Abstract
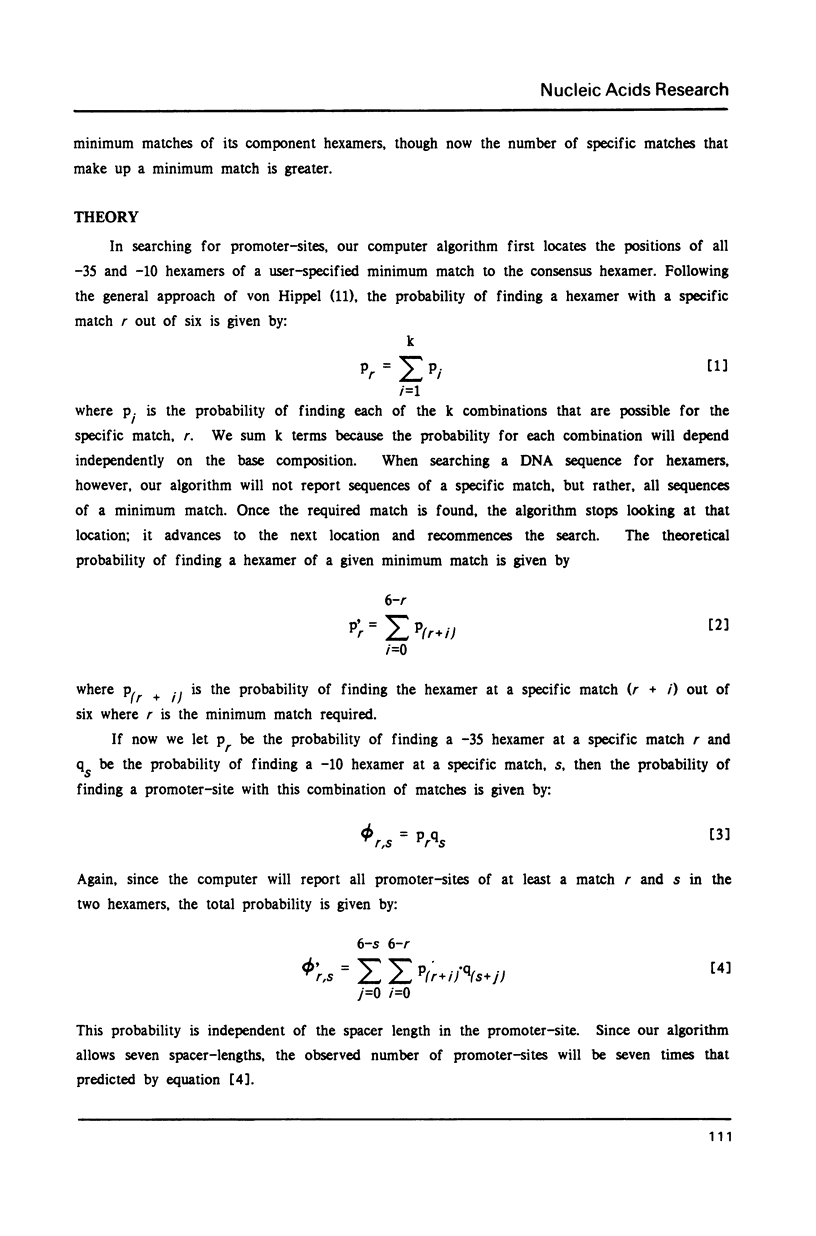
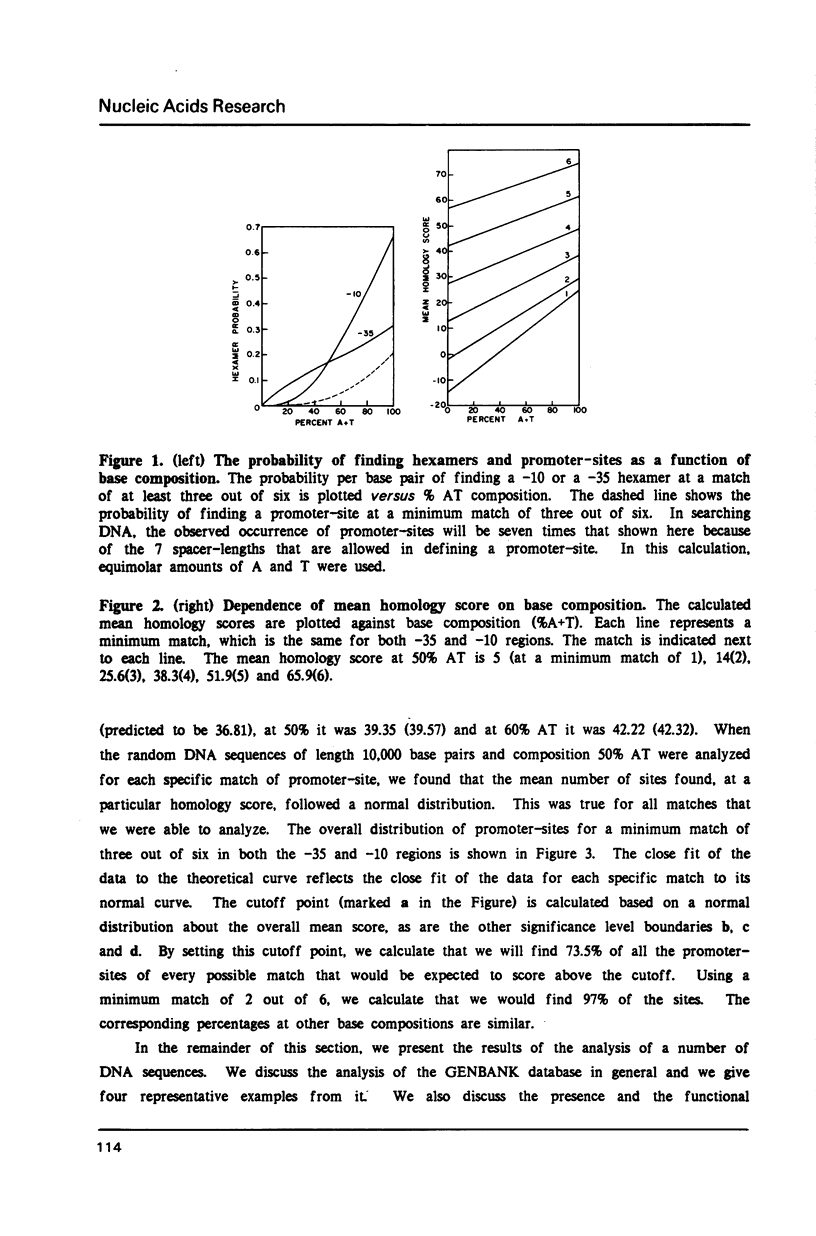
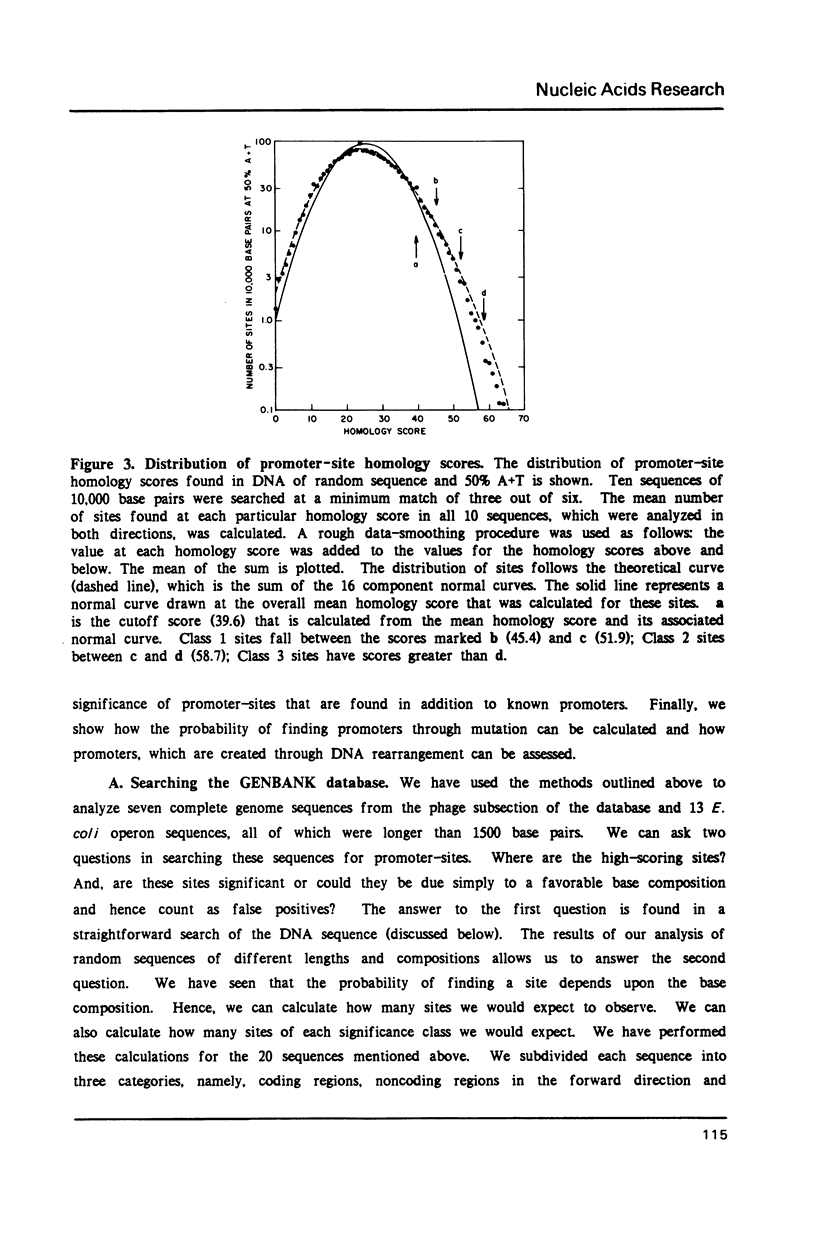
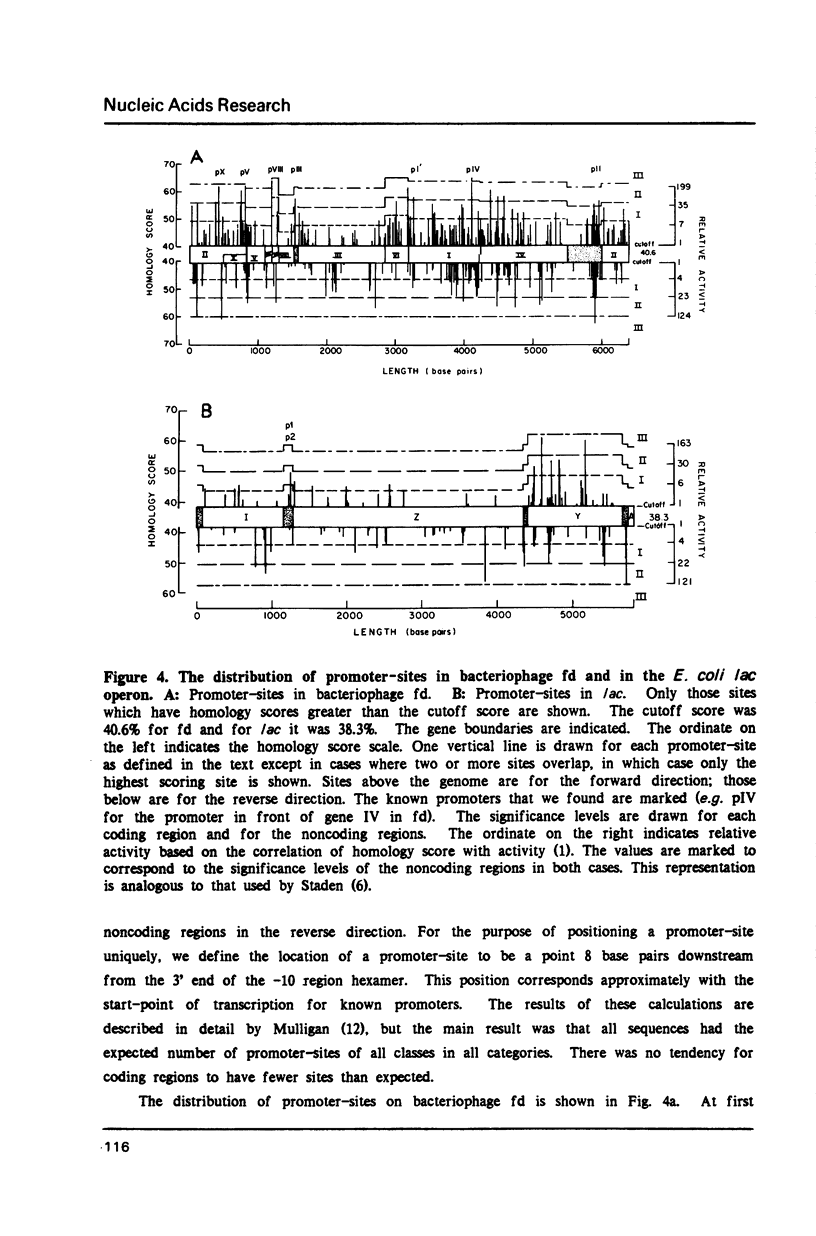
We show that the occurrence and homology score (1) of promoter-sites in DNA depends upon the base composition of the DNA. We used simple probability theory to calculate the mean homology score expected for all promoter-sites that had a specific match in the canonical hexamers. By using the square root of this mean score as a measure of significance, we objectively classify all promoter-sites which are reported. We tested the theoretical approach in two ways. First, we used the program (PROMSEARCH) to analyze approximately 150,000 base pairs of random sequence DNA with different base compositions and we found excellent agreement with the theoretical predictions. Our second test was the analysis of a number of sequences drawn from the GENBANK DNA sequence database. We have analyzed 20 bacterial and bacteriophage sequences, which consisted of at least one operon, for promoter-sites. We found no absolute preference for promoter-sites within noncoding regions. We show the results of analyzing the phages lambda, T7 and fd, and the E. coli lac operon. The major known promoters in these sequences were all found correctly. We discuss the question of the location of a number of minor promoter-sites and show how PROMSEARCH can be used to help identify the correct location of the promoter. This approach can be applied to the search for any DNA site and should allow greater objectivity when comparing DNA sequences for meaningful subsequences.
