Abstract
Dendritic cells (DCs) regulate T cell function by promoting either tolerance or activation, and in the latter case, by directing response quality. New imaging tools now permit direct visualization of the relevant DC-T cell interactions in vivo and have provided a new perspective on the dynamics of these crucial cellular contacts. Here we discuss the insights generated by these analyses and the controversies/unanswered questions that need to be addressed in future work.
Introduction
1. Progressive dynamic changes in DC-T interactions during early immune responses
Proper functioning of the immune system is clearly dependent on the correct localization of cells within and outside of lymphoid tissues, on the trafficking of cells between distinct tissue sites, and on the physical interaction of various myeloid and lymphoid cell subpopulations, accompanied by exchange of information through local cytokine secretion and direct membrane molecule binding events. All of these are dynamic events that occur over time spans ranging from seconds to days. Collecting quantitative information on these attributes of the immune system has been a problem for the field until recently due to the lack of suitable tools for conducting spatio-temporal studies with adequate resolution in vivo.
With respect to DCs, for the first 30 years after their discovery [1], examination of their interaction with T cells was confined to static immunohistochemical studies and in vitro time-lapse imaging. Classic in vitro experiments with guinea pig cells indicated that antigen-unspecific interactions a few minutes but that associations between antigen-specific T cells and lasted for antigen-bearing DCs could last for hours [2], but the relevance of these observations to in vivobiology remained unknown.
This situation changed dramatically a decade ago with the first application of advanced microscopy tools (confocal and especially 2-photon methods) to the direct dynamic visualization of DCs and T cells in lymph nodes. Initial work with LN explants and later studies using intravital imaging approaches revealed that antigen-specific interactions between T cells and DCs occur in several distinct phases [3-5], (Figure 1). First, T cells scan DCs for relevant antigenic epitopes. When they have found an antigen-bearing DC they form long-lasting and stable interactions that often persist for several hours, in agreement with the guinea pig results. There is some evidence that T cells can integrate suboptimal signals before passing a threshold that leads to prolonged stopping and DC contact, whereas with optimally activated DCs and high levels of strong agonist ligands, T cells stop and engage the first antigen-bearing DC they encounter [6]. Irrespective of whether there is a transient interaction phase preceding the stable contact period, all studies suggest that after 24-30 hours of interaction, the T cells disengage, often in concert with the onset of proliferation [3-5, 7], followed by rapid migration within the LN and transient interactions with DCs. CD4+ T cells in particular seem to require a prolonged phase of stable interactions with DCs for full activation [8]. How these progressive changes in the dynamics of T-DC association play out in situations in which the interaction leads to tolerance rather than immunity is an area of controversy. Using DEC205-OVA for antigen delivery one study suggested that CD8+ T-cells fail to establish long-lasting interactions with DC during tolerogenic conditions whereas others using the same antigen delivery CD4+ T cells showed similar stable long-lasting interactions of priming and tolerance but these interactions were further prolonged (>) during priming [9, 10].
