effectors including phosphatidylinositol 3-kinase (PI3K), phospholipase C-γ (PLC-γ), Src, Src homology-2 (SH2)-containing protein tyrosine phosphatase 2 (Shp2), signal transducer and activator of transcription 3 (STAT3), and the adapter proteins v-crk avian sarcoma virus CT10 oncogene homolog-like (Crk-L), growth factor receptor bound protein 2 (Grb2), SH2 domain containing transforming protein 1 (Shc1), and growth factor receptor bound protein 2-associated protein 1 (Gab1) (Zhang and Vande Woude, 2003). Gab1 is a member of the family of docking proteins that includes the insulin receptor substrates IRS-1, IRS-2, and IRS-3, FGF receptor substrate-2 (FRS-2/SNT1), Drosophila Daughter of Sevenless (DOS), the p62 Dok subfamily, as well as Gab2 and Gab3 (Gu et al., 1998; Wolf et al., 2002; Zhao et al., 1999). Family members are typically phosphorylated by receptor or receptor-associated tyrosine kinases and contribute to downstream signal specificity and amplification (Pawson and Scott, 1997). Gab1 contains an amino-terminal pleckstrin homology domain that binds the plasma membrane lipid phosphatidylinositol 3,4,5-triphosphate (Maroun et al., 1999; Rodrigues et al., 2000), a carboxyl-terminal proline-rich Met binding domain (MBD), as well as potential binding sites for SH2 and SH3 domains (Holgado-Madruga et al., 1996; Schaeper et al., 2000; Weidner et al., 1996). Gab1 binds directly to phospho-Y1349 in Met through the MBD domain (Nguyen et al., 1997; Weidner et al., 1996) and indirectly through Grb2 bound to phospho-Y1356 in Met (Bardelli et al., 1997; Fixman et al., 1997; Nguyen et al., 1997). Several tyrosine residues in Gab1 become phosphorylated in response to stimulation by cytokines and growth factors (e.g. IL-3, IL-6, erythropoietin, thrombopoietin, HGF, epidermal growth factor, nerve growth factor, platelet-derived growth factor, insulin and SCF), and following activation of T- and B-cell-antigen receptors, G-protein coupled receptors, and the complement component C1q receptor (gC1q-R/p32) (Braun et al., 2000).
Gab1 functions in a network with other intracellular signaling molecules including Grb2, PI3K, Shc, PLC-γ, Crk-L, and Shp-2 (Nishida and Hirano, 2003). Shp-2 has two tandem SH2 domains amino-terminal to a phosphatase domain and is a predominant Gab1-associated molecule in mitogen-stimulated cells (Feng and Pawson, 1994; Neel and Tonks, 1997). The binding of Shp-2 SH2 domains to other proteins and Shp-2 tyrosyl phosphorylation have been shown to independently activate its phosphatase activity (Lechleider et al., 1993; Vogel and Ullrich, 1996). Shp-2 regulates Ras signaling downstream of growth factor and cytokine receptors, affecting mitogenesis, cell adhesion and migration (Dance et al., 2008). Aberrant Shp-2 function has been linked to several malignancies (Chan, 2008); for example activating Shp-2 mutations have been identified in individuals with Noonan syndrome, a developmental disorder associated with juvenile myelomonocytic leukemia(Wang et al., 2009). Like Gab1, Shp-2 participates in signaling proximal to a variety of hematopoietic and non-hematopoietic cytokine and growth factor receptors (Chan, 2008) through mechanisms that are not yet fully defined.
Several lines of evidence suggest that Gab1 is a critical mediator of HGF signaling. The transforming potential of Tpr-Met, an oncogenic variant of Met, correlates with its ability to associate with and phosphorylate Gab1 (Bardelli et al., 1997; Fixman et al., 1997). Genetic studies also indicate an essential role for Gab1 in Met signaling in vivo (Itoh et al., 2000; Sachs et al., 2000; Schaeper et al., 2007). Gab1-deficient embryos die in utero and display abnormal development of liver and placenta as well as defects in the migration of myogenic precursors into the limb bud, features which are very similar to those observed in mice lacking HGF or Met (Sachs et al., 2000). Moreover, overexpression of Gab1 in Madin-Darby canine kidney (MDCK) epithelial cells is sufficient to promote HGF-induced branching tubulogenesis and scattering (Weidner et al., 1996; Furge et al., 2000).
To investigate Gab1 function in hematopoietic cells, we reconstituted Gab1 expression in the HGF-responsive myeloid cell line 32D, which lacks endogenous expression of Gab1 family members known to interact directly with Met (Gab1, IRS-1 and IRS-2) (Sun et al., 1995; Wang et al., 1993). We show that in myeloid cells, Gab1 constitutively enhances adhesion and motility, enhances HGF mitogenic potency by coupling Met to Shp-2, and is required for HGF-induced morphogenic differentiation.
MATERIALS AND METHODS
Reagents
Cell culture supplies were from Gibco BRL (Grand Island, NY, USA). Human plasma fibronectin was purchased from Becton Dickinson (The Oak Park, Bedford, MA, USA). Recombinant mouse IL-3 was purchased from Sigma-Aldrich (St. Louis, MO, USA). The truncated HGF isoform HGF/NK1, which possesses the same biological activities as full length HGF, was expressed recombinantly in E. coli, purified and refolded as previously described (Stahl et al., 1997). Anti-Gab1 and anti-phosphotyrosine (pTyr) antibodies were from Upstate Biotechnology (Lake Placid, NY, USA). Anti-Met, and anti-Shp-2 antibodies were purchased from Santa Cruz Biotechnology (Santa Cruz, CA, USA). Anti-p38, Erk1, Erk2, JNK, and Akt/PKB antibodies were purchased from New England Biolabs (Beverly, MD, USA). Reporter HRP-anti-mouse/rabbit antibodies were from Amersham (Buckinghamshire, England).
Cell Culture and Transfection
A clone of murine IL-3-dependent 32D cells expressing the human Met receptor (32D/Met) that has been previously described (Day et al., 1999) was maintained in RPMI 1640 medium supplemented with 15% heat-inactivated fetal bovine serum (FBS) and 5% WEHI-3B-conditioned medium as source of IL-3. 32D/Met/Gab1 cells were generated by co-transfection of 32D/Met cells with pBAT-Flag-Gab1 mouse Gab1 cDNA (Weidner et al., 1996) and a histidinol-resistance-encoding cDNA by electroporation as previously described (Day et al., 1999). Cells were simultaneously selected in 2 mM histidinol (Sigma) and 750 ug/ml G418 (Mediatech, Inc., Manassa, VA) for Gab1 and Met expression respectively. In parallel, an empty vector was transfected into 32D/Met cells as a control and results obtained using these cells were equivalent in all respects to those obtained using the parental 32D/Met cells. Gab1 expression in stable clonal cell lines was detected by immunoblotting.
Immunoprecipitation and Immunoblotting
Cells were starved in RPMI/0.8% bovine serum albumin (BSA) for 3 hours before receiving 300 ng/ml of HGF/NK1 or 5 ng/ml of IL-3 for 10 min at 37°C. Cells lysis, immunoprecipitation, immunoblotting and detection by enhanced chemiluminescence were performed as described (Timms et al., 1998). Immunoprecipitations utilized 4 μg antibody/mg cell lysate, while immunoblots utilized 1:1000 dilution of the primary antibody in 0.1% BSA/phosphate-buffered saline (PBS)/0.05% Tween. Analysis of Erk1 and Erk2 phosphorylation by immunoblotting was performed according to the manufacturer's instructions with 1:1000 dilution of a monoclonal antibody that recognizes Erk1 and Erk2 only when phosphorylated at Thr202/Tyr204 (New England Biolabs). Analysis of JNK1 phosphorylation by immunoblotting was performed with 1:1000 dilution of a polyclonal antibody that recognizes JNK only when phosphorylated at Thr183/Tyr185, according to the manufacturer's instructions (New England Biolabs). Analysis of Akt/PKB phosphorylation was performed by immunoblotting with phospho-specific antibody to Ser473 (New England Biolabs) according to the manufacturer's instructions.
Mitogenicity, Motility and Cell Adhesion Assays
Mitogenic assays were performed as previously described (Soon et al., 1999; Stahl et al., 1997). Briefly, transfectants of 32D cells were washed twice with PBS and 1×105 cells were plated onto each well of 24-well plates in RPMI 1640/15% heat-inactivated FBS without IL-3. Recombinant human HGF (gift from Dr. G. Vande Woude, Van Andel Institute, Grand Rapids, MI) was added at the indicated concentrations. When indicated, wortmannin (100nM) or LY294002 (0.5 μM) was included. After 44 hours in culture, the cells were pulsed with 1μCi of [3H]-thymidine (New England Nuclear) for another 4 hours and harvested with a cell harvester (Skatron). Incorporation of [3H]-thymidine was determined by liquid scintillation counting.
For proliferation assays with Shp-2 dominant negative mutants, cells were resuspended at 107/0.5 ml in RPMI/10%FBS, incubated 10 min with 10 μg of the indicated expression vectors. Cells were then electroporated at 300 V/960 μF, and transferred to fresh RPMI/15% heat-inactivated FBS/5%WEHI-3B-conditioned medium. Three hours post-transfection, mitogenic assays were performed as described above and cells were harvested 20 hours after transfection. The transfection efficiency was assessed by Western blot analysis of Shp-2 protein expression on parallel samples.
32D/Met and 32D/Met/Gab1 cell migration was assayed using a modified Boyden chamber with 5μm pore size Nucleopore filters (Corning) as described previously (Day et al., 1999). The assay was performed in Dulbecco's Modified Eagle Medium/0.1% BSA. HGF/NK1 was added to the lower chamber at the indicated concentrations and 6×105 32D/Met or 32D/Met/Gab1 cells were applied to the upper chamber and incubated 8 hours at 37°C. The number of migrated cells in the lower chamber was determined with a cell counter (Coulter).
