Abstract
Background
Campylobacter jejuni is a leading cause of foodborne bacterial enterocolitis worldwide. Investigation of immunopathology is hampered by a lack of suitable vertebrate models. We have recently shown that gnotobiotic mice as well as conventional IL-10−/− animals are susceptible to C. jejuni infection and develop intestinal immune responses. However, clinical symptoms of C. jejuni infection were rather subtle and did not reflect acute bloody diarrhea seen in human campylobacteriosis.
Methodology/Principal Findings
In order to overcome these limitations we generated gnotobiotic IL-10−/− mice by quintuple antibiotic treatment starting right after weaning. The early treatment was essential to prevent these animals from chronic colitis. Following oral infection C. jejuni colonized the gastrointestinal tract at high levels and induced acute enterocolitis within 7 days as indicated by bloody diarrhea and pronounced histopathological changes of the colonic mucosa. Immunopathology was further characterized by increased numbers of apoptotic cells, regulatory T-cells, T- and B-lymphocytes as well as elevated TNF-α, IFN-γ, and MCP-1 concentrations in the inflamed colon. The induction of enterocolitis was specific for C. jejuni given that control animals infected with a commensal E. coli strain did not display any signs of disease. Most strikingly, intestinal immunopathology was ameliorated in mice lacking Toll-like-receptors-2 or -4 indicating that C. jejuni lipoproteins and lipooligosaccharide are essential for induction and progression of immunopathology.
Conclusion/Significance
Gnotobiotic IL-10−/− mice develop acute enterocolitis following C. jejuni infection mimicking severe episodes of human campylobacteriosis and are thus well suited to further dissect mechanisms underlying Campylobacter infections in vivo.
Introduction
Campylobacter (C.) jejuni is a leading cause of bacterial-induced enteritis worldwide. The zoonotic pathogen forms part of the commensal flora in many wild and domestic animals. Broiler chicks are primarily involved in transmitting this pathogen to humans [1], [2], [3]. Clinical symptoms of human campylobacteriosis vary from mild malaise to severe ulcerative enterocolitis requiring hospitalization in severely (immuno-) compromized patients. In most cases, however, infection of humans is self-limiting [4]. In the acute stage of C. jejuni induced enterocolitis, infected patients present with bloody diarrhea, abdominal cramps and fever. Histological examination of affected intestinal tissue sites reveals crypt abscesses, ulcerations, and elevated immune cell numbers in the colon in situ [5], [6], [7], [8]. In small subgroups of patients the acute phase is followed by serious sequelae, including Guilliane-Barré-Syndrome, Miller-Fischer-Syndrome and reactive arthritis [9], [10]. Despite the high prevalence of C. jejuni induced disease and its distinctive socioeconomic impact, molecular and cellular events leading to campylobacteriosis are still poorly understood [11]. A very recent review article from Ó Cróinin and Backert highlighted various pathogenicity factors and host cell determinants proposed to be involved in establishing C. jejuni infection and triggering disease [12]. Eventhough colonization factors of C. jejuni are well known, paucity of understanding molecular mechanisms underlying C. jejuni induced immunopathology is mainly due to the scarcity of suitable experimental in vivo models of human infection [13]. Mice are generally highly convenient for studying pathogenicity of bacteria and immunopathology. Most murine C. jejuni infection models analyzed so far suffer from sporadic colonization and the lack of intestinal immunopathology. Mice harboring a conventional commensal gut microbiota can often not be stably colonized by C. jejuni [6], [13]. Eventhough this phenomenon of “colonization resistance” in immunocompetent mice has been known for decades the mechanistic basis of this physiological barrier and how it can be overcome by commensal or pathogenic bacteria is still incompletely defined [14], [15], [16]. Some studies with isolator raised germfree mice underlined the substantial role of the murine microbiota in colonization resistance against C. jejuni. Following oral infection, C. jejuni colonized the entire gastrointestinal tract of these animals and induced clinical signs of disease including granulocyte infiltrates and bloody diarrhea, as well as humoral immune responses [17], [18], [19]. Despite the increased susceptibility to C. jejuni infection, germfree mice do not represent a suitable experimental model of C. jejuni infections due to the absence of an intact immune system [20], [21]. More recent investigations of our group revealed that colonization resistance is mainly caused by the host-specific intestinal microbiota composition and can be overcome under certain circumstances: Following peroral C. jejuni infection gnotobiotic wildtype mice, gnotobiotic mice reconstituted with a human microbiota and animals fed a Western style cafeteria diet could be readily colonized by the pathogen and displayed pro-inflammatory immune responses within the colon [22], [23]. In addition, acute as well as chronic inflammation within the small or large intestine has been shown to facilitate C. jejuni infection due to higher intestinal enterobacteria loads such as E. coli. In a very recent study we could clearly demonstrate that artificially elevating the intestinal E. coli burden by feeding live bacteria to adult mice harboring a conventional gut microbiota (and thus rendering them resistant to C. jejuni infection) abolished colonization resistance and C. jejuni infection induced pro-inflammatory immune responses within the infected gut [24]. However, severe clinical symptoms of acute enterocolitis such as bloody diarrhea in human campylobacteriosis were missing in so far generated animal models. Very recently we demonstrated that infection of 3-weeks-old infant mice right after weaning, but not adult 3-months-old animals harboring an age-dependent conventional intestinal microbiota developed acute enterocolitis within 6 days p.i. as indicated by bloody diarrhea, colonic shortening and increased apoptotic cell numbers in the colon mucosa. Similar to human campylobacteriosis, disease was self-limited and resolved within two weeks [25].
We here demonstrate that following C. jejuni infection gnotobiotic IL-10−/− mice develop acute enterocolitis as in human campylobacteriosis. Antibiotic treatment right after weaning prevented IL-10−/− mice from development of chronic colitis which usually becomes overt between 3 to 6 months of age, depending on housing conditions and the specific gut microbiota composition [26]. Toll like receptors (TLR) mediate essential signaling pathways involved in innate and adaptive host responses to commensal and pathogenic bacteria. Bacterial lipoproteins (LP) and lipooligosaccharides (LOS) are recognized by TLRs 2 and -4, respectively [27]. Detailed investigation of the interaction between C. jejuni and individual TLRs in vivo and in the intestines in situ are rather scarce. In different human, murine and avian cell lines C. jejuni was shown to activate TLR-2, TLR-4 and TLR-9 via MyD88 [28], [29], [30], [31], [32]. We have recently demonstrated that TLR-4 and -9 signaling are essential for induction of intestinal inflammation by C. jejuni in gnotobiotic mice which develop rather subtle symptoms [22]. The crucial role of TLRs and defined bacterial molecules in C. jejuni mediated intestinal immunopathology was now independently confirmed for lipoproteins and lipooligosaccharide in gnotobiotic IL-10−/− mice lacking TLRs 2 or 4.
Results
Acute Enterocolitis in C. jejuni Infected Gnotobiotic IL-10−/− Mice
We have recently shown that 5 to 6 months old IL-10−/− mice harboring a conventional intestinal microbiota and suffering from chronic colitis could be stably infected with C. jejuni at intermediate levels in approximately 70% of cases, but symptoms were rather subtle and, hence, ”classical” clinical signs of severe human camplyobacteriosis such as bloody diarrhea were missing. In this study, we eradicated the chronic colitogenic stimulus in IL-10−/− mice by quintuple antibiotic treatment for 4–5 months starting right after weaning. These gnotobiotic IL-10−/− mice were then perorally challenged either with C. jejuni B2 or a commensal E. coli strain lacking any relevant pathogenicity factors such as stx 1 and 2, catA, hlyA, cspA, katP, and astA which had been isolated from the colonic lumen of a conventional wildtype mouse [24] (see Materials and Methods). C. jejuni reference strain B2 originates from a patient with severe enteritis, is invasive in vitro and has been extensively investigated in earlier studies [33]. Naïve, uninfected gnotobiotic IL-10−/− mice without any signs of intestinal inflammation served as negative controls.
