Abstract
Background
The clinicopathologic significance of lower uterine segment involvement (LUSI) in endometrial cancer patients remains unclear. Although LUSI has been reported to be a prognostic indicator, literature is limited.
Methods
We studied 481 surgically staged endometrioid endometrial cancers with disease confined to the uterus (FIGO 1988 stage I or II). Primary outcomes were overall survival (OS) and disease-free survival (DFS). The relationships between LUSI and OS and DFS were assessed using the Kaplan–Meier method and Cox proportional hazard models. The t test or Fisher exact test was used for evaluating relationships between variables of interest.
Results
LUSI was present in 223 cases (46.4%), and was associated with both decreased disease free survival (P = 0.02) and overall survival (P = 0.01) in univariate analysis. Multivariate analysis confirmed the association between LUSI and increased risk for recurrence [hazard ratio (HR) 2.27; 95% confidence interval (95% CI) 1.09–4.7; P = 0.03] and increased mortality (HR 1.76; 95% CI 1.12–2.78; P = 0.01).
Conclusions
LUSI in patients with early-stage endometrioid endometrial cancer is associated with decreased survival.
Endometrial cancer is the most common gynecologic malignancy and the fourth most common cancer among women in developed countries.1 It is estimated that approximately 43,470 women in the United States will be diagnosed with uterine cancer, and 7950 will die of their disease in 2010.2,3 The incidence of endometrial cancer has remained constant with little improvement in mortality rates from 1995 to 2008.3
As the majority of patients present with early-stage disease, endometrial cancer generally carries an overall good prognosis with an estimated 5-year survival of approximately 85%.3 Treatment typically consists of surgery followed by individualized adjuvant therapy. Patients with advanced stage or recurrent disease have a much graver prognosis and less consistent treatment options.
Although stage is directive in decisions for treatment and remains the major predictor of outcomes, other factors not incorporated into the staging system, such as lymphovascular space involvement, grade, and age independently influence survival.4–8 These well-established prognostic factors in early-stage endometrial cancer are included in many clinical management algorithms. Despite incorporation of these additional prognostic markers into treatment strategies, 10–20% of early-stage endometrial cancers will still recur, emphasizing the need for more predictive prognostic biomarkers.6,9
There have been several reports on the potential value of lower uterine segment involvement (LUSI) in the prognosis and management of early-stage endometrial cancer patients. These published studies are retrospective and were, in general, underpowered to identify small or modest-sized effects.10–14 One study also included patients with nodal metastatic disease that could mask the prognostic value of LUSI in early-stage patients.12 However, LUSI does appear to be an important predictor of lymph node involvement for patients with endometrioid histology, and a recently reported large multi-institutional study of Israeli patients with 1988 FIGO stage I endometrioid endometrial cancer suggested that LUSI is a poor prognostic factor, conferring a significantly higher risk of distal recurrence and death.15–17 Confirming the prognostic value of LUSI could potentially alter the current management of many of these patients who frequently only undergo surveillance postoperatively without adjuvant therapy. The significance of tumor within the lower uterine segment gained further attention with recent work, suggesting that endometrial carcinoma originating solely from within the lower uterine segment may be associated with Lynch syndrome in contrast to tumors that involved the uterine corpus.18 Such a finding may have important implications for genetic testing and/or counseling of these individuals.
Our aims were: (1) to evaluate the association of LUSI with clinical outcomes of surgically staged endometrioid endometrial cancer patients with disease confined to the uterus and (2) to evaluate the relationship of LUSI and the Lynch syndrome molecular feature, MSI, in this same group of patients.
