We assessed 18–24-month postpartum disease progression risk among women in a randomized trial assessing efficacy and safety of prophylactic maternal antiretrovirals (ARVs). Interrupting prolonged triple-ARV prophylaxis had no effect on HIV-1 progression following cessation (compared to zidovudine until delivery with single-dose nevirapine without postpartum prophylaxis).
Abstract
Background. Antiretroviral (ARV) prophylaxis effectively reduces mother-to-child transmission of human immunodeficiency virus type 1 (HIV). However, it is unclear whether stopping ARVs after breastfeeding cessation affects maternal HIV disease progression. We assessed 18–24-month postpartum disease progression risk among women in a randomized trial assessing efficacy and safety of prophylactic maternal ARVs.
Methods. From 2005 to 2008, HIV–infected pregnant women with CD4+ counts of 200–500/mm3 were randomized to receive either triple ARV (zidovudine, lamivudine, and lopinavir/ritonavir during pregnancy and breastfeeding) or AZT/sdNVP (zidovudine until delivery with single-dose nevirapine without postpartum prophylaxis). Maternal disease progression was defined as the combined endpoint of death, World Health Organization clinical stage 4 disease, or CD4+ counts of <200/mm3.
Results. Among 824 randomized women, 789 had at least 1 study visit after cessation of ARV prophylaxis. Following delivery, progression risk up to 24 months postpartum in the triple ARV arm was significantly lower than in the AZT/sdNVP arm (15.7% vs 28.3%; P = .001), but the risks of progression after cessation of ARV prophylaxis (rather than after delivery) were not different (15.0% vs 13.8% 18 months after ARV cessation). Among women with CD4+ counts of 200–349/mm3 at enrollment, 24.0% (95% confidence interval [CI], 15.7–35.5) progressed with triple ARV, and 23.0% (95% CI, 17.8–29.5) progressed with AZT/sdNVP, whereas few women in either arm (<5%) with initial CD4+ counts of ≥350/mm3 progressed.
Conclusions. Interrupting prolonged triple ARV prophylaxis had no effect on HIV progression following cessation (compared with AZT/sdNVP). However, women on triple ARV prophylaxis had lower progression risk during the time on triple ARV. Given the high rate of progression among women with CD4+ cells of <350/mm3, ARVs should not be discontinued in this group.
Clinical Trials Registration. ISRCTN71468410.
Although breastfeeding is essential for child survival in low-resource settings, it carries a significant risk of human immunodeficiency virus type 1 (HIV) transmission for infants born to HIV–infected mothers, especially mothers with late-stage HIV disease. The Kesho Bora project [1] included a multicenter, randomized controlled trial assessing the efficacy and safety of maternal antiretrovirals (ARVs) during pregnancy, delivery, and breastfeeding to reduce mother-to-child HIV transmission (MTCT) among HIV–infected African pregnant women with CD4+ T-lymphocyte cell counts of 200–500/mm3. Women were randomized to receive a combination of zidovudine (AZT), lamivudine (3TC), and lopinavir/ritonavir (LPV/r) initiated during pregnancy and continued during breastfeeding (triple-ARV prophylaxis) or AZT until delivery with single-dose nevirapine (sd-NVP) at the onset of labor without postpartum prophylaxis [1]. We previously reported a greater overall MTCT prevention efficacy of the triple ARV prophylactic regimen when continued during breastfeeding and a lack of serious adverse events in the first 12 months postpartum [2]. However, the longer-term impact of triple ARV prophylaxis cessation on maternal health after prolonged administration during pregnancy and breastfeeding has not been evaluated. Trials of intermittent vs continuous ARV treatment showed higher rates of disease progression among persons with treatment interruptions [3–5]. The present paper focuses on maternal AIDS-free survival among women enrolled in the Kesho Bora randomized controlled trial up to 18–24 months postdelivery.
METHODS
Detailed objectives and methods of the Kesho Bora study have been reported elsewhere [1]. Briefly, HIV–infected, ARV-naive pregnant women at 5 study sites in Burkina-Faso, Kenya, and South Africa from January 2005 to August 2008 were offered enrollment in the Kesho Bora study. Women with contraindications for initiation of ARVs were excluded (ie, known allergy to ARVs or benzodiazepines; treatment with drugs that interact with ARVs; or severe [grade >2] [6] anemia, neutropenia, or liver or renal failure). The study protocol was approved by the ethical and regulatory committees in Burkina Faso, Kenya, and South Africa and at the World Health Organization (WHO) and the US Centers for Disease Control and Prevention.
Pregnant women at 20–32 weeks of gestation with WHO clinical stages 1, 2, or 3 and CD4 + cells of 200–500/mm3 were randomized to initiate between 28–34 weeks gestation either triple ARV prophylaxis (triple ARV) consisting of twice daily AZT 300 mg, 3TC 150 mg, and LPV/r 400 mg/100 mg until cessation of breastfeeding (to a maximum of 6.5 months postpartum; or 1 month if not breastfeeding) or standard MTCT prophylaxis (AZT/sdNVP) consisting of AZT (300 mg twice daily) alone until delivery, with a single dose of AZT (600 mg) and NVP (200 mg) at the onset of labor without postnatal prophylaxis of the mother or breastfeeding infant [1]. From December 2006, following updated WHO recommendations [7], 1 week of AZT + 3TC postpartum was added for women randomized to the AZT/sdNVP arm. Computer-generated block randomization was used, stratifying by study site and planned mode of infant feeding. Patients and study investigators were not blinded to treatment allocation.
All infants received a single dose of 0.6 mL oral NVP suspension. From December 2006 onward, they also received 1 week of AZT (4 mg/kg twice daily) from birth [1, 2].
All mothers were counseled on infant feeding as per 2003 WHO guidelines. Those opting for replacement feeding from birth received free formula for 6 months; those opting for breastfeeding were counseled to exclusively breastfeed and wean over a 2-week period with complete cessation before the child reached age 6 months.
Women were seen every 2 weeks from enrollment until 8 weeks after delivery, monthly until 1 year, and every 3 months thereafter. Although the original Kesho Bora protocol included a follow-up of 24 months after delivery, the Project Coordinating Committee (PCC) decided in January 2008 to shorten follow-up to 18 months after delivery/birth to focus on the main endpoint of the trial, notably infant HIV-free survival at age 1 year [1].
At each scheduled visit, clinical events, adherence to ARVs, infant feeding practices, and nutritional status (Body Mass Index [BMI]: weight/height2) were recorded using standardized case report forms. Blood samples were collected at regular intervals from mothers for toxicity monitoring, CD4 count, viral load (VL), and ARV drug-resistance testing.
