Abstract
Willem Takken and colleagues argue for the expansion of insecticide monotherapy in malaria control by taking lessons from agriculture and including more sustainable integrated vector management strategies.
Summary Points
The effectiveness of insecticide-treated bed nets and indoor insecticide sprays to control adult mosquito vectors is being threatened by the spread of insecticide resistance.
We argue for expanding beyond “insecticide monotherapy” to more sustainable integrated vector management strategies that use optimal suites of control tactics.
Experience in agriculture suggests that such integrated approaches can provide more effective and durable pest management.
This shift will require increased investment in research and translational science.
Failure to act risks a resurgence of malaria and erosion of community support and donor commitment.
Vector Control and the Emerging Insecticide Resistance Crisis
The 2011 World Malaria Report [1] showed welcome progress in the fight against the world's most important vector-borne disease. In the last 10 years, the estimated incidence of malaria has fallen by 17% globally, with malaria-specific mortality rates reduced by 25%. Central to these gains, especially in Africa, has been the massive scale-up of chemical insecticide interventions against malaria mosquito vectors. Current malaria vector control relies almost exclusively on killing adult mosquitoes with chemical insecticides deployed as either insecticide-treated nets (ITNs) or indoor residual sprays (IRS). However, these technologies use a limited arsenal of insecticides originally developed for agriculture, and their efficacy is threatened by the spread of insecticide resistance [1]–[3]. In 2010, 27 countries in sub-Saharan Africa reported mosquitoes resistant to pyrethroids [1]. Such resistance is alarming because pyrethroids are the only class of insecticides approved for use on ITNs and account for two-thirds of the total product (by area) used in IRS for malaria control [4]. Evidence suggests that resistance is beginning to reduce control [5],[6]. Implementation of alternative management strategies is needed to slow and reverse this trend.
Parallels with Agriculture
In the middle of the last century, the development of cheap and effective synthetic chemical insecticides revolutionized crop protection. Widespread use of broad-spectrum insecticides reduced pest damage substantially in many systems, prompting discussion of pest eradication, similar to some current discussions of eradication ofmalaria. However, rapid evolution of insecticide resistance, pest resurgence due to disruption of biological control, and harmful environmental side effects quickly revealed the limitations of “pesticide monotherapy” [7]–[9].
The search to find new chemical insecticides continued, stimulated by the transient efficacy of products in use and increased restrictions on available insecticides because of their toxicity to people and other non-target organisms. Meanwhile, academic and government researchers explored ways to reduce reliance on insecticides. In crop systems where insecticide use was actually exacerbating pest problems, researchers combined diverse tools such as pest monitoring and forecasting, conservation of natural pest control, habitat manipulation, and resistant host plants, and thereby limited pesticide use to situations where it was necessary [10]. This approach, called integrated pest management (IPM) [10], reduces the risk of insecticide resistance. IPM is knowledge intensive, relying heavily on farmers' understanding and monitoring of local conditions. Its development therefore engendered a culture of farmer participation and decision-making, providing a balance to the former top-down, technology-driven approach. While not a panacea, IPM is now a cornerstone of many production systems in both developed and developing countries [10]–[14]. Even new technologies, such as genetically engineered crops, can be more effective and sustainable when used with other tactics in IPM [12],[15].
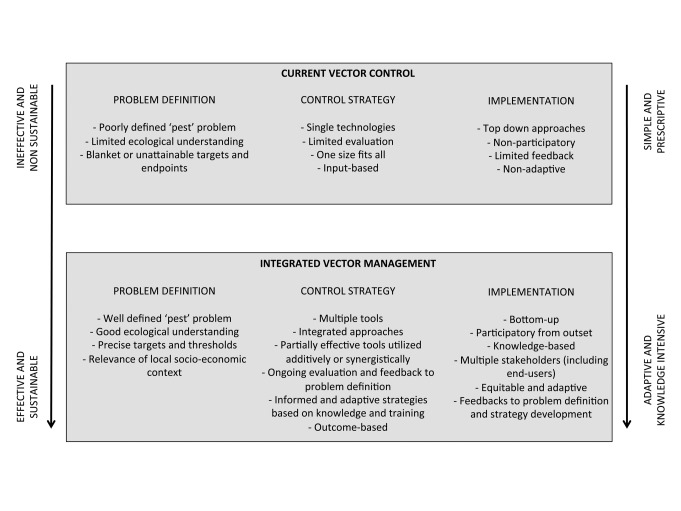
Current malaria vector control has more in common with the agricultural practices of the 1950s than contemporary IPM (Figure 1). There is a reliance locally on single technologies associated with fast-acting insecticides used in ways that impose intense selection pressure for resistance.