Full text
PDF










Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Botchan P. An electron microscopic comparison of transcription on linear and superhelical DNA. J Mol Biol. 1976 Jul 25;105(1):161–176. doi: 10.1016/0022-2836(76)90201-1. [DOI] [PubMed] [Google Scholar]
- Delius H., Westphal H., Axelrod N. Length measurements of RNA synthesized in vitro by Escherichia coli RNA polymerase. J Mol Biol. 1973 Mar 15;74(4):677–687. doi: 10.1016/0022-2836(73)90056-9. [DOI] [PubMed] [Google Scholar]
- Dunn J. J., Studier F. W. Complete nucleotide sequence of bacteriophage T7 DNA and the locations of T7 genetic elements. J Mol Biol. 1983 Jun 5;166(4):477–535. doi: 10.1016/s0022-2836(83)80282-4. [DOI] [PubMed] [Google Scholar]
- Goad W. B., Kanehisa M. I. Pattern recognition in nucleic acid sequences. I. A general method for finding local homologies and symmetries. Nucleic Acids Res. 1982 Jan 11;10(1):247–263. doi: 10.1093/nar/10.1.247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gragerov A. I., Smirnov OYu, Mekhedov S. L., Nikiforov V. G., Chuvpilo S. A., Korobko V. G. DNA rearrangements generating artificial promoters. FEBS Lett. 1984 Jun 25;172(1):64–66. doi: 10.1016/0014-5793(84)80874-1. [DOI] [PubMed] [Google Scholar]
- Grisolia V., Riccio A., Bruni C. B. Structure and function of the internal promoter (hisBp) of the Escherichia coli K-12 histidine operon. J Bacteriol. 1983 Sep;155(3):1288–1296. doi: 10.1128/jb.155.3.1288-1296.