Abstract
Trypanosoma brucei rhodesiense (Tbr) and T. b. gambiense (Tbg), causative agents of Human African Trypanosomiasis (sleeping sickness) in Africa, have evolved alternative mechanisms of resisting the activity of trypanosome lytic factors (TLFs), components of innate immunity in human serum that protect against infection by other African trypanosomes. In Tbr, lytic activity is suppressed by the Tbr-specific serum-resistance associated (SRA) protein. The mechanism in Tbg is less well understood but has been hypothesized to involve altered activity and expression of haptoglobin haemoglobin receptor (HpHbR). HpHbR has been shown to facilitate internalization of TLF-1 in T.b. brucei (Tbb), a member of the T. brucei species complex that is susceptible to human serum. By evaluating the genetic variability of HpHbR in a comprehensive geographical and taxonomic context, we show that a single substitution that replaces leucine with serine at position 210 is conserved in the most widespread form of Tbg (Tbg group 1) and not found in related taxa, which are either human serum susceptible (Tbb) or known to resist lysis via an alternative mechanism (Tbr and Tbg group 2). We hypothesize that this single substitution contributes to reduced uptake of TLF and thus may play a key role in conferring serum resistance to Tbg group 1. In contrast, similarity in HpHbR sequence among isolates of Tbg group 2 and Tbb/Tbr provides further evidence that human serum resistance in Tbg group 2 is likely independent of HpHbR function.
Author Summary
Human African Trypanosomiasis, or sleeping sickness, is caused by two different parasites: Trypanosoma brucei gambiense (Tbg) and T. b. rhodesiense (Tbr). Each parasite employs a different mechanism to resist trypanosome lytic factor (TLF), the active innate immune component of human serum. In Tbg group 1, which causes the vast majority of disease cases, the mechanism is thought to involve the reduced activity of a receptor involved in binding and internalizing TLF. In this study, we investigate genetic variation in this receptor across a broad geographic sample of Tbg and closely related trypanosomes to test whether unique polymorphisms in the receptor from Tbg may explain its altered function. We identified a single mutation in all copies of the receptor gene sequenced from Tbg but not in any other closely related species. This finding suggests that this single mutation could play a key role in conferring human infectivity to Tbg. Given the possible consequences for drug development and diagnostics, we suggest that future functional studies target this mutation to fully elucidate its role.
Introduction
Trypanosomiasis, a deadly disease of humans and livestock in sub-Saharan Africa, is caused by protozoan parasites of the genus Trypanosoma, which are transmitted between mammalian hosts by insect vectors of the genus Glossina (tsetse). Human-infective members of the Trypanosoma brucei complex cause the human form of the disease, Human African Trypanosomiasis (HAT), or sleeping sickness. T. b. rhodesiense (Tbr) causes an acute form of HAT in eastern Africa, while T. b. gambiense group 1 (Tbg1) causes a chronic form of the disease in western and central Africa and accounts for over 90% of reported cases (Figure 1a). T. b. gambiense group 2 (Tbg2), a rare form described from West Africa in the 1970s and 1980s, also causes human disease but the trait of human-infectivity is not stable [1], [2], [3]. The final member of the brucei complex, T. b. brucei (Tbb), is not infective to humans, but, together with other animal trypanosome species, causes the livestock wasting disease, Nagana, across a range that overlaps with that of the human-infective parasites.
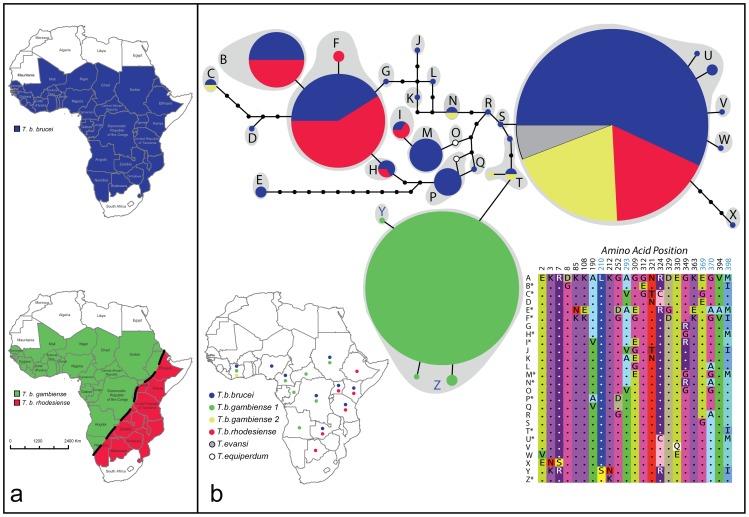
Figure 1. Trypanosoma brucei distribution, sampling scheme and relationships among HpHbR DNA and amino acid sequences.