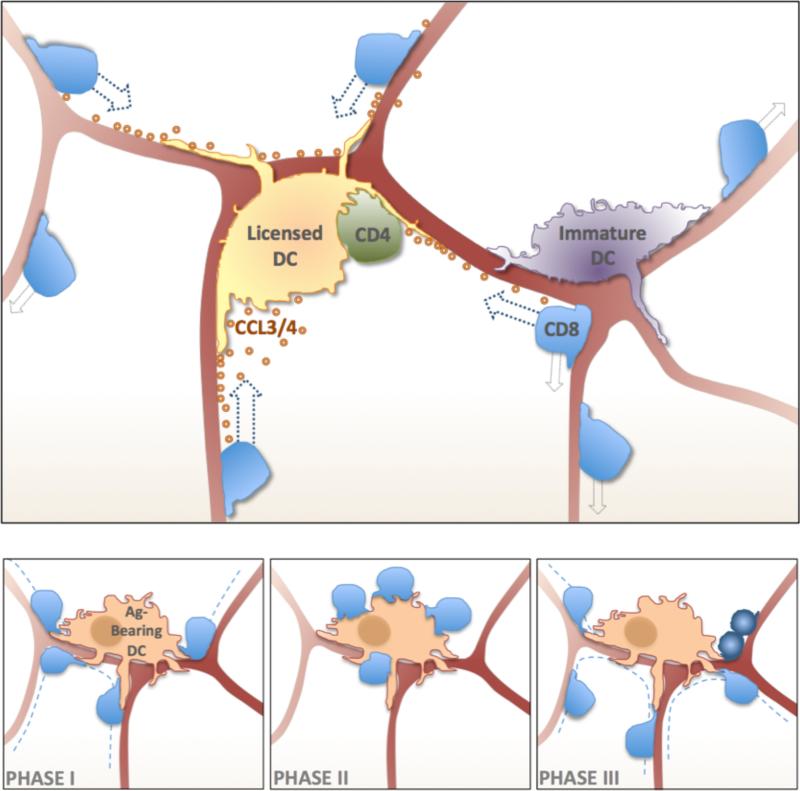
Figure 1. Dynamics of T cell DC Interactions in Lymphoid Tissues.
Upper Panel - T cells and DCs migrate or are positioned on the FRC network (burgundy interconnected lines), respectively. Antigen-reactive CD4 T cells cooperate with the DCs to produce CCL3/4 chemokines that cause directed chemotactic migration of CD8 T cells that have expressed CCR5 under the influence of inflammatory factors in the lymph node (random walk migration – light-grey arrows; chemotactic migration - dark-blue arrows). Lower Panels – T cells undergo three phases of interactions with Ag-bearing DCs. During Phase-I T cells migrate (dashed lines) searching for Ag- bearing DCs and undergo short-term interactions (left panel). Long-term stable contacts are formed during Phase-II (middle panel). Activated T cells resume rapid migration and undergo cellular division (dark-blue cells) during Phase-III (right panel).
Interestingly, T cells are not the only lymphocytes that scan DCs for antigen shortly after entering the LN parenchyma from the HEVs. The application of intravital 2P imaging allowed direct tracking of B cells as they emerged from HEV, revealing that naive B cells also probe DCs on their way to the follicle. If they encounter antigen, they can form functional, activation-promoting associations that last many times longer than the few minutes seen for antigen-unspecific contacts between these cell types [11].
2.How to find the right DC in an efficient manner
The initial imaging studies of naive T cell migration in LNs analyzed displacement vs. time behavior and concluded that these lymphocytes followed a random walk pattern within the paracortical region [12]. Calculations based on the rate of T cell migration and the volume swept by the dendrites of a DC indicated th there was a 95% likelihood that a single antigen-specific T cell would encounter at least 1 of a few hundred antigen-bearing DCs during a single passage through a LN, suggesting that random interactions would suffice for effective initiation of adaptive immune responses. However, these studies assumed that random walk behavior by the T cells meant free movement within an unstructured space, whereas many earlier anatomic and histological analyses revealed a rather well-ordered stromal architecture in LN that had to be taken into account in understanding the movement of lymphocytes and the placement of DCs within the node. By developing a novel means of revealing the previously non-fluorescent and hence, invisible stromal components of the LN at the same time as imaging T cells, Bajenoff et al. identified the fibroblastic reticular cell (FRC) network as a fine meshwork on which naive T cells moved during their passage in the LN from HEV to lymphatic exits [13], (Figure 1). While T cell migration within the LN is random when viewed on a large spatial scale, on a smaller scale the movements of naive T cells are guided and limited by the FRC network, which also defines the extent of the T cell zone [14].
Dendritic cells are positioned on the same FRC network, a finding that provides a structural explanation for the observations of the seemingly fixed, distributed positioning of LN resident DCs and the migration, then stable positioning of newly arriving DCs in LNs [15]. The latter behavior appears to correspond to the new DCs finding niches on the incompletely occupied FRC network. This concentration of DCs on the same FRC network that constrains and guides T cell migration provides enhanced efficiency of interaction between these critical cell populations as compared to purely random distribution and movement of both cell types within the node structure (figure 1).