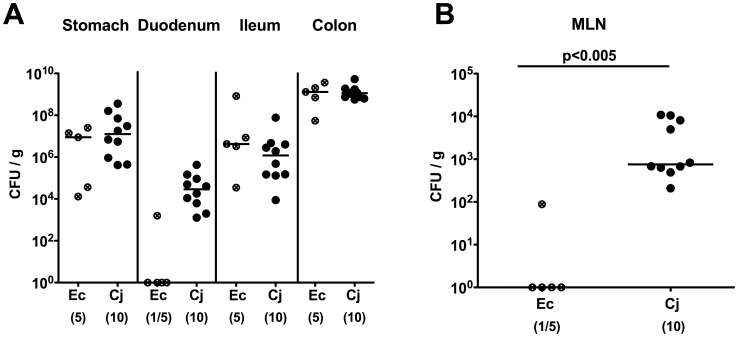
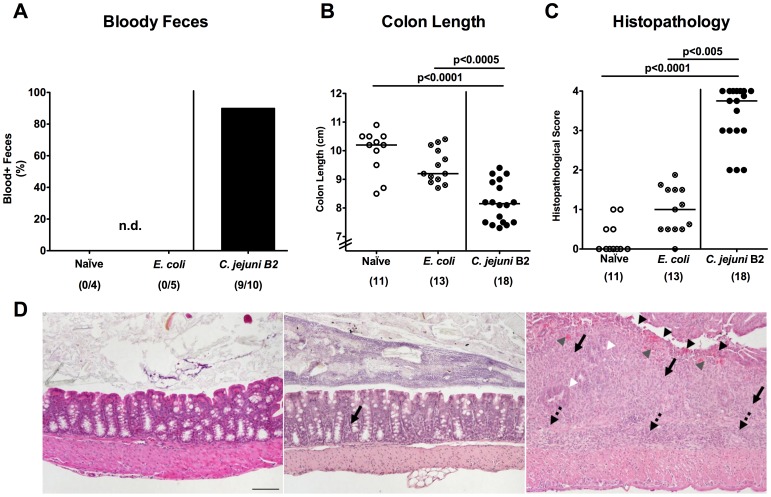
Seven days following peroral infection C. jejuni B2 and E. coli stably colonized the gastrointestinal tract of gnotobiotic IL-10−/− mice at comparably high levels of approximately 109 colony forming units (CFU) per gram ( Fig. 1A ). Interestingly C. jejuni B2, but not E. coli, translocated to mesenteric lymphnodes draining the large intestine at day 7 p.i. ( Fig. 1B ). Strikingly, C. jejuni B2 infected animals were decisively compromized by wasting enterocolitis as indicated by diarrhea, occurrence of blood in 80% of fecal samples in infected mice ( Fig. 2A ) and a significant reduction of the mean colonic length by approximately 20% within 7 days p.i. ( Fig. 2B ). Of note, approximately 30% of C. jejuni B2 infected animals had deceased before necropsy between day 4 and 7 p.i. (not shown). E. coli infected mice, however, were virtually unaffected and clinically comparable to naïve controls ( Fig. 2 ). Acute C. jejuni B2 induced enterocolitis was further confirmed by distinct histopathological changes of the colonic mucosa as indicated by significantly higher histopathological scores in C. jejuni B2 infected as compared to naive and E. coli infected gnotobiotic IL-10−/− mice ( Fig. 2C ). Acute ulcerative colitis in diseased IL-10−/− mice was characterized by ulcerations of and bleeding into the colonic mucosa as well as diffuse mucosal and submucosal infiltrates, and, in additon, by loss of goblet cells and crypt drop-outs at day 7 p.i. ( Fig. 2D ). Taken together, acute enterocolitis in gnotobiotic IL-10−/− mice following C. jejuni, but not E. coli infection mimicks clinical symptoms of severe human campylobacteriosis and is specifically induced by the pathogen.
Figure 1. C. jejuni B2 colonization and translocation in gnotobiotic IL-10−/− mice.
Gnotobiotic IL-10−/− mice were generated by antibiotic treatment and orally infected either with C. jejuni B2 (solid circles) or an apathogenic E. coli strain (crossed circles) derived from the commensal gut microbiota of conventional colonized wildtype mice as described in Methods. C. jejuni B2 and E. coli loads were determined in luminal samples taken from different compartments of the gastrointestinal tract (A) and mesenteric lymphnodes (MLN) (B) at day 7 p.i. (CFU, colony forming units). Numbers of animals harboring the respective bacterial species out of the total number of analyzed animals are given in parentheses. Medians (black bars) and significance levels (P-values) determined by Mann-Whitney-U test are indicated. Data shown are representative for three independent experiments.
Figure 2. C. jejuni induces acute enterocolitis in gnotobiotic IL-10−/− mice.
Gnotobiotic IL-10−/− mice were generated by antibiotic treatment and orally infected either with C. jejuni B2 (solid circles; black bar) or a commensal E. coli strain (crossed circles) derived from the commensal gut microbiota of conventional colonized wildtype mice as described in Methods. Uninfected gnotobiotic IL-10−/− mice served as negative controls (open circles). (A) Relative rate of blood-positive stool samples of infected IL-10−/− and uninfected control mice were determined by haemoccult test at day 7 p.i. (see Methods). Numbers of animals with haemcoocult positive stool samples out of the total number of analyzed animals are given in parentheses (n.d., not detectable). (B) Absolute colon lengths and (C) histopathological changes applying a standardized histopathological score in HE-stained colonic paraffin sections were determined at day 7 p.i., illustrated by (D) representative photomicrographs out of three independent experiments (100x magnification, scale bar 100 µm) derived from naïve (left), E. coli- (middle) and C. jejuni B2- (right) infected animals. Solid arrows indicate mucosal, dotted arrows submucosal infiltrates, black arrow heads ulcerations, white arrow heads loss of goblet cells, and gray arrow heads mucosal bleeding. Numbers of analyzed animals are given in parentheses. Medians (black bars) and significance levels (P-values) determined by Mann-Whitney-U test are indicated. Data shown were pooled from at least three independent experiments.