a. Approximate geographic distribution of the animal-restricted parasite T. b. brucei (blue) and the human-infective parasites T. b. gambiense groups 1 and 2 (Tbg1, green; Tbg2, yellow) and T. b. rhodesiense (Tbr; red) which cause human African trypanosomiasis. b. A haplotype network (top) shows the relationships among unique HpHbR alleles (represented by colored circles (sampled) or black dots (unsampled)) and highlights the differentiation of Tbg1 from other taxa at this locus. Each line in the network represents one nucleotide change. Circle size is proportional to allele frequency. Colored sections of the circles indicate the relative frequency with which a particular allele was recovered from different taxa within the subgenus Trypanozoon (key at bottom left). Dots on the map indicate the country where isolates of each taxon, as shown by color, were collected. Grey shading in the network joins alleles with an identical inferred amino acid sequence. Unique amino acid sequences found in this study are identified by a capital letter in the haplotype network and a corresponding letter in the alignment of variable positions (bottom right). Reference sequence A, previously identified in Tbb strain Lister 427 (Kieft et al. 2010) was not recovered in this study. Asterisks indicate amino acid sequences found in more than one isolate. Amino acids in the alignment are represented with standard single letter codes and color-coded for ease of comparison across sequences. The two amino acid sequences recovered from Tbg1 (Y and Z) share a single substitution at position 210 that was not found in any other taxa. Amino acid positions labeled in blue correspond to positions previously identified as playing a possible role in altered activity of HpHbR in Tbg1 (Kieft et al. 2010).
Humans possess an innate resistance to some trypanosomes through the action of trypanosome lytic factors (TLFs) in their serum [4]. TLF-1 is a high-density lipoprotein complex that includes the active toxin apolipoprotein L-I (apoL-I) in association with haptoglobin-related protein (Hpr). In the primary immune pathway [5], [6], [7], TLF-1 is bound and internalized via a haptoglobin haemoglobin receptor (HpHbR) on the surface of susceptible trypanosomes. Uptake of TLF-1 is followed by disruption of the lysosomal membrane by apoL-I and eventual cell lysis. While Tbb is susceptible to lysis by human TLF-1, Tbr, Tbg1 and Tbg2 are resistant. In Tbr, the serum-resistance associated (SRA) protein confers resistance to TLF-1 [8] by binding directly to apoL-I after it has been internalized into the cell, inhibiting its lysosome-lytic capacity [9]. Tbg1 and Tbg2, on the other hand, lack the gene encoding SRA and are thought to have evolved an independent mechanism to prevent lysis by TLF.
In Tbg2, apoL-I is also internalized, but lysis is prevented by an unidentified mechanism [10]. In Tbg1, the mechanism is better understood and appears to involve reduced expression and altered function of the parasite HpHbR [11]. Sequencing of a few isolates of Tbg and Tbb led Kieft et al. [11] to suggest that mutations in HpHbR may have altered TLF-1 binding in Tbg1. Specifically, the authors identified five non-synonymous substitutions shared by the four sequenced isolates of Tbg1, but not present in two Tbb isolates. This work has helped to narrow the universe of possible structural differences in HpHbR that could, for example, eventually be exploited to design novel drugs to overcome Tbg1 resistance. However, the small number of isolates examined to date is not sufficient to determine whether the mutations are really Tbg1-specific. While genetic variation in Tbg1 is extremely limited [12], [13], the remainder of the T. brucei complex exhibits relatively high variation, most of which does not partition into neatly defined geographic or taxonomic units [14], [15], [16], [17]. Thus, characterizing the genetic differences that contribute to a critical epidemiological trait such as human infectivity requires that those differences be evaluated in a comprehensive geographical and taxonomic context.