However, even a random walk on the FRC network did not seem able to provide for optimal function in adaptive immunity when two distinct antigen-specific T cell must find the same antigen-bearing DC, as is the case for CD4+ T cells helping CD8+ T cells [16, 17]. The calculation cited above for the good efficiency of antigen-bearing DC identification during a single pass through a LN for a single antigen-specific T cell changes dramatically if a second T cell must find not just any of the antigen-bearing DCs, but exactly the same DC as another rare specific T cell. Some mechanism for reducing the search space seemed necessary and chemokines were a likely candidate for the cues allowing such selective migration and cell-cell contact. Indeed, Castellino et al., using a combination of classical immunological tools and intravital 2P imaging, showed that interaction between a DC and a CD4+ T cell leads to local CCL3/4 production [18]. Naive CD8+ T cells in inflamed LNs are able to follow this chemokine guidance and quickly locate a licensed DC in preference over a random, non-engaged DC in the same paracortical LN volume (Figure 1). This principle of chemokine guidance also can involve CD8 T cells ‘communicating’ with each other [19]. The experimental method used for the analysis of Castellino et al. did not allow a direct evaluation of whether this ‘GPS’ system in the LN operates during the initial searching phase 1/2 only or also comes into play after long-lasting T cell DC contacts during the later phase 3 of T cell priming. This question is of substantial importance because of the evidence that CD4+ and CD8+ T cells preferentially respond to antigen presenting by distinct DCs.[20, 21] and that the initial encounters of T cells with DCs might ‘stop’ the relevant CD4+ and CD8+ T cells on different DCs. This would require a later phase in which the chemokine guidance cues allow relocalization of one or the other T cell on the relevant DC, associated with a period of time in which ‘helpless’ CD8+ Tcells that have already interacted with antigen can be rescued and ‘helped’. This is an important issue for additional investigation.
As a final comment on the subject of stromal guidance, the recent reports of changes in chemokine expression by [22] or even physical disruption of the FRC network [23] in response to various pathogens raises new questions regarding the way in which infectious agents modulate immune responses, given the role of the stromal network in control of critical cell-cell interactions in lymphoid tissues, not only LNs but also spleen [24].
3. DC compartmentalization and specialization to optimize the immune response
One common limitation of the above studies is the use of transferred peptide-pulsed DCs, often neither separated or even characterized with respect to DC subset content, no less analyzed in the context of natural infections and antigen presentation. Infectious agents can play a major role in determining the number, nature, and location of antigen-presenting DCs. For example, using dynamic imaging after VV infection it was found that virus infection is not randomly distributed throughout the LN, but it is mainly restricted to cells in the subcapsular sinus (SCS) area, particularly the CD169+ macrophages [25]. In addition, there are clearly DCs in the same region that acquire antigen, either through direct infection or as a result of cross-presentation of antigen from infected macrophages and other cell types. Antigen-specific T cells and to some degree non-specific T cells seem to translocate to the SCS where they encounter these infected DCs and undergo priming. This work demonstrated that T cell priming does not randomly occur within the T zone, but rather takes place in specific compartments of the LN connected to the entry routes and the target cells of the analyzed pathogen.
When antigen-bearing, migrating DC from the periphery enters the LN via the afferent lymphatics, they also don't randomly distribute in the LN to find their position somewhere on the FRC network. Migratory dermal DC colonize a specific area that is distinct from the region occupied by slower migrating LC [26]. Similarly, resident DC subpopulations also seem to localize in specific subregions of the LN; for example, CD8+ DC are predominantly found in the inter- and subfollicular region [27], (Figure 2). Currently, it is unclear if chemokine cues help the DC to find their places, or if the stromal network itself supports attachment of specific DC subpopulations in certain based on adhesive interactions.