Inflammatory Immune Responses in C. jejuni Infected Gnotobiotic IL-10−/− Mice
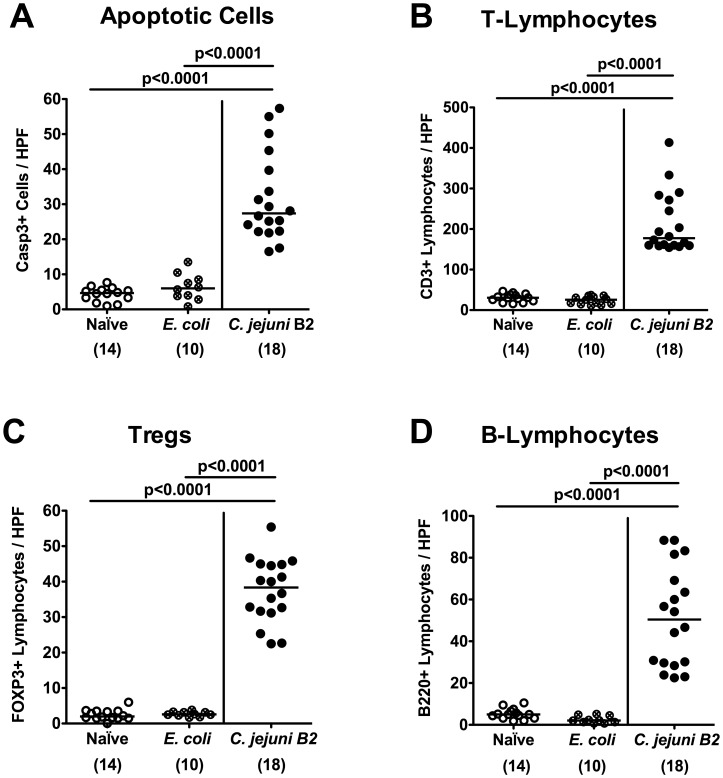
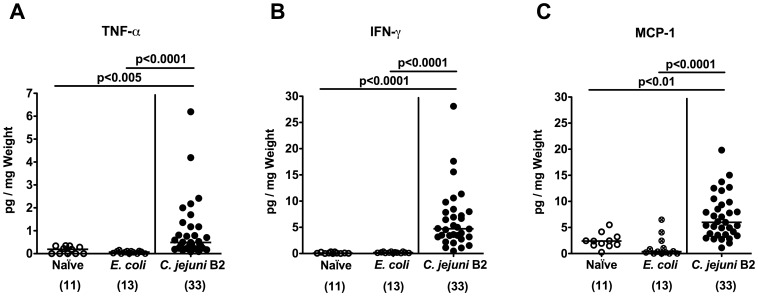
To further elucidate whether C. jejuni infection of gnotobiotic IL-10−/− mice induced pro-inflammatory immune responses, we analyzed apoptotic and distinct immune cell populations in the colonic mucosa in situ. C. jejuni B2 infected gnotobiotic IL-10−/− mice displayed multifold (between 5 to 15 times) higher numbers of apoptotic cells, T- and B-lymphocytes as well as regulatory T-cells in the colonic mucosa at day 7 p.i. as compared to E. coli infected and naive control animals ( Fig. 3 ). The immune cell responses were accompanied by increased expression of pro-inflammatory cytokines and mediators in the colon. Levels of TNF-α, IFN-γ, and MCP-1 were significantly higher in ex vivo colonic biopsies derived from C. jejuni B2 infected mice at day 7 p.i. as compared to E. coli infected and naive controls ( Fig. 4 ). Again, pro-inflammatory immune cell responses were absent in E. coli infected as well as in un-infected, naïve gnotobiotic IL-10−/− mice ( Fig. 3 , Fig. 4 ).
Figure 3. Inflammatory and immune cell responses following C. jejuni infection of gnotobiotic IL-10−/− mice.
Gnotobiotic IL-10−/− mice were generated by antibiotic treatment and orally infected either with C. jejuni B2 (solid circles) or an apathogenic E. coli strain (crossed circles) derived from the commensal gut microbiota of conventional colonized wildtype mice as described in Methods. Uninfected gnotobiotic IL-10−/− mice served as negative controls (open circles). The average numbers of apoptotic cells (positive for caspase-3, panel A), T-lymphocytes (positive for CD3, panel B), regulatory T-cells (Tregs, positive for FOXP3, panel C) and B-lymphocytes (positive for B220, panel D) from at least six high power fields (HPF, 400x magnification) per animal were determined microscopically in immunohistochemically stained colon sections at day 7 p.i. Numbers of analyzed animals are given in parentheses. Means (black bars) and levels of significance (P-values) determined by the Student’s t-test are indicated. Data shown are pooled from at least three independent experiments.
Figure 4. Pro-inflammatory cytokine responses in the colon of C. jejuni infected gnotiotic IL-10−/− mice.
Gnotobiotic IL-10−/− mice were generated by antibiotic treatment and orally infected with either C. jejuni B2 (solid circles) or an apathogenic E. coli strain (crossed circles) derived from the commensal gut microbiota of conventional colonized wildtype mice as described in Methods. Uninfected gnotobiotic IL-10−/− mice served as negative controls (open circles). Concentrations (pg per mg colon) of (A) TNF-α, (B) IFN-γ, and (C) MCP-1 were determined in supernatants of ex vivo colonic cultures at day 7 p.i. by cytometric bead array (CBA). Numbers of analyzed animals are given in parentheses. Means (black bars) and levels of significance (P-values) as compared to the respective group (determined by Student’s t-test) are indicated. Data shown are pooled from at least three independent experiments.
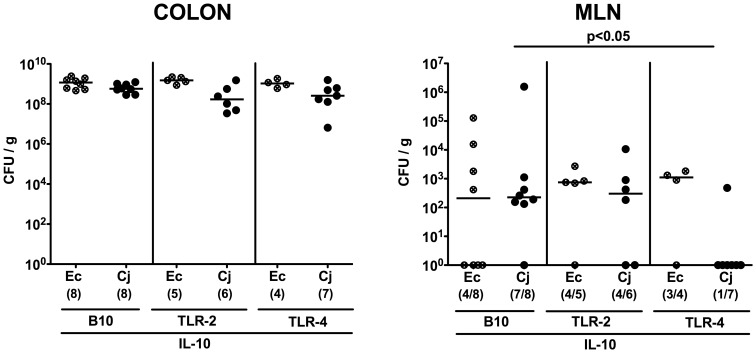
C. jejuni Colonization and Translocation in Gnotobiotic IL-10−/− Mice Lacking TLR-2 or TLR-4
To gain further insights into the immunopathological mechanisms underlying acute C. jejuni induced enterocolitis in vivo we crossed IL-10−/− mice with animals deficient in TLR-2 or -4 recognizing bacterial lipoproteins and lipooligosaccharide, respectively (all in B10 background, see Methods), and generated gnotobiotic double-deficient mice by quintuple antibiotic treatment for 4–5 months starting right after weaning. In the naïve condition, neither gnotobiotic TLR-2 IL-10 nor TLR-4 IL-10 double deficient mice displayed any clinical or histopathological signs of intestinal inflammation at all (not shown). At day 7 following oral C. jejuni B2 or E. coli infection gnotobiotic mice of either genotype harbored comparable loads of the respective bacterial species in their colon lumen ( Fig. 5A ). Thus, neither TLR-2 nor TLR-4 deficiency affected colonization capacity of C. jejuni B2 in gnotobiotic IL-10−/− animals.
Figure 5. TLR-dependent C. jejuni colonization and translocation in gnotobiotic IL-10−/− mice.
Gnotobiotic IL-10−/− (B10 background) as well as TLR-2−/− IL-10−/− and TLR-4−/− IL-10−/− mice were generated by antibiotic treatment and orally infected either with C. jejuni B2 (Cj; solid symbols) or a commensal E. coli strain (Ec; crossed symbols) as described in Methods. C. jejuni B2 and E. coli loads were determined in luminal colonic samples (A) and mensenteric lymphnodes (MLN) (B) at day 7 p.i. (CFU, colony forming units). Numbers of animals harboring the respective bacterial species out of the total number of analyzed animals are given in parentheses. Medians (black bars) and significance levels (P-values) determined by Mann-Whitney-U test are indicated. Data shown are representative for three independent experiments.
Whereas in 87.5% and 66.7% of IL-10−/− and TLR-2 IL-10 double deficient mice, respectively, live C. jejuni B2 were detected in MLNs, only in 14.3% of TLR-4 IL-10 double deficient animals the pathogen could be cultured from MLNs taken from at day 7 p.i. ( Fig. 5B ). Interestingly, no bacterial translocation from the intestinal tract to other extra-intestinal organs such as spleen, liver, kidney or cardiac blood occurred at day 7 p.i., irrespective of the genotype of infected mice (not shown).