In the present study, we tested if the five non-synonymous substitutions previously hypothesized to alter HpHbR activity in Tbg1 [11] are both conserved in Tbg1 isolates and also absent from other T. brucei subspecies by examining HpHbR gene variation in T. brucei s.l. sampled across the entire range of the species complex. By narrowing the pool of substitutions that are specific to Tbg1, we expect to facilitate future functional studies aimed at understanding the contribution of HpHbR to conferring human serum resistance.
Methods
Sampling
Isolates of Tbb, Tbg1, Tbg2 and Tbr, were selected to incorporate representative genetic diversity from the entire geographic range of the T. brucei complex (Table S1, Figure 1b). When available, we included isolates of all co-occurring taxa from each country sampled (Figure 1b).
PCR amplification and sequencing
For each isolate, DNA was extracted as described in [17]. PCR was performed using primers designed from Tbb (TREU927) and Tbg1 (Dal972) TriTrypDB database sequences (Tb927.6.440 and Tbg972.6.120, respectively) to amplify a 1297 base pair (bp) fragment that encompassed the entire HpHbR gene (HpHbR_F 5′ CGGGAAAGTTGTACGCAAG, HpHbR_R2 5′ CGACCACTTAATGTTACGAGG). For each PCR, 2–4 µL of a 1∶10 dilution of DNA extract were used. PCR reactions were performed using the reagents provided with GoTaq® DNA Polymerase and Green Master Mix. Difficult templates were amplified using Failsafe PCR 2X PreMixes Buffers (Epicentre Biotechnologies, Madison WI). All PCR reactions used the following cycle: Initial denaturation 95°C for 2 min, 50 cycles of 95°C for 35 s, 58°C for 35 s, and 72°C for 1 min 20 s and a final extension at 72°C for 7 min. PCR success was verified with 1% agarose gel electrophoresis. PCR products were purified and then sequenced (Yale DNA Analysis Facility) using two internal primers located in the middle of the sequence (HpHbR_F2in 5′ TGCTCGAGATATTCCTCAAG, HpHbR_Rin 5′ CTCCCACTGAAGCATTAGAC). The sequenced fragment included 22 nucleotides upstream of the HpHbR start codon, the entire HpHbR gene and 62 nucleotides downstream of the HpHbR stop codon.
Sequence analysis and phasing of alleles
Sequences generated using the internal primers overlapped by approximately 200 bp permitting the assembly of an entire contiguous sequence of the HpHbR gene. Contiguous sequences were constructed and chromatograms from each isolate were manually examined for double peaks using the CLCBio DNA Workbench 5.7 (Cambridge, MA). Sites with double peaks were assigned the appropriate nucleotide ambiguity code. Sequences were aligned manually using MacClade 4.08 [18].
Samples with double peaks were considered heterozygotes. We used the programs SeqPhase [19] to format files and PHASE 2.1.1 [20] to resolve individual alleles from heterozygous sequences. To assess evidence for recombinant alleles and to relax the assumption of a stepwise mutation model, we employed the recombination model (MR) and the parent-independent models, respectively. Each run used 1000 iterations and a burnin of 500 generations and thinning interval = 1. The dataset was run twice with different random starting seeds and checked for consistency. The replicate with the best average goodness-of-fit was selected for subsequent analyses.
Phylogenetic analysis and amino acid alignment
Nucleotide DNA sequence alignments were generated from phased alleles in MacClade 4.08. Haplotype networks were constructed in the program TCS [21]. DNA sequences were translated to amino acids and aligned in MacClade 4.08. Non-coding regions were removed from sequences and amino acid sequences were compared to those generated by [11].