The HIV plasma VL was measured at enrollment, delivery, and 18 months after delivery using a quantitative HIV RNA real-time polymerase chain reaction assay with a lower detection limit of 300 copies/mL (Generic HIV Charge Virale; Biocentric) [8]. Viral loads below the limit of detection were assigned the value 1 on log10 scale. HIV drug resistance mutations were evaluated in all maternal specimens collected within 6 weeks of triple ARV cessation from Bobo-Dioulasso and the 2 South African sites and in a sample of specimens obtained from women in the AZT + sdNVP arm at 2 or 6 weeks postpartum. Sequence analysis was performed on all specimens with VLs > 1000 copies/mL, and drug resistance patterns were predicted using the Agence Nationale de Recherche sur le SIDA et les hepatites algorithm (http://www.hivfrenchresistance.org). The sequences reported include the entire protease region and at least the first 330 codons of the reverse transcriptase region of the pol gene. Group comparisons were made using Student's t test and χ2 test (95% level; 2-sided tests). Kaplan-Meier product-limit estimates and log rank tests were used to assess and compare progression of maternal HIV disease, based on the intention-to-treat principle. Time to progression was first estimated from delivery. However, because disease progression was not expected to occur during the period of triple ARV prophylaxis, analyses were repeated using a start date equivalent to the date of cessation of ARV prophylaxis for the triple ARV group.
Definitions of progression were based on the WHO antiretroviral therapy (ART) eligibility endpoints: death, WHO clinical stage 3 or 4 opportunistic disease, or having at least 1 CD4+ count of <200/mm3 or <350/mm3. Two physicians who were members of the PCC (I. V. and J. R.) reviewed each woman's clinical history to assess whether clinical progression occurred according to the defined endpoints. A woman could reach different endpoints during follow-up, but time to progression was considered as time to the first endpoint. Only events occurring between cessation of ARV prophylaxis and therapeutic ART initiation (if any) were considered (women were censored at ARV initiation for therapeutic reasons or for a new pregnancy or study exit). The following two definitions of progression were used for separate analyses:
Among all women: 1a. clinical progression to WHO clinical stage 4 or death;1b. clinical or immunological progression (death, stage 4, or at least 1 CD4+ count of <200/mm3).
Among women enrolled with a CD4+ count of ≥350/mm3 who were asymptomatic: progression to stage 3 or a CD4+ count of <350/mm3.
Risk factors for progression were analyzed using univariate and multivariate analysis (logistic regression). All characteristics of participants described in Table 1 were first tested in univariate analysis. Variables significantly associated with disease progression in univariate analysis were tested in a logistic regression model after fitting known risk factors for progression (VL, CD4+ count, and age at enrollment).
Table 1.
Characteristics of Mothers According to Study Arm
| Characteristics | Triple ARV (n = 384) | AZT/sdNVP (n = 405) | P Value |
|---|---|---|---|
| Study site | |||
| Bobo Dioulasso, Burkina Faso | 115 (30.0) | 122 (30.1) | .98 |
| Kwadabeka, South-Africa | 91 (23.7) | 91 (22.5) | |
| Mombasa, Kenya | 113 (29.3) | 120 (29.6) | |
| Nairobi, Kenya | 19 (5.0) | 24 (5.9) | |
| Somkhele, South-Africa | 46 (12.0) | 48 (11.9) | |
| Age in years, median (IQR) | 27.0 (24.0–31.0) | 27.0 (23.0–31.0) | .70 |
| Education | |||
| Never attended school | 53 (13.8) | 63 (15.6) | .41 |
| Completed primary school | 127 (33.1) | 146 (36.0) | |
| At least some secondary school education | 204 (53.1) | 196 (48.4) | |
| Occupation | |||
| Unemployed | 258 (67.2) | 294 (72.6) | .22 |
| Self-employed | 73 (19.0) | 68 (16.8) | |
| Salaried job | 53 (13.8) | 43 (10.6) | |
| Marital status | |||
| Married, monogamous | 152 (39.6) | 181 (44.7) | .15 |
| Married, polygamous | 40 (10.4) | 51 (12.6) | |
| Not married, regular partner | 173 (45.0) | 161 (39.7) | |
| Single | 19 (5.0) | 12 (3.0) | |
| Gravidity | |||
| Primigravida | 66 (17.2) | 70 (17.3) | .97 |
| Socioeconomic scorea | .44 | ||
| 1st quintile (lowest score) | 75 (19.5) | 76 (18.8) | |
| 2nd quintile | 70 (18.2) | 86 (21.2) | |
| 3rd quintile | 72 (18.8) | 89 (22.0) | |
| 4th quintile | 89 (23.2) | 77 (19.0) | |
| 5th quintile | 78 (20.3) | 77 (19.0) | |
| Duration of use of ARVs: | |||
| From enrollment until delivery, in weeks, median (IQR) | 6.0 (4.0–8.0) | 6.0 (4.0–9.0) | .07 |
| After delivery, in weeks, median (IQR) | 18.7 (5.9–27.3) | NA | |
| Breastfeeding (BF) | |||
| Ever BF | 287 (75.5) | 314 (78.3) | .36 |
| BF durationb (if BF), in weeks, median (IQR) | 21.4 (9.7–25.6) | 20.0 (9.1–25.8) | .79 |
| CD4+ count in cells/mm3c, median (IQR) | |||
| At enrollment | 334 (283–408) | 339 (268–408) | .55 |
| At delivery | 465 (383–603) | 415 (331–531) | <.0001 |
| At 6 months after delivery | 479 (367–597) | 377 (292–474) | <.0001 |
| At 12 months after delivery | 401 (320–519) | 380 (289–475) | .004 |
| At 18 months after delivery | 396 (301–493) | 362 (273–467) | .008 |
| At 24 months after delivery | 396 (289–490) | 331 (263–425) | .04 |
| Maternal viral loadd (VL) | |||
| At enrollment, log10e, median (IQR) | 4.19 (3.65–4.75) | 4.20 (3.56–4.74) | .97 |
| Undetectable VL (<300 copies/mL) | 18 (4.7) | 14 (3.5) | .38 |
| At delivery | |||
| log10e, median (IQR) | 0.00 (0.00–2.85) | 3.10 (2.10–3.73) | <.0001 |
| Undetectable VL | 221 (63.3) | 111 (29.9) | <.0001 |
| At 18 months postpartum | |||
| log10e, median (IQR) | 4.50 (3.97–5.10) | 4.53 (3.78–5.11) | .75 |
| Undetectable VL | 17 (5.4) | 12 (4.1) | .28 |
| Body mass indexf in Kg/m2, median (IQR) | |||
| At enrollment | 26.4 (24.1–29.4) | 26.0 (23.5–29.0) | .18 |
| At delivery | 24.2 (22.0–27.3) | 24.1 (21.9–27.0) | .66 |
| At 6 months after delivery | 24.0 (21.5–27.7) | 23.6 (21.3–26.9) | .43 |
| At 12 months after delivery | 24.4 (21.6–28.0) | 23.5 (21.2–26.8) | .04 |
| At 18 months after delivery | 24.6 (21.5–28.8) | 23.7 (21.3–27.0) | .07 |
| At 24 months after delivery | 23.7 (20.9–27.6) | 23.0 (20.8–25.5) | .22 |
Data are no. (%) unless otherwise specified.