Figure 1. Features of current vector control strategies compared with potential integrated vector management (IVM).
The arrows indicate trends representative of the contrasting strategies. Progression towards IVM has the potential to increase the effectiveness and sustainabilty of control, but requires more diverse and knowledge-intensive approaches.
The pending resistance crisis creates an urgent need to develop and implement integrated, multi-tactic strategies for vector control that parallel IPM in agriculture. We call this “integrated vector management” (IVM), which we define as the optimal use of diverse tools, tactics, and resources to reduce transmission of disease by vectors. The potential of IVM has been discussed previously (e.g., [16],[17]), and tacit recognition of the approach already exists in World Health Organization (WHO) policy [18],[19]. The transition to more sustainable IVM will, however, require increased efforts in several key areas.
Quantifying the Problem
One of the foundations of IPM and thus IVM is to quantify the “pest” or “vector” problem and define the targets for control. For malaria this might seem straightforward—“control mosquitoes and reduce disease as much as possible”. Yet, it is surprising how little is understood about how local vector ecology contributes to infection. A typical list of unknowns could include the temporal and spatial distribution of biting, rate of parasite development, local variation in vector competence, sites where mosquitoes rest, the causes and rate of adult mosquito mortality, the nature of density-dependent regulation, and sometimes even which vector species is most important [20],[21]. Equally little is understood regarding the impact of insecticide resistance on vectorial capacity and malaria epidemiology [22],[23]. These unknown factors influence the approaches and strategies required to reduce malaria transmission in a particular setting. For example, while a 30% reduction in infectious bites might substantially reduce disease prevalence in a low transmission environment, even a 90% reduction might not be sufficient in a high transmission environment [24]. Effective IVM requires a better understanding of local vector and transmission ecology with appropriate targets for control defined in ways analogous to economic thresholds of pest density used widely to guide pest control decisions in agriculture.
Conventional Chemicals
Highly lethal insecticides like pyrethroids knock down and kill mosquitoes rapidly after contact. This lethality can provide excellent disease control, yet it also selects intensely for resistance. Development of replacement insecticides is one recognized strategy to address this problem [25]. However, the insecticide target product profiles prescribed by the WHO Pesticide Evaluation Scheme (WHOPES) set a high bar with respect to rapid killing, high persistence, and low mammalian toxicity. This, together with protracted regulatory procedures, means new insecticides are still many years off [3]. Moreover, novel chemistry will not prevent resistance evolution [26]. Resistance management strategies used in agriculture such as insecticide combinations and rotations require two or more insecticides with diverse modes of action to avoid cross-resistance [27], yet this diversity is not commonly available for vector control [28]. This problem is compounded when the same insecticide active ingredients are used in both agriculture and vector control [29],[30]. In the only controlled trial of resistance management strategies for malaria mosquito vectors we know of, rotations or mosaics did not delay pyrethroid resistance [26],[31].
In addition, ITNs and IRS only target mosquitoes inside domestic dwellings, leaving potentially significant fractions of the vector community untouched. While outdoor biting tends to be less epidemiologically important than indoor biting, it still contributes to transmission [32],[33]. Thus, even in the absence of resistance, it is unlikely that ITNs and IRS will be sufficiently effective to meet the goal of long-term malaria suppression in intense transmission settings.
Additional Tools
Current vector control relies on killing mosquitoes quickly with neurotoxins. However, more subtle approaches, such as slow-acting insecticides that shorten adult mosquito longevity, could also reduce transmission while imposing less intense selection for resistance [24],[34]. Alternative modes of action that impair olfaction, flight, energy metabolism, or immunity could further contribute to reduced vectorial capacity (e.g., see [35]). Such “sub-lethal insecticides” would represent genuinely new additions to the mosquito control tool kit that extend beyond the current fast-acting insecticide paradigm [36].