Results
Sampling and allelic phase inference
We collected 1296 bp of sequence from each of 65 T. brucei isolates: 32 from Tbb, 15 from Tbg1, five from Tbg2 and 13 from Tbr. In addition, we generated sequence for one isolate each of Trypanosoma equiperdum and Trypanosoma evansi (Table S1), both of which are also members of the subgenus Trypanozoon but are not human infective (reviewed in [3]).
Of the 67 isolates sequenced in this study, 30 were heterozygous at the locus sequenced. PHASE 2.1.1 inferred a total of 34 alleles present in the 67 isolates. For all heterozygotes, allele pairs had Bayesian posterior probabilities of 1.0 across replicate runs, indicating that no alternative allele sequences could be inferred from the heterozygotes.
Nucleotide variation within and among T. brucei strains
The 34 alleles recovered in this study exhibited a total of 40 variable sites, of which four were located outside the HpHbR coding region. Each of these four sites occurred in a distinct allele (f2, c, u3, z1) across a total of five isolates (Boula (Tbg1), STIB338 (Tbr), STIB386 (Tbg2), STIB777AE (Tbb), and KP13 (Tbb)). The remaining 36 variable sites were found within the coding region of HpHbR (File S1).
Most allelic diversity (28 alleles) was found in isolates of Tbb, Tbr and Tbg2 and much of this diversity was common to two or more taxa. Five of the seven alleles recovered from Tbr were identical to those found in Tbb. Likewise, four of the five alleles recovered from Tbg2 were also identical to alleles in Tbb. The most common allele in this study (u1; Table S1) was recovered from Tbb, Tbr and Tbg2. In contrast to these observations, we recovered four distinct alleles from Tbg1, but none of these were shared with any member of the subgenus Trypanozoon. Allelic diversity in Tbg1 was relatively low. Allele z1 (identical to the Tbg1 sequence reported in Kieft et al. [2010]) was the most common Tbg1 variant and was recovered from 26 of 30 sampled chromosomes. The remaining Tbg1 alleles differed by only one nucleotide from this common variant, z1. Trypanosoma equiperdum sequences were more similar to Tbb and Tbr sequences, though both alleles from T. equiperdum were unique. In T. evansi, alleles were identical to the most common allele found in Tbb, Tbr and Tbg2 (Fig. 1b).
Protein coding differences inferred from nucleotide sequence
The HpHbR protein consists of 403 amino acids. In silico translation of the DNA sequences of the 34 alleles described above yielded 25 unique protein sequences (Figure 1, Figure S1). Notably, the single nucleotide difference in HpHbR that distinguished all isolates of Tbg1 from all other T. brucei isolates sampled in this study was non-synonymous, resulting in the replacement of leucine with serine at position 210 (L210S; Figure 1b, Figure S1). With one exception, all Tbg1 isolates possessed two copies of HpHbR that coded for just this single amino acid sequence (Z). In the exception, isolate ITMAP020578, one allele coded for amino acid sequence Z and the second allele coded for a second peptide (Y) differing from Z by just one amino acid at position 212. All other variation in HpHbR amino acid sequences partitioned to differences within and among Tbb, Tbr and Tbg2.
Discussion
The primary goal of this study was to examine the genetic diversity of HpHbR in a broad geographical and taxonomic context to better characterize the mutations that potentially give rise to differences in HpHbR function and that may contribute to the phenotype of human serum resistance observed in Tbg1. An earlier study of HpHbR genetic diversity in a limited sample of parasite isolates identified five non-synonymous substitutions shared by Tbg1, but not found in Tbb, suggesting that these five differences could play an important functional role [11]. By sampling more broadly across the subgenus Trypanozoon and across Africa, we have demonstrated that just one of these substitutions (L210S) is conserved in Tbg1 and also absent from the most closely related trypanosome taxa, all of which are either susceptible to human serum (Tbb) or known to possess an alternative resistance mechanism (Tbr or Tbg2). Although our sample size remains relatively limited compared to the vast number of parasites distributed widely across Africa, the extremely low genetic diversity observed in Tbg1 HpHbR is consistent with prior population genetic studies [12], [13], [17] and we hypothesize that the mutation L210S is likely fixed in the taxon. This could be extended to field-circulating Tbg1 by using either allele specific PCR primers or a restriction fragment length polymorphism (RFLP) that targets the single nucleotide substitution (e.g., enzyme PleI).