MATERIALS AND METHODS
Study Participants and Clinical Data
Washington University prospectively gathers clinical and demographic information for endometrial cancer patients treated at our facility. Blood and tumor specimens are collected on participating subjects at the time of study enrollment. Tissue specimen and data collection began in 1991 and continues with active curation of clinical data. We chose to retrospectively study patients enrolled between 1991 and 2007 to ensure ≥2 years follow-up time for outcome data analysis. Eligibility criteria included women 18 years or older with 1988 FIGO stage IA–IIB endometrioid endometrial cancer who underwent a full surgical staging procedure and for whom follow-up data are available. All participants consented to molecular analyses and follow-up as part of a Washington University Medical Center Human Studies Committee-approved protocol (93-0828). Of the 956 patients included in our database, 149 (15.7%) were excluded because of lack of outcome data, 143 (15%) because of nonendometrioid or mixed histology, and 10 (1%) because of presence of a synchronous primary. Of the remaining 654 subjects with endometrioid tumors, 88 (13.5%) did not undergo a lymph node dissection during their surgery and 85 (13%) did not have pathologic assessment for LUSI.
Pathologic evaluation of all tissue specimens was performed by gynecologic pathologists at our institution. A subject was categorized as LUSI positive if tumor involvement of the lower uterine segment was documented by either histologic or gross pathologic description within the pathology report. Demographic and follow-up data were extracted from the research database and hospital records. Accurate surgical stage for each subject was confirmed during data collection for our study according to the FIGO 1988 criteria.19
Adjuvant therapy was administered on an individual basis at the discretion of the treating physician, and consisted of chemotherapy, radiation therapy, hormonal therapy, or some combination of these treatment modalities. At completion of treatment subjects were typically followed at 3-month intervals for the first two years, at six-month intervals for 3 additional years, and yearly thereafter. Standard surveillance at our institution included physical examination and Pap test for at least 3 years after initial treatment with performance of additional imaging studies and directed biopsies as indicated. All recurrent and progressive disease was histologically and/or radiographically confirmed.
MSI Typing
Tissue specimens and blood were obtained at the time of surgery, snap frozen, and stored at −70°C. DNA was isolated from high neoplastic tumor tissues as previously described.20–23
Microsatellite analysis was performed as previously described by our group.20,21 Standard definitions for MSI were used.24 Low-level MSI and microsatellite stable tumors were designated MSI negative for the purposes of this study.
Statistical Analyses
Power was estimated assuming that the 5-year overall survival for early-stage endometrial cancer is greater than 90%.3 An 80% power to detect a 10% or greater difference in overall survival between the 2 groups required a minimum of 318 patients using a 2-tailed chi-square test with significance defined as P < 0.05. Primary outcomes were overall survival (OS) and disease-free survival (DFS). OS was defined as the time from surgery to date of death from any cause. Survivors were censored at the date of last contact. DFS was defined as the time from surgery to disease recurrence or progression. OS and DFS were estimated by Kaplan-Meier product limit method and compared using univariate Cox proportional hazards models. Multivariate Cox models were fitted to adjust for the possible effects of other covariates on OS and DFS using backward elimination techniques. In the analysis of DFS, Gray’s competing risk methods were also used as a sensitivity analysis to account for the potential competing effect of death.25 Secondary outcome was presence of tumor MSI. Although not diagnostic, MSI status is a highly sensitive test for Lynch syndrome. A high MSI result by immunohistochemistry will detect approximately 95% of all cases.26 If no association is found between MSI status and LUSI, then LUSI is also unlikely to be associated with the Lynch syndrome. Relationships between LUSI and other variables of interest were assessed using t test or Fisher exact test as appropriate. All analyses were 2-sided, and significance was set at a P value of 0.05. Statistical analyses were performed using SAS (SAS Institutes, Cary, NC), as well as the cmprsk R (http://biowww.dfci.harvard.edu/~gray) statistical packages for competing risk analysis.
RESULTS
The demographic and clinicopathologic characteristics of the 481 subjects included in this study are presented in Table 1. The median age at diagnosis was 62 years (range, 22–92 years). Most patients were stage IB (n = 222, 46.2%). The mean number of pelvic and para-aortic lymph nodes removed were 18.3 (range, 0–42) and 5.3 (range, 0–23), respectively. Median follow-up time was 53.6 months (range, 0.2–165.3). A total of 80 deaths (16.6%) and 36 recurrences (7.5%) occurred during the study period. The majority of patients (79%) did not receive adjuvant therapy. The most common adjuvant modality used was radiation therapy alone (83.2%).