Adhesion assays were performed as described previously (Richard et al., 1995). Briefly, 0.8ml of PBS containing 16 μg /ml of human fibronectin (FN) (Collaborative Research, Becton Dickinson) was distributed in 35 mm plates. Following 5 hours incubation at room temperature the wells were air dry for 2 hours. Cells (4×105/35 mm dish) were either seeded in complete medium (RPMI1640/15% heat-inactivated FBS/5% WEHI-3B conditioned medium as a source of IL-3) in the absence or in the presence of HGF/NK1 (300 ng/ml) or were washed twice with PBS, IL-3-starved 3 hours in RPMI1640/15% heat-inactivated FBS, and then seeded in RPMI1640/15% heat-inactivated FBS, with or without IL-3 (5 ng/ml). After incubation at 37°C for the indicated time periods, nonadherent cells were removed by three consecutive rinses with Ca2+- and Mg2+-containing PBS. Adherent cells were harvested with 0.1% EDTA and both the suspended and the attached cells were counted with a cell counter. Percent adhesion was calculated as the ratio of the adherent cells/(adherent cells + suspended cells) ×100. Statistical significance was determined by Student's t test using GraphPad Prism software; values are expressed as mean +/- standard deviation and significance (p < 0.05) is indicated throughout the text.
Light Microscopy
Living cells were examined by light microscopy after 9 days in culture in RPM1/15%FBS/5%WEHI-3B-conditioned medium with or without HGF (300 ng/ml). The cultures were split every 3 days. The dishes were examined with an inverted microscope (Zeiss Axiovert 135TV) and images were recorded with a CCD video camera.
Quantitative Real Time RT-PCR
To confirm gene expression changes observed in microarray studies, total RNA from 32D/Met and 32D/Met/Gab1 cells treated with HGF/NK1 (300 ng/ml) and heparin (1 μg/ml) for 6h was isolated with the RNeasy mini kit (Quiagen Inc., Valencia, CA). cDNA was synthesized with SuperScript™III First-Strand Synthesis System (Invitrogen) and amplified using a TaqMan® Gene Expression Assay (Applied Biosystems) on an ABI PRISM®7000. For each sample, gene expression and the internal control, 18S rRNA, was determined using the comparative threshold cycle (CT) method. Each gene was examined in three replicates and was repeated with at least two independent preparations of RNA. A two-tailed Student's t test was used to determine significant difference (p <0.01) between the transcriptional level of each gene in a 32D/Met/Gab1 clone and the corresponding 32D/Met clone. The primer/probe sets used (Applied Biosystems) were Mm00484678_m1 for GATA-1 (GenBank NM_008089) and Mm00492300_m1 for GATA-2 (GenBank NM_008090).
RESULTS
Gab1 Engages Multiple Effectors in Cytokine-Stimulated 32D Cells
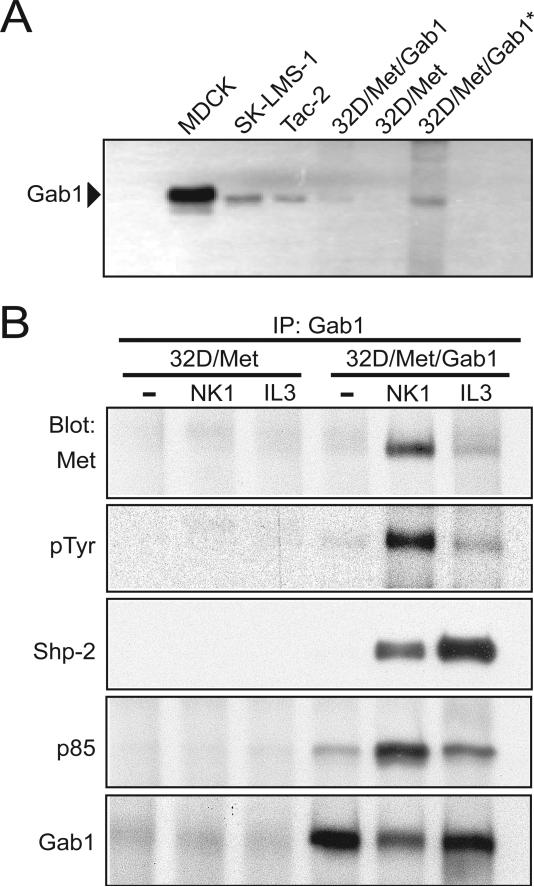
We previously characterized Met signaling in 32D cells by creating stable human Met transfectants (32D/Met) (Day et al., 1999). The 32D/Met cell line was then transfected with a murine Gab1 cDNA and individual clones from antibiotic resistance-selected mass cultures were derived (32D/Met/Gab1). Two clones expressing different Gab1 levels, one with low expression (1W3) and one with 2.5-fold higher expression (1W5), as determined by quantitative real-time-PCR (data not shown) and immunoblot analysis (Fig. 1A), were selected for further study. Where indicated, the truncated HGF isoform HGF/NK1 (denoted hereafter as NK1), which possesses the same biological activities as full-length HGF (Stahl et al., 1997), was used to activate Met.
Figure 1. Ectopically-expressed Gab1 engages Met and IL-3R effectors in 32D/Met cells.
A. Gab1 expression in various cell lines. Fifty μg or 100 μg (*) of cell lysate per lane were subjected to immunoblot analysis with anti-Gab1 antibody. Cell lines: MDCK canine kidney epithelial cells, SK-LMS-1 human leiomyosarcoma cells, Tac-2 mouse mammary epithelial cells, 32D/Met cells and 32D/Met/Gab1 cell clone 1W5. B. Anti-Gab1 immunoprecipitation of lysates prepared from serum deprived 32D/Met or 32D/Met/Gab1 cell clone 1W5 left untreated (-) or stimulated with NK1 (300 ng/ml) or IL-3 (6 ng/ml) for 10 min at 37°C. From top to bottom, NK1-stimulated association of Gab1 with Met (Weidner et al., 1996); NK1- and IL-3-stimulated Gab1 phosphorylation (IB: pTyr); NK1- and IL-3-induced association of Gab1 with Shp-2 (IB: Shp-2) and p85/PI3K (IB: p85); and the amount of Gab1 protein present in each lane.
Gab1 is a predominant phosphotyrosyl-containing protein following HGF stimulation of epithelial cells (Nguyen et al., 1997). Upon phosphorylation, Gab1 associates with the protein tyrosine phosphatase Shp-2 and with the p85 subunit of phoshatidylinositol 3-kinase (PI3K) downstream of the IL-3 (Takahashi-Tezuka et al., 1998) and HGF receptors (Maroun et al., 1999; Nguyen et al., 1997; Schaeper et al., 2000). To determine whether these signaling proteins associated with ectopically expressed Gab1 in NK1- or IL-3 -stimulated 32D/Met/Gab1 cells, anti-Gab1 immunoprecipitation was followed by immunoblotting with anti-Met, anti-pTyr, anti-Shp-2, anti-p85, or anti-Gab1 (Fig. 1B). NK1 stimulated the rapid physical association of Gab1 with activated Met (Fig. 1B). Treatment with either NK1 or IL-3 resulted in tyrosyl phosphorylation of Gab1 and significantly increased the physical association of Gab1 with both Shp-2 and p85 (Fig. 1B). A much lower level of constitutive Gab1-p85 association was also detected (Fig. 1B), consistent with a prior report of constitutive Gab1-Grb2-PI3K association in A431 cells (Holgado-Madruga et al., 1996). Thus Gab1, when ectopically expressed in 32D cells, couples the HGF and IL-3 receptors with multiple signaling pathways similar to those described in epithelial and other hematopoietic cell types (Nishida and Hirano, 2003; Sarmay et al., 2006).
Gab1 Enhances Cell Motility and Adhesion to Fibronectin
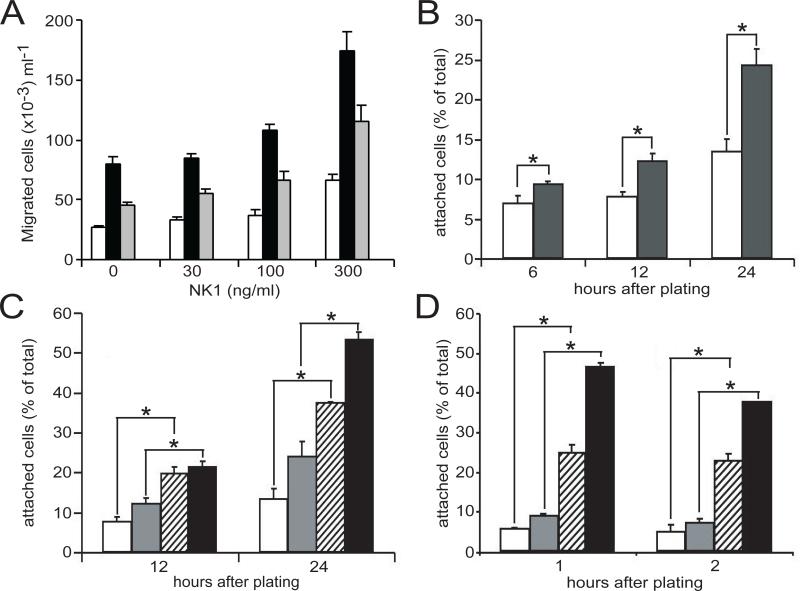
HGF-stimulated migration of epithelial, mesenchymal, and hematopoietic cells occurs during embryogenesis, tissue repair, inflammation, tumor invasiveness and metastasis. We investigated the role of Gab1 in HGF-stimulated motility by comparing the motile responses of serum-deprived 32D/Met and 32D/Met/Gab1 cells using a modified Boyden chamber assay. As shown in Figure 2, the basal motility of both parental and 32D/Met/Gab1 cells was enhanced by NK1 stimulation in a dose-dependent fashion. However, when expressed as fold increase relative to untreated cells, NK1 stimulated the motility of both cell lines with equal potency. Interestingly, in the absence of NK1, the spontaneous or basal motility of 32D/Met/Gab1 cells was 3-fold higher than that of the parental cell line (p < 0.05). This effect was also directly related to Gab1 protein abundance: a lower Gab1 expressing clone (1W3) displayed an intermediate basal motility when compared to the 1W5 clone and 32D/Met (Fig. 2A). These data indicate that Gab1 expression enhances cell motility generally, independent of HGF-stimulation in 32D cells.
Figure 2. Gab1 constitutively enhances migration and stimulates adhesion of 32D/Met cells.