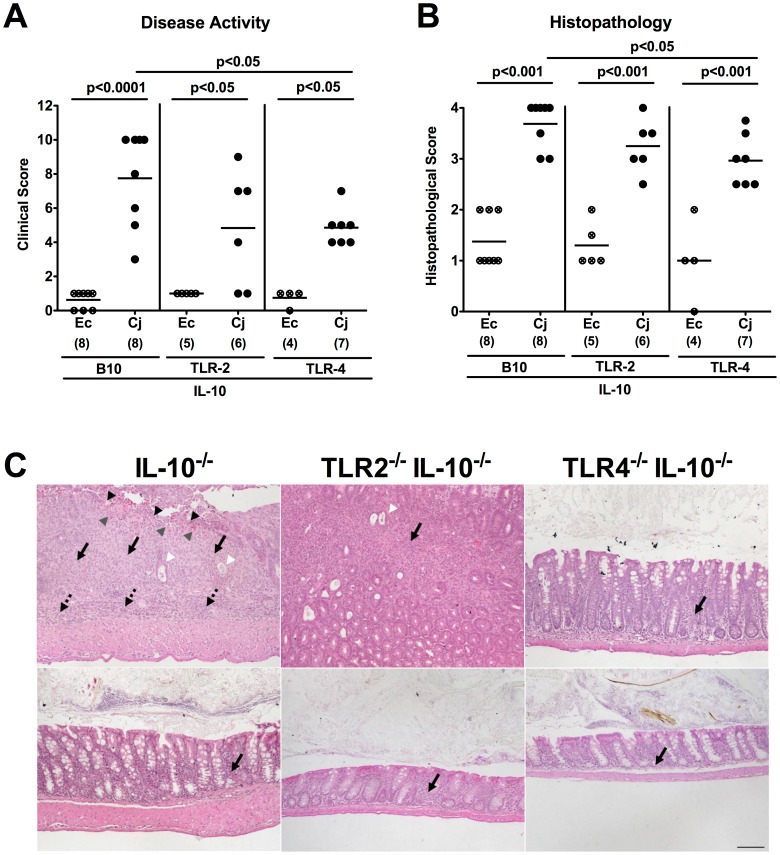
Acute Enterocolitis in C. jejuni Infected Gnotobiotic IL-10−/− Mice Lacking TLR-2 or TLR-4
At day 7 p.i., C. jejuni B2 infected TLR-4 IL-10 double deficient mice exhibited less severe acute enterocolitis when compared to IL-10−/− mice as indicated by significantly lower clinical scores assessing clinical condition, stool consistency and occurrence of blood in the stool. Additionally, histopathological changes of colonic mucosa in infected TLR-4 IL-10 double deficient mice were less distinct compared to IL-10−/− controls whereas in IL-10−/− animals lacking TLR-2 only a trend towards lower clinical and histopathological scores could be observed. This trend, however, did not reach statistical significance due to a relatively high standard deviation in TLR-2 IL-10 double deficient mice ( Fig. 6A-C ). Thus, TLR-4 and less distinctly TLR-2 mediate acute enterocolitis in gnotobiotic IL-10−/− mice following C. jejuni B2 infection.
Figure 6. C. jejuni induces TLR-dependent acute enterocolitis in gnotobiotic IL-10−/− mice.
Gnotobiotic IL-10−/− (B10 background) as well as TLR-2−/− IL-10−/− and TLR-4−/− IL-10−/− mice were generated by antibiotic treatment and orally infected either with C. jejuni B2 (Cj; solid symbols) or a commensal E. coli strain (Ec; crossed symbols) derived from the commensal gut microbiota of conventional colonized wildtype mice as described in Methods. (A) Disease activity of infected mice and (B) histopathological changes in HE-stained colonic paraffin sections applying a standardized clinical and histopathological score, respectively, were determined at day 7 p.i., illustrated by (C) representative photomicrographs out of three independent experiments (100x magnification, scale bar 100 µm) derived from C. jejuni B2- (upper panel) and E. coli- (lower panel) infected IL-10−/− (left panel), TLR-2−/− IL-10−/− (middle panel), and TLR-4−/− IL-10−/− (right panel) mice. Solid arrows indicate mucosal, dotted arrows submucosal iinfiltrates, black arrow heads ulcerations, white arrow heads crypt drop-out, and gray arrow heads mucosal bleeding. Numbers of analyzed animals are given in parentheses. Medians (black bars) and significance levels (P-values) determined by Mann-Whitney-U test are indicated. Data shown are representative for three independent experiments.
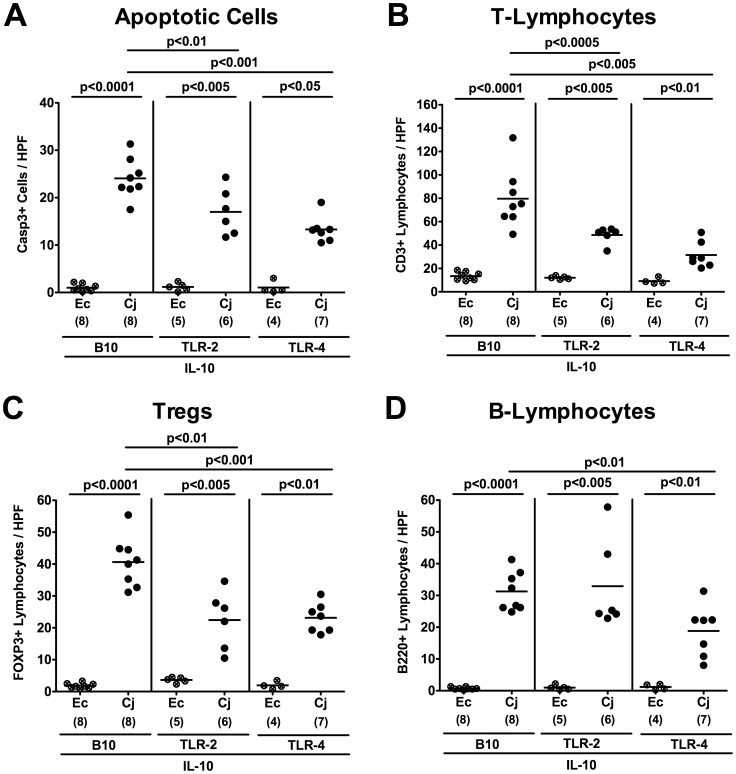
Inflammatory Immune Responses in C. jejuni Infected Gnotobiotic IL-10−/− Mice Lacking TLR-2 or TLR-4
In humans, C. jejuni induces the recruitment of pro-inflammatory immune cell populations to sites of inflammation in the colon [7], [8]. Given that quantitative assessment of apoptotic and infiltrating immune cells in the colonic mucosa is more sensitive than applying the used histopathological score, we performed in situ immunohistochemical staining of paraffin colonic sections. At day 7 p.i., C. jejuni infected IL-10−/− mice displayed two-fold higher numbers of apoptotic cells, T-lymphocytes and Tregs as compared to IL-10−/− mice additionally lacking TLR-2 or TLR-4 ( Fig. 7A-C ). Furthermore, TLR-4 IL-10 double deficient mice exhibited lower B-lymphocyte numbers in their colonic mucosa at day 7 p.i. as compared to IL-10−/− controls ( Fig. 7D ).
Figure 7. TLR-dependent inflammatory and immune cell responses following C. jejuni infection of gnotobiotic IL-10−/− mice.