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harr R., Häggström M., Gustafsson P. Search algorithm for pattern match analysis of nucleic acid sequences. Nucleic Acids Res. 1983 May 11;11(9):2943–2957. doi: 10.1093/nar/11.9.2943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawley D. K., McClure W. R. Compilation and analysis of Escherichia coli promoter DNA sequences. Nucleic Acids Res. 1983 Apr 25;11(8):2237–2255. doi: 10.1093/nar/11.8.2237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes S., Szybalski W. Control of short leftward transcripts from the immunity and ori regions in induced coliphage lambda. Mol Gen Genet. 1973 Nov 22;126(4):275–290. doi: 10.1007/BF00269438. [DOI] [PubMed] [Google Scholar]
- Hopkins J. D. A new class of promoter mutations in the lactose operon of Escherichia coli. J Mol Biol. 1974 Aug 25;87(4):715–724. doi: 10.1016/0022-2836(74)90080-1. [DOI] [PubMed] [Google Scholar]
- Horowitz H., Platt T. Identification of trp-p2, an internal promoter in the tryptophan operon of Escherichia coli. J Mol Biol. 1982 Apr 5;156(2):257–267. doi: 10.1016/0022-2836(82)90327-8. [DOI] [PubMed] [Google Scholar]
- Hsieh T., Wang J. C. Physicochomecial studies on interactions between DNA and RNA polymerase. Isolation and mapping of a T7 DNA fragment containing the early promoters for Escherichia coli RNA polymerase. Biochemistry. 1976 Dec 28;15(26):5776–5783. doi: 10.1021/bi00671a014. [DOI] [PubMed] [Google Scholar]
- Jackson E. N., Yanofsky C. Internal promoter of the tryptophan operon of Escherichia coli is located in a structural gene. J Mol Biol. 1972 Aug 21;69(2):307–313. doi: 10.1016/0022-2836(72)90232-x. [DOI] [PubMed] [Google Scholar]