Abbreviations: ARV, antiretroviral; AZT/sdNVP, zidovudine until delivery with single-dose nevirapine without postpartum prophylaxis; IQR, interquartile range; NA, not applicable; Triple ARV, zidovudine, lamivudine, and lopinavir/ritonavir during pregnancy and breastfeeding.
a Calculated by multiple correspondence analysis using 8 household assets (electricity, refrigerator, radio, television, telephone/cell phone, source of water, type of toilets, type of fuel). Quintiles were defined within each country (Burkina Faso, Kenya, South Africa). Higher socioeconomic scores denote people with more assets.
b Available for 280 of 287 in the triple ARV group and 309 of 314 in the AZT/sdNVP group.
c Available for 384 women at randomization, 361 of 384 at delivery, 363 of 382 at 6 months after delivery, 347 of 359 at 12 months, 303 out of 340 at 18 months, and 76 of 96 at 24 months in the triple ARV group and 405, 377 of 405, 360 of 385, 330 of 341, 286 of 329, and 73 of 93 in the AZT/sdNVP group.
d Available for 382 of 384 women at randomization, 349 of 384 at delivery, and 313 out of 325 at 18 months in the triple ARV group and 403 of 405, 371 of 405, and 293 of 306 in the AZT/sdNVP group.
e Viral loads below the detection level (<300 copies/mL) were assigned a value of 1 copy/mL (0 in log10 scale).
f Available for 379 of 384 women at randomization, 373 of 384 at delivery, 370 of 383 at 6 months after delivery, 351 of 369 at 12 months, 303 out of 351 at 18 months, and 83 out of 94 at 24 months in the triple ARV group and 402 of 405, 382 of 405, 369 of 393, 352 of 372, 299 of 345, and 85 of 95 in the AZT/sdNVP group.
RESULTS
A total of 824 women were enrolled, with 412 enrolled in the triple ARV arm and 412 in the AZT/sdNVP arm. Of these, 789 (384 in the triple ARV arm and 405 in the AZT/sdNVP arm) had at least 1 study visit after cessation of ARV prophylaxis. At enrollment, maternal demographic, clinical, and virological parameters were comparable between arms (Table 1).
Rates of follow-up to 18 months after delivery were 92.1% and 89.0% in the triple-ARV arm and AZT/sdNVP arm, respectively; at 24 months, they were 86.1% and 84.7%, respectively.
Women received ARV prophylaxis for a median of 24.7 weeks in the triple ARV arm (6.0 weeks before and 18.7 weeks after delivery) and 6.0 weeks (before delivery) in the AZT/sdNVP arm (227 of 405 stopped prophylaxis at delivery; 178 of 405 received 1 week of AZT/3TC after delivery).
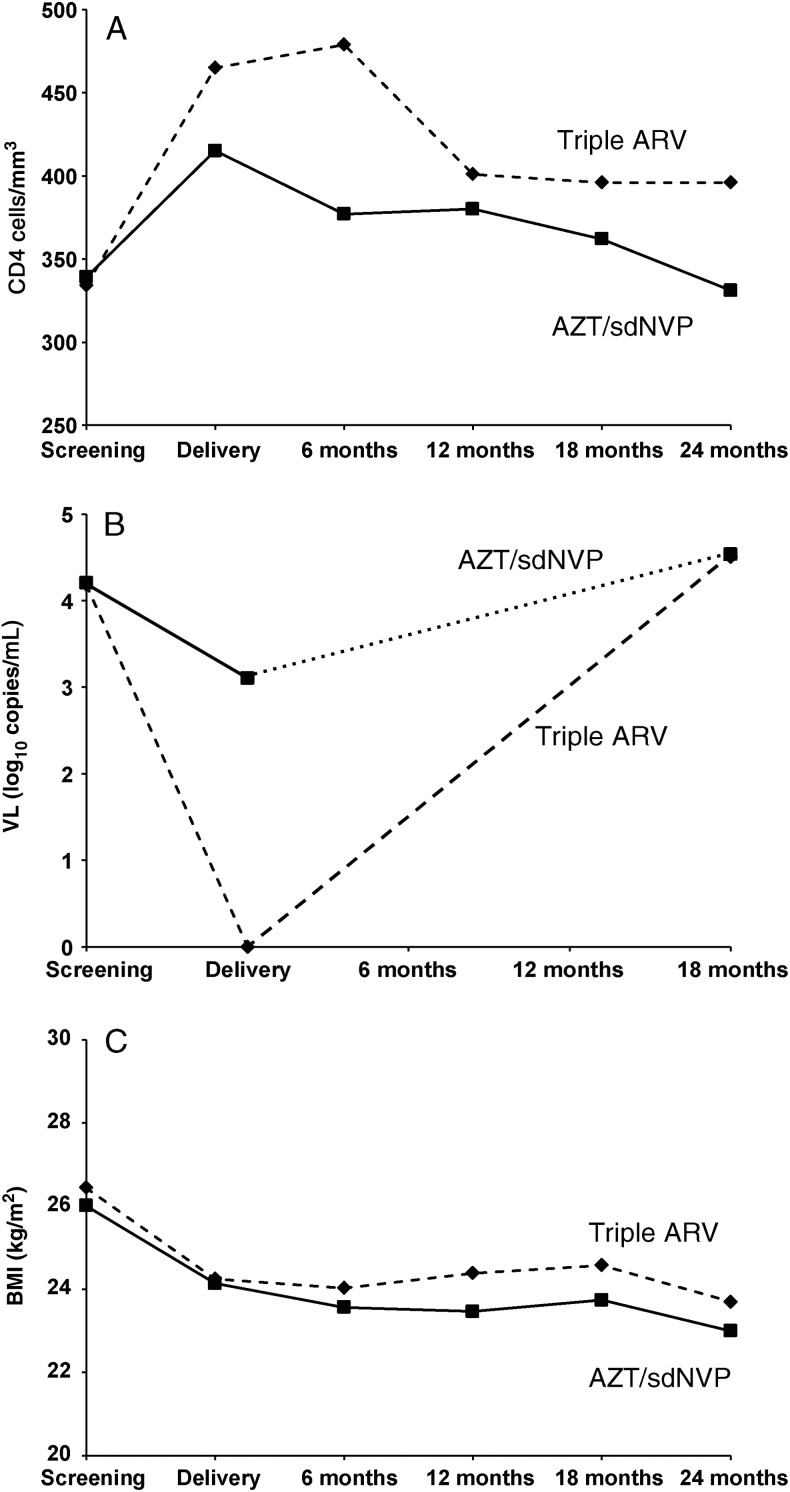
The median CD4 counts increased in both arms (more in the triple ARV than in AZT/sdNVP arm) from enrollment to ARV prophylaxis cessation and decreased thereafter to about baseline levels (Table 1 Figure 1A).
Figure 1.
A, Evolution of median CD4+ count (cells/mm3) up to 24 months after delivery. B, Evolution of the median viral load (log10 copies/mL) up to 18 months after delivery. C, Evolution of median body mass index (kg/m2) up to 24 months after delivery. Abbreviations: AZT/sdNVP, zidovudine until delivery with single-dose nevirapine without postpartum prophylaxis; Triple ARV, zidovudine, lamivudine, and lopinavir/ritonavir during pregnancy and breastfeeding.
By the time of delivery, the median VL had decreased by 1.1 log in the AZT/sdNVP arm and by 4.2 log in the triple ARV arm; the rate of undetectable VL in the triple ARV arm was twice that in the AZT/sdNVP arm (P < .0001). However, the VLs at 18 months returned to baseline levels at enrollment in both study arms (Table 1; Figure 1B).
The BMI was lower in the AZT/sdNVP arm at all times postpartum, although differences were significant only at 12 months (Table 1; Figure 1C).