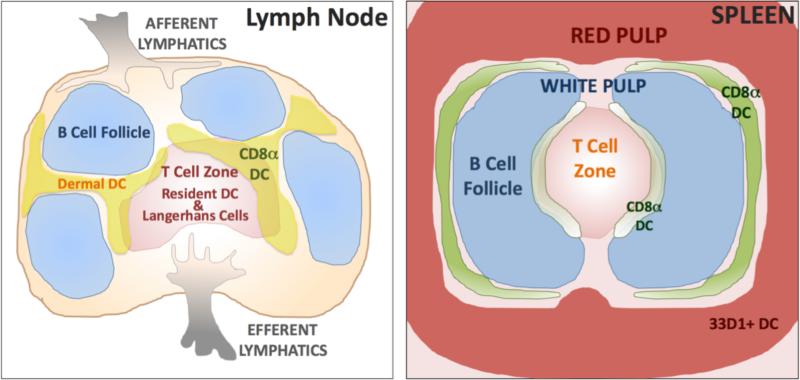
Figure 2. DC Compartmentalization in Lymphoid Tissues.
Schematic representation of DC subset localization within skin-draining LN (left panel) and spleen (right panel). Even though all migratory DCs appear to enter the draining LN through the interfollicular region, dermal DC are thought to be positioned near the B cell follicles and the Langerhans cells to be positioned deeper in the T cell zone with other resident DCs. Resident CD8α+ DCs have also recently been shown to have distinct compertmenalization in skin-draining LN. Differences in resident DC subset localization have also been noted in the spleen, with specific regionalization of CD8α+ and 33D1+ DC between the white and red pulp.
Such specific localization is likely to relate to distinct functions exerted by different DC subpopulations. For example, Dudziak et al. have shown that there is a separation of labor among different DC subsets [20]. While some DC subpopulations seem to be particularly good at priming CD4+ T cells (these DCs are identified by the 33D1 mAb), others (marked by DEC-205 expression) seem to be specialized for priming model that lacks the CD8+ DC population due to a deficiency in BatF3 expression and that seems to have a defect in priming antiviral CD8+ T cell responses [28]. While such DC specialization makes sense in terms of activating different T cell subsets and promoting functionally relevant effector responses, it raises an interesting question given our knowledge of the need for CD4-CD8 communication via a DC intermediary in optimizing memory stage, if not early effector, cytotoxic responses [29-31].On which DC is cognate CD4+ help delivered? Or is the functional separation of DC not as stringent during actual infections as opposed to what has been observed with targeted antigen delivery? A similar issue can be raised with respect to activation of naive vs. memory CD8+ T cells. Migratory DC have been reported to have only a minimal ability to activate memory CD8+ T cells while they are fully capable of priming naive CD8+ T cells [32]. The reason for this finding remains elusive but imaging methods should certainly help to shed light on this issue. In this regard, it is worth noting that nearly all the literature on T cell migration and T cell-DC interactions in LNs involve naive cells; only recently has one study compared naive vs. memory CD8+ T cells interactions with DC in situ [33]. Using Toxoplasma infection the authors found that memory CD8+ T cells seem to more rapidly engage infected APC at the site of entry in the LN - the SCS - and form long-lasting interactions with DC similar to those of naive CD8+ T cells. The question of differential interaction of these same two T cell subpopulations with migratory DC has not been addressed.