A. The migration of 32D/Met cells (open bars), 32D/Met/Gab1clone 1W5 (black bars), and 32D/Met/Gab1 clone 1W3 (gray bars) was measured using modified Boyden chambers. NK1 concentrations are indicated on the X-axis. Results are expressed as number of migrating cells/ml of medium in the bottom chamber and are representative of three or more experiments. All differences observed within dose groups are significant (p < 0.05). B. Growing cells were plated on FN-coated dishes in the absence (open bars) or presence (black bars) of NK1 (300ng/ml). At the indicated time points attached and suspended cells were counted. The Y-axis shows the number of attached cells expressed as percentage of the total number of cells in the plate to compensate for proliferation that could occur during the assay period. C. 32D/Met cell adhesion in the absence (open bars) or presence (gray bars) of NK1 (300 ng/ml), and 32D/Met/Gab1 clone 1W5 adhesion in the absence (striped bars) of presence (black bars) of NK1. D. IL-3 deprived 32D/Met (open bars), IL-3-treated 32D/Met cells (gray bars), IL-3-deprived 32D/Met/Gab1 cell clone 1W5 (striped bars) and IL-3 treated 32D/Met/Gab1 cell clone 1W5 (black bars) were incubated in FN-coated dishes for the indicated time periods at 37°C, and suspended and attached cells were counted. Data are expressed as percent attached cells of triplicate samples, and are representative of three or more experiments. Error bars indicate standard deviation; where no error bars are visible, the error is too small to be shown. In all panels, asterisks indicate statistically significant differences between the bracketed groups.
Previous studies revealed that HGF augments the adhesion of Met-positive B-cell lines to fibronectin (FN) by activating integrin α4β1 (Van Der Voort et al., 1997; Weimar et al., 1997). In 32D/Met cells, NK1 significantly augmented adhesion to immobilized FN in a time-dependent manner (Fig. 2B; p < 0.05). When 32D/Met/Gab1 or 32D/Met cells, grown in serum and IL-3, were allowed to attach to FN-coated wells for 12 to 24 hours in the presence or absence of NK1, Gab1 expression further enhanced adhesion (Fig. 2C; p < 0.05). Gab1 expression appeared to increase the basal level of cell adhesion without increasing the fold-stimulation associated with NK1 treatment, suggesting that Gab1 affected cell adhesion constitutively. However, previous work has shown that cytokines such as IL-3, which is present in WEHI growth medium supplement in our assay, induce adhesion of CD34+ hematopoietic progenitors. In particular, IL-3 has been implicated in “inside-out” signaling through the activation of integrin function in hematopoietic progenitors (Levesque et al., 1995; Levesque et al., 1996). To determine whether IL-3 had enhanced the attachment of Gab1-expressing 32D cells, adhesion studies were performed with IL-3-deprived cells in the presence of serum, with or without added IL-3 (5 ng/ml). Consistent with a previous report (Levesque et al., 1995), very few IL-3-deprived 32D/Met cells attached to FN, and adhesion was modestly activated upon addition of IL-3 (Fig. 2D). In contrast, Gab1 expression was associated with a dramatic increase in the adhesion of IL-3-deprived, untreated cells (Fig. 2D; p < 0.05), indicating that IL-3 signaling was only partly responsible for the increased adhesion of 32D/Met/Gab1 cells. Thus the increased adhesion observed in NK1-stimulated 32D/Met/Gab1 cells (Fig. 2C) resulted from constitutive Gab1 activity combined with Gab1-mediated signaling downstream of the IL-3 and HGF receptors.
To determine whether constitutive enhancement of cell attachment and motility by Gab1 occurred by promoting increased expression cell surface integrins, we compared the surface levels of the α4β1 and α5β1 integrin subunits in Gab1-expressing and parental 32D cells by FACS analysis using specific monoclonal antibodies. Equivalent levels of these integrins were found in both cell lines (data not shown), indicating that Gab1 enhances cell adhesion in response to the engagement of existing integrin receptors.
Gab1 Enhances HGF Mitogenicity in Hematopoietic Cells
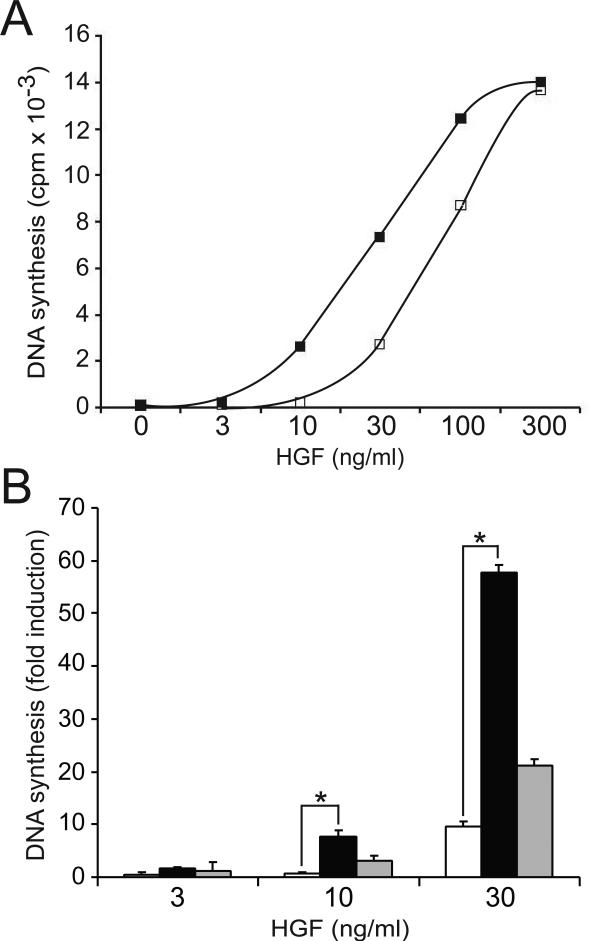
HGF is a potent mitogen for epithelial cells, endothelial cells and myeloid progenitors (Day et al., 1999). To investigate the role of Gab1 in HGF-mediated mitogenesis, we compared the levels of HGF-stimulated DNA synthesis in 32D/Met and 32D/Met/Gab1 cells. As shown in Figure 3A, Gab1 expression markedly enhanced HGF-stimulated S-phase entry in a dose-dependent manner. At physiologically relevant HGF doses, 32D/Met/Gab1 cells exhibited a 3- to 5-fold greater mitogenic response than cells expressing the same level of Met but lacking Gab1 (Fig. 3A; p < 0.05). Comparing HGF-induced DNA synthesis in the two 32D/Met/Gab1 clones revealed that Gab1 amplification of HGF mitogenicity was directly related to the level of Gab1 protein expression (Fig 3B; p < 0.05 at both 10 ng/ml and 30 ng/ml HGF). These results are consistent with reported correlations between Gab1 overexpression and tumorigenesis in epithelial cells (Seiden-Long et al., 2008; Kameda et al., 2001).
Figure 3. Gab1 expression enhances HGF mitogenesis in 32D/Met cells.
A. DNA synthesis in 32D/Met cells (open squares), and in 32D/Met/Gab1 cell clone 1W5 (filled squares) is shown in response to increasing concentrations of HGF. Results are expressed as mean CPM + SD of triplicate measurements and are representative of three or more experiments. B. DNA synthesis by 32D/Met cells (open bars), 32D/Met/Gab1 clone 1W5 (black bars), and clone 1W3 (gray bars), in response to increasing concentrations of HGF. Ordinate indicates average fold increase in [3H]-thymidine uptake compared to unstimulated cells. Asterisks indicate statistically significant differences between the bracketed groups.
Gab1-Enhanced Mitogenicity is not Mediated by MAPKs, PI3K or Akt
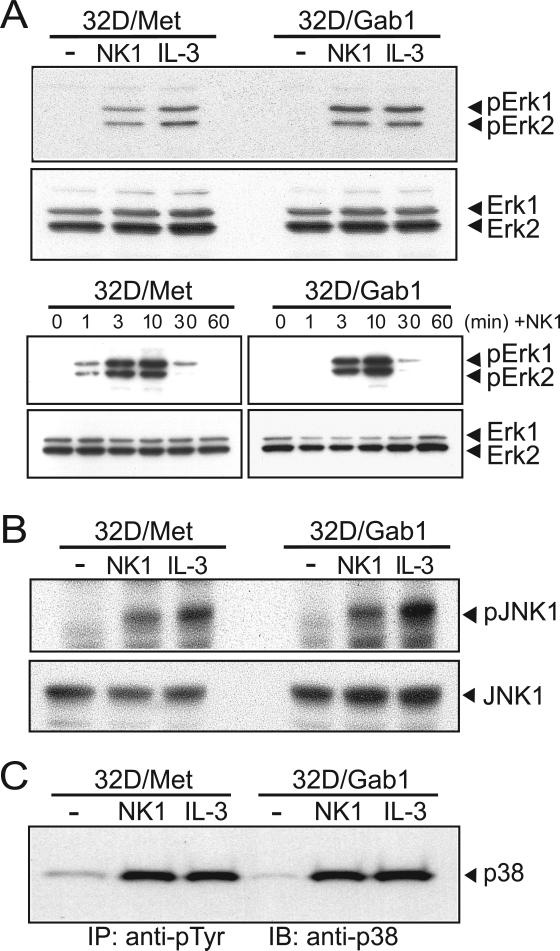
To identify pathways that might mediate Gab1-enhanced growth factor signaling, we assessed the activation state of several known effectors of mitogenicity. We first examined the phosphorylation of extracellular signal-regulated kinase (Erk)-1 (Heisterkamp et al., 1999) and Erk2 upon NK1 and IL-3 stimulation of 32D/Met and 32D/Met/Gab1cells. No significant enhancement of Erk activation was detected in 32D/Met/Gab1 cells compared to the parental cells initially, or over 60 minutes of NK1 stimulation (Fig. 4A). c-Jun N-terminal kinase (JNK) is involved in Met-induced transformation (Rodrigues et al., 1997) and is activated downstream of phospho-Gab1-Crk interaction (Garcia-Guzman et al., 2000). We observed equivalent NK1- and IL-3-stimulated induction of phospho-JNK in both 32D/Met/Gab1 and 32D/Met cell lines (Fig. 4B). Similarly, Gab1 expression had no detectable effect on cytokine-stimulated p38 MAPK phosphorylation (Fig. 4C). Thus MAPKs were unlikely to mediate Gab1-enhanced mitogenesis in hematopoietic cells.
Figure 4. Gab1 expression does not alter HGF-induced activation of MAP kinases.