In addition, chemical insecticides that act against the adult vectors are not the only available tools. Physical barriers such as house screens [37], habitat management to reduce vector breeding site quality [38], microbial larvicides [39], and manipulation of nectar sources [40] could contribute to reduced disease transmission. Other tools in development such as fungal biopesticides [41], odor-baited traps [42], manipulation or release of parasites [43], and genetically modified [44],[45] or transinfected mosquitoes [46] could add to the list.
Individually, many of these technologies face today the same constraints that alternatives to insecticides faced in crop protection: marketing and regulatory systems for new products favored broad spectrum, fast-acting, lethal insecticides that provided stand alone, albeit unsustainable, solutions to pest problems. Against this model, subtler alternative methods cannot compete, except in an IPM/IVM context, where the benefit comes from the sum of the parts. It is important that regulatory frameworks are amenable to IVM to encourage research and development (R&D) and prevent barriers to ultimate commercialization.
Integrated Strategies and Sustainable Implementation
Developing effective IVM will require better understanding of the impact of control tactics individually and in various combinations [39],[47]–[49]. Again, there is surprisingly little relevant research. Yet, different combinations of tools could deliver the same end points with strategies optimized over time and space.
Development of IVM will also require substantial money and effort. It has been estimated that effective delivery of ITN or IRS measures will require 40%–61% of projected national malaria control program budgets [50]. This is in sharp contrast to the 4% of the global malaria R&D budget that is currently spent on vector control [51]. Given the historic and contemporary significance of vector control in reducing malaria [52], this level of funding is inadequate. Experience from agriculture suggests that with appropriate engagement and education, even complex knowledge-intensive practices can be successfully implemented. Extensive IPM programs in many developing countries indicate that such strategies are best developed and implemented via bottom-up approaches engaging end users from the outset in research and development [53],[54]. Embracing this philosophy can bolster vector control and move it away from top-down prescriptions towards adaptive, surveillance-, and evidence-based strategies that vary in space and time depending on local conditions. As with IPM, IVM can be best advanced by engaging the end users and working in partnerships to generate shared knowledge and solutions relevant to the local context. This strategy is necessary not only to develop effective solutions, but also to avert the risks of donor and community fatigue. There is no “quick fix” for sustainable vector control, or for eradication of malaria.
Conclusions
Ensuring continued advance in malaria control requires rethinking how we manage vector populations. Current strategies rely heavily on repeated application of single neurotoxic insecticides that quickly kill adult mosquitoes. This narrow paradigm is beginning to fail, as it did in agriculture, as well as in previous malaria eradication campaigns of the '50s and '60s. We should not abandon ITNs and IRS; these can be useful in IVM just as insecticides are in IPM. But experience with IPM in agriculture suggests that integrated approaches have the potential to provide more effective and durable pest management. To achieve the equivalent for malaria control requires additional tools in the armory, a better understanding of the impact of individual tools and their interactions, appropriate training for end users, and design of novel integrated strategies that maximize impact and fit the local ecological and socioeconomic context. Given the current lack of any clear alternative to the current insecticide paradigm, researchers, policy makers, and funding agencies need to act now to support this more diverse and adaptive ap proach. It is unlikely that any single tactic or combination of tactics will provide a permanent solution. Vector control programs must proactively and continuously innovate to optimize and sustain impact.
Abbreviations
- IPM
integrated pest management
- IRS
indoor residual sprays
- ITN
insecticide-treated net
- IVM
integrated vector management
- R&D
research and development
- WHO
World Health Organization
Footnotes
The authors have declared that no competing interests exist.
This essay was supported in part by the Research and Policy for Infectious Disease Dynamics (RAPIDD) program and by Wageningen University through a travel grant to WT. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Provenance: Not commissioned; externally peer reviewed.