4. Future perspectives
Dynamic imaging has revealed a great deal about the modes of T-DC interaction in situ. We now appreciate the key role of stromal elements in guiding T cell movement and relevance of the colocalization of DCs on the same stromal network for efficient interactions of the two cell types during the initial scanning process taking place at the onset of an immune response. Chemokine cues have been shown to bias movement on the stromal network to further optimize relevant interactions. The association of T cells with DCs changes over time in a manner that reflects antigen density, quality, and state of DC maturation. There are hints of regional specialization within the LN, with distinct DC subsets occupying preferential niches that may correlate with different biological responses (CD8+ cytotoxic responses, humoral immunity, CD4+ help and perhaps Th1 vs. Th2 or Th17 generation). With the identification of specific markers for DC subpopulations (DNGR-1, XCR-1), new fluorescent mouse models are being established that will allow in situ imaging of these populations and direct comparison of the cellular interactions in which they engage [27, 34]. Trackable infectious agents used in concert with such DC-marked hosts and properly labeled T cells will permit analysis of the entire course of cell-cell interactions during responses to pathogens. Finally, the development and use of reporters for signaling and transcriptional events will move imaging from a mere description of dynamics, as important as these are, to one in which function at a molecular level is connected to the dynamic behaviors our imaging tools have now revealed.
References
- 1.Steinman RM, Cohn ZA. Identification of a novel cell type in peripheral lymphoid organs of mice. I. Morphology, quantitation, tissue distribution. J Exp Med. 1973;137:1142–1162. doi: 10.1084/jem.137.5.1142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lipsky PE, Rosenthal AS. Macrophage-lymphocyte interaction. II. Antigen-mediated physical interactions between immune guinea pig lymph node lymphocytes and syngeneic macrophages. J Exp Med. 1975;141:138–154. doi: 10.1084/jem.141.1.138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Stoll S, Delon J, Brotz TM, Germain RN. Dynamic imaging of T cell-dendritic cell interactions in lymph nodes. Science. 2002;296:1873–1876. doi: 10.1126/science.1071065. [DOI] [PubMed] [Google Scholar]
- 4.Miller MJ, Wei SH, Parker I, Cahalan MD. Two-photon imaging of lymphocyte motility and antigen response in intact lymph node. Science. 2002;296:1869–1873. doi: 10.1126/science.1070051. [DOI] [PubMed] [Google Scholar]
- 5.Mempel TR, Henrickson SE, Von Andrian UH. T-cell priming by dendritic cells in lymph nodes occurs in three distinct phases. Nature. 2004;427:154–159. doi: 10.1038/nature02238. [DOI] [PubMed] [Google Scholar]
- 6.Henrickson SE, Mempel TR, Mazo IB, Liu B, Artyomov MN, Zheng H, Peixoto A, Flynn MP, Senman B, Junt T, Wong HC, Chakraborty AK, von Andrian UH. T cell sensing of antigen dose governs interactive behavior with dendritic cells and sets a threshold for T cell activation. Nat Immunol. 2008;9:282–291. doi: 10.1038/ni1559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bousso P, Robey E. Dynamics of CD8+ T cell priming by dendritic cells in intact lymph nodes. Nat Immunol. 2003;4:579–585. doi: 10.1038/ni928. [DOI] [PubMed] [Google Scholar]
- 8.Celli S, Lemaitre F, Bousso P. Real-time manipulation of T cell-dendritic cell interactions in vivo reveals the importance of prolonged contacts for CD4+ T cell activation. Immunity. 2007;27:625–634. doi: 10.1016/j.immuni.2007.08.018. [DOI] [PubMed] [Google Scholar]