- Kalnins A., Otto K., Rüther U., Müller-Hill B. Sequence of the lacZ gene of Escherichia coli. EMBO J. 1983;2(4):593–597. doi: 10.1002/j.1460-2075.1983.tb01468.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanehisa M. Use of statistical criteria for screening potential homologies in nucleic acid sequences. Nucleic Acids Res. 1984 Jan 11;12(1 Pt 1):203–213. doi: 10.1093/nar/12.1part1.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kravchenko V. V., Vasilenko S. K., Grachev M. A. A rightward promoter to the left of the att site of lambda phage DNA: possible participant in site-specific recombination. Gene. 1979 Nov;7(3-4):181–195. doi: 10.1016/0378-1119(79)90045-3. [DOI] [PubMed] [Google Scholar]
- Landsmann J., Kröger M., Hobom G. The rex region of bacteriophage lambda: two genes under three-way control. Gene. 1982 Nov;20(1):11–24. doi: 10.1016/0378-1119(82)90083-x. [DOI] [PubMed] [Google Scholar]
- Minkley E. G., Pribnow D. Transcription of the early region of bacteriophage T7: selective initiation with dinucleotides. J Mol Biol. 1973 Jun 25;77(2):255–277. doi: 10.1016/0022-2836(73)90335-5. [DOI] [PubMed] [Google Scholar]
- Mulligan M. E., Hawley D. K., Entriken R., McClure W. R. Escherichia coli promoter sequences predict in vitro RNA polymerase selectivity. Nucleic Acids Res. 1984 Jan 11;12(1 Pt 2):789–800. doi: 10.1093/nar/12.1part2.789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okamoto T., Sugimoto K., Sugisaki H., Takanami M. DNA regions essential for the function of a bacteriophage fd promoter. Nucleic Acids Res. 1977 Jul;4(7):2213–2222. doi: 10.1093/nar/4.7.2213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pirrotta V., Ineichen K., Walz A. An unusual RNA polymerase binding site in the immunity region of phage lambda. Mol Gen Genet. 