Of 271 women enrolled in Burkina Faso or South Africa in the triple ARV arm, 152 had specimens collected within 6 weeks of ARV cessation; 72 could be sequenced, and 1 resistance mutation was detected (1.4%) (Table 2). Of 75 specimens from 264 women exposed to AZT/sdNVP, 41 could be sequenced; nonnucleoside reverse transcriptase inhibitor (NNRTI) resistance mutations were found in 7 of 29 women (24.1%) who stopped ARVs at delivery and 0 of 12 women who received 1 week of AZT + 3TC after delivery.
Table 2.
Comparison of Genotypic Drug Resistance Between Study Arms
| Mothers | Triple ARV | AZT/sd-NVP |
|---|---|---|
| Total enrolled in Burkina and South-Africa | 271 | 264 |
| Sample available <6 weeks after cessation of ARV | 152 | 75 controls |
| VL >1000 copies/mL | 90 | 48 |
| Could be sequenced | 72 | 41 |
| Total with resistance | 1/72 | 7/41a |
| NNRTI resistance | K101E | K103N: n = 3; V106L: n = 3; Y188C: n = 1 |
Abbreviations: ARV, antiretroviral; AZT/sdNVP, zidovudine until delivery with single-dose nevirapine without postpartum prophylaxis; NNRTI, nonnucleoside reverse transcriptase inhibitor; Triple ARV, zidovudine, lamivudine, and lopinavir/ritonavir during pregnancy and breastfeeding; VL, viral load.
a Zero of 12 women who received an AZT/3TC tail for 1 week after delivery; 7 out of 29 women who received no tail.
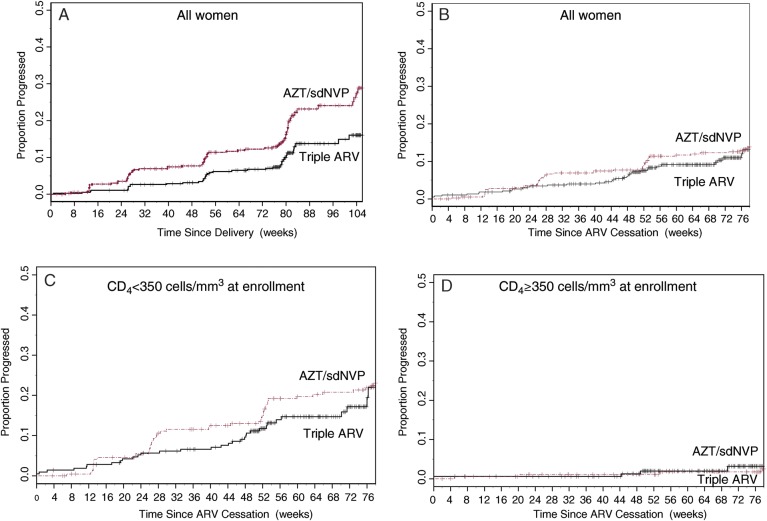
The numbers of progression-defining events are shown in Table 3. The rates of progression to either death, stage 4 disease, or a CD4+ count of <200 cells/mm3 are given in Table 4 and Figure 2. The rate of progression up to 24 months after delivery was significantly lower in the triple ARV arm than in the Abbreviations: AZT/sdNVP arm (44.5% reduction; P = .001), but the rates of progression did not differ when cessation of ARV prophylaxis was taken as the date of origin rather than the date of delivery.
Table 3.
Maternal HIV Disease Progression–Classifying Events Occurring up to 2 Years After Deliverya
| Events | Triple ARV, No. (%) [cumulative] | AZT/sdNVP, No. (%) [cumulative] |
|---|---|---|
| All women | n = 384 | n = 405 |
| Death | 2 (0.5) [2] | 5 (1.2) [5] |
| WHO stage 4 disease | 2 (0.5) [4] | 7 (1.7) [11] |
| At least 1 CD4+ count of <200 cells/mm3 | 39 (10.2) [40] | 68 (16.8) [71] |
| Started on ART (whether or not having reached >1 of the above endpoints) | 39 (10.2) [55] | 65 (16.1) [88] |
| Women with CD4+ count of ≥350 cells/mm3 at entry | n = 169 | n = 181 |
| Death | 1 (0.6) [1] | 0 (0.0) [0] |
| WHO stage 4 disease | 0 (0.0) [1] | 2 (1.1) [2] |
| WHO stage 3 disease | 11 (6.5) [12] | 15 (8.3) [15] |
| At least 1 CD4+ count of <350 cells/mm3 | 43 (25.4) [49] | 62 (34.3) [65] |
| Started on ART (whether or not having reached one or more of the above endpoints) | 7 (4.1) [49] | 8 (4.4) [65] |
Abbreviations: ART, antiretroviral therapy; AZT/sdNVP, zidovudine until delivery with single-dose nevirapine without postpartum prophylaxis; HIV-1, human immunodeficiency virus type 1; Triple ARV, zidovudine, lamivudine, and lopinavir/ritonavir during pregnancy and breastfeeding; WHO, World Health Organization.
aOne woman could have >1 event.
Table 4.
Cumulative Rates of Progression
| Triple ARV From Delivery |
Triple ARV From ARV Cessation |
AZT/sdNVP |
|||||
|---|---|---|---|---|---|---|---|
| Endpoint | Time (weeks) | Events/At Riska (cumulative) | Rate (95% CI) | Events/At Riska (cumulative) | Rate (95% CI) | Events/At Riska (cumulative) | Rate (95% CI) |
| Among All Women | |||||||
| Death or WHO stage 4 | 0 | 0/384 | 0.0 | 0/384 | 0.0 | 0/405 | 0.0 |
| 26 | 0/381 | 0.0 | 0/368 | 0.0 | 3/381 | 0.8 (.2–2.3) | |
| 52 | 2/359 | 0.6 (.1–2.2) | 3/270 | 0.9 (.3–2.7) | 8/332 | 2.1 (1.1–4.2) | |
| 78 | 3/278 | 0.8 (.3–2.6) | 4/68 | 2.3 (.6–8.3) | 10/273 | 2.8 (1.5–5.1) | |
| 104 | 4/69 | 2.1 (.6–7.3) | NA | NA | 11/69 | 3.5 (1.8–6.4) | |
| Log-rank P value (triple ARV vs AZT/sdNVP) stratified for intent to BF and study site | .07 | .24 | |||||
| Death, WHO stage 4, or CD4+ count of <200/mm3 | 0 | 0/384 | 0.0 | 0/382 | 0.0 | 0/405 | 0.0 |
| 26 | 8/378 | 2.1 (1.0–4.1) | 11/358 | 2.9 (1.6–5.2) | 20/374 | 5.1 (3.3–7.8) | |
| 52 | 17/355 | 4.5 (2.8–7.2) | 25/265 | 7.0 (4.8–10.2) | 37/330 | 9.7 (7.1–13.1) | |
| 78 | 29/275 | 8.0 (5.6–11.4) | 36/66 | 15.0 (10.3–21.6) | 51/265 | 13.8 (10.7–17.8) | |
| 104 | 40/66 | 15.7 (11.1–21.9) | NA | NA | 71/65 | 28.3 (22.0–35.8) | |
| Log-rank P value (triple-ARV vs AZT/sdNVP) stratified for intent to BF and study site | .001 | .49 | |||||
| Triple-ARV |