- 9.Hugues S, Fetler L, Bonifaz L, Helft J, Amblard F, Amigorena S. Distinct T cell dynamics in lymph nodes during the induction of tolerance and immunity. Nat Immunol. 2004;5:1235–1242. doi: 10.1038/ni1134. [DOI] [PubMed] [Google Scholar]
- 10.Shakhar G, Lindquist RL, Skokos D, Dudziak D, Huang JH, Nussenzweig MC, Dustin ML. Stable T cell-dendritic cell interactions precede the development of both tolerance and immunity in vivo. Nat Immunol. 2005;6:707–714. doi: 10.1038/ni1210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Qi H, Egen JG, Huang AY, Germain RN. Extrafollicular activation of lymph node B cells by antigen-bearing dendritic cells. Science. 2006;312:1672–1676. doi: 10.1126/science.1125703. [DOI] [PubMed] [Google Scholar]
- 12.Miller MJ, Wei SH, Cahalan MD, Parker I. Autonomous T cell trafficking examined in vivo with intravital two-photon microscopy. Proc Natl Acad Sci U S A. 2003;100:2604–2609. doi: 10.1073/pnas.2628040100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bajenoff M, Egen JG, Koo LY, Laugier JP, Brau F, Glaichenhaus N, Germain RN. Stromal cell networks regulate lymphocyte entry, migration, and territoriality in lymph nodes. Immunity. 2006;25:989–1001. doi: 10.1016/j.immuni.2006.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bajenoff M, Germain RN. B-cell follicle development remodels the conduit system and allows soluble antigen delivery to follicular dendritic cells. Blood. 2009;114:4989–4997. doi: 10.1182/blood-2009-06-229567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lindquist RL, Shakhar G, Dudziak D, Wardemann H, Eisenreich T, Dustin ML, Nussenzweig MC. Visualizing dendritic cell networks in vivo. Nat Immunol. 2004;5:1243–1250. doi: 10.1038/ni1139. [DOI] [PubMed] [Google Scholar]
- 16.Smith CM, Wilson NS, Waithman J, Villadangos JA, Carbone FR, Heath WR, Belz GT. Cognate CD4(+) T cell licensing of dendritic cells in CD8(+) T cell immunity. Nat Immunol. 2004;5:1143–1148. doi: 10.1038/ni1129. [DOI] [PubMed] [Google Scholar]
- 17.Keene JA, Forman J. Helper activity is required for the in vivo generation of cytotoxic T lymphocytes. J Exp Med. 1982;155:768–782. doi: 10.1084/jem.155.3.768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Castellino F, Huang AY, Altan-Bonnet G, Stoll S, Scheinecker C, Germain RN. Chemokines enhance immunity by guiding naive CD8+ T cells to sites of CD4+ T cell-dendritic cell interaction. Nature. 2006;440:890–895. doi: 10.1038/nature04651. [DOI] [PubMed] [Google Scholar]
- 19.Hugues S, Scholer A, Boissonnas A, Nussbaum A, Combadiere C, Amigorena S, Fetler L. Dynamic imaging of chemokine-dependent CD8+ T cell help for CD8+ T cell responses. Nat Immunol. 2007;8:921–930. doi: 10.1038/ni1495. [DOI] [PubMed] [Google Scholar]
- 20.Dudziak D, Kamphorst AO, Heidkamp GF, Buchholz VR, Trumpfheller C, Yamazaki S, Cheong C, Liu K, Lee HW, Park CG, Steinman RM, Nussenzweig MC. Differential antigen processing by dendritic cell subsets in vivo. Science. 2007;315:107–111. doi: 10.1126/science.1136080. [DOI] [PubMed] [Google Scholar]
- 21.Schnorrer P, Behrens GM, Wilson NS, Pooley JL, Smith CM, El-Sukkari D, Davey G, Kupresanin F, Li M, Maraskovsky E, Belz GT, Carbone FR, Shortman K, Heath WR, Villadangos JA. The dominant role of CD8+ dendritic cells in cross-presentation is not dictated by antigen capture. Proc Natl Acad Sci U S A. 2006;103:10729–10734. doi: 10.1073/pnas.0601956103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mueller SN, Hosiawa-Meagher KA, Konieczny BT, Sullivan BM, Bachmann MF, Locksley RM, Ahmed R, Matloubian M. Regulation of homeostatic chemokine expression and cell trafficking during immune responses. Science. 2007;317:670–674. doi: 10.1126/science.1144830. [DOI] [PubMed] [Google Scholar]