To the extent that the unique substitution in Tbg1 HpHbR prevents the uptake of TLF-1, this single amino acid change may play a key role in conferring serum resistance to this parasite. A role for HpHbR in facilitating lytic activity of human serum was originally established by experiments demonstrating that loss of HpHbR in Tbb (through RNA interference or gene knockout) conferred resistance to TLF-mediated lysis [22]. Later work demonstrated that Tbb selected to be TLF-1-resistant exhibited reduced HpHbR expression. Furthermore, the ectopic expression of Tbg1 HpHbR (using an allele identical to the most common Tbg1 allele identified in our study) in these serum resistant Tbb was not sufficient to restore human serum susceptibility, providing evidence for the altered function of Tbg1 HpHbR [11]. Our data indicate that this altered function likely stems from the L210S mutation in Tbg1, a substitution that effects an approximate 20-fold reduction in the affinity of HpHbR for HpHb [23]. Given that L210S appears to be the single mutation that distinguishes Tbg1 HpHbR from the HpHbR of all closely related members of the Trypanozoon subgenus, we hypothesize that this single mutation could play a major role in the serum resistance of Tbg1. However, this mutation is unlikely to be the sole factor. As noted previously, reduced expression levels of HpHbR are also likely to play a role in Tbg1 serum resistance [10], [11]. Also, while HpHbR is likely to be the main route of entry into the cell for TLF-1, poorly characterized alternative routes appear to exist for both TLF-1 and TLF-2, a second HDL particle that also exhibits trypanolytic activity [6]. Finally, an in vitro study has demonstrated that, regardless of receptor function, Tbg1 may be inherently resistant to apoL-1, the active trypanolytic factor in human serum [10]. While HpHbR may only be one component of Tbg1 serum resistance, the possible benefit of designing new drugs targeted to this receptor variant warrants further functional study to fully circumscribe its effect on serum resistance.
In contrast to Tbg1, the mechanism of Tbg2 resistance to human serum is thought to be independent of HpHbR, based on the finding that HpHbR from Tbg2 internalizes TLF-1 at a rate similar to that observed in Tbb and Tbr [10]. While that study included just a single strain of Tbg2 (STIB386), our results, which include data for several additional strains, suggest that this conclusion is likely to hold more broadly in Tbg2. Sequencing of HpHbR indicated that several isolates of Tbg2 shared sequence identity with isolates of both Tbb and Tbr, while exhibiting no overlap with isolates of Tbg1, a result that is consistent with previous surveys of neutral genetic markers [13], [17]. The genetic similarity of HpHbR observed among a large collection of isolates of Tbb, Tbr, and Tbg2 suggests that the function of HpHbR in Tbg2 is more likely to reflect that of Tbb and Tbr than Tbg1 and further supports the conclusion that Tbg2 serum resistance is independent of HpHbR. Our study surveyed only five strains of Tbg2, but even these five strains exhibited substantially more diversity than Tbg1 at both the nucleotide and amino acid level. The genetic variability of HpHbR in Tbg2 reiterates the fact that Tbg2, unlike Tbg1, is not genetically homogeneous and suggests that future studies should consider this diversity when examining functional differences among parasite subgroups.
Supporting Information
Amino acid alignment of the complete HpHbR gene. Amino acids are identified by a unique color and a single letter abbreviation at the top of the figure. Letters at left identify unique amino acid sequences shown in Figure 1b. An asterisk (*) indicates that multiple isolates share the same amino acid sequence (see Table S1). Variable positions are highlighted below the alignment.
(EPS)
This file contains the DNA sequences from each of the alleles identified in this study. Allele sequences were inferred from direct sequences in silico using the program PHASE 2.1.1 (Stephens et al. 2001).
(FAS)
Subgenus Trypanozoon isolate taxonomic classification, collection information and characterization of genetic variation at the HpHbR locus.