AZT/sdNVP |
||||||
|---|---|---|---|---|---|---|---|
| Endpoint | Time (weeks) | Events/At Riska (cumulative) | Rate (95% CI) | Events/At Riska (cumulative) | Rate (95% CI) | Log-Rank P Value Stratified for Intent to BF and Study Site |
|
| According to CD4+ Cell Count at Entry (origin: cessation of ARVs) | |||||||
| CD4+ count <350 cells/mm3 | |||||||
| Death, WHO stage 4, or CD4+ count of <200 cells/mm3 | 0 | 0/214 | 0.0 | 0/224 | 0.0 | ||
| 26 | 11/197 | 5.2 (2.9–9.2) | 18/203 | 8.3 (5.3–12.8) | |||
| 52 | 23/145 | 11.3 (7.6–16.5) | 35/169 | 16.6 (12.2–22.4) | |||
| 78 | 32/25 | 24.0 (15.8–35.5) | 47/131 | 23.0 (17.8–29.5) | .24 | ||
| CD4+ count of ≥350 cells/mm3 | |||||||
| Death, WHO stage 4, or CD4+ count of <200 cells/mm3 | 0 | 0/168 | 0.0 | 0/181 | 0.0 | ||
| 26 | 0/161 | 0.0 | 2/171 | 1.1 (.3–4.4) | |||
| 52 | 2/120 | 1.4 (.4–5.5) | 2/161 | 1.1 (.3–4.4) | |||
| 78 | 4/41 | 4.9 (1.6–14.5) | 4/134 | 2.5 (.9–6.4) | .47 | ||
| Triple ARV |
AZT/sdNVP |
||||||
|---|---|---|---|---|---|---|---|
| Endpoint | Time (weeks) | Events/At Riska (cumulative) | Rate (95% CI) | Events/At Riska (cumulative) | Rate (95% CI) | Log-Rank P Value Stratified for Intent to BF and Study Site |
|
| Among Women with CD4+ Count of ≥350 cells/mm3 at Entry (endpoint: 2010 criteria for ART; origin: cessation of ARVs) | |||||||
| Death, WHO stage 3 or 4, or CD4+ count of <350 cells/mm3 | 0 | 0/156 | 0.0 | 0/173 | 0.0 | ||
| 26 | 9/144 | 5.9 (3.1–11.0) | 21/148 | 12.5 (8.3–18.5) | |||
| 52 | 26/95 | 17.8 (12.5–25.1) | 34/132 | 20.5 (15.1–27.5) | |||
| 78 | 36/29 | 31.8 (23. 2–42.6) | 45/100 | 28.1 (21.8–35.8) | .95 | ||
Abbreviations: ART, antiretroviral therapy; ARV, antiretroviral; AZT/sdNVP, zidovudine until delivery with single-dose nevirapine without postpartum prophylaxis; BF, breastfeed; CI, confidence interval; NA, not applicable; Triple ARV, zidovudine, lamivudine, and lopinavir/ritonavir during pregnancy and breastfeeding; WHO, World Health Organization.
a Cumulative number of events up to the given time / number of women still at risk of progression at the given time.
Figure 2.
Rates of progression to death, World Health Organization stage 4 disease, or at least 1 CD4+ count of <200 cells/mm3 for all women from delivery (A), all women from cessation of ARV prophylaxis (B), women with CD4+ count of <350 cells/mm3 at enrollment (from cessation of prophylaxis) (C), and women with CD4+ count of >350 cells/mm3 at enrollment (from cessation of prophylaxis (D). Abbreviations: AZT/sdNVP, zidovudine until delivery with single-dose nevirapine without postpartum prophylaxis; Triple ARV, zidovudine, lamivudine, and lopinavir/ritonavir during pregnancy and breastfeeding.
When the analysis was restricted to women with a CD4+ count of 200–350 cells/mm3 at enrollment, clinical or immunological progression 18 months after cessation of ARV prophylaxis reached 24.0% (95% confidence interval [CI],: 15.7–35.5) in the triple ARV arm and 23.0% (95% CI, 17.8–29.5) in the AZT/sdNVP arm (P = .24). In contrast, only a small number of women (4 in each arm) with an initial CD4+ count of ≥350 cells/mm3 progressed to death, stage 4 disease, or at least 1 CD4+ count of <200 cells/mm3 within 18 months of stopping prophylaxis. Among women with a CD4+ count of ≥350 cells/mm3 at enrollment, rates of progression (to death, stage 3 or 4 disease, or a CD4+ count of <350 cells/mm3) 18 months after cessation of ARV prophylaxis were 31.8% (95% CI, 23.2–42.6) and 28.1% (95% CI, 21.8–55.8) in the AZT/sdNVP and triple ARV arms, respectively (P = .96).
Factors associated with HIV-1 progression within 18 months after cessation of ARV prophylaxis in univariate and multivariate analyses (Table 5) included duration of receipt of ARVs after delivery (the longer the ARV prophylaxis, the shorter the duration of follow-up after cessation of prophylaxis; thus the probability of progression during follow-up was reduced); older age, and lower CD4 count and higher VL at enrollment (all well-known risk factors for HIV-1 disease progression). Factors associated with progression in univariate but not in multivariate analysis included gravidity (association explained by older maternal age among multigravida women) and never breastfeeding (association explained by a shorter duration of prophylaxis in nonbreastfeeders, in whom prophylaxis stopped soon after delivery). Prophylaxis arm was not associated with progression during the 18 months after cessation of ARV prophylaxis, both in univariate and multivariate analyses.
Table 5.