TABLE 1.
Demographic, clinicopathologic, and molecular characteristics
| LUSI + (n = 223) |
LUSI − (n = 258) |
p | |||
|---|---|---|---|---|---|
| N | % | N | % | ||
| Age (years) | 0.43 | ||||
| Mean ± standard deviation | 62.5 ± 11.7 | 63.4 ± 11.3 | |||
| BMI (kg/m2) | 0.18 | ||||
| Mean ± standard deviation | 36.0 ± 11.0 | 34.4 ± 9.6 | |||
| Race | 0.62 | ||||
| White | 199 | 89.2 | 227 | 87.9 | |
| Black | 24 | 10.8 | 30 | 11.7 | |
| Other/not specified | 0 | 0 | 1 | 0.4 | |
| Stage | <0.001 | ||||
| IA | 24 | 10.7 | 88 | 34.1 | |
| IB | 101 | 45.3 | 121 | 46.9 | |
| IC | 49 | 22 | 46 | 17.8 | |
| II | 49 | 22 | 3 | 1.2 | |
| Grade | 0.51 | ||||
| 1 | 124 | 55.6 | 156 | 53 | |
| 2 | 67 | 30.1 | 74 | 33.7 | |
| 3 | 31 | 13.9 | 28 | 13.3 | |
| Not evaluable | 1 | 0.4 | 0 | 0 | |
| Adjuvant treatment | 0.32 | ||||
| Yes | 48 | 21.5 | 47 | 18.2 | |
| No | 172 | 77.2 | 210 | 81.4 | |
| Not specified | 3 | 1.3 | 1 | 0.4 | |
| LVSI | <0.01 | ||||
| Present | 67 | 30.0 | 42 | 16.3 | |
| Absent | 156 | 70.0 | 215 | 83.3 | |
| Not specified | 0 | 0 | 1 | 0.4 | |
| MSI statusa | 0.36 | ||||
| Neg/low | 127 | 70.2 | 116 | 64.1 | |
| High | 53 | 29.3 | 61 | 33.7 | |
| No data | 1 | 0.5 | 4 | 2.2 | |
BMI body mass index, LUSI lower uterine segment involvement, MSI microsatellite instability, LVSI lymphovascular space involvement
MSI data available for 357 tumors
A total of 223 subjects had evidence of LUSI (46.4%). Of these, 208 tumors met histologic criteria for documentation of LUSI (93.3%), whereas 15 were identified by gross description alone (6.7%). Microsatellite instability analysis was successfully performed on 357 subjects (74.2%).
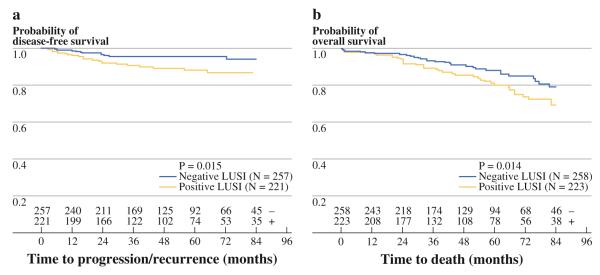
In univariate analyses, the presence of LUSI was significantly associated with decreased DFS (HR 2.46; 95% CI 1.19–5.08; P = 0.02) and OS (HR 1.75; 95% CI 1.12–2.76; P = 0.01) (Table 2, Fig. 1). Grade and LVSI were associated with decreased OS and DFS. Age was only associated with decreased OS. MSI was not associated with either primary outcome and was not included in subsequent analyses.
TABLE 2.