Gnotobiotic IL-10−/− (B10 background) as well as TLR-2−/− IL-10−/− and TLR-4−/− IL-10−/− mice were generated by antibiotic treatment and orally infected either with C. jejuni B2 (Cj; solid symbols) or a commensal E. coli strain (Ec; crossed symbols) as described in Methods. The average numbers of apoptotic cells (positive for caspase-3, panel A), T-lymphocytes (positive for CD3, panel B), regulatory T-cells (Tregs, positive for FOXP3, panel C) and B-lymphocytes (positive for B220, panel D) from at least six high power fields (HPF, 400x magnification) per animal were determined microscopically in immunohistochemically stained colon sections at day 7 p.i. Numbers of analyzed animals are given in parentheses. Means (black bars) and levels of significance (P-values) determined by the Student’s t-test are indicated. Data shown are representative for three independent experiments.
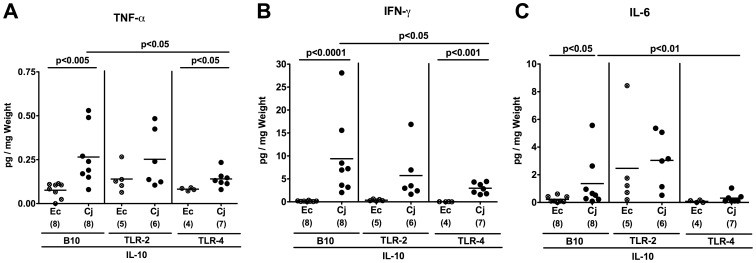
The less distinct apoptotic mucosal changes and colonic immune cell infiltration observed in TLR-4 IL-10 double deficient versus IL-10−/− mice were accompanied by significantly less secretion of pro-inflammatory cytokines such as TNF-α, IFN-γ, and IL-6 in ex vivo colonic biopsies taken at day 7 p.i. ( Fig. 8 ). Again, oral E. coli infection did not induce intestinal inflammation in gnotobiotic animals irrespective of the investigated genotypes ( Fig. 7 , 8 ).
Figure 8. TLR-dependent pro-inflammatory cytokine responses in the colon of C. jejuni infected gnotobiotic IL-10−/− mice.
Gnotobiotic IL-10−/− (B10 background) as well as TLR-2−/− IL-10−/− and TLR-4−/− IL-10−/− mice were generated by antibiotic treatment and orally infected either with C. jejuni B2 (Cj; solid symbols) or a commensal E. coli strain (Ec; crossed symbols) as described in Methods. Concentrations (pg per mg colon) of (A) TNF-α, (B) IFN-γ, and (C) IL-6 were determined in supernatants of ex vivo colon cultures at day 7 p.i. by cytometric bead array (CBA). Numbers of analyzed animals are given in parentheses. Means (black bars) and levels of significance (P-values) as compared to the respective group (determined by Student’s t-test) are indicated. Data shown are representative for three independent experiments.
Taken together, in this study we present a murine C. jejuni induced acute intestinal inflammation in gnotobiotic IL-10 deficient mice characterized by acute enterocolitis leading to death in some uneventful cases thus mimicking severe episodes of human camplyobacteriosis as seen in immunocompromized patients. Furthermore, TLR-4 signaling of C. jejuni lipooligosaccharide, and to a less distinct extent, TLR-2 signaling of lipoprotein mediates acute immunopathology following C jejuni infection. The presented model proves useful for further dissecting the immunopathological mechanisms underlying Campylobacter infections in vivo and to elucidate the interplay between intestinal pathogens, the commensal intestinal microbiota and the innate as well as adaptive immune system of the host.
Discussion
Despite the high prevalence of campylobacteriosis in humans and its associated socioeconomic burden molecular mechanisms underlying immunopathology of C. jejuni infection in the host are still incompletely understood. This is mainly due to the strong colonization resistance of mice leading to sporadic colonization (if at all) and the absence of disease defining clinical manifestations. Previous studies of our group underlined the role of the host microbiota and its composition in maintaining colonization resistance. Using gnotobiotic wildtype mice in which the microbiota had been completely eradicated by antibiotic treatment or gnotobiotic mice reconstituted with a human microbiota oral C. jejuni infection resulted in stable gastrointestinal colonization and pro-inflammatory immune responses in the colon [22], [23]. Furthermore, we could demonstrate that conventional mice suffering from chronic colitis due to IL-10 deficiency could be stably infected with C. jejuni at intermediate levels for two weeks [24]. Severe clinical symptoms of human campylobacteriosis such as acute ulcerative enterocolitis seen in immunocompromized patients, however, were missing in these animal models. Given that firstly, C. jejuni infection of gnotobiotic mice resulted in stable colonization and, secondly, production of IL-10 and other anti-inflammatory mediators are involved in limiting C. jejuni induced immunopathology, we generated IL-10−/− mice lacking any intestinal bacteria following quintuple antibiotic treatment for 5 months. One key feature of the novel infection model presented here is the very early beginning of antibiotic treatment in IL-10−/− mice: Eradicating any commensal bacteria right after weaning abolished the main trigger for inducing and perpetuating chronic colitis in IL-10−/− animals. Of note, following antibiotic treatment gnotobiotic IL-10−/− mice displayed neither any clinical nor immunopathological signs of large intestinal inflammation prior infection. Following C. jejuni infection, however, gnotobiotic IL-10−/− mice developed acute enterocolitis leading to death within one week in severe cases. Acute inflammation was characterized by rectal bleeding, histopathological signs of ulcerative colitis, increased numbers of apoptotic cells and increased recruitment of innate immune as well as effector cells in the colonic mucosa. In addition, an increased secretion of pro-inflammatory cytokines such as TNF-α, IFN-γ and MCP-1 was observed in ex vivo colonic cultures taken from C. jejuni infected mice. These findings are well in line with earlier studies using epithelial cell lines and bone marrow-derived dendritic cells. C. jejuni was shown to up-regulate chemokines involved in inflammatory responses such as MCP-1 and induced high level secretion of TNF-α and IFN-γ in vitro [34], [35].
Importantly, age- and sex-matched gnotobiotic IL-10−/− mice infected with a commensal E. coli strain (isolated from the colonic lumen of a wildtype mouse harboring a conventional gut microbiota and lacking any relevant pathogenicity factors) did not display any disease symptoms. Thus, the induced acute enterocolitis was pathogen specific and not due to infection with any (Gram-negative) bacterial species. Interestingly, acute enterocolitis was accompanied by translocation of live C. jejuni into mesenteric lymph nodes of all infected animals whereas E. coli virtually did not spread to extraintestinal compartments.
IL-10−/− mice have been used to study C. jejuni colonization and enteritis before. Bell et al. showed that C. jejuni induced a distinct inflammatory response in IL-10−/− mice [36]. Whereas Mansfield and colleagues demonstrated that enteritis developed after more than 28 days upon C. jejuni infection in IL-10−/− mice housed under specific pathogen free conditions (and depending on their genetic background) [37], [38], in our study, C. jejuni induced a rapid ulcerative colitis in gnotobiotic IL-10−/− mice. Using germfree IL-10−/− NF-κBEGFP mice raised under isolator-conditions, Lippert et al. highlighted the role of NF-κB in C. jejuni induced pathogenesis [39]. Following infection, germfree IL-10−/− NF-κBEGFP mice displayed mild symptoms at day 5 and ulcerative colitis at day 14 following C. jejuni strain 81–176 infection. In our C. jejuni infected gnotobiotic IL-10−/− mice acute enterocolitis had developed earlier (between day 4 and 7 p.i.) which might be due to differences in the C. jejuni strains used and due to the fact that our gnotobiotic animals exhibited a functional NF-kB pathway. Furthermore, germfree mice raised and housed under isolator conditions display an abnormally developed gut-associated lymphoid tissue [20], [21] and, hence, might not represent a suitable model for human C. jejuni infection [22].