Factors Associated With Progression to Death, World Health Organization Stage 4 Disease, or at Least 1 CD4+ Count of <200 cells/mm3 Within 18 Months of Cessation of Antiretroviral Prophylaxis
| n/N (%) | OR (95% CI) | P Value | Adjusted ORa (95% CI) | P Valuea | |
|---|---|---|---|---|---|
| Study site | .58 | ||||
| Bobo Dioulasso, Burkina Faso | 27/236 (11.4) | 2.07 (.62–6.89) | |||
| Kwadabeka, South-Africa | 23/182 (12.6) | 1.37 (.57–3.43) | |||
| Mombasa, Kenya | 22/233 (9.4) | 1.11 (.45–2.83) | |||
| Nairobi, Kenya | 7/43 (16.3) | 1.54 (.62–3.92) | |||
| Somkhele, South-Africa | 8/93 (8.6) | 1.00 | |||
| Treatment arm | .17 | ||||
| Triple ARV | 36/382 (9.4) | 1.00 | |||
| ZDV/sdNVP | 51/405 (12.6) | 1.38 (.86–2.23) | |||
| Age, years | .05 | .08 | |||
| <25 | 18/252 (7.1) | 1.00 | 1.00 | ||
| 25–34 | 58/459 (12.6) | 1.88 (1.05–3.40) | 1.85 (1.04–3.29) | ||
| ≥35 | 11/76 (14.5) | 2.20 (.92–5.21) | 2.03 (.87–4.73) | ||
| Education | .58 | ||||
| Never attended school | 16/116 (13.8) | 1.32 (.68–2.54) | |||
| Completed primary school | 28/272 (10.3) | 0.95 (.56–1.62) | |||
| At least some secondary school education | 43/399 (10.8) | 1.00 | |||
| Occupation | .40 | ||||
| Unemployed | 58/550 (10.5) | 1.14 (.52–2.56) | |||
| Self-employed | 20/141 (14.2) | 1.60 (.65–4.00) | |||
| Salaried job | 9/96 (9.4) | 1.00 | |||
| Marital status | .12 | ||||
| Married, monogamous | 35/333 (10.5) | 1.00 | |||
| Married, polygamous | 13/91 (14.3) | 1.42 (.68–2.94) | |||
| Not married, regular partner | 32/332 (9.6) | 0.91 (.53–1.55) | |||
| Single | 7/31 (22.6) | 2.48 (.90–6.63) | |||
| Gravidity | .02 | ||||
| Multigravida | 80/651 (12.3) | 2.58 (1.12–6.26) | |||
| Primigravida | 7/136 (5.1) | 1.00 | |||
| Socioeconomic score | |||||
| 1st quintile | 19/151 (12.6) | 1.17 (.55–2.48) | .88 | ||
| 2nd quintile | 19/155 (12.3) | 1.13 (.54–2.40) | |||
| 3rd quintile | 15/161 (9.3) | 0.83 (.38–1.84) | |||
| 4th quintile | 17/165 (10.3) | 0.93 (.43–2.01) | |||
| 5th quintile | 17/155 (11.0) | 1.00 | |||
| Breastfeeding (BF) | .01 | ||||
| Never BF | 31/188 (16.5) | 1.91 (1.16–3.15) | |||
| Ever BF | 56/599 (9.3) | 1.00 | |||
| BMI at delivery, kg/mm2 | .28 | ||||
| <22 | 27/190 (14.2) | 1.53 (.79–3.00) | |||
| 22–27 | 41/402 (10.2) | 1.05 (.57–1.94) | |||
| >27 | 19/195 (9.7) | 1.00 | |||
| CD4+ count at enrollment, cells/mm3 | <.001 | <.001 | |||
| <350 | 79/438 (18.0) | 9.38 (4.30–21.29) | 8.23 (3.89–17.43) | ||
| ≥350 | 8/349 (2.3) | 1.00 | 1.00 | ||
| VL at enrollment, log10 copies/mL | <.001 | .004 | |||
| <3.5 | 8/162 (4.9) | 1.00 | 1.00 | ||
| 3.5–3.9 | 10/146 (6.8) | 1.42 (.50–4.07) | 1.31 (.49–3.52) | ||
| 4.0–4.4 | 21/191 (11.0) | 2.38 (.97–6.03) | 2.19 (.92–5.25) | ||
| 4.5–4.9 | 20/158 (12.7) | 2.79 (1.12–7.15) | 2.27 (.94–5.48) | ||
| ≥5 | 28/126 (22.2) | 5.50 (2.28–13.71) | 4.32 (1.83–10.21) | ||
| Missing | 0/4 (0) | ||||
| Duration of ARV use, weeks | |||||
| From enrollment until delivery | .13 | ||||
| ≤4 | 31/213 (14.6) | 1.00 | |||
| 4–8 | 38/365 (10.4) | 0.68 (.40–1.17) | |||
| >8 | 18/209 (8.6) | 0.55 (0.29–1.06) | |||
| After delivery | .08 | .03 | |||
| <6 | 64/501 (12.8) | 1.00 | 1.00 | ||
| 6–24 | 13/131 (9.9) | 0.75 (.38–1.46) | 0.88 (.45–1.72) | ||
| ≥25 | 10/155 (6.5) | 0.47 (.22–.98) | (0.20–.82) |
Abbreviations: ARV, antiretroviral; AZT/sdNVP, zidovudine until delivery with single-dose nevirapine without postpartum prophylaxis; BMI, body mass index; CI, confidence interval; OR, odds ratio; Triple ARV, zidovudine, lamivudine, and lopinavir/ritonavir during pregnancy and breastfeeding; VL, viral load.
a Only for variables included in the model.
DISCUSSION
Prior report from the Kesho Bora study demonstrated that triple ARV prophylaxis was efficacious in preventing MTCT during pregnancy, delivery, and breastfeeding without evidence of excess serious adverse events in the first year after delivery [2]. However, there were concerns that the cessation of a triple ARV regimen after administration for as long as 10 months (from 28 weeks gestation through 6 months postpartum) could lead to a sustained VL rebound, enhanced CD4+ cell count decline, or faster progression of HIV disease. We now present evidence that progression in women is similar after interruption of a triple ARV to that after receipt of an AZT/sdNVP regimen.
Kesho Bora is 1 of 2 randomized controlled trials [9] comparing AZT/sdNVP regimen (not expected to have an impact on the course of HIV disease) to a longer triple ARV regimen (up to 10 months), which, given the duration, could have an impact not only on the prevention of MTCT but also on disease progression in the mother. The study offered a unique opportunity to measure the effect, if any, of interrupting a long triple ARV prophylaxis regimen in comparison with an AZT/sdNVP prophylaxis regimen stopping around delivery.
Questions regarding the potential effect of time-limited triple ARV prophylaxis on maternal health were raised in 2006 when results of 3 trials comparing the outcomes of continuous ART with different strategies for interrupting and restarting treatment (in an attempt to decrease costs and toxicity of ART) in nonpregnant, treatment-eligible adults were published. These three trials (SMART [3], Trivacan [4], and DART [5]) were prematurely halted following interim analyses that showed a higher incidence of severe diseases (mainly opportunistic infections) in patients receiving interrupted treatment compared with those receiving continuous treatment.
In the treatment interruption trials, only participants with advanced HIV disease requiring long-term treatment were enrolled. In the context of MTCT prevention, all women with advanced HIV disease should start and continue ART for life, as per national and international guideline [7]. A triple ARV MTCT prophylaxis regimen (as per the Kesho Bora study protocol) is therefore only given to women who do not yet require treatment, with the sole objective to reduce MTCT risk during pregnancy, delivery, and breastfeeding, and thus the regimen is given only for a limited time. Because the stage of HIV disease is different in women receiving ART versus MTCT prophylaxis, it is not possible to extrapolate from treatment interruption trials to the use of triple ARV regimens for MTCT prophylaxis.
With the exception of the frequent emergence of maternal resistance to NVP after MTCT prophylaxis consisting of a single-dose of NVP (which is markedly diminished with receipt of the AZT/3TC “tail”), there have been no worrying reports to date from long-term monitoring of women who received short-course ARV regimens for MTCT prevention. Women from the PACTG 076 study were followed for >4 years postpartum, and no difference was observed between AZT and placebo in CD4+ cells counts, VL, resistance, or clinical progression [10]. Several studies found no difference in progression to AIDS in women who are pregnant (and receiving ARV prophylaxis, mostly AZT alone) vs not pregnant after adjusting for CD4+ cell counts and ART [11–13]. Women from the PACTG 185 study (AZT prophylaxis for 86% of women) were followed to 18 months postpartum, and CD4+ cell counts and VL trajectories were the same regardless of whether the women continued or discontinued ARVs—all women showed a slight increase in VLs postpartum [14, 15]. Similar data showing an increase of postpartum VL were reported in the pre-ARV era [16]. In the Women and Infants Transmission Study (WITS), women (two-thirds on AZT alone prophylaxis) who had an index pregnancy and no subsequent pregnancies had a similar disease progression rate to women who had a subsequent pregnancy with ARV prophylaxis [17]. These available data do not indicate that ARV use (especially mono- or dual prophylaxis) during pregnancy and discontinued soon after delivery before subsequently initiating therapy when required for a woman's own health is associated with adverse outcomes, but there has been little study of the effect of triple ARV MTCT prophylaxis used for extended periods after delivery (up to 6 months of breastfeeding) and then stopped.