A. Serum-deprived 32D/Met or 32D/Met/Gab1 cell clone 1W5 were left untreated or stimulated for 10 min at 37°C (upper two panels), or stimulated for 1-60 min as indicated (lower two panels), then lysed with detergent containing buffer. Lysate samples were resolved by SDS-PAGE and immunoblotted with antibodies against phospho-Erk1/2 (upper panel in each set) or total Erk1/2 (lower panel in each set). B. Serum-deprived 32D/Met or 32D/Met/Gab1 cell clone 1W5 were left untreated or stimulated for 10 min at 37°C, then lysed. Lysate samples were resolved by SDS-PAGE and immunoblotted with the antibodies against phosphor-JNK and JNK (upper and lower panels, respectively) C. Anti-phosphotyrosine immunoprecipites of lysates prepared from 32D/Met or 32D/Met/Gab clone 1W5 treated as described in Panel A were resolved by SDS-PAGE and immunoblotted with anti-p38 antibody.
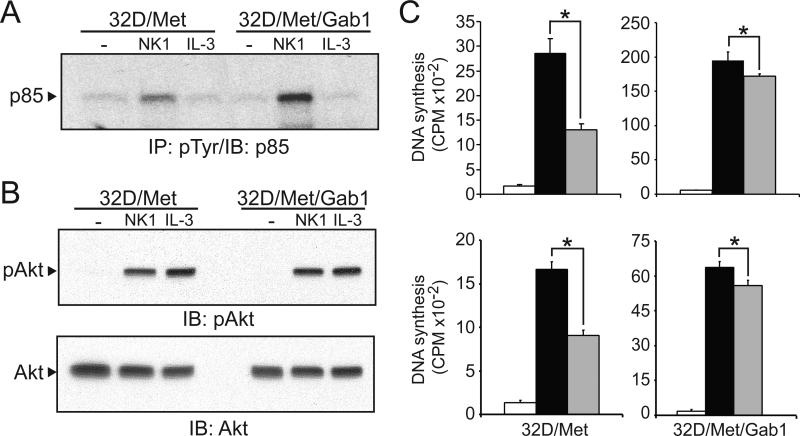
PI3K activation is required for HGF-induced mitogenesis in epithelial cells (Rahimi et al., 1996). HGF also activates PI3K in 32D/Met cells (Day et al., 1999), coincident with Gab1 binding to the 85kDa regulatory subunit of PI3K (Maroun et al., 1999). We compared NK1- and IL-3-driven PI3K recruitment in 32D/Met/Gab1 and 32D/Met cells by immunoprecipitating with anti-pTyr and immunoblotting for PI3K p85. As shown in Figure 5A, significantly more p85/PI3K was recruited in NK1-stimulated 32D/Met/Gab1 cells than in parental cells, suggesting that PI3K might mediate Gab1-enhanced HGF mitogenicity. We also examined whether Gab1 expression affected Akt activation downstream of activated PI3K by immunoblotting lysates from growth factor stimulated cells with an antibody that specifically recognizes Akt phosphoserine 473. As shown in Figure 5B, no difference in Akt activation was detected in 32D/Met/Gab1 relative to 32D/Met cells. Thus, while more PI3K was recruited to activated Met/effector complexes, this did not result in increased Akt activity.
Figure 5. PI3K does not mediate Gab1-enhanced HGF mitogenicity.
A. Serum-deprived 32D/Met or 32D/Met/Gab1 cell clone 1W5 were left untreated (-) or stimulated with NK1 (300 ng/ml) or IL-3 (6 ng/ml). Cell extracts were immunoprecipitated with anti-pTyr, resolved by SDS-PAGE and immunoblotted with anti p85/PI3K antibody. B. Cell lysates were analyzed by immunoblotting with anti-phosphoAkt/PKB (upper panel) or anti-Akt/PKB (lower panel). C. Upper panel: DNA synthesis by 32D/Met and 32D/Met/Gab1 cell clone 1W5 left untreated (open bars), or treated with HGF (30ng/ml) without Wortmannin (black bars) or with Wortmannin (gray bars; 100 nM; upper panels). Lower panel: as in upper panel, except gray bars represent treatment with LY294002 (0.5 μM). Results are expressed as mean CPM ± SD of quadruplicate samples and are representative of three experiments. In all panels, asterisks indicate statistically significant differences between the bracketed groups.
To determine whether p85/PI3K activation was required for Gab1-mediated hematopoietic cell proliferation, HGF-stimulated cells were treated with the PI3K inhibitors Wortmannin and LY294002 at doses known to block PI3K activity (Soon et al., 1999). Wortmannin treatment (100 nM) inhibited the proliferation of 32D/Met cells by 50%, but had little effect on 32D/Met/Gab1 cell proliferation (Fig. 5C). Similarly, the PI3K inhibitor LY294002 (0.5 μM) markedly inhibited HGF-stimulated mitogenesis in 32D/Met cells (p < 0.05), but had only marginal impact on Gab1 expressing cells (Fig. 5C). The failure of both PI3K inhibitors to block Gab1-enhanced HGF mitogenesis suggests that Gab1 acts downstream or independently of PI3K under these conditions.
Shp-2 Mediates Gab1-Enhanced HGF Mitogenicity
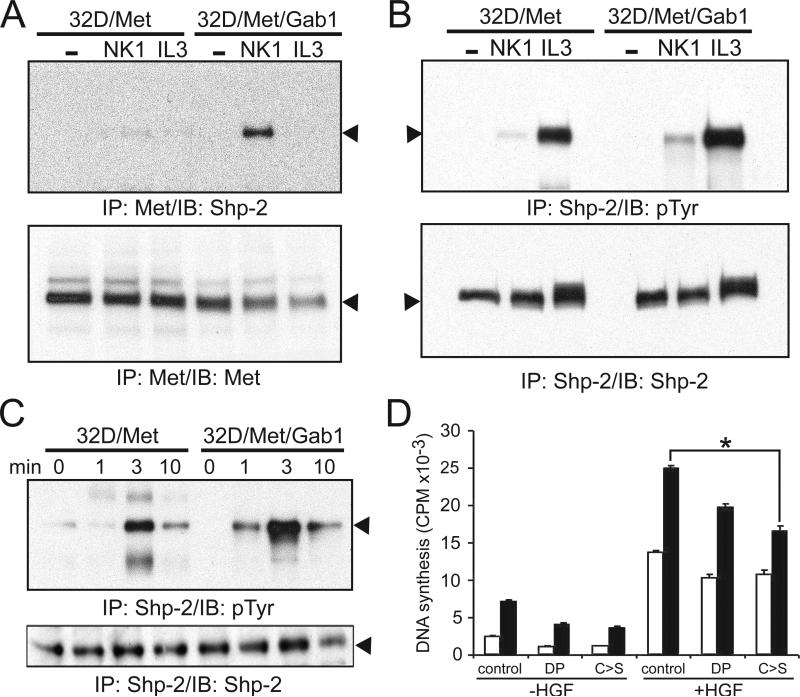
The tyrosine phosphatase Shp-2 is associated with Gab1 and positively regulates 32D cell proliferation (Kim et al., 1998; Ward et al., 1999). We examined the physical association of Shp-2 with activated Met by immunoblotting anti-Met immunoprecipitates for Shp-2 protein (Fig. 6). NK1-stimulated Shp-2/Met association was barely detectable in 32D/Met cells, but clearly evident in 32D/Met/Gab1 cells (Fig. 6A). NK1-stimulated Shp-2 activation, as represented by its tyrosyl phosphorylation, was also more readily observed in 32D/Met/Gab1 cells than in parental cells (Fig. 6B). A brief time course of NK1-induced Shp-2 activation highlighted this difference (Fig. 6C). These results, as well as genetic evidence of an essential role for Shp-2 in the proliferation and differentiation of hematopoietic progenitors (Qu et al., 1997; Qu et al., 1998), prompted us to further characterize Shp-2 in Gab1-mediated mitogenicity. To this end, two well-characterized catalytically inactive mutants of Shp-2, Shp-2ΔP and Shp-2CS, were tested for their ability to impair HGF-stimulated mitogenesis in 32D/Met/Gab1 cells. Shp-2ΔP carries a 31-amino acid internal deletion within the protein tyrosine phosphatase domain (Bennett et al., 1994), and Shp-2CS has a cysteine to serine point mutation at amino acid 459, an essential catalytic cysteine (Bennett et al., 1996). In light of the obvious obstacle to generating stable cell clones with suppressed Shp-2 signaling, the mutant Shp-2 constructs were transiently transfected into 32D/Met and 32D/Met/Gab1 cells, and HGF-stimulated DNA synthesis was measured in each mass culture. As anticipated, the Shp-2 mutants had little effect on 32D/Met cells (Fig. 6D, open bars). In contrast, 32D/Met/Gab1 cells were significantly more impaired by the Shp-2 mutants, particularly the Shp-2CS mutant (Fig. 6D; p < 0.05). Together with evidence that Gab1 increased Met/Shp-2 association and Shp-2 activation, these results strongly implicate Shp-2 in transducing Gab1-enhanced HGF proliferative signaling.
Figure 6. Shp-2 mediates Gab1-enhanced HGF mitogenicity.
A. Serum-deprived 32D/Met or 32D/Met/Gab1 cell clone 1W5 were treated as described in Results. Cell extracts were immunoprecipitated with anti-Met antibody and immunoblotted either with anti-Shp-2 (upper panel, arrowhead indicates Shp-2) or with anti-Met (lower panel, arrowhead indicates Met) as a control for sample loading. B. Tyrosyl-phosphorylated Shp-2 (upper panel) and the amount of total Shp-2 protein present in each lane (lower panel; arrowheads indicate Shp-2). C. Time course of NK1 stimulated Shp-2 phosphorylation. Samples were prepared after NK1 stimulation for the indicated time periods as described in Results. Arrowheads indicate Shp-2. D. 32D/Met (open bars) and 32D/Met/Gab1 cell clone 1W5 (black bars), transfected with the indicated expression vectors, were serum-deprived, stimulated with HGF (30 ng/ml) as indicated, and assayed for 3H-thymidine incorporation into DNA. Transfection efficiency was assessed by immunoblot analysis of Shp-2 protein expression on parallel samples. Asterisks indicate statistically significant differences between the bracketed groups.