Toll like receptors comprize essential signaling pathways involved in innate and adaptive host responses to pathogenic infections [27], but detailed in vivo studies on the interplay of TLRs with C. jejuni in the intestinal tract are scarce. In gnotobiotic mice generated by antibiotic treatment we showed previously that detection of C. jejuni lipooligoaccharide and CpG-DNA by host TLR-4 and TLR-9, respectively, was essential for mediating pro-inflammatory immune responses upon C. jejuni infection [22]. Clinical and histopathological signs of inflammation, however, were rather subtle in these mice. In the presented study, we could clearly demonstrate that signaling of bacterial lipooligoaccharide and, less distinctly, lipoproteins by TLR-4 and -2, respectively, was essentially involved in mediating acute enterocolitis in infected gnotobiotic IL-10 deficient mice. This is well in line with in vitro studies showing that C. jejuni activates TLR-2 and TLR-4 in different human, murine and avian cell lines [28], [29], [30], [31], [32]. Whereas following C. jejuni infection IL-10−/− mice lacking TLR-4 were significantly less compromized as compared to IL-10−/− controls, a not significant, but clear trend towards a better clinical outcome and less distinct histopathological changes seen in the colonic mucosa could be observed in the heterogenous cohort of TLR-2 IL-10 double deficient animals. In both, IL-10−/− mice lacking TLR-2 or TLR-4, amelioration of inflammation was associated with significantly lower numbers of apoptotic cells and T lymphocytes in the colon in situ. These diminished cellular responses were accompanied by lower secretion of pro-inflammatory cytokines such as TNF-α, IFN-γ and IL-6 in ex vivo colonic biopsies derived from C. jejuni infected TLR-4 IL-10 double deficient mice. In addition, increases in Tregs were less distinct in the colon of TLR deficient animals further indicating that TLRs -2 and -4 play essential roles in initiating inflammation by pro-inflammatory signals.
Furthermore, virtually no translocation of live C. jejuni to mesenteric lymphnodes in infected TLR-4 IL-10 double deficient, but not IL-10−/− mice indicates an uncompromized intestinal epithelial barrier function in the former. The immunopathological impact of TLR-4 dependent signaling of lipooligosaccharide in human campylobacteriosis is even more underlined by the fact that humans are up to 1000 times more sensitive to TLR-4 ligands than rodents [40]. Taken together, we here present a novel C. jejuni infection model mimicking acute enterocolitis in human campylobacteriosis. The course of disease mimics the situation seen in immunocompromized humans. Furthermore, the observed non self-limited acute ulcerative colitis following C. jejuni infection of gnotobiotic IL-10 deficient mice underlines the crucial role of the host specific microbiota in protecting against invading pathogens and underlines the importance of the anti-inflammatory cytokine IL-10 in C. jejuni induced immunopathology.
We conclude that TLR-4-, and to a lesser extent TLR-2-, signaling play important roles in mediating C. jejuni induced acute enterocolitis. The presented study provides valuable tools to gain further insights into the immunological and molecular interplays between C. jejuni and innate immunity in human campylobacteriosis.
Materials and Methods
Ethics Statement
Animal experiments were conducted according to the European Guidelines for animal welfare (2010/63/EU) with approval of the commission for animal experiments headed by the “Landesamt für Gesundheit und Soziales” (LaGeSo, Berlin, Germany; Registration numbers: G0173/07 and G135/10). Animal welfare was monitored twice daily by assessment of clinical conditions.
Mice
IL-10−/− mice (in C57BL/10 background, B10) were bred and maintained in the facilities of the “Forschungsinstitut für Experimentelle Medizin” (FEM, Charité - Universitätsmedizin, Berlin, Germany), under specific pathogen-free (SPF) conditions. In order to gain double-deficient animals, TLR-2−/− and TLR-4−/− mice (in B10 background each) were crossed to IL-10−/− mice and backcrossed at least 7 generations before use.
To eradicate the commensal gut flora, mice were transferred to sterile cages and treated by adding ampicillin (1 g/L; Ratiopharm), vancomycin (500 mg/L; Cell Pharm), ciprofloxacin (200 mg/L; Bayer Vital), imipenem (250 mg/L; MSD), and metronidazole (1 g/L; Fresenius) to the drinking water ad libitum as described earlier [41] starting at 3 weeks of age right after weaning. Age matched female mice were subjected to the quintuple antibiotic treatment for 4 months before the infection experiment.
Bacterial Strains
The E. coli strain used is a commensal isolate derived from our conventionally colonized, naive C57BL/6j wildtype mice and identical with E. coli M described earlier [42]. The genome does not contain known virulence factors of pathogenic E. coli such as stx, 1 and 2, catA, hlyA, cspA, katP and astA. This was confirmed in a reference laboratory by PCR analysis. The C. jejuni strain B2 was kindly provided by Prof. Dr. Uwe Groß, University of Göttingen (Germany). This strain was isolated from a patient suffering from bloody diarrhea, has shown to be invasive in vitro, and its main properties have been described in detail by Tareen et al. [33].
C. jejuni and E. coli Infection of Mice
Two days prior to infection the antibiotic cocktail was withdrawn and changed to sterilized tap water. Right before oral infection, sterility of each mouse was confirmed by transferring individual fecal samples to thioglycollate enrichment broths. Then, mice were infected with approximately 109 viable CFU of C. jejuni strains B2 by gavage in a total volume of 0.3 mL PBS on two consecutive days as described earlier in detail [22]. For infection with a Gram-negative control species, live E. coli were isolated from naïve, healthy 3 months old C57BL/10 mice by culture on MacConkey media. This commensal E. coli strain was subcultivated on blood agar and mice with approximately 109 viable CFU of E. coli by gavage in a total volume of 0.3 mL PBS on two consecutive days in parallel to the respective C. jejuni B2 groups.
Clinical Score
To assess clinical signs of C. jejuni induced infection on a daily basis, a standardized cumulative clinical score (maximum 12 points; modified according to [43] addressing the occurrence of blood in feces (0 points: no blood; 2 points: microscopic detection of blood by the Guajac method using Haemoccult, Beckman Coulter/PCD, Krefeld, Germany; 4 points: overt blood visible), diarrhea (0: formed feces; 2: pasty feces; 4: liquid feces), and the clinical aspect (0: normal; 2: ruffled fur, less locomotion; 4: isolation, severely compromized locomotion, pre-final aspect) was used.
Sampling Procedures, Determination of Colon Length and Histopathology
Mice were sacrificed by isofluran treatment (Abbott, Germany). From each mouse samples derived from the gastrointestinal tract (stomach, duodenum, ileum, colon) were collected in parallel for histological, microbiological and immunobiological analyses. Cardiac blood and tissue samples from mesenteric lymphnodes, spleen, liver and the gastrointestinal tract were asserved under sterile conditions. Histopathological changes were determined in colon samples immediately fixed in 5% formalin and embedded in paraffin. Sections (5 µm) were stained with respective antibodies for immunohistochemistry.
Immunohistochemistry
In situ immunohistochemical analysis of colonic paraffin sections were performed as described previously [22], [44], [45]. Primary antibodies against cleaved caspase-3 (Asp175, Cell Signaling, USA, 1∶200), CD3 (#N1580, Dako, Denmark, dilution 1∶10), FOXP-3 (FJK-16s, eBioscience, 1∶100), and B220 (eBioscience, San Diego, CA, USA, 1∶200) were used. For each animal, the average number of positive stained cells within at least six independent high power fields (HPF, 400x magnification) were determined microscopically and subjected to statistical analysis as indicated.