References
- 1.WHO. World malaria report 2011. Geneva: World Health Organization; 2011. [Google Scholar]
- 2.Yewhalaw D, Wassie F, Steurbaut W, Spanoghe P, Van Bortel W, et al. Multiple insecticide resistance: an impediment to insecticide-based malaria vector control program. PLoS ONE. 2011;6:e16066. doi: 10.1371/journal.pone.0016066. doi: 10.1371/journal.pone.0016066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ranson H, N'Guessan R, LInes J, Moiroux N, Nkuni Z, et al. Pyrethroid resistance in African anopheline mosquitoes: what are the implications for malaria control? Trends Parasitol. 2011;27:91–98. doi: 10.1016/j.pt.2010.08.004. [DOI] [PubMed] [Google Scholar]
- 4.Van den Berg H, Zaim M, Yadav RS, Soares A, Ameneshewa B, et al. Global trends in the use of insecticides to control vector-borne diseases. Environ Health Perspect. 2012;120:577–582. doi: 10.1289/ehp.1104340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chanda E, Hemingway J, Kleinschmidt I, Rehman AM, Ramdeen V, et al. Insecticide resistance and the future of malaria control in Zambia. PLoS ONE. 2011;6:e24336. doi: 10.1371/journal.pone.0024336. doi: 10.1371/journal.pone.0024336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Trape JF, Tall A, Diagne N, Ndiath O, Ly AB, et al. Malaria morbidity and pyrethroid resistance after the introduction of insecticide-treated bednets and artemisinin-based combination therapies: a longitudinal study. Lancet Infect Dis. 2011;11:925–932. doi: 10.1016/S1473-3099(11)70194-3. [DOI] [PubMed] [Google Scholar]
- 7.Pimentel D, Andow DA. Pest management and pesticide impacts. Insect Sci Applic. 1984;5:141–149. [Google Scholar]
- 8.Van Lenteren JC. Insects, man and environment: who will survive? In: Hansen JA, editor. Environmental concerns. An interdisciplinary exercise. London: Elsevier Sci. Publ; 1991. pp. 191–210. [Google Scholar]
- 9.Council NR. Pesticide resistance: strategies and tactics for management. Washington (D.C.): National Academy Press; 1986. [Google Scholar]
- 10.Kogan F. Integrated pest management: historical perspectives and contemporary developments. Annu Rev Entomol. 1998;42:243–270. doi: 10.1146/annurev.ento.43.1.243. [DOI] [PubMed] [Google Scholar]
- 11.Lewis WJ, Lenteren JCv, Phatak SC, Tumlinson JH. A total system approach to sustainable pest management. Proc Natl Acad Sci U S A. 1998;94:12243–12248. doi: 10.1073/pnas.94.23.12243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Naranjo SE, Ellsworth PC. Fifty years of the integrated control concept: moving the model and implementation forward in Arizona. Pest Manag Sci. 2009;65:1267–1286. doi: 10.1002/ps.1861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gomiero T, Pimentel D, Paoletti MG. Is there a need for a more sustainable agriculture? CRC Crit Rev Plant Sci. 2011;30:6–23. [Google Scholar]
- 14.Weddle PW, Welter SC, Thomson D. History of IPM in California pears-50 years of pesticide use and the transition to biologically intensive IPM. Pest Manag Sci. 2009;65:1287–1292. doi: 10.1002/ps.1865. [DOI] [PubMed] [Google Scholar]
- 15.Tabashnik BE, Sisterson MS, Ellsworth PC, Dennehy TJ, Antilla L, et al. Suppressing resistance to Bt cotton with sterile insect releases. Nat Biotechnol. 2010;28:1304–1307. doi: 10.1038/nbt.1704. [DOI] [PubMed] [Google Scholar]
- 16.Beier JC, Keating J, Githure JI, MacDonald MB, Impoinvil DE, et al. Integrated vector management for malaria control. Malar J. 2008;7(Suppl 1) doi: 10.1186/1475-2875-7-S1-S4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Van den Berg H, Takken W. A framework for decision-making in integrated vector management to prevent disease. Trop Med Int Health. 2007;12:1230–1238. doi: 10.1111/j.1365-3156.2007.01905.x. [DOI] [PubMed] [Google Scholar]
- 18.WHO. WHO position statement on integrated vector management. Wkly Epidemiol Rec. 2008;83:177–181. [PubMed] [Google Scholar]
- 19.WHO. Handbook on integrated vector management. Geneva: World Health Organization; 2010. 78 [Google Scholar]