Univariate analysis of LUSI and survival
| Disease-free survival |
Overall survival |
|||||
|---|---|---|---|---|---|---|
| Hazard ratio | 95% CI | p | Hazard ratio | 95% CI | p | |
| Patient characteristics | ||||||
| Age | 1.01 | 0.98–1.04 | 0.44 | 1.03 | 1.01–1.05 | 0.01 |
| BMI | 0.99 | 0.96–1.03 | 0.77 | 1.01 | 0.99–1.04 | 0.37 |
| Racea | 2.18 | 0.94–5.01 | 0.07 | 1.36 | 0.73–2.51 | 0.33 |
| Adjuvant therapyb | 1.35 | 0.61–2.99 | 0.46 | 1.17 | 0.69–1.99 | 0.55 |
| Tumor characteristics | ||||||
| LUSI | 2.46 | 1.19–5.08 | 0.02 | 1.75 | 1.12–2.76 | 0.01 |
| Stage | ||||||
| IA vs. II | – c | – c | 0.99 | 0.39 | 0.19–0.83 | 0.01 |
| IB vs. II | 0.54 | 0.22–1.30 | 0.17 | 0.55 | 0.30–0.99 | 0.05 |
| IC vs. II | 0.84 | 0.32–2.22 | 0.73 | 0.69 | 0.35–1.36 | 0.28 |
| Grade | ||||||
| 2 vs. 1 | 3.26 | 1.35–7.87 | <0.01 | 1.65 | 1.00–2.72 | 0.05 |
| 3 vs. 1 | 7.71 | 3.15–18.87 | <0.001 | 2.61 | 1.47–4.64 | <0.01 |
| LVSI | 3.43 | 1.73–6.79 | <0.001 | 1.67 | 1.04–2.68 | 0.03 |
| MSI high | 1.38 | 0.65–2.9 | 0.41 | 0.95 | 0.57–1.59 | 0.85 |
BMI body mass index, LUSI lower uterine segment involvement, LVSI lymphovascular space involvement, MSI microsatellite instability
Reference category is “white”
Reference category is “none”
Unable to estimate due to no events in category “IA”
FIG. 1.
Reduced disease-free (A) and overall survival (B) for patients with LUSI. Numbers at bottom indicate number of LUSI positive (+) and LUSI negative (−) survivors at each time point. There was no data regarding recurrence in 3 subjects
Covariates with a P of ≤0.2 were included in the multivariate analysis. The presence of LUSI was confirmed as an independent prognostic factor for both worse DFS (HR 2.27; 95% CI 1.09–4.71; P = 0.03) and OS (HR 1.76; 95% CI 1.12–2.78; P = 0.01) (Table 3). Grade remained significantly associated with DFS and OS. Age was associated with decreased OS, and nonwhite race was associated with decreased DFS. A subanalysis of only stage I patients demonstrated LUSI to remain significantly associated with decreased OS (HR 1.67, 95% CI 1.02–2.72; P = 0.04).
TABLE 3.
Multivariate analysis of LUSI and survival
| Disease-free survival |
Overall survival |
|||||
|---|---|---|---|---|---|---|
| Hazard ratio |
95% CI | p | Hazard ratio |
95% CI | p | |
| LUSI | 2.27 | 1.09–4.71 | 0.03 | 1.76 | 1.12–2.78 | 0.01 |
| Grade | ||||||
| 2 vs. 1 | 3.69 | 1.47–9.26 | <0.01 | 1.67 | 0.99–2.79 | 0.05 |
| 3 vs. 1 | 7.86 | 3.08–20.05 | <0.0001 | 2.56 | 1.42–4.60 | <0.01 |
| Race | 2.05 | 0.86–4.78 | 0.09 | – a | – a | – a |
| Age | – a | – a | – a | 1.02 | 1.00–1.04 | 0.03 |
LUSI lower uterine segment involvement
Not included in final model
DISCUSSION
To the best of our knowledge, this represents the largest single-institution study evaluating the role of LUSI in endometrioid endometrial cancer, and our cohort included the largest number of LUSI positive patients of any published study. We demonstrated that the presence of LUSI is associated with decreased disease-free and overall survival in patients with surgically staged endometrioid endometrial cancer confined to the uterus (1988 FIGO stages I and II).