- 23.Scandella E, Bolinger B, Lattmann E, Miller S, Favre S, Littman DR, Finke D, Luther SA, Junt T, Ludewig B. Restoration of lymphoid organ integrity through the interaction of lymphoid tissue-inducer cells with stroma of the T cell zone. Nat Immunol. 2008;9:667–675. doi: 10.1038/ni.1605. [DOI] [PubMed] [Google Scholar]
- 24.Bajenoff M, Glaichenhaus N, Germain RN. Fibroblastic reticular cells guide T lymphocyte entry into and migration within the splenic T cell zone. J Immunol. 2008;181:3947–3954. doi: 10.4049/jimmunol.181.6.3947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hickman HD, Takeda K, Skon CN, Murray FR, Hensley SE, Loomis J, Barber GN, Bennink JR, Yewdell JW. Direct priming of antiviral CD8+ T cells in the peripheral interfollicular region of lymph nodes. Nat Immunol. 2008;9:155–165. doi: 10.1038/ni1557. [DOI] [PubMed] [Google Scholar]
- 26.Kissenpfennig A, Henri S, Dubois B, Laplace-Builhe C, Perrin P, Romani N, Tripp CH, Douillard P, Leserman L, Kaiserlian D, Saeland S, Davoust J, Malissen B. Dynamics and function of Langerhans cells in vivo: dermal dendritic cells colonize lymph node areas distinct from slower migrating Langerhans cells. Immunity. 2005;22:643–654. doi: 10.1016/j.immuni.2005.04.004. [DOI] [PubMed] [Google Scholar]
- 27.Dorner BG, Dorner MB, Zhou X, Opitz C, Mora A, Guttler S, Hutloff A, Mages HW, Ranke K, Schaefer M, Jack RS, Henn V, Kroczek RA. Selective expression of the chemokine receptor XCR1 on cross-presenting dendritic cells determines cooperation with CD8+ T cells. Immunity. 2009;31:823–833. doi: 10.1016/j.immuni.2009.08.027. [DOI] [PubMed] [Google Scholar]
- 28.Hildner K, Edelson BT, Purtha WE, Diamond M, Matsushita H, Kohyama M, Calderon B, Schraml BU, Unanue ER, Diamond MS, Schreiber RD, Murphy TL, Murphy KM. Batf3 deficiency reveals a critical role for CD8alpha+ dendritic cells in cytotoxic T cell immunity. Science. 2008;322:1097–1100. doi: 10.1126/science.1164206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sun JC, Bevan MJ. Defective CD8 T cell memory following acute infection without CD4 T cell help. Science. 2003;300:339–342. doi: 10.1126/science.1083317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shedlock DJ, Shen H. Requirement for CD4 T cell help in generating functional CD8 T cell memory. Science. 2003;300:337–339. doi: 10.1126/science.1082305. [DOI] [PubMed] [Google Scholar]
- 31.Obar JJ, Molloy MJ, Jellison ER, Stoklasek TA, Zhang W, Usherwood EJ, Lefrancois L. CD4+ T cell regulation of CD25 expression controls development of short-lived effector CD8+ T cells in primary and secondary responses. Proc Natl Acad Sci U S A. 2010;107:193–198. doi: 10.1073/pnas.0909945107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Belz GT, Bedoui S, Kupresanin F, Carbone FR, Heath WR. Minimal activation of memory CD8+ T cell by tissue-derived dendritic cells favors the stimulation of naive CD8+ T cells. Nat Immunol. 2007;8:1060–1066. doi: 10.1038/ni1505. [DOI] [PubMed] [Google Scholar]
- 33.Chtanova T, Han SJ, Schaeffer M, van Dooren GG, Herzmark P, Striepen B, Robey EA. Dynamics of T cell, antigen-presenting cell, and pathogen interactions during recall responses in the lymph node. Immunity. 2009;31:342–355. doi: 10.1016/j.immuni.2009.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sancho D, Mourao-Sa D, Joffre OP, Schulz O, Rogers NC, Pennington DJ, Carlyle JR, Reis e Sousa C. Tumor therapy in mice via antigen targeting to a novel, DC-restricted C-type lectin. J Clin Invest. 2008;118:2098–2110. doi: 10.1172/JCI34584. [DOI] [PMC free article] [PubMed] [Google Scholar]