1980;180(2):369–376. doi: 10.1007/BF00425850. [DOI] [PubMed] [Google Scholar]
- Prosen D. E., Cech C. L. Bacteriophage T7 E promoter: identification and measurement of kinetics of association with Escherichia coli RNA polymerase. Biochemistry. 1985 Apr 23;24(9):2219–2227. doi: 10.1021/bi00330a016. [DOI] [PubMed] [Google Scholar]
- Reich J. G., Drabsch H., Däumler A. On the statistical assessment of similarities in DNA sequences. Nucleic Acids Res. 1984 Jul 11;12(13):5529–5543. doi: 10.1093/nar/12.13.5529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg M., Court D. Regulatory sequences involved in the promotion and termination of RNA transcription. Annu Rev Genet. 1979;13:319–353. doi: 10.1146/annurev.ge.13.120179.001535. [DOI] [PubMed] [Google Scholar]
- Rosenvold E. C., Calva E., Burgess R. R., Szybalski W. In vitro transcription from the b2 region of bacteriophage lambda. Virology. 1980 Dec;107(2):476–487. doi: 10.1016/0042-6822(80)90314-1. [DOI] [PubMed] [Google Scholar]
- Sanger F., Coulson A. R., Hong G. F., Hill D. F., Petersen G. B. Nucleotide sequence of bacteriophage lambda DNA. J Mol Biol. 1982 Dec 25;162(4):729–773. doi: 10.1016/0022-2836(82)90546-0. [DOI] [PubMed] [Google Scholar]