Our results are reassuring regarding the risk of interrupting triple ARV MTCT prophylaxis, although there was no group with continuous ART to compare with, which was the case in treatment interruption trials. Our follow-up to 18–24 months after delivery (12–18 months after triple ARV MTCT prophylaxis has stopped) may have been somewhat short to detect adverse effects of ARV interruption. However, the only other MTCT trial assessing triple ARV prophylaxis continued during breastfeeding vs prophylaxis stopping around delivery had a follow-up restricted to 48 weeks after delivery [9].
Our analyses also suggest that the triple ARV prophylaxis is not only safe in terms of HIV progression but may also be beneficial for maternal health during the period the mother is receiving the regimen—especially if she had a CD4+ count of <350/mm3. The risk of HIV progression seems to be delayed until cessation of the prophylaxis.
The high rate of progression observed in women with CD4+ counts of 200–350 cells/mm3 (25%–30%) is comparable with the rate observed in Haitian women with CD4+ counts in the same range. In Haiti, 40% progressed to death, stage 4 disease, or a CD4+ count of <200 cells/mm3 after a follow-up of 21 months [18]. These observations support the 2010 change in WHO ART guidelines [19] to consider all HIV-infected patients with CD4+ counts of <350 cells/mm3 as eligible for ART. In addition to preserving maternal health, this change has the potential to prevent the vast majority of cases of MTCT of HIV. Indeed, data from Zambia indicate that 92% of maternal deaths and 88% of perinatal or postnatal transmissions occurred among the 68% of women who would have met the new criteria for ART [20]. In contrast, the rate of progression in women with CD4+ counts of 350–500 cells/mm3 was low after cessation of ARVs, supporting current WHO recommendations that triple ARV prophylaxis can be interrupted after breastfeeding cessation, with initiation of ART when the woman meets criteria for initiation of therapy. However, it is important to note that 25% of such women did reach the current CD4+ threshold for treatment within 2 years postpartum. Antepartum care should be viewed as an opportunity to bring women into care for their own health, and assurance of careful and close follow-up of women after giving birth and maintenance of their retention in care is critical to maximize maternal (and child) health.
Other risks of triple ARV prophylaxis include the emergence of HIV resistance against ARVs used for prophylaxis and potential effects on infant health. An analysis of HIV drug resistance evaluating resistance within 6 weeks of cessation of ARVs detected a rate of NNRTI resistance of 24% in women exposed to AZT/sdNVP prophylaxis without the 1 week postpartum AZT/3TC tail and 0 of 12 in women exposed to AZT/sdNVP with the AZT/3TC tail; 1 resistance mutation was detected in 72 specimens sequenced from women exposed to triple ARV (1.4%), suggesting a relatively low risk of resistance emergence in mothers who received the triple ARV prophylaxis.
Notes
Financial support. Financial support was provided by Agence Nationale de Recherche sur le Sida et les hépatites virales; Department for International Development; European and Developing Countries Clinical Trials Partnership; Thrasher Research Fund; Belgian Directorate General for International Cooperation; US Centers for Disease Control and Prevention; Eunice Kennedy Shriver National Institute of Child Health and Human Development; and UNDP/UNFPA/World Bank/WHO Special Programme of Research, Development and Research Training in Human Reproduction.
Potential conflicts of interest All authors: No reported conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Appendix
Contributors
Isabelle de Vincenzi was the overall study coordinator and wrote the manuscript with support from Tim Farley, Marie-Louise Newell, Jennifer Read, Philippe Van de Perre, Stanley Luchters and Lynne Mofenson.
The Kesho Bora Study Group
Study Sites
(1) Bobo Dioulasso, Burkina Faso (Centre Muraz): Nicolas Meda (Principal Investigator), Paulin Fao, Odette Ky-Zerbo, and Clarisse Gouem (study coordinators); Paulin Somda, Hervé Hien, Patrice Elysée Ouedraogo, Dramane Kania, Armande Sanou, Ida Ayassou Kossiwavi, Bintou Sanogo, Moussa Ouedraogo, and Issa Siribie (investigators); Diane Valéa (laboratory coordinator), Sayouba Ouedraogo and Roseline Somé (data managers); and François Rouet (inter-site laboratory coordination).
(2) Durban, South Africa (University of KwaZulu Natal): Nigel Rollins (principal investigator); and Lynne McFetridge and Kevi Naidu (study coordinators).
(3) Mombasa, Kenya (International Centre for Reproductive Health): Stanley Luchters and Marcel Reyners (principal investigators); Eunice Irungu (study coordinator); Christine Katingima, Mary Mwaura, and Gina Ouattara (investigators); Kishor Mandaliya and Sammy Wambua (laboratory coordinators); and Mary Thiongo (data manager).
(4) Nairobi, Kenya (Network for AIDS Researchers in East and Southern Africa): Ruth Nduati (principal investigator); Judith Kose (study coordinator); Ephantus Njagi (laboratory coordinator); and Peter Mwaura (data manager).
(5) Somkhele, South Africa (Africa Centre for Health and Population Studies, University of KwaZulu Natal): Marie-Louise Newell (principal investigator); Stephen Mepham (study coordinator); Johannes Viljoen (laboratory coordinator); Ruth Bland (investigator); and Londiwe Mthethwa (data manager).
Supporting Institutions
(1) Agence Nationale de Recherches sur les SIDA et les Hépatites Virales, France: Brigitte Bazin and Claire Rekacewicz (Sponsor Representatives).
(2) Centers for Disease Control and Prevention, Atlanta, Georgia: Allan Taylor (sponsor representative and coinvestigator); and Nicole Flowers, Michael Thigpen, Mary Glenn Fowler and Denise Jamieson (coinvestigators).
(3) Eunice Kennedy Shriver National Institute of Child Health and Human Development, National Institutes of Health, Bethesda, Maryland: Lynne M. Mofenson (sponsor representative); and Jennifer S. Read (coinvestigator).
(4) Institut de Recherche pour le Développement (IRD), Montpellier, France: Kirsten Bork, Cécile Cames, and Amandine Cournil (nutrition coordination).
(5) International Centre for Reproductive Health (ICRH), Ghent University, Ghent, Belgium: Patricia Claeys, Marleen Temmerman, and Stanley Luchters (sponsor representatives).
(6) Université Montpellier 1, EA 4205 “Transmission, Pathogenèse et Prévention de l'infection par le VIH”; and CHU Montpellier, Laboratoire de Bactériologie-Virologie, Montpellier, France: Philippe Van de Perre, Pierre Becquart (until December 2006), Vincent Foulongne, and Michel Segondy (laboratory coordination).
Study Coordination
World Health Organization, Geneva, Switzerland: Isabelle de Vincenzi (study coordinator); Philippe Gaillard (site coordinator); Tim Farley (project manager); Ndema Habib (study statistician); and Sihem Landoulsi (study analyst).