Understanding the prognostic value of LUSI could be beneficial both in adjuvant therapy planning, and for patient counseling. New data suggest there is no survival benefit derived from full surgical staging in early-stage endometrial cancer.27,28 It seems likely that in the near future patients will undergo simple hysterectomy with bilateral oophorectomy as primary treatment rather than full surgical staging. Molecular markers for risk stratification in the management of endometrial cancer patients could become a valuable prognostic tool. In the meantime, new algorithms that take full advantage of information obtained within the hysterectomy specimen should be considered and, as molecular marker data emerge, considered in conjunction with validated clinicopathologic markers. Whether adjuvant radiotherapy or other therapeutic interventions would be indicated for patients with LUSI remains unclear and would require further investigation.
We did not find evidence of an association between LUSI and the molecular feature of the Lynch syndrome, MSI. Although not specifically powered to find such difference, our study sample was considerably larger than any previously published data regarding the topic.18 Westin et al. evaluated 35 patients with endometrial carcinoma of the lower uterine segment and found that 10 (29%) were confirmed to have Lynch syndrome by the presence of MSI and either immunohistochemical evidence of loss of MLH1 with lack of MLH1 methylation or loss of either MSH2 and/or MSH6 protein expression. However, the results of the studies were derived from 2 distinct study designs that focused on different patient populations. We studied a cohort of endometrial cancer patients and classified each case by presence or absence of lower uterine segment involvement. The study by Westin and colleagues was focused on evaluating differences in DNA mismatch repair status in patients with tumors that appeared to originate solely from the lower uterine segment, without involvement of the uterine corpus proper.
Few studies addressing the prognostic importance of LUSI have been reported. A large multicenter Israeli study of 769 patients, among which 138 had LUSI, suggested LUSI is a poor prognostic factor conferring a significant decreased overall survival (HR = 2.3; 95% CI 1.3–3.9; P = 0.003).17 However, only half (50%) of the subjects had undergone systematic surgical staging, and many cases could have had unrecognized advanced stage disease. Several studies did not find LUSI to be prognostic, but all were retrospective and limited by small sample sizes.10–12,14 A Turkish study (N = 106) suggests that patients with LUSI should be considered high risk, as LUSI was associated with increased myometrial invasion, presence of lymph vascular space involvement, and positive cytology.13
As our study population was derived from a single large tertiary care center, results may not be generalizable to other populations. Differences between pathology departments may also vary in regard to the definition of LUSI, emphasizing the need for multi-institutional studies on this topic. We did not account for variation of treatment strategies and modalities over the 16-year study period. Only 15% of the original cohort identified was excluded in the final analysis because of incomplete outcome data.
Our findings support LUSI as a negative prognostic factor influencing survival. This is important in the counseling of these patients who may do worse than women with LUSI negative tumors of similar stage. We did not address whether LUSI warrants adjuvant therapy. Such an important question, as well as determining what type of treatment should be recommended, is beyond the scope of our study. New algorithms utilizing information, such as presence or absence of LUSI, from hysterectomy specimens in unstaged patients may become the standard method of triaging their medical management. Prospective multi-institutional studies are needed to confirm our research findings.
ACKNOWLEDGMENT
The authors wish to acknowledge the support of the Biostatistics Core, Siteman Comprehensive Cancer Center and NCI Cancer Center Support Grant P30 CA091842. Supported by RO1 CA71754 (P.J.G.), Barnes-Jewish Foundation 00161-0806 (P.J.G.) and 1T32HD055172-01A2 (N.T.K.)
Footnotes
DISCLOSURE Kizer et al. have nothing to disclose.