Gab1 Regulates GATA Expression in HGF-treated Myeloid Cells
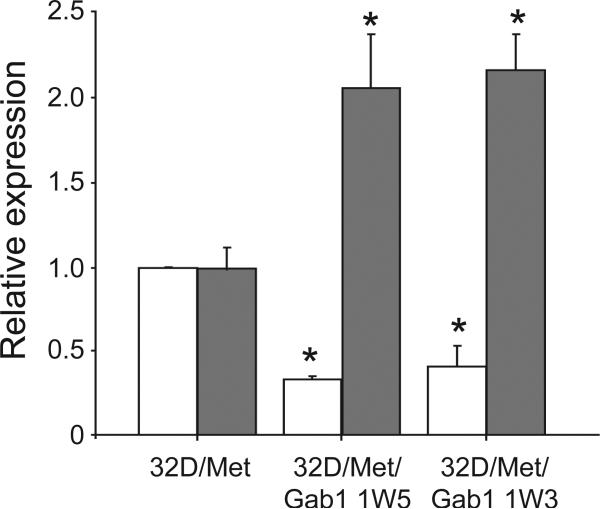
To identify genes modulated specifically by Gab1 signaling downstream of activated Met, the gene expression profiles of 32D/Met and 32D/Met/Gab1 cells stimulated with NK1 for 6 hours were compared using a 3000 gene mouse cDNA microarray. This time point was chosen to optimally identify genes related to stable, long-term phenotypic changes such as differentiation and the maintenance of pluripotency. A subset of genes exhibiting 2-fold or greater modulation and satisfying stringent statistical tests for significance were then analyzed by quantitative RT-PCR under identical conditions. The RT-PCR results were normalized to Met transcript level, which were equivalent among the parental and Gab1 clonal cell lines and were unchanged over the 6 hours treatment period (data not shown). This analysis confirmed a significant reduction of GATA-1 transcript (p < 0.0001) and concomitant increase in GATA-2 transcript (p < 0.002) abundance in 32D/Met/Gab1 cells relative to the parental cell line (Fig. 7).
Figure 7. Gab1 mediates Met-driven GATA-1/2 expression changes.
Relative abundance of GATA-1 (open bars) and GATA-2 transcripts (gray bars) in 32D/Met cells, 32D/Met/Gab1 clone 1W3 and 32D/Met/Gab1 clone 1W5. For each gene the mean expression level in the 32D/Met/Gab1 clones was significantly different compared to the corresponding 32D/Met clone. Error bars that indicate standard error of the mean (SEM). Asterisks indicate statistically significant differences (p<0.05) between 32D/Met and Gab1 transfected lines for expression of GATA-1 or GATA-2.
Gab1 is Required for HGF-induced Morphogenesis in 32D Cells
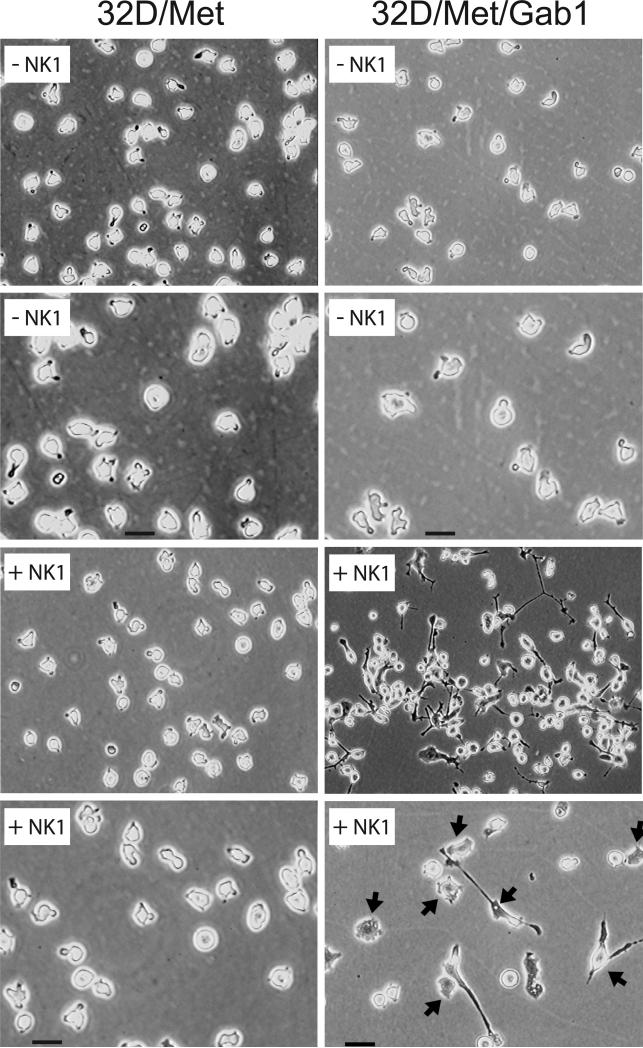
The established role of Gab1 in HGF-induced epithelial morphogenesis, and the evidence presented here of alterations in the adhesive, motile and growth properties of Gab1 transfectants prompted us to investigate whether long-term culture of 32D/Met/Gab1 cells in the presence of HGF might stimulate morphological differentiation. As shown in Figure 8, 32D/Met cells, in the presence or absence of NK1, as well as untreated 32D/Met/Gab1 cells, displayed the normal appearance of suspended myeloid blast cells. Untreated 32D/Met/Gab1 cells were slightly larger than 32D/Met cells and had a greater ratio of cytoplasmic to nuclear volume as determined by FACS analysis (data not shown). Interestingly, long-term propagation of 32D/Met/Gab1 cells with HGF induced dramatic morphological changes: cell adhesion and spreading, increased cell size, extension of lamellipodia and long filopodia (Fig. 8). In general the changes resembled the acquisition of an early macrophage-like phenotype, suggesting that Gab1 expression is required to initiate HGF-driven differentiation in hematopoietic progenitors.
Figure 8. Gab1 mediates NK1-stimulated morphogenesis.
Morphology of parental 32D/Met (left panels) or 32D/Met/Gab1 clone 1W5 (right panels) left untreated (-NK1) or treated with NK1 (300 ng/ml) for 9 days (+NK1). The photomicrographs in the second and bottom rows are 4-fold higher magnification images of the samples shown immediately above them (bar = 20 μm). Cells were grown in RPMI 1640/15%FBS/5%WEHI-3B-conditioned medium and photographed using phase contrast microscopy .
DISCUSSION
Activation of the HGF receptor triggers the recruitment of several intracellular adaptor proteins. Among these, Gab1 is a critical mediator of embryonic development, epithelial morphogenesis and oncogenic transformation. The role of Gab1 in the regulation of hematopoiesis by HGF, however, remains poorly understood. In studying the role of Gab1-mediated signaling in 32D myeloid cells, we found that Gab1 expression did not specifically enhance Met-driven cell motility, but constitutively increased cell motility 3-fold relative to Gab1-negative cells. Gab1 expression was also associated with substantially increased cell-substrate adhesion in the absence of obvious changes in cell-surface integrin expression. Although additional studies will be needed to completely define the molecular mechanism of Gab1-induced cell migration and adhesion reported here, we speculate that Gab1 regulates both “inside-out” and “outside-in” integrin signaling. Gab1 mediates signaling in the IL-3, EPO, thrombopoietin and SCF pathways, all of which activate hematopoietic cell adhesion to FN through α4β1 and α5β1 integrins (Levesque et al., 1995). Gab1 may promote this “inside-out” integrin signaling by recruiting Crk-L, which can activate integrin-mediated 32D cell adhesion through the guanine nucleotide exchange factor C3G and the GTPase Rap1 (Arai et al., 1999; Sakkab et al., 2000). Our observation of Gab1-enhancement of IL-3-induced cell adhesion in hematopoietic cells is consistent with this model. Gab1-enhanced adhesion of cytokine-deprived cells suggests that it also regulates “outside-in” integrin signaling. This could occur by binding FAK through a constitutive association with Grb2 and/or Shp-2 (Oh et al., 1999; Sattler et al., 1997; Inagaki et al., 2000). In support of this hypothesis, the related protein Gab2 was shown to mediate migration and adhesion in Ba/F3 cells, and Gab2 mutants reduced PI3K activation induced by β1-integrin ligation (Yu et al., 2002).
Consistent with prior studies of non-hematopoietic cells (Winnay et al., 2000; Holgado-Madruga et al., 1996; Korhonen et al., 1999), our results provide direct evidence that Gab1 enhances Met-driven mitogenicity in 32D myeloid progenitor cells. In contrast with some cell systems (Itoh et al., 2000; Takahashi-Tezuka et al., 1998), but consistent with some others (Winnay et al., 2000; Holgado-Madruga et al., 1996), this did not occur through enhanced MAPK pathway activation, supporting the likelihood that MAPK mediation of Gab1 mitogenic signaling is cell-specific. Gab1 enhanced mitogenicity in myeloid cells did not involve PI3K, in contrast with the general importance of PI3K to Gab1 signaling in epithelial cells (Royal and Park, 1995; Niemann et al., 1998; Maroun et al., 1999). Gab1 expression in 32D cells enhanced both Met/Shp-2 binding and Shp-2 phosphorylation and results obtained using dominant negative Shp-2 mutants further suggest that Shp-2 is a critical effector of Gab1-mediated HGF/Met mitogenesis in these cells.
Gene profiling and quantitative RT-PCR analysis linked Gab1 expression in HGF-stimulated 32D myeloid cells to a significant shift in expression of GATA transcription factors: GATA-1 transcript levels were significantly reduced with a concomitant increase in GATA-2 transcript abundance. The observed shift to GATA-2 expression downstream of activated Met is consistent with the enhanced HGF mitogenic potency displayed in these cells, and suggests that Gab1 may play an important role downstream of activated growth factor receptors in hematopoiesis. Of the six mammalian members of the evolutionary conserved GATA transcription factor family, GATA-1, -2 and -3 are primarily involved in hematopoiesis cell lineage determination, whereas GATA-4, -5 and -6 are involved in the development of meso- and endo-dermally-derived tissues (Harigae, 2006). Although GATA-1 and -2 share some roles in erythroid differentiation (Takahashi et al., 2000), GATA-2 expression is highest in stem cell progenitors and decreases with erythroid maturation, whereas GATA-1 is upregulated over the course of differentiation (Leonard et al., 1993; Mouthon et al., 1993; Nagai et al., 1994; Labbaye et al., 1995). GATA-2 is required for hematopoiesis and GATA-2 haploinsufficiency in genetically engineered mice results in hematopoietic stem cell defects (Ling et al., 2004). Consistent with a general role in promoting pluripotency, GATA-2 is required to maintain the immaturity of preadipocytes (Tong et al., 2005) and patients with aplastic anemia display severely reduced GATA-2 transcript levels (Fujimaki et al., 2001).