- 20.The malERA Consultative Group on Vector Control. A research agenda for malaria eradication: vector control. PLoS Med. 2011;8:e1000401. doi: 10.1371/journal.pmed.1000401. doi: 10.1371/journal.pmed.1000401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ferguson HM, Dornhaus A, Beeche A, Borgemeister C, Gottlieb M, et al. Ecology: a prerequisite for malaria elimination and eradication. PLoS Med. 2010;7:e1000303. doi: 10.1371/journal.pmed.1000303. doi: 10.1371/journal.pmed.1000303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rivero A, Vezilier J, Weill M, Read AF, Gandon S. ) Insecticide control of vector-borne diseases: when is insecticide resistance a problem? PLoS Pathog. 2010;6:e1001000. doi: 10.1371/journal.ppat.1001000. doi: 10.1371/journal.ppat.1001000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jones C, Sanou A, Guelbeogo W, Sagnon NF, Johnson P, et al. Aging partially restores the efficacy of malaria vector control in insecticide-resistant populations of Anopheles gambiae s.l. from Burkina Faso. Malar J. 2012;11:24. doi: 10.1186/1475-2875-11-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Koella JC, Lynch PA, Thomas MB, Read AF. Towards evolution-proof malaria control with insecticides. Evol Appl. 2009;2:469–480. doi: 10.1111/j.1752-4571.2009.00072.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hemingway J, Beaty BJ, Rowland M, Scott TW, Sharp BL. The innovative vector control consortium: improved control of mosquito-borne diseases. Trends Parasitol. 2006;22:308–312. doi: 10.1016/j.pt.2006.05.003. [DOI] [PubMed] [Google Scholar]
- 26.Read AF, Lynch PA, Thomas MB. How to make evolution-proof insecticides for malaria control. PLoS Biol. 2009;7:e1000058. doi: 10.1371/journal.pbio.1000058. doi: 10.1371/journal.pbio.1000058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Denholm I, Rowland MW. Tactics for managing pesticide resistance in arthropods - theory and practice. Annu Rev Entomol. 1992;37:91–112. doi: 10.1146/annurev.en.37.010192.000515. [DOI] [PubMed] [Google Scholar]
- 28.Nauen R. Insecticide resistance in disease vectors of public health importance. Pest Manag Sci. 2007;63:628–633. doi: 10.1002/ps.1406. [DOI] [PubMed] [Google Scholar]
- 29.Baleta A. Insecticide resistance threatens malaria control in Africa. Lancet. 2009;374:1581–1582. doi: 10.1016/s0140-6736(09)61933-4. [DOI] [PubMed] [Google Scholar]
- 30.Yadouleton A, Asidi A, Djouaka R, Baraima J, Agossou C, et al. Development of vegetable farming: a cause of the emergence of insecticide resistance in populations of Anopheles gambiae in urban areas of Benin. Malar J. 2009;14:103. doi: 10.1186/1475-2875-8-103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Penilla RP, Rodriguez AD, Hemingway J, Torres JL, Arredondo-Jimenez JI, et al. Resistance management strategies in malaria vector mosquito control. Baseline data for a large scale field trial against Anopheles albimanus in Mexico. Med Vet Entomol. 1998;12:217–233. doi: 10.1046/j.1365-2915.1998.00123.x. [DOI] [PubMed] [Google Scholar]
- 32.Reddy MR, Overgaard HJ, Abaga S, Reddy VP, Caccone A, et al. Outdoor host seeking behaviour of Anopheles gambiae mosquitoes following initiation of malaria vector control on Bioko Island, Equatorial Guinea. Malar J. 2011;10:184. doi: 10.1186/1475-2875-10-184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Killeen GF, Moore SJ. Target product profiles for protecting against outdoor malaria transmission. Malar J. 2012;11:17. doi: 10.1186/1475-2875-11-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Thomas M, Read A. Can fungal biopesticides control malaria? Nat Rev Microbiol. 2007;5:377–383. doi: 10.1038/nrmicro1638. [DOI] [PubMed] [Google Scholar]
- 35.Blanford S, Shi WP, Christian R, Marden JH, Koekemoer LL, et al. Lethal and pre-lethal effects of a fungal biopesticide contribute to substantial and rapid control of malaria vectors. PLoS ONE. 2011;6:e23591. doi: 10.1371/journal.pone.0023591. doi: 10.1371/journal.pone.0023591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Takken W, Knols BG. Malaria vector control: current and future strategies. Trends Parasitol. 2009;25:101–104. doi: 10.1016/j.pt.2008.12.002. [DOI] [PubMed] [Google Scholar]
- 37.Kirby MJ, Njie M, Dilger E, Lindsay SW. Importance of eaves to house entry by anopheline, but not culicine, mosquitoes. J Med Entomol. 2009;46:505–510. doi: 10.1603/033.046.0314. [DOI] [PubMed] [Google Scholar]