REFERENCES
- 1.Curado MP, Edwards B, Shin HR, editors. Cancer incidence in five continents. vol. IX. Lyon, France: 2008. pp. 1–837. IARC Scientific Publication no. 160. [Google Scholar]
- 2.Horner MJ, Ries LAG, Krapcho M, Neyman N, Aminou R, Howlader N, et al., editors. SEER Cancer Statistics Review, 1975–2006. National Cancer Institute; Bethesda, MD: 2009. Available at http://seer.cancer.gov/csr/1975_2006/, based on November 2008 SEER data submission, posted to the SEER web site. [Google Scholar]
- 3.Jemal A, Siegel E, Xu J, Ward E. Cancer statistics, 2010. CA Cancer J Clin. 2010;60:277–300. doi: 10.3322/caac.20073. [DOI] [PubMed] [Google Scholar]
- 4.Creasman WT, Morrow CP, Bundy BN, Homesley HD, Graham JE, Heller PB. Surgical pathologic spread patterns of endometrial cancer. A gynecologic oncology group study. Cancer. 1987;60:2035–41. doi: 10.1002/1097-0142(19901015)60:8+<2035::aid-cncr2820601515>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- 5.Boronow RC, Morrow CP, Creasman WT, Disaia PJ, Silverberg SG, Miller A, et al. Surgical staging in endometrial cancer: clinical-pathologic findings of a prospective study. Obstet Gynecol. 1984;63:825–32. [PubMed] [Google Scholar]
- 6.Morrow CP, Bundy BN, Kurman RJ, Creasman WT, Heller P, Homesley HD, et al. Relationship between surgical pathological risk factors and outcome in clinical stage I and II carcinoma of the endometrium: a Gynecologic Oncology Group study. Gynecol Oncol. 1991;40:55–6. doi: 10.1016/0090-8258(91)90086-k. [DOI] [PubMed] [Google Scholar]
- 7.Disaia PJ, Creasman WT, Boronow RC, Blessing JA. Risk factors and recurrent patterns in Stage I endometrial cancer. Am J Obstet Gynecol. 1995;151:1009–15. doi: 10.1016/0002-9378(85)90371-0. [DOI] [PubMed] [Google Scholar]
- 8.Briet LM, Hollema H, Reesink N, Aalders JG, Mourits MJ, ten Hoor KA, et al. Lymphvascular space involvement: an independent prognostic factor in endometrial cancer. Gynecol Oncol. 2005;96:799–804. doi: 10.1016/j.ygyno.2004.11.033. [DOI] [PubMed] [Google Scholar]
- 9.Creutzberg CL, van Putten WL, Koper PC, Lybeert ML, Jobsen JJ, Wárlám-Rodenhuis CC, et al. Surgery and postoperative radiotherapy versus surgery alone for patients with stage-1 endometrial carcinoma: multicentre randomised trial. PORTEC Study Group. Post Operative Radiation Therapy in Endometrial Carcinoma. Lancet. 2000;355:1404–11. doi: 10.1016/s0140-6736(00)02139-5. [DOI] [PubMed] [Google Scholar]
- 10.Lavie O, Uriev L, Gdalevich M, Barak F, Peer G, Auslender R, et al. The outcome of patients with stage I endometrial cancer involving the lower uterine segment. Int J Gynecol Cancer. 2008;18:1079–83. doi: 10.1111/j.1525-1438.2007.01150.x. [DOI] [PubMed] [Google Scholar]
- 11.Brown AK, Madom L, Moore R, Granai CO, DiSilvestro P. The prognostic significance of lower uterine segment involvement in surgically staged endometrial cancer patients with negative nodes. Gynecol Oncol. 2007;105:55–8. doi: 10.1016/j.ygyno.2006.10.058. [DOI] [PubMed] [Google Scholar]
- 12.Phelan C, Montag AG, Rotmensch J, Waggoner SE, Yamada SD, Mundt AJ. Outcome and management of pathological stage I endometrial carcinoma patients with involvement of the lower uterine segment. Gynecol Oncol. 2001;83:513–7. doi: 10.1006/gyno.2001.6407. [DOI] [PubMed] [Google Scholar]
- 13.Dilek S, Dede M, Gezginc K, Yenen MC, Göktolga U, Ulutin HC, et al. Does the localization of tumour at stage I endometrial endometrioid adenocarcinoma have an impact on invasion of the tumour and individualization of the surgical procedure? Eur J Gynaecol Oncol. 2008;29:138–40. [PubMed] [Google Scholar]