Funding
The Bobo-Dioulasso site was funded by l'Agence Nationale de Recherches sur le Sida et les Hépatites Virales (ANRS) and UNDP/UNFPA/World Bank/WHO Special Programme of Research, Development and Research Training in Human Reproduction (WHO/HRP),
The Mombasa site was funded by ANRS, WHO/HRP, European and Developing Countries Clinical Trials Partnership (EDCTP), Thrasher Research Fund, and Belgian Directorate General for International Cooperation.
The Nairobi site was funded by the Centers for Disease Control and Prevention (CDC) and the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) through a cooperative agreement,
The South-African sites were funded by the Department for International Development (DFID), EDCTP, UNICEF, and WHO/HRP.
The Nutrition and laboratory coordination were funded by ANRS.
The overall coordination and external monitoring was funded by WHO/HRP.
Representatives of ANRS, CDC, NICHD, and WHO/HRP were involved in study design and collection, analysis, and interpretation of data.
The findings and conclusions in this report are those of the authors and do not necessarily represent the views of the World Health Organization, the Centers for Disease Control and Prevention, or the National Institutes of Health
References
- 1.The Kesho Bora Study Group. Safety and effectiveness of antiretroviral drugs during pregnancy, delivery and breastfeeding for prevention of mother-to-child transmission of HIV-1: the Kesho Bora Multicentre Collaborative Study rationale, design, and implementation challenges. Contemp Clin Trials. 2011;1:74–85. doi: 10.1016/j.cct.2010.09.008. [DOI] [PubMed] [Google Scholar]
- 2.The Kesho Bora Study Group. Triple antiretroviral compared with zidovudine and single-dose nevirapine prophylaxis during pregnancy and breastfeeding for prevention of mother-to-child transmission of HIV-1 (Kesho Bora study): a randomised controlled trial. Lancet Infect Dis. 2011;11:171–80. doi: 10.1016/S1473-3099(10)70288-7. [DOI] [PubMed] [Google Scholar]
- 3.The Strategies for Management of Antiretroviral Therapy (SMART) Study Group. CD4+ count-guided interruption of antiretroviral treatment. N Engl J Med. 2006;355:2283–96. doi: 10.1056/NEJMoa062360. [DOI] [PubMed] [Google Scholar]
- 4.Danel C, Moh R, Minga A, et al. for the Trivacan ANRS 1269 trial group. CD4-guided structured antiretroviral treatment interruption strategy in HIV-infected adults in west Africa (Trivacan ANRS 1269 trial): a randomised trial. Lancet. 2006;367:1981–9. doi: 10.1016/S0140-6736(06)68887-9. [DOI] [PubMed] [Google Scholar]
- 5.DART Trial Team. Routine versus clinically driven laboratory monitoring of HIV antiretroviral therapy in Africa (DART): a randomised non-inferiority trial. Lancet. 2010;375:123–31. doi: 10.1016/S0140-6736(09)62067-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Division of AIDS tables for grading severity of adverse experiences. 1992. Available at: http://rsc.tech-res.com/safetyandpharmacovigilance/gradingtables.aspx. Accessed 20 December 2011. [Google Scholar]
- 7.World Health Organization. Antiretroviral drugs for treating pregnant women and preventing HIV infection in infants: Towards universal access. World Health Organization, 2006; Available at: http://www.who.int/hiv/pub/mtct/antiretroviral/en/index.html . Accessed 20 December 2011. [Google Scholar]
- 8.Rouet F, Foulongne V, Viljoen J, et al. Comparison of the generic HIV viral load assay with the Amplicor HIV-1 Monitor v1.5 and Nuclisens HIV-1 EasyQ v1.2 techniques for plasma HIV-1 RNA quantification of non-B subtypes. The Kesho Bora Preparatory Study. J Virol Methods. 2010;163:253–7. doi: 10.1016/j.jviromet.2009.10.005. [DOI] [PubMed] [Google Scholar]
- 9.Chasela C, Hudgens M, Jamieson D, et al. Maternal or infant antiretroviral drugs to reduce HIV-1 transmission. N Engl J Med. 2010;362:2271–81. doi: 10.1056/NEJMoa0911486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bardeguez A, Shapiro D, Mofenson L, et al. Effect of cessation of zidovudine prophylaxis to reduce vertical transmission on maternal HIV disease progression and survival. J Acquir Immune Defic Syndr. 2003;32:170–81. doi: 10.1097/00126334-200302010-00009. [DOI] [PubMed] [Google Scholar]
- 11.Saada M, Le Chenadec J, Berrebi A, et al. Pregnancy and progression to AIDS: results of the French prospective cohorts. SEROGEST and SEROCO Study Groups. AIDS. 2000;14:2355–60. doi: 10.1097/00002030-200010200-00017. [DOI] [PubMed] [Google Scholar]
- 12.Buskin SE, Diamond C, Hopkins SG. HIV-infected pregnant women and progression of HIV disease. Arch Intern Med. 1998;158:1277–8. doi: 10.1001/archinte.158.11.1277. [DOI] [PubMed] [Google Scholar]
- 13.Bessinger R, Clark R, Kissinger K, et al. Pregnancy is not associated with the progression of HIV disease in women attending an HIV outpatient program. Am J Epidemiol. 1998;147:434–40. doi: 10.1093/oxfordjournals.aje.a009468. [DOI] [PubMed] [Google Scholar]
- 14.Watts H, Lambert J, Stiehm R, et al. Progression of HIV disease among women following delivery. J Acquir Immune Defic Syndr. 2003;33:585–93. doi: 10.1097/00126334-200308150-00006. [DOI] [PubMed] [Google Scholar]
- 15.Watts DH, Lu M, Thompson B, et al. Treatment interruption after pregnancy: effects on disease progression and laboratory findings. Infect Dis Obstet Gyneco. 2009 doi: 10.1155/2009/456717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Burns DN, Landesman S, Minkoff H, et al. The influence of pregnancy on human immunodeficiency virus type 1 infection: antepartum and postpartum changes in human immunodeficiency virus type 1 viral load. Am J Obstet Gynecol. 1998;8:355–9. doi: 10.1016/s0002-9378(98)80025-2. [DOI] [PubMed] [Google Scholar]
- 17.Minkoff H, Hershow R, Watts H, et al. The relationship of pregnancy to human immunodeficiency virus disease progression. Am J Obstet Gynecol. 2003;189:552–9. doi: 10.1067/s0002-9378(03)00467-8. [DOI] [PubMed] [Google Scholar]
- 18.Severe P, Jean Juste MA, Ambroise A, et al. Early versus standard ART for HIV-infected adults in Haiti. N Engl J Med. 2010;363:257–65. doi: 10.1056/NEJMoa0910370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.World Health Organization. Antiretroviral drugs for treating pregnant women and preventing HIV infection in infants: Towards universal access. World Health Organization, 2010; Available at: http://www.who.int/hiv/pub/mtct/antiretroviral2010/en/index.html . Accessed 20 December 2011. [PubMed] [Google Scholar]
- 20.Kuhn L, Aldrovandi G, Sinkala M, et al. Potential impact of new World Health Organization criteria for antiretroviral treatment for prevention of mother to child HIV transmission. AIDS. 2010;24:1374–7. [PMC free article] [PubMed] [Google Scholar]