Gab1-mediated upregulation of GATA-2 could also contribute to hematopoietic neoplasia and malignant transformation. Signaling via Gab1 is known to promote oncogenesis in solid tumors (Bardelli et al., 1997; Seiden-Long et al., 2008). Evidence of Gab1 expression in several leukemic cell lines (Lecoq-Lafon et al., 1999) suggests that upregulated GATA-2 resulting from inappropriate activation of Met and/or other pathways may facilitate the onset and progression of hematopoietic neoplasias. Moreover, a recent mutational screen of patients with chronic myeloid leukemia during blast transformation revealed two gain-of-function mutations in the coding region of GATA-2 (Zhang et al., 2008). The predominant L359V substitution within zinc finger domain 2 of GATA-2 was found in eight cases with myelomonoblastic features. Further studies indicated that L359V not only increased GATA-2 transactivation activity but also enhanced its inhibitory effects on the activity of PU.1, an important regulator of myelopoiesis (Zhang et al., 2008).
We show that Gab1 expression is required for myeloid cells to display long-term Met-driven morphogenesis, manifested by increased cell volume, adhesion and spreading of these normally spherical suspended cells. Prior studies have shown that Gab1-Shp-2 association is required for epithelial morphogenesis downstream of Met (Maroun et al., 2000) and that Shp-2 regulates cell adhesion, migration and cytoskeletal architecture driven through other cues (Yu et al., 1998; Manes et al., 1999; Oh et al., 1999; Inagaki et al., 2000). More recently, Gab2-deficient mice were found to display defective hematopoiesis, due to an intrinsic requirement of this adaptor for hematopoietic cell response to early-acting cytokines (Zhang et al., 2007).Our results are consistent with the latter findings and further suggest the HGF may promote hematopoietic precursor cell responsiveness to early-acting cytokines by linking Gab1 (and potentially other Gab family members) to Shp-2 immediately downstream of activated Met. We further show that the Gab1/Shp-2 axis is likely to contribute to Met-driven cell spreading and morphogenesis in myeloid cells through the differential regulation of GATA transcription factors. It is also possible, in light of existing evidence of GATA and Shp-2 gene defects in hematologic malignancies, and the known role of HGF in promoting hematopoietic precursor self-renewal, that aberrant Met/Gab1/Shp-2/GATA-2 signaling may contribute myeloid neoplasias.
ACKNOWLEDGMENTS
We thank Dr. M. Sachs for the pBat-Flag-Gab1, Dr. B. Neel for the pJ3Shp-2ΔP and pJ3Shp-2CS expression vectors, Dr. G. Vande Woude for recombinant human HGF, Dr. M. Udey for helpful comments, Mr. N. Ellmore for technical assistance and Ms. V. Kapoor for FACS analysis. This research was supported by the Intramural Research Program of the NIH, National Cancer Institute, Center for Cancer Research, Bethesda, MD 20892 USA.
ABBREVIATIONS
- HGF
Hepatocyte growth factor
- Gab-1
Grb2 associated binding protein 1
- GATA
signal transducer and activator of transcription
- Shp-2
(SH2)-containing protein tyrosine phosphatase 2
- IL-3
interleukin-3
- SCF
stem cell factor
- PI3K
phosphatidylinositol 3-kinase
- PLC-gamma
phospholipase C-gamma
- SH2
Src homology-2
- Crk
cellular regulator of kinase
- Crk-L
Crk-like protein
- Shc1
SH2 domain containing transforming protein 1
- Gab1
growth factor receptor bound protein 2-associated protein 1
- FRS-2/SNT1
FGF receptor substrate-2
- DOS
Daughter of Sevenless
- MBD
Met binding domain
- Erk
Extracellular Signal-Regulated Kinase
- Tpr-Met
Translocated promoter region-Met
- MDCK
Madin-Darby canine kidney
- JNK
c-Jun N-terminal kinase
- EPO
erythropoietin
- SCF
stem cell factor
- FAK
focal adhesion kinase
- FN
fibronectin
- PKB
protein kinases B
REFERENCES
- Arai A, Nosaka Y, Kohsaka H, Miyasaka N, Miura O. CrkL activates integrin-mediated hematopoietic cell adhesion through the guanine nucleotide exchange factor C3G. Blood. 1999;93:3713–3722. [PubMed] [Google Scholar]
- Bardelli A, Longati P, Gramaglia D, Stella MC, Comoglio PM. Gab1 coupling to the HGF/Met receptor multifunctional docking site requires binding of Grb2 and correlates with the transforming potential. Oncogene. 1997;15:3103–3111. doi: 10.1038/sj.onc.1201561. [DOI] [PubMed] [Google Scholar]
- Bennett AM, Hausdorff SF, O'Reilly AM, Freeman RM, Neel BG. Multiple requirements for SHPTP2 in epidermal growth factor-mediated cell cycle progression. Mol Cell Biol. 1996;16:1189–1202. doi: 10.1128/mcb.16.3.1189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett AM, Tang TL, Sugimoto S, Walsh CT, Neel BG. Protein-tyrosinephosphatase SHPTP2 couples platelet-derived growth factor receptor beta to Ras. Proc Natl Acad Sci U S A. 1994;91:7335–7339. doi: 10.1073/pnas.91.15.7335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun L, Ghebrehiwet B, Cossart P. gC1q-R/p32, a C1q-binding protein, is a receptor for the InlB invasion protein of Listeria monocytogenes. EMBO J. 2000;19:1458–1466. doi: 10.1093/emboj/19.7.1458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan G. The tyrosine phosphatase Shp2 (PTPN11) in cancer. Cancer and metastasis reviews. 2008;27:179–192. doi: 10.1007/s10555-008-9126-y. [DOI] [PubMed] [Google Scholar]
- Dance M, Montagner A, Salles JP, Yart A, Raynal P. The molecular functions of Shp2 in the Ras/Mitogen-activated protein kinase (ERK1/2) pathway. Cellular Signalling. 2008;20:453–459. doi: 10.1016/j.cellsig.2007.10.002. [DOI] [PubMed] [Google Scholar]
- Day RM, Cioce V, Breckenridge D, Castagnino P, Bottaro DP. Differential signaling by alternative HGF isoforms through c-Met: activation of both MAP kinase and PI 3-kinase pathways is insufficient for mitogenesis. Oncogene. 1999;18:3399–3406. doi: 10.1038/sj.onc.1202683. [DOI] [PubMed] [Google Scholar]
- Feng GS, Pawson T. Phosphotyrosine phosphatases with SH2 domains: regulators of signal transduction. Trends Genet. 1994;10:54–58. doi: 10.1016/0168-9525(94)90149-x. [DOI] [PubMed] [Google Scholar]
- Fixman ED, Holgado-Madruga M, Nguyen L, Kamikura DM, Fournier TM, Wong AJ, Park M. Efficient cellular transformation by the Met oncoprotein requires a functional Grb2 binding site and correlates with phosphorylation of the Grb2-associated proteins, Cbl and Gab1. J Biol Chem. 1997;272:20167–20172. doi: 10.1074/jbc.272.32.20167. [DOI] [PubMed] [Google Scholar]
- Fujimaki S, Harigae H, Sugawara T, Takasawa N, Sasaki T, Kaku M. Decreased expression of transcription factor GATA-2 in haematopoietic stem cells in patients with aplastic anaemia. Br J Haematol. 2001;113:52–57. doi: 10.1046/j.1365-2141.2001.02736.x. [DOI] [PubMed] [Google Scholar]
- Furge KA, Zhang YW, Vande Woude GF. Met receptor tyrosine kinase: enhanced signaling through adapter proteins. Oncogene. 2000;19:5582–5589. doi: 10.1038/sj.onc.1203859. [DOI] [PubMed] [Google Scholar]
- Galimi F, Bagnara GP, Bonsi L, Cottone E, Follenzi A, Simeone A, Comoglio PM. Hepatocyte growth factor induces proliferation and differentiation of multipotent and erythroid hemopoietic progenitors. J Cell Biol. 1994;127:1743–1754. doi: 10.1083/jcb.127.6.1743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Guzman M, Larsen E, Vuori K. The proto-oncogene c-Cbl is a positive regulator of Met-induced MAP kinase activation: a role for the adaptor protein Crk. Oncogene. 2000;19:4058–4065. doi: 10.1038/sj.onc.1203750. [DOI] [PubMed] [Google Scholar]
- Gu H, Pratt JC, Burakoff SJ, Neel BG. Cloning of p97/Gab2, the major SHP2-binding protein in hematopoietic cells, reveals a novel pathway for cytokine-induced gene activation. Mol Cell. 1998;2:729–740. doi: 10.1016/s1097-2765(00)80288-9. [DOI] [PubMed] [Google Scholar]
- Harigae H. GATA transcription factors and hematological diseases. Tohoku Journal of Experimental Medicine. 2006;210:1–9. doi: 10.1620/tjem.210.1. [DOI] [PubMed] [Google Scholar]
- Heisterkamp J, van HR, IJzermans JN. Critical temperature and heating time for coagulation damage: implications for interstitial laser coagulation (ILC) of tumors. Lasers Surg Med. 1999;25:257–262. doi: 10.1002/(sici)1096-9101(1999)25:3<257::aid-lsm10>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- Holgado-Madruga M, Emlet DR, Moscatello DK, Godwin AK, Wong AJ. A Grb2-associated docking protein in EGF- and insulin-receptor signalling. Nature. 1996;379:560–564. doi: 10.1038/379560a0. [DOI] [PubMed] [Google Scholar]