- 14.Gemer O, Uriev L, Harkovsky T, Peled R, Ben-Dor D, Barak F, et al. Significance of lower uterine segment involvement in women with stage I endometrial adenocarcinoma. J Reprod Med. 2004;49:703–6. [PubMed] [Google Scholar]
- 15.Madom LM, Brown AK, Lui F, Moore RG, Granai CO, Disilvestro PA. Lower uterine segment involvement as a predictor for lymph node spread in endometrial carcinoma. Gynecol Oncol. 2007;107:75–8. doi: 10.1016/j.ygyno.2007.05.026. [DOI] [PubMed] [Google Scholar]
- 16.Creasman WT, Morrow CP, Bundy BN, Homesley HD, Graham JE, Heller PB. Surgical pathologic spread patterns of endometrial cancer. Cancer. 1997;60:2035–41. doi: 10.1002/1097-0142(19901015)60:8+<2035::aid-cncr2820601515>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- 17.Gemer O, Gdalevich M, Voldarsky M, Barak F, Arie A Ben, Schneider D, et al. Lower uterine segment involvement is associated with adverse outcome in patients with stage I endometrioid endometrial cancer: Results of a multicenter study. Eur J Surg Oncol. 2009;35:865–9. doi: 10.1016/j.ejso.2008.10.007. [DOI] [PubMed] [Google Scholar]
- 18.Westin SN, Lacour RA, Urbauer DL, Luthra R, Bodurka DC, Lu KH, et al. Carcinoma of the lower uterine segment: a newly described association with Lynch Syndrome. J Clin Oncol. 2008;26:5965–71. doi: 10.1200/JCO.2008.18.6296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.FIGO Announcements, stages-1988 Revision. Gynecol Oncol. 1989;35:125. [Google Scholar]
- 20.Peiffer SL, Herzog TJ, Tribune DJ, Mutch DG, Gersell DJ, Goodfellow PJ. Allelic loss of sequences from the long arm of chromosome 10 and replication errors in endometrial cancers. Cancer Res. 1995;55:1922–6. [PubMed] [Google Scholar]
- 21.Kowalski LD, Mutch DG, Herzog TJ, Rader JS, Goodfellow PJ. Mutational analysis of MLH1 and MSH2 in 25 prospectively-acquired RER+ endometrial cancers. Genes Chromosomes Cancer. 1997;19:219–27. doi: 10.1002/(sici)1098-2264(199703)18:3<219::aid-gcc8>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- 22.Miller SA, Dykes DD, Polesky HF. A simple salting out procedure for extracting DNA from human nucleated cells. Nucleic Acids Res. 1988;16:1215. doi: 10.1093/nar/16.3.1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lahiri DK, Nurnberger JI., Jr A rapid non-enzymatic method for the preparation of HMW DNA from blood for RFLP analysis. Nucleic Acids Res. 1991;19:5444. doi: 10.1093/nar/19.19.5444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Boland CR, Thibodeau SN, Hamilton SR, Sidransky D, Eshleman JR, Burt RW, et al. A National Cancer Institute Workshop on Microsatellite Instability for cancer detection and familial predisposition: development of international criteria for the determination of microsatellite instability in colorectal cancer. Cancer Res. 1998;58:5248–57. [PubMed] [Google Scholar]
- 25.Fine JP, Gray RJ. A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc. 1999;94:496–509. [Google Scholar]
- 26.Hendriks YMC, Wagner A, Morreau H, Menko F, Stormorken A, Quehenberger F, et al. Cancer risk in hereditary nonpolyposis colorectal cancer due to MSH6 mutations: impact on counseling and surveillance. Gastroenterology. 2004;127:17–25. doi: 10.1053/j.gastro.2004.03.068. [DOI] [PubMed] [Google Scholar]
- 27.ASTEC Study Group et al. Efficacy of systematic pelvic lymphadenectomy in endometrial cancer (MRS ASTEC trial): a randomized study. Lancet. 2009;373:125–36. doi: 10.1016/S0140-6736(08)61766-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Panici P Benedetti, Basile S, Maneschi F, Lissoni A Alberto, Signorelli M, Scambia G, et al. Systematic pelvic lymphadenectomy vs no lymphadenectomy in early-stage endometrial carcinoma: randomized clinical trial. J Natl Cancer Inst. 2008;100:1707–16. doi: 10.1093/jnci/djn397. [DOI] [PubMed] [Google Scholar]