Abstract
Fragile X syndrome (FXS) is a common form of mental disability and one of the known causes of autism. The mutation responsible for FXS is a large expansion of the trinucleotide CGG repeats which leads to DNA methylation of the fragile X mental retardation gene 1 (FMR1) and transcriptional silencing, resulting in the absence of fragile X mental retardation protein (FMRP), an mRNA binding protein. Although it is widely known that FMRP is critical for metabotropic glutamate receptor (mGluR)-dependent long-term depression (LTD), which has provided a general theme for developing pharmacological drugs for FXS, specific downstream targets of FMRP may also be of therapeutic value. Since alterations in potassium channel expression level or activity could underlie neuronal network defects in FXS, here we describe recent findings on how these channels might be altered in mouse models of FXS and the possible therapeutic avenues for treating FXS.
Keywords: fragile X syndrome, FMRP, mGluR theory, potassium channels
Introduction
Fragile X syndrome (FXS), with an incidence of 1 in 5,000 males [1], causes mild to severe mental disability, often accompanied by autism-like behaviors, developmental delay, increased susceptibility to seizures, and macroorchidism in males [2]. A key advance for understanding FXS was the cloning of the fragile X mental retardation gene 1 (FMR1) [3*] located at Xq27.3, the diagnostic fragile site on the X chromosome [4], and the generation of the fmr1 knockout (KO) mouse line [5*]. In affected individuals, expansion of a CGG repeat (>200) located in the 5’ untranslated region (UTR) of FMR1 [6] leads to hypermethylation of both the CGG repeats and the FMR1 promoter, transcriptional silencing, and loss of its protein product fragile X mental retardation protein (FMRP) [7, 8]. In addition, a small number of deletions and missense mutations in the FMR1 gene have been linked to FXS [9-11]. Multiple symptoms seen in FXS patients, including the altered spine morphology [12-14], are recapitulated in fmr1 KO mice [15, 16], which also display compromised learning, abnormal behavior and altered synaptic plasticity [17]. The fmr1 KO mouse is therefore a useful system for mechanistic studies of FXS.
FMRP is ubiquitously expressed in mammalian tissues [18], and its abundance in the brain and testes is consistent with FXS symptoms [18, 19]. FMRP is expressed primarily in neurons in the brain [18] and can bind target mRNAs directly or indirectly [20]. FMRP has multiple RNA-binding motifs including two K homology domains (KH1 and KH2) and the arginine-glycine-glycine (RGG) box [21**], whose affinity for certain mRNAs may be regulated by the methylation status of the arginines in the RGG box [22]. In addition to these conserved domains, other regions of FMRP have also been implicated in protein-protein interactions that are important for its function [21**].
Multiple U-rich pentamers reside in both the coding region and 3’UTR of some FMRP target mRNAs [23], and a U-rich region in the 5’UTR of hASH1 also binds FMRP [24]. The C-terminal RGG box recognizes the G quadruplex [25**, 26] likely present in targets such as the FMRP, MAP1b, and Sema3F mRNAs [21**]. Another secondary structure known as the kissing complex binds the KH2 domain in vitro [27]. Moreover, FMRP also binds to the superoxide dismutase 1 (Sod1) mRNA through a novel RNA structure termed Sod1 stem loops interacting with FMRP (SoSLIP) [28], which interacts with the RGG box-containing C-terminal domain and competes with G quadruplex for FMRP binding [28].
The dense and immature dendritic spines associated with FXS [12-14] indicate that FMRP regulates dendritic development and function. Because FMRP is localized to dendrites and spines, it could regulate local protein synthesis to modulate spine development and synaptic plasticity [20]. Indeed, many of the FMRP target mRNAs localize to dendrites [21**], and FMRP may regulate mRNA localization [29], stability [30], or translation [31, 32] in central neurons [33, 34].
FMRP inhibits translation of most of its target mRNAs, which has been demonstrated in rabbit reticulocyte lysate [35], in Xenopus laevis oocytes [36] and in immortalized cells from an fmr1 KO mouse [37]. In addition, brains and synaptosomes from fmr1 KO mice have an overabundance of FMRP targets such as Map1b, Arc, and CamKIIα [38, 32], and they have the CamKIIα, PSD-95, and GluR1/2 mRNAs shifted to actively translating polyribosomes [31]. Surprisingly, FMRP seems to upregulate the translation of Sod1 mRNA by strengthening SoSLIP’s ability to activate translation [28]. Thus far, only a small number of mRNAs have been verified as FMRP targets [21**], while the molecular mechanisms for FMRP regulation of translation remain to be elucidated.
FMRP repression of its targets may be relieved to mediate dynamic regulation – a process that may involve phosphorylation regulation of FMRP [39-41], which contains a highly conserved serine (human Ser500, murine Ser499, Drosophila Ser406) that is phosphorylated [39] to enable FMRP repression of translation [39, 42*, 43**]. Phosphorylated FMRP is associated with stalled ribosomes, whereas unphosphorylated FMRP allows ribosomes to proceed with translation [39], and may also associate with Dicer [44].
Targeted treatments for neurodevelopmental disorders such as FXS have become a feasible therapeutic strategy following the development of appropriate animal models [45**, 46**], such as the fmr1 KO mice. Recent studies of FXS have opened new avenues of investigation leading to the rational design of potential therapeutics for FXS. Besides FMRP, the metabotropic glutamate receptor 5 (mGluR5) signaling pathway may provide a target for the treatment of FXS. Recent studies have also implicated abnormal potassium channel activity in FXS, which will be discussed later in this review. Hence, mGluR5 and potassium channels have emerged as targets for developing novel therapeutic reagents for FXS.
Current strategy for targeted treatment based on the mGluR theory
Because group I mGluR-dependent long-term depression (mGluR-LTD) is a major form of synaptic plasticity involving synaptic regulation of local protein synthesis of dendritically localized mRNAs, mGluR-LTD and the antagonistic regulation by FMRP have been studied extensively. Given that mGluR-LTD is enhanced in the hippocampus of fmr1 KO mice [47**, 48], Huber et al. [47**] proposed that FMRP limits LTD by inhibiting mGluR-dependent translation of dendritic mRNAs encoding the hypothetical “LTD protein(s)”, so that FMRP synthesis induced by mGluR5 would provide a brake to prevent runaway synaptic protein synthesis. The increase in cerebral protein synthesis in fmr1 KO mice [49, 50*] may lead to the internalization of α-amino-3-hydroxyl-4-isoxazole propionic acid receptors (AMPAR), a key step in mGluR-LTD [51**, 52**]. In addition, dephosphorylation of FMRP following mGluR activation correlates with the release of translational inhibition of FMRP target mRNAs [40, 42*], with protein phosphatase 2A (PP2A) and ribosomal protein S6 kinase 1 (S6K1) as the primary phosphatase and kinase [40, 41