- Inagaki K, Yamao T, Noguchi T, Matozaki T, Fukunaga K, Takada T, Hosooka T, Akira S, Kasuga M. SHPS-1 regulates integrin-mediated cytoskeletal reorganization and cell motility. EMBO J. 2000;19:6721–6731. doi: 10.1093/emboj/19.24.6721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itoh M, Yoshida Y, Nishida K, Narimatsu M, Hibi M, Hirano T. Role of Gab1 in heart, placenta, and skin development and growth factor- and cytokine-induced extracellular signal-regulated kinase mitogen-activated protein kinase activation. Mol Cell Biol. 2000;20:3695–3704. doi: 10.1128/mcb.20.10.3695-3704.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kameda H, Risinger JI, Han BB, Seung JB, Barrett JC, Glasgow WC, Eling TE. Identification of epidermal growth factor receptor- Grb2-associated binder-1-SHP-2 complex formation and its functional loss during neoplastic cell progression. Cell Growth and Differentiation. 2001;12:307–318. [PubMed] [Google Scholar]
- Kim SO, Jiang J, Yi W, Feng GS, Frank SJ. Involvement of the Src homology 2-containing tyrosine phosphatase SHP-2 in growth hormone signaling. J Biol Chem. 1998;273:2344–2354. doi: 10.1074/jbc.273.4.2344. [DOI] [PubMed] [Google Scholar]
- Korhonen JM, Said FA, Wong AJ, Kaplan DR. Gab1 mediates neurite outgrowth, DNA synthesis, and survival in PC12 cells. J Biol Chem. 1999;274:37307–37314. doi: 10.1074/jbc.274.52.37307. [DOI] [PubMed] [Google Scholar]
- Kosone T, Takagi H, Horiguchi N, Toyoda M, Sohara N, Kakizaki S, Sato K, Nishiyama U, Kuwaki T, Mori M. Hepatocyte growth factor accelerates thrombopoiesis in transgenic mice. Laboratory Investigation. 2007;87:284–291. doi: 10.1038/labinvest.3700514. [DOI] [PubMed] [Google Scholar]
- Labbaye C, Valtieri M, Barberi T, Meccia E, Masella B, Pelosi E, Condorelli GL, Testa U, Peschle C. Differential expression and functional role of GATA-2, NF-E2, and GATA-1 in normal adult hematopoiesis. J Clin Invest. 1995;95:2346–2358. doi: 10.1172/JCI117927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lechleider RJ, Sugimoto S, Bennett AM, Kashishian AS, Cooper JA, Shoelson SE, Walsh CT, Neel BG. Activation of the SH2-containing phosphotyrosine phosphatase SH-PTP2 by its binding site, phosphotyrosine 1009, on the human platelet-derived growth factor receptor. J Biol Chem. 1993;268:21478–21481. [PubMed] [Google Scholar]
- Lecoq-Lafon C, Verdier F, Fichelson S, Chretien S, Gisselbrecht S, Lacombe C, Mayeux P. Erythropoietin induces the tyrosine phosphorylation of GAB1 and its association with SHC, SHP2, SHIP, and phosphatidylinositol 3-kinase. Blood. 1999;93:2578–2585. [PubMed] [Google Scholar]
- Leonard M, Brice M, Engel JD, Papayannopoulou T. Dynamics of GATA transcription factor expression during erythroid differentiation. Blood. 1993;82:1071–1079. [PubMed] [Google Scholar]
- Levesque JP, Haylock DN, Simmons PJ. Cytokine regulation of proliferation and cell adhesion are correlated events in human CD34+ hemopoietic progenitors. Blood. 1996;88:1168–1176. [PubMed] [Google Scholar]
- Levesque JP, Leavesley DI, Niutta S, Vadas M, Simmons PJ. Cytokines increase human hemopoietic cell adhesiveness by activation of very late antigen (VLA)-4 and VLA-5 integrins. J Exp Med. 1995;181:1805–1815. doi: 10.1084/jem.181.5.1805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ling KW, Ottersbach K, van Hamburg JP, Oziemlak A, Tsai FY, Orkin SH, Ploemacher R, Hendriks RW, Dzierzak E. GATA-2 plays two functionally distinct roles during the ontogeny of hematopoietic stem cells. J Exp Med. 2004;200:871–882. doi: 10.1084/jem.20031556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manes S, Mira E, Gomez-Mouton C, Zhao ZJ, Lacalle RA, Martinez A. Concerted activity of tyrosine phosphatase SHP-2 and focal adhesion kinase in regulation of cell motility. Mol Cell Biol. 1999;19:3125–3135. doi: 10.1128/mcb.19.4.3125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maroun CR, Holgado-Madruga M, Royal I, Naujokas MA, Fournier TM, Wong AJ, Park M. The Gab1 PH domain is required for localization of Gab1 at sites of cell-cell contact and epithelial morphogenesis downstream from the met receptor tyrosine kinase. Mol Cell Biol. 1999;19:1784–1799. doi: 10.1128/mcb.19.3.1784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maroun CR, Moscatello DK, Naujokas MA, Holgado-Madruga M, Wong AJ, Park M. A conserved inositol phospholipid binding site within the pleckstrin homology domain of the Gab1 docking protein is required for epithelial morphogenesis. J Biol Chem. 1999;274:31719–31726. doi: 10.1074/jbc.274.44.31719. [DOI] [PubMed] [Google Scholar]
- Maroun CR, Naujokas MA, Holgado-Madruga M, Wong AJ, Park M. The tyrosine phosphatase SHP-2 is required for sustained activation of extracellular signal-regulated kinase and epithelial morphogenesis downstream from the met receptor tyrosine kinase. Mol Cell Biol. 2000;20:8513–8525. doi: 10.1128/mcb.20.22.8513-8525.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mouthon MA, Bernard O, Mitjavila MT, Romeo PH, Vainchenker W, Mathieu-Mahul D. Expression of tal-1 and GATA-binding proteins during human hematopoiesis. Blood. 1993;81:647–655. [PubMed] [Google Scholar]
- Nagai T, Harigae H, Ishihara H, Motohashi H, Minegishi N, Tsuchiya S, Hayashi N, Gu L, Andres B, Engel JD. Transcription factor GATA-2 is expressed in erythroid, early myeloid, and CD34+ human leukemia-derived cell lines. Blood. 1994;84:1074–1084. [PubMed] [Google Scholar]
- Neel BG, Tonks NK. Protein tyrosine phosphatases in signal transduction. Curr Opin Cell Biol. 1997;9:193–204. doi: 10.1016/s0955-0674(97)80063-4. [DOI] [PubMed] [Google Scholar]
- Nguyen L, Holgado-Madruga M, Maroun C, Fixman ED, Kamikura D, Fournier T, Charest A, Tremblay ML, Wong AJ, Park M. Association of the multisubstrate docking protein Gab1 with the hepatocyte growth factor receptor requires a functional Grb2 binding site involving tyrosine 1356. J Biol Chem. 1997;272:20811–20819. doi: 10.1074/jbc.272.33.20811. [DOI] [PubMed] [Google Scholar]
- Niemann C, Brinkmann V, Spitzer E, Hartmann G, Sachs M, Naundorf H, Birchmeier W. Reconstitution of mammary gland development in vitro: requirement of c-met and c-erbB2 signaling for branching and alveolar morphogenesis. J Cell Biol. 1998;143:533–545. doi: 10.1083/jcb.143.2.533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishida K, Hirano T. The role of Gab family scaffolding adapter proteins in the signal transduction of cytokine and growth factor receptors. Cancer Science. 2003;94:1029–1033. doi: 10.1111/j.1349-7006.2003.tb01396.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh ES, Gu H, Saxton TM, Timms JF, Hausdorff S, Frevert EU, Kahn BB, Pawson T, Neel BG, Thomas SM. Regulation of early events in integrin signaling by protein tyrosine phosphatase SHP-2. Mol Cell Biol. 1999;19:3205–3215. doi: 10.1128/mcb.19.4.3205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ovali E, Ratip S, Kibaroglu A, Tekelioglu Y, Cetiner M, Karti S, Aydin F, Bayik M, Akoglu T. Role of hepatocyte growth factor in the development of dendritic cells from CD34+ bone marrow cells. Haematologica. 2000;85:464–469. [PubMed] [Google Scholar]
- Pawson T, Scott JD. Signaling through scaffold, anchoring, and adaptor proteins. Science. 1997;278:2075–2080. doi: 10.1126/science.278.5346.2075. [DOI] [PubMed] [Google Scholar]
- Qu CK, Shi ZQ, Shen R, Tsai FY, Orkin SH, Feng GS. A deletion mutation in the SH2-N domain of Shp-2 severely suppresses hematopoietic cell development. Mol Cell Biol. 1997;17:5499–5507. doi: 10.1128/mcb.17.9.5499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qu CK, Yu WM, Azzarelli B, Cooper S, Broxmeyer HE, Feng GS. Biased suppression of hematopoiesis and multiple developmental defects in chimeric mice containing Shp-2 mutant cells. Mol Cell Biol. 1998;18:6075–6082. doi: 10.1128/mcb.18.10.6075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahimi N, Tremblay E, Elliott B. Phosphatidylinositol 3-kinase activity is required for hepatocyte growth factor-induced mitogenic signals in epithelial cells. J Biol Chem. 1996;271:24850–24855. doi: 10.1074/jbc.271.40.24850. [DOI] [PubMed] [Google Scholar]
- Richard C, Liuzzo JP, Moscatelli D. Fibroblast growth factor-2 can mediate cell attachment by linking receptors and heparan sulfate proteoglycans on neighboring cells. J Biol Chem. 1995;270:24188–24196. doi: 10.1074/jbc.270.41.24188. [DOI] [PubMed] [Google Scholar]
- Rodrigues GA, Falasca M, Zhang Z, Ong SH, Schlessinger J. A novel positive feedback loop mediated by the docking protein Gab1 and phosphatidylinositol 3-kinase in epidermal growth factor receptor signaling. Mol Cell Biol. 2000;20:1448–1459. doi: 10.1128/mcb.20.4.1448-1459.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodrigues GA, Park M, Schlessinger J. Activation of the JNK pathway is essential for transformation by the Met oncogene. EMBO J. 1997;16:2634–2645. doi: 10.1093/emboj/16.10.2634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Royal I, Park M. Hepatocyte growth factor-induced scatter of Madin-Darby canine kidney cells requires phosphatidylinositol 3-kinase. J Biol Chem. 1995;270:27780–27787. doi: 10.1074/jbc.270.46.27780. [DOI] [PubMed] [Google Scholar]