- 38.Imbahale S, Mweresa C, Takken W, Mukabana W. Development of environmental tools for anopheline larval control. Parasit Vectors. 2011;4:130. doi: 10.1186/1756-3305-4-130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fillinger U, Ndenga B, Githeko A, Lindsay SW. Integrated malaria vector control with microbial larvicides and insecticide-treated nets in western Kenya: a controlled trial. Bull World Health Org. 2009;87:655–665. doi: 10.2471/BLT.08.055632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Muller GC, Beier JC, Traore SF, Toure MB, Traore MM, et al. Successful field trial of attractive toxic sugar bait (ATSB) plant-spraying methods against malaria vectors in the Anopheles gambiae complex in Mali, West Africa. Malar J. 2010;9:210. doi: 10.1186/1475-2875-9-210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Scholte EJ, Ng'habi K, Kihonda J, Takken W, Paaijmans K, et al. An entomopathogenic fungus for control of adult African malaria mosquitoes. Science. 2005;308:1641–1642. doi: 10.1126/science.1108639. [DOI] [PubMed] [Google Scholar]
- 42.Okumu FO, Killeen GF, Ogoma S, Biswaro L, Smallegange RC, et al. Development and field evaluation of a synthetic mosquito lure that is more attractive than humans. PLoS ONE. 2010;5:e8951. doi: 10.1371/journal.pone.0008951. doi: 10.1371/journal.pone.0008951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cirimotich CM, Dong YM, Clayton AM, Sandiford SL, Souza-Neto JA, et al. Natural microbe-mediated refractoriness to Plasmodium infection in Anopheles gambiae. Science. 2011;332:855–858. doi: 10.1126/science.1201618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Windbichler N, Menichelli M, Papathanos PA, Thyme SB, Li H, et al. A synthetic homing endonuclease-based gene drive system in the human malaria mosquito. Nature. 2011;473:212–215. doi: 10.1038/nature09937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Isaacs AT, Li FW, Jasinskiene N, Chen XG, Nirmala X, et al. Engineered resistance to Plasmodium falciparum development in transgenic Anopheles stephensi. PLoS Pathog. 2011;7:e1002017. doi: 10.1371/journal.ppat.1002017. doi: 10.1371/journal.ppat.1002017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hughes GL, Koga R, Xue P, Fukatsu T, Rasgon JL. Wolbachia infections are virulent and inhibit the human malaria parasite Plasmodium falciparum in Anopheles gambiae. PLoS Pathog. 2011;7:e1002043. doi: 10.1371/journal.ppat.1002043. doi: 10.1371/journal.ppat.1002043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kleinschmidt I, Schwabe C, Shiva M, Segura JL, Sima V, et al. Combining indoor residual spraying and insecticide-treated net interventions. Am J Trop Med Hyg. 2009;81:519–524. [PMC free article] [PubMed] [Google Scholar]
- 48.Hancock PA. Combining fungal biopesticides and insecticide-treated bednets to enhance malaria control. PLoS Comput Biol. 2009;5:e1000525. doi: 10.1371/journal.pcbi.1000525. doi: 10.1371/journal.pcbi.1000525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Van den Berg H, Takken W. Evaluation of integrated vector management. Trends Parasitol. 2009;25:71–76. doi: 10.1016/j.pt.2008.11.005. [DOI] [PubMed] [Google Scholar]
- 50.Kiszewski A, Johns B, Schapira A, Delacollette C, Crowell V, et al. Estimated global resources needed to attain international malaria control goals. Bull World Health Org. 2007;85:623–630. doi: 10.2471/BLT.06.039529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.PATH. Staying the Course? Malaria research and development in a time of economic uncertainty. Seattle: PATH (Program for Appropriate Technology in Health); 2011. 98 [Google Scholar]
- 52.Alonso PL, Brown G, Arevalo-Herrera M, Binka F, Chitnis C, et al. PLoS Med. Vol. 8. doi:10.1371/journal.pmed.1000406; 2011. A research agenda to underpin malaria eradication. p. e1000406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Van den Berg H, Jiggins J. Investing in farmers: the impacts of farmer field schools in relation to integrated pest management. World Development. 2007;35:663–686. [Google Scholar]
- 54.Brooks S, Loevinsohn M. Shaping agricultural innovation systems responsive to food insecurity and climate change. Nat Resour Forum. 2011;35:185–200. [Google Scholar]