(DOCX)
Acknowledgments
We are grateful to Richard Echodu for providing the T. b. brucei sample from Arua, Uganda. We are very grateful to Pascal Grébaut (CIRAD-IRD/LRCT, Montpellier) and Anne Clarisse Lekane (IRD, Montpellier) for sharing isolates for our analyses.
Footnotes
The authors have declared that no competing interests exist.
This work was supported by a grant from National Institutes of Health (www.nih.gov: AI068932, AI094615) to S. Aksoy and A. Caccone, a Fogarty Center award (D43TW004381) to S. Aksoy and A. Caccone, the YIBS Molecular Systematics and Conservation Genetics Lab and the Science, Technology and Research Scholars (STARS) Program for undergraduates administered by Yale University (http://science.yalecollege.yale.edu/stars-home). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Mehlitz D, Zillmann U, Scott CM, Godfrey DG. Epidemiological studies on the animal reservoir of Gambiense sleeping sickness. Part III. Characterization of Trypanozoon stocks by isoenzymes and sensitivity to human serum. Tropenmed Parasitol. 1982;33:113–118. [PubMed] [Google Scholar]
- 2.Agbo EC, Majiwa PA, Claassen EJ, Roos MH. Measure of molecular diversity within the Trypanosoma brucei subspecies Trypanosoma brucei brucei and Trypanosoma brucei gambiense as revealed by genotypic characterization. Exp Parasitol. 2001;99:123–131. doi: 10.1006/expr.2001.4666. [DOI] [PubMed] [Google Scholar]
- 3.Gibson W. Resolution of the species problem in African trypanosomes. Int J Parasitol. 2007;37:829–838. doi: 10.1016/j.ijpara.2007.03.002. [DOI] [PubMed] [Google Scholar]
- 4.Hajduk SL, Moore DR, Vasudevacharya J, Siqueira H, Torri AF, et al. Lysis of Trypanosoma brucei by a toxic subspecies of human high density lipoprotein. J Biol Chem. 1989;264:5210–5217. [PubMed] [Google Scholar]
- 5.Pays E, Vanhollebeke B. Human innate immunity against African trypanosomes. Curr Opin Immunol. 2009;21:493–498. doi: 10.1016/j.coi.2009.05.024. [DOI] [PubMed] [Google Scholar]
- 6.Vanhollebeke B, Pays E. The trypanolytic factor of human serum: many ways to enter the parasite, a single way to kill. Mol Microbiol. 2010;76:806–814. doi: 10.1111/j.1365-2958.2010.07156.x. [DOI] [PubMed] [Google Scholar]
- 7.Wheeler RJ. The trypanolytic factor-mechanism, impacts and applications. Trends Parasitol. 2010;26:457–464. doi: 10.1016/j.pt.2010.05.005. [DOI] [PubMed] [Google Scholar]
- 8.Xong HV, Vanhamme L, Chamekh M, Chimfwembe CE, Van Den Abbeele J, et al. A VSG expression site-associated gene confers resistance to human serum in Trypanosoma rhodesiense. Cell. 1998;95:839–846. doi: 10.1016/s0092-8674(00)81706-7. [DOI] [PubMed] [Google Scholar]
- 9.Vanhamme L, Paturiaux-Hanocq F, Poelvoorde P, Nolan DP, Lins L, et al. Apolipoprotein L-I is the trypanosome lytic factor of human serum. Nature. 2003;422:83–87. doi: 10.1038/nature01461. [DOI] [PubMed] [Google Scholar]
- 10.Capewell P, Veitch NJ, Turner CM, Raper J, Berriman M, et al. Differences between Trypanosoma brucei gambiense groups 1 and 2 in their resistance to killing by trypanolytic factor 1. PLoS Negl Trop Dis. 2011;5:e1287. doi: 10.1371/journal.pntd.0001287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kieft R, Capewell P, Turner CM, Veitch NJ, MacLeod A, et al. Mechanism of Trypanosoma brucei gambiense (group 1) resistance to human trypanosome lytic factor. Proc Natl Acad Sci U S A. 2010;107:16137–16141. doi: 10.1073/pnas.1007074107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Koffi M, De Meeus T, Bucheton B, Solano P, Camara M, et al. Population genetics of Trypanosoma brucei gambiense, the agent of sleeping sickness in Western Africa. Proc Natl Acad Sci U S A. 2009;106:209–214. doi: 10.1073/pnas.0811080106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Simo G, Njiokou F, Tume C, Lueong S, De Meeus T, et al. Population genetic structure of Central African Trypanosoma brucei gambiense isolates using microsatellite DNA markers. Infect Genet Evol. 2010;10:68–76. doi: 10.1016/j.meegid.2009.09.019. [DOI] [PubMed] [Google Scholar]