- Sachs M, Brohmann H, Zechner D, Muller T, Hulsken J, Walther I, Schaeper U, Birchmeier C, Birchmeier W. Essential role of Gab1 for signaling by the c-Met receptor in vivo. J Cell Biol. 2000;150:1375–1384. doi: 10.1083/jcb.150.6.1375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakkab D, Lewitzky M, Posern G, Schaeper U, Sachs M, Birchmeier W, Feller SM. Signaling of hepatocyte growth factor/scatter factor (HGF) to the small GTPase Rap1 via the large docking protein Gab1 and the adapter protein CRKL. J Biol Chem. 2000;275:10772–10778. doi: 10.1074/jbc.275.15.10772. [DOI] [PubMed] [Google Scholar]
- Sarmay G, Angyal A, Kertesz A, Maus M, Medgyesi D. The multiple function of Grb2 associated binder (Gab) adaptor/scaffolding protein in immune cell signaling. Immunology Letters. 2006;104:76–82. doi: 10.1016/j.imlet.2005.11.017. [DOI] [PubMed] [Google Scholar]
- Sattler M, Salgia R, Shrikhande G, Verma S, Uemura N, Law SF, Golemis EA, Griffin JD. Differential signaling after beta1 integrin ligation is mediated through binding of CRKL to p120(CBL) and p110(HEF1). J Biol Chem. 1997;272:14320–14326. doi: 10.1074/jbc.272.22.14320. [DOI] [PubMed] [Google Scholar]
- Schaeper U, Gehring NH, Fuchs KP, Sachs M, Kempkes B, Birchmeier W. Coupling of Gab1 to c-Met, Grb2, and Shp2 mediates biological responses. J Cell Biol. 2000;149:1419–1432. doi: 10.1083/jcb.149.7.1419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaeper U, Vogel R, Chmielowiec J, Huelsken J, Rosario M, Birchmeier W. Distinct requirements for Gab1 in Met and EGF receptor signaling in vivo. Proceedings of the National Academy of Sciences. 2007;104:15376–15381. doi: 10.1073/pnas.0702555104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seiden-Long I, Navab R, Shih W, Li M, Chow J, Zhu CQ, Radulovich N, Saucier C, Tsao MS. Gab1 but not Grb2 mediates tumor progression in Met overexpressing colorectal cancer cells. Carcinogenesis. 2008;29:647–655. doi: 10.1093/carcin/bgn009. [DOI] [PubMed] [Google Scholar]
- Soon L, Flechner L, Gutkind JS, Wang LH, Baserga R, Pierce JH, Li W. Insulin-like growth factor I synergizes with interleukin 4 for hematopoietic cell proliferation independent of insulin receptor substrate expression. Mol Cell Biol. 1999;19:3816–3828. doi: 10.1128/mcb.19.5.3816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stahl SJ, Wingfield PT, Kaufman JD, Pannell LK, Cioce V, Sakata H, Taylor WG, Rubin JS, Bottaro DP. Functional and biophysical characterization of recombinant human hepatocyte growth factor isoforms produced in Escherichia coli. Biochem J. 1997;326(Pt 3):763–772. doi: 10.1042/bj3260763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun XJ, Wang LM, Zhang Y, Yenush L, Myers MG, Jr., Glasheen E, Lane WS, Pierce JH, White MF. Role of IRS-2 in insulin and cytokine signalling. Nature. 1995;377:173–177. doi: 10.1038/377173a0. [DOI] [PubMed] [Google Scholar]
- Taher TE, van d, V, Smit L, Keehnen RM, Schilder-Tol EJ, Spaargaren M, Pals ST. Cross-talk between CD44 and c-Met in B cells. Curr Top Microbiol Immunol. 1999;246:31–37. doi: 10.1007/978-3-642-60162-0_4. [DOI] [PubMed] [Google Scholar]
- Takahashi S, Shimizu R, Suwabe N, Kuroha T, Yoh K, Ohta J, Nishimura S, Lim KC, Engel JD, Yamamoto M. GATA factor transgenes under GATA-1 locus control rescue germline GATA-1 mutant deficiencies. Blood. 2000;96:910–916. [PubMed] [Google Scholar]
- Takahashi-Tezuka M, Yoshida Y, Fukada T, Ohtani T, Yamanaka Y, Nishida K, Nakajima K, Hibi M, Hirano T. Gab1 acts as an adapter molecule linking the cytokine receptor gp130 to ERK mitogen-activated protein kinase. Mol Cell Biol. 1998;18:4109–4117. doi: 10.1128/mcb.18.7.4109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timms JF, Carlberg K, Gu H, Chen H, Kamatkar S, Nadler MJ, Rohrschneider LR, Neel BG. Identification of major binding proteins and substrates for the SH2-containing protein tyrosine phosphatase SHP-1 in macrophages. Mol Cell Biol. 1998;18:3838–3850. doi: 10.1128/mcb.18.7.3838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tong Q, Tsai J, Tan G, Dalgin G, Hotamisligil GS. Interaction between GATA and the C/EBP family of transcription factors is critical in GATA-mediated suppression of adipocyte differentiation. Mol Cell Biol. 2005;25:706–715. doi: 10.1128/MCB.25.2.706-715.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Der Voort R, Taher TEI, Keehnen RMJ, Smit L, Groenink M, Pals ST. Paracrine regulation of germinal center b cell adhesion through the c- met-hepatocyte growth factor/scatter factor pathway. Journal of Experimental Medicine. 1997;185:2121–2131. doi: 10.1084/jem.185.12.2121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogel W, Ullrich A. Multiple in vivo phosphorylated tyrosine phosphatase SHP-2 engages binding to Grb2 via tyrosine 584. Cell Growth Differ. 1996;7:1589–1597. [PubMed] [Google Scholar]
- Wang LM, Myers MG, Jr., Sun XJ, Aaronson SA, White M, Pierce JH. IRS-1: essential for insulin- and IL-4-stimulated mitogenesis in hematopoietic cells. Science. 1993;261:1591–1594. doi: 10.1126/science.8372354. [DOI] [PubMed] [Google Scholar]
- Wang S, Yu WM, Zhang W, McCrae KR, Neel BG, Qu CK. Noonan syndrome/leukemia-associated gain-of-function mutations in SHP-2 phosphatase (PTPN11) enhance cell migration and angiogenesis. Journal of Biological Chemistry. 2009;284:913–920. doi: 10.1074/jbc.M804129200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward AC, Smith L, de Koning JP, van AY, Touw IP. Multiple signals mediate proliferation, differentiation, and survival from the granulocyte colony-stimulating factor receptor in myeloid 32D cells. J Biol Chem. 1999;274:14956–14962. doi: 10.1074/jbc.274.21.14956. [DOI] [PubMed] [Google Scholar]
- Weidner KM, Di CS, Sachs M, Brinkmann V, Behrens J, Birchmeier W. Interaction between Gab1 and the c-Met receptor tyrosine kinase is responsible for epithelial morphogenesis. Nature. 1996;384:173–176. doi: 10.1038/384173a0. [DOI] [PubMed] [Google Scholar]
- Weimar IS, de JD, Muller EJ, Nakamura T, van Gorp JM, de Gast GC, Gerritsen WR. Hepatocyte growth factor/scatter factor promotes adhesion of lymphoma cells to extracellular matrix molecules via alpha 4 beta 1 and alpha 5 beta 1 integrins. Blood. 1997;89:990–1000. [PubMed] [Google Scholar]
- Weimar IS, Miranda N, Muller EJ, Hekman A, Kerst JM, de Gast GC, Gerritsen WR. Hepatocyte growth factor/scatter factor (HGF/SF) is produced by human bone marrow stromal cells and promotes proliferation, adhesion and survival of human hematopoietic progenitor cells (CD34+). Exp Hematol. 1998;26:885–894. [PubMed] [Google Scholar]
- Winnay JN, Bruning JC, Burks DJ, Kahn CR. Gab-1-mediated IGF-1 signaling in IRS-1-deficient 3T3 fibroblasts. J Biol Chem. 2000;275:10545–10550. doi: 10.1074/jbc.275.14.10545. [DOI] [PubMed] [Google Scholar]
- Wolf I, Jenkins BJ, Liu Y, Seiffert M, Custodio JM, Young P, Rohrschneider LR. Gab3, a new DOS/Gab family member, facilitates macrophage differentiation. Mol Cell Biol. 2002;22:231–244. doi: 10.1128/MCB.22.1.231-244.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu DH, Qu CK, Henegariu O, Lu X, Feng GS. Protein-tyrosine phosphatase Shp-2 regulates cell spreading, migration, and focal adhesion. J Biol Chem. 1998;273:21125–21131. doi: 10.1074/jbc.273.33.21125. [DOI] [PubMed] [Google Scholar]
- Yu WM, Hawley TS, Hawley RG, Qu CK. Role of the docking protein Gab2 in beta(1)-integrin signaling pathway-mediated hematopoietic cell adhesion and migration. Blood. 2002;99:2351–2359. doi: 10.1182/blood.v99.7.2351. [DOI] [PubMed] [Google Scholar]
- Zhang SJ, Ma LY, Huang QH, Li G, Gu BW, Gao XD, Shi JY, Wang YY, Gao L, Cai X, Ren RB, Zhu J, Chen Z, Chen SJ. Gain-of-function mutation of GATA-2 in acute myeloid transformation of chronic myeloid leukemia. Proc Natl Acad Sci U S A. 2008;105:2076–2081. doi: 10.1073/pnas.0711824105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Diaz-Flores E, Li G, Wang Z, Kang Z, Haviernikova E, Rowe S, Qu CK, Tse W, Shannon KM, Bunting KD. Abnormal hematopoiesis in Gab2 mutant mice. Blood. 2007;110:116–124. doi: 10.1182/blood-2006-11-060707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang YW, Vande Woude GF. HGF/SF-met signaling in the control of branching morphogenesis and invasion. J Cell Biochem. 2003;88:408–417. doi: 10.1002/jcb.10358. [DOI] [PubMed] [Google Scholar]
- Zhao C, Yu DH, Shen R, Feng GS. Gab2, a new pleckstrin homology domain-containing adapter protein, acts to uncouple signaling from ERK kinase to Elk-1. J Biol Chem. 1999;274:19649–19654. doi: 10.1074/jbc.274.28.19649. [DOI] [PubMed] [Google Scholar]