- Schmid M. B., Roth J. R. Internal promoters of the his operon in Salmonella typhimurium. J Bacteriol. 1983 Feb;153(2):1114–1119. doi: 10.1128/jb.153.2.1114-1119.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siebenlist U., Simpson R. B., Gilbert W. E. coli RNA polymerase interacts homologously with two different promoters. Cell. 1980 Jun;20(2):269–281. doi: 10.1016/0092-8674(80)90613-3. [DOI] [PubMed] [Google Scholar]
- Smith T. F., Waterman M. S., Burks C. The statistical distribution of nucleic acid similarities. Nucleic Acids Res. 1985 Jan 25;13(2):645–656. doi: 10.1093/nar/13.2.645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staden R. Computer methods to locate signals in nucleic acid sequences. Nucleic Acids Res. 1984 Jan 11;12(1 Pt 2):505–519. doi: 10.1093/nar/12.1part2.505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stahl S. J., Chamberlin M. J. An expanded transcriptional map of T7 bacteriophage. Reading of minor T7 promoter sites in vitro by Escherichia coli RNA polymerase. J Mol Biol. 1977 Jun 5;112(4):577–601. doi: 10.1016/s0022-2836(77)80165-4. [DOI] [PubMed] [Google Scholar]
- Studier F. W., Rosenberg A. H. Genetic and physical mapping of the late region of bacteriophage T7 DNA by use of cloned fragments of T7 DNA. J Mol Biol. 1981 Dec 15;153(3):503–525. doi: 10.1016/0022-2836(81)90405-8. [DOI] [PubMed] [Google Scholar]
- Travers A. A. Conserved features of coordinately regulated E. coli promoters. Nucleic Acids Res. 1984 Mar 26;12(6):2605–2618. doi: 10.1093/nar/12.6.2605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vollenweider H. J., Szybalski W. Electron microscopic mapping of RNA polymerase binding to coliphage lambda DNA. J Mol Biol. 1978 Aug 15;123(3):485–498. doi: 10.1016/0022-2836(78)90092-x. [DOI] [PubMed] [Google Scholar]