- 14.Gibson WC, de C. Marshall TF, Godfrey DG. Numerical analysis of enzyme polymorphism: a new approach to the epidemiology and taxonomy of trypanosomes of the subgenus Trypanozoon. Adv Parasitol. 1980;18:175–246. doi: 10.1016/s0065-308x(08)60400-5. [DOI] [PubMed] [Google Scholar]
- 15.Tait A, Barry JD, Wink R, Sanderson A, Crowe JS. Enzyme variation in T. brucei ssp. II. Evidence for T. b. rhodesiense being a set of variants of T. b. brucei. Parasitology. 1985;90(Pt 1):89–100. doi: 10.1017/s0031182000049040. [DOI] [PubMed] [Google Scholar]
- 16.MacLeod A, Welburn S, Maudlin I, Turner CM, Tait A. Evidence for multiple origins of human infectivity in Trypanosoma brucei revealed by minisatellite variant repeat mapping. J Mol Evol. 2001;52:290–301. doi: 10.1007/s002390010157. [DOI] [PubMed] [Google Scholar]
- 17.Balmer O, Beadell JS, Gibson W, Caccone A. Phylogeography and taxonomy of Trypanosoma brucei. PLoS Negl Trop Dis. 2011;5:e961. doi: 10.1371/journal.pntd.0000961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Maddison DR, Maddison WP. MacClade4: Analysis of phylogeny and character evolution. 4.08 ed. Sunderland, MA: Sinauer Associates; 2006. [Google Scholar]
- 19.Flot JF. seqphase: a web tool for interconverting phase input/output files and fasta sequence alignments. Mol Ecol Resour. 2011;10:162–166. doi: 10.1111/j.1755-0998.2009.02732.x. [DOI] [PubMed] [Google Scholar]
- 20.Stephens M, Smith NJ, Donnelly P. A new statistical method for haplotype reconstruction from population data. Am J Hum Genet. 2001;68:978–989. doi: 10.1086/319501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Clement M, Posada D, Crandall KA. TCS: a computer program to estimate gene genealogies. Mol Ecol. 2000;9:1657–1659. doi: 10.1046/j.1365-294x.2000.01020.x. [DOI] [PubMed] [Google Scholar]
- 22.Vanhollebeke B, De Muylder G, Nielsen MJ, Pays A, Tebabi P, et al. A haptoglobin-hemoglobin receptor conveys innate immunity to Trypanosoma brucei in humans. Science. 2008;320:677–681. doi: 10.1126/science.1156296. [DOI] [PubMed] [Google Scholar]
- 23.Higgins M, Tkachenko O, Brown S, Reed J, Carrington M. Structure of the trypanosome haptoglobin haemoglobin receptor and its implications for receptor function and innate immunity. Nat Struct Mol Biol. In Review doi: 10.1073/pnas.1214943110. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Amino acid alignment of the complete HpHbR gene. Amino acids are identified by a unique color and a single letter abbreviation at the top of the figure. Letters at left identify unique amino acid sequences shown in Figure 1b. An asterisk (*) indicates that multiple isolates share the same amino acid sequence (see Table S1). Variable positions are highlighted below the alignment.
(EPS)
This file contains the DNA sequences from each of the alleles identified in this study. Allele sequences were inferred from direct sequences in silico using the program PHASE 2.1.1 (Stephens et al. 2001).
(FAS)
Subgenus Trypanozoon isolate taxonomic classification, collection information and characterization of genetic variation at the HpHbR locus.
(DOCX)