Cytokine Detection in Colon Culture Supernatants
Colon biopsies were cut longitudinally, washed in PBS and strips of 1 cm2 placed in 24-flat-bottom well culture plates (Nunc, Wiesbaden, Germany) containing 500 µL serum-free RPMI 1640 medium supplemented with penicillin (100 U/mL) and streptomycin (100 µg/mL; PAA Laboratories). After 18 h at 37°C, culture supernatants were tested for TNF-α, IFN-γ, MCP-1 and IL-6 by the Mouse Inflammation Cytometric Bead Array (CBA; BD Biosciences) on a BD FACSCanto II flow cytometer (BD Biosciences).
Microbiota Analyses
Cultural analyses, biochemical identification and molecular detection of luminal bacterial communities from (stomach, duodenum, ileum, and) colon as well as feces were performed as previously described [22], [41], [42], [44]. For bacterial translocation experiments, mesenteric lymphnodes draining the small intestine, spleen, liver (1 cm2) and cardiac blood (1 mL) were transferred into thioglycollate enrichment broths under sterile conditions and cultivated for 7 days. Turbid broths were streaked onto sheep blood, MacConkey as well as Karmali agar in order to detect translocated live E. coli and C. jejuni followed by species identification via biochemical and molecular methods [22]. Given that bacterial growth in enrichment broths cannot be quantitated, the relative rates of translocating C. jejuni or E. coli are expressed in %.
Statistical Analysis
Mean values, medians, standard deviations, and levels of significance were determined using appropriate tests as indicated (two-tailed Student’s t-Test, Mann-Whitney-U Test). Two-sided probability (P) values ≤0.05 were considered significant. All experiments were repeated at least twice.
Acknowledgments
We thank Michaela Wattrodt, Ursula Rüschendorf, Gernot Reifenberger, Uwe Lohmann, and the staff of the animal research facility for excellent technical assistance and animal breeding. We are grateful to Simone Spieckermann for immunohistochemistry staining of colon sections.
Footnotes
Competing Interests: Co-authors Stefan Bereswill and Markus M. Heimesaat are PLoS ONE Editorial Board members. This does not alter the authors' adherence to all the PLoS ONE policies on sharing data and materials.
Funding: This work was supported by grants from the German Research Foundation (DFG) to UBG (GO363/12-1, CampyGerm; SFB633, TP A7), SB (SFB633, TP A7), AK (SFB633, TP Z1), MMH (SFB633, TP B6), LMH and BO (SFB633, Immuco), and from the German Federal Ministery of Education and Research (BMBF) to SB (“Lab in a hanky” projects TP1.1 and TP 8.2). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Hermans D, Pasmans F, Messens W, Martel A, Van Immerseel F, et al. Poultry as a Host for the Zoonotic Pathogen Campylobacter jejuni. Vector Borne Zoonotic Dis. 2012. [DOI] [PubMed]
- 2.Lane JA, Mehra RK, Carrington SD, Hickey RM. The food glycome: a source of protection against pathogen colonization in the gastrointestinal tract. Int J Food Microbiol. 2010;142:1–13. doi: 10.1016/j.ijfoodmicro.2010.05.027. [DOI] [PubMed] [Google Scholar]
- 3.Young KT, Davis LM, Dirita VJ. Campylobacter jejuni: molecular biology and pathogenesis. Nat Rev Microbiol. 2007;5:665–679. doi: 10.1038/nrmicro1718. [DOI] [PubMed] [Google Scholar]
- 4.Havelaar AH, van Pelt W, Ang CW, Wagenaar JA, van Putten JP, et al. Immunity to Campylobacter: its role in risk assessment and epidemiology. Crit Rev Microbiol. 2009;35:1–22. doi: 10.1080/10408410802636017. [DOI] [PubMed] [Google Scholar]
- 5.Janssen R, Krogfelt KA, Cawthraw SA, van Pelt W, Wagenaar JA, et al. Host-pathogen interactions in Campylobacter infections: the host perspective. Clin Microbiol Rev. 2008;21:505–518. doi: 10.1128/CMR.00055-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kist M, Bereswill S. Campylobacter jejuni. Contrib Microbiol. 2001;8:150–165. doi: 10.1159/000060405. [DOI] [PubMed] [Google Scholar]
- 7.van Spreeuwel JP, Duursma GC, Meijer CJ, Bax R, Rosekrans PC, et al. Campylobacter colitis: histological immunohistochemical and ultrastructural findings. Gut. 1985;26:945–951. doi: 10.1136/gut.26.9.945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Walker RI, Caldwell MB, Lee EC, Guerry P, Trust TJ, et al. Pathophysiology of Campylobacter enteritis. Microbiol Rev. 1986;50:81–94. doi: 10.1128/mr.50.1.81-94.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Allos BM. Association between Campylobacter infection and Guillain-Barre syndrome. J Infect Dis. 1997;176:S125–128. doi: 10.1086/513783. [DOI] [PubMed] [Google Scholar]
- 10.Butzler JP. Campylobacter, from obscurity to celebrity. Clin Microbiol Infect. 2004;10:868–876. doi: 10.1111/j.1469-0691.2004.00983.x. [DOI] [PubMed] [Google Scholar]
- 11.Sun X, Threadgill D, Jobin C. Campylobacter jejuni induces colitis through activation of mammalian target of rapamycin signaling. Gastroenterology 142: 86–95 e85. 2012. [DOI] [PMC free article] [PubMed]
- 12.Ó Cróinin T, Backert S. Host epithelial cell invasion by Campylobacter jejuni: trigger or zipper mechanism? Front Cell Inf Microbio 2: 25. 2012. doi 10.3389/fcimb.2012.00025. [DOI] [PMC free article] [PubMed]
- 13.Dorrell N, Wren BW. The second century of Campylobacter research: recent advances, new opportunities and old problems. Curr Opin Infect Dis. 2007;20:514–518. doi: 10.1097/QCO.0b013e3282a56b15. [DOI] [PubMed] [Google Scholar]
- 14.Stecher B, Chaffron S, Kappeli R, Hapfelmeier S, Freedrich S, et al. Like will to like: abundances of closely related species can predict susceptibility to intestinal colonization by pathogenic and commensal bacteria. PLoS Pathog. 2010;6:e1000711. doi: 10.1371/journal.ppat.1000711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Stecher B, Hardt WD. Mechanisms controlling pathogen colonization of the gut. Curr Opin Microbiol. 2011;14:82–91. doi: 10.1016/j.mib.2010.10.003. [DOI] [PubMed] [Google Scholar]
- 16.Stecher B, Hardt WD. The role of microbiota in infectious disease. Trends Microbiol. 2008;16:107–114. doi: 10.1016/j.tim.2007.12.008. [DOI] [PubMed] [Google Scholar]
- 17.Yrios JW, Balish E. Pathogenesis of Campylobacter spp. in athymic and euthymic germfree mice. Infect Immun. 1986;53:384–392. doi: 10.1128/iai.53.2.384-392.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jesudason MV, Hentges DJ, Pongpech P. Colonization of mice by Campylobacter jejuni. Infect Immun. 1989;57:2279–2282. doi: 10.1128/iai.57.8.2279-2282.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Youssef M, Corthier G, Goossens H, Tancrede C, Henry-Amar M, et al. Comparative translocation of enteropathogenic Campylobacter spp. and Escherichia coli from the intestinal tract of gnotobiotic mice. Infect Immun. 1987;55:1019–1021. doi: 10.1128/iai.55.4.1019-1021.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Savidge TC, Smith MW, James PS, Aldred P. Salmonella-induced M-cell formation in germ-free mouse Peyer's patch tissue. Am J Pathol. 1991;139:177–184. [PMC free article] [PubMed] [Google Scholar]
- 21.Shroff KE, Cebra JJ. Development of mucosal humoral immune responses in germ-free (GF) mice. Adv Exp Med Biol. 1995;371A:441–446. doi: 10.1007/978-1-4615-1941-6_92. [DOI] [PubMed] [Google Scholar]
- 22.Bereswill S, Fischer A, Plickert R, Haag LM, Otto B, et al. Novel Murine Infection Models Provide Deep Insights into the "Menage a Trois" of Campylobacter jejuni, Microbiota and Host Innate Immunity. PLoS One. 2011;6:e20953. doi: 10.1371/journal.pone.0020953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bereswill S, Plickert R, Fischer A, Kühl AA, Loddenkemper C, et al. What you eat is what you get: Novel Campylobacter models in the quadrangle relationship between nutrition, obesity, microbiota and susceptibiliy to infection. Eur J Microbiol Immunol. 2011;1:237–248. doi: 10.1556/EuJMI.1.2011.3.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Haag LM, Fischer A, Otto B, Plickert R, Kühl AA, et al. Intestinal Microbiota Shifts towards Elevated Commensal Escherichia coli Loads Abrogate Colonization Resistance against Campylobacter jejuni in Mice, PLoSONE. 2012;7:e35988. doi: 10.1371/journal.pone.0035988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Haag LM, Fischer A, Otto B, Grundmann U, Kühl AA, et al. Campylobacter jejuni infection of infant mice: acute enterocolitis is followed by asymptomatic intestinal and extra-intestinal immune response. Eur J Microbiol Immunol. 2012;2:2–11. doi: 10.1556/EuJMI.2.2012.1.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wohlgemuth S, Keller S, Kertscher R, Stadion M, Haller D, et al. Intestinal steroid profiles and microbiota composition in colitic mice. Gut Microbes. 2011;2:159–166. doi: 10.4161/gmic.2.3.16104. [DOI] [PubMed] [Google Scholar]
- 27.Janeway CA, Jr, Medzhitov R. Innate immune recognition. Annu Rev Immunol. 2002;20:197–216. doi: 10.1146/annurev.immunol.20.083001.084359. [DOI] [PubMed] [Google Scholar]
- 28.de Zoete MR, Keestra AM, Roszczenko P, van Putten JP. Activation of human and chicken toll-like receptors by Campylobacter spp. Infect Immun. 2010;78:1229–1238. doi: 10.1128/IAI.00897-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rathinam VA, Appledorn DM, Hoag KA, Amalfitano A, Mansfield LS. Campylobacter jejuni-induced activation of dendritic cells involves cooperative signaling through Toll-like receptor 4 (TLR4)-MyD88 and TLR4-TRIF axes. Infect Immun. 2009;77:2499–2507. doi: 10.1128/IAI.01562-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Watson RO, Galan JE. Signal transduction in Campylobacter jejuni-induced cytokine production. Cell Microbiol. 2005;7:655–665. doi: 10.1111/j.1462-5822.2004.00498.x. [DOI] [PubMed] [Google Scholar]
- 31.Friis LM, Keelan M, Taylor DE. Campylobacter jejuni drives MyD88-independent interleukin-6 secretion via Toll-like receptor 2. Infect Immun. 2009;77:1553–1560. doi: 10.1128/IAI.00707-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.van Mourik A, Steeghs L, van Laar J, Meiring HD, Hamstra HJ, et al. Altered linkage of hydroxyacyl chains in lipid A of Campylobacter jejuni reduces TLR4 activation and antimicrobial resistance. J Biol Chem. 2010;285:15828–15836. doi: 10.1074/jbc.M110.102061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tareen AM, Dasti JI, Zautner AE, Gross U, Lugert R. Campylobacter jejuni proteins Cj0952c and Cj0951c affect chemotactic behaviour towards formic acid and are important for invasion of host cells. Microbiology. 2010;156:3123–3135. doi: 10.1099/mic.0.039438-0. [DOI] [PubMed] [Google Scholar]
- 34.Hu L, Hickey TE. Campylobacter jejuni induces secretion of proinflammatory chemokines from human intestinal epithelial cells. Infect Immun. 2005;73:4437–4440. doi: 10.1128/IAI.73.7.4437-4440.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rathinam VA, Hoag KA, Mansfield LS. Dendritic cells from C57BL/6 mice undergo activation and induce Th1-effector cell responses against Campylobacter jejuni. Microbes Infect. 2008;10:1316–1324. doi: 10.1016/j.micinf.2008.07.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bell JA, St Charles JL, Murphy AJ, Rathinam VA, Plovanich-Jones AE, et al. Multiple factors interact to produce responses resembling spectrum of human disease in Campylobacter jejuni infected C57BL/6 IL-10−/− mice. BMC Microbiol. 2009;9:57. doi: 10.1186/1471-2180-9-57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mansfield LS, Patterson JS, Fierro BR, Murphy AJ, Rathinam VA, et al. Genetic background of IL-10(−/−) mice alters host-pathogen interactions with Campylobacter jejuni and influences disease phenotype. Microb Pathog. 2008;45:241–257. doi: 10.1016/j.micpath.2008.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mansfield LS, Bell JA, Wilson DL, Murphy AJ, Elsheikha HM, et al. C57BL/6 and congenic interleukin-10-deficient mice can serve as models of Campylobacter jejuni colonization and enteritis. Infect Immun. 2007;75:1099–1115. doi: 10.1128/IAI.00833-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lippert E, Karrasch T, Sun X, Allard B, Herfarth HH, et al. Gnotobiotic IL-10; NF-kappaB mice develop rapid and severe colitis following Campylobacter jejuni infection. PLoS One. 2009;4:e7413. doi: 10.1371/journal.pone.0007413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Warren HS, Fitting C, Hoff E, Adib-Conquy M, Beasley-Topliffe L, et al. Resilience to bacterial infection: difference between species could be due to proteins in serum. J Infect Dis. 2010;201:223–232. doi: 10.1086/649557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Heimesaat MM, Bereswill S, Fischer A, Fuchs D, Struck D, et al. Gram-negative bacteria aggravate murine small intestinal Th1-type immunopathology following oral infection with Toxoplasma gondii. J Immunol. 2006;177:8785–8795. doi: 10.4049/jimmunol.177.12.8785. [DOI] [PubMed] [Google Scholar]
- 42.Heimesaat MM, Fischer A, Jahn HK, Niebergall J, Freudenberg M, et al. Exacerbation of murine ileitis by Toll-like receptor 4 mediated sensing of lipopolysaccharide from commensal Escherichia coli. Gut. 2007;56:941–948. doi: 10.1136/gut.2006.104497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Siegmund B, Fantuzzi G, Rieder F, Gamboni-Robertson F, Lehr HA, et al. Neutralization of interleukin-18 reduces severity in murine colitis and intestinal IFN-gamma and TNF-alpha production. Am J Physiol Regul Integr Comp Physiol. 2001;281:R1264–1273. doi: 10.1152/ajpregu.2001.281.4.R1264. [DOI] [PubMed] [Google Scholar]
- 44.Heimesaat MM, Fischer A, Siegmund B, Kupz A, Niebergall J, et al. Shift towards pro-inflammatory intestinal bacteria aggravates acute murine colitis via Toll-like receptors 2 and 4. PLoS One. 2007;2:e662. doi: 10.1371/journal.pone.0000662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Heimesaat MM, Nogai A, Bereswill S, Plickert R, Fischer A, et al. MyD88/TLR9 mediated immunopathology and gut microbiota dynamics in a novel murine model of intestinal graft-versus-host disease. Gut. 2010;59:1079–1087. doi: 10.1136/gut.2009.197434. [DOI] [PubMed] [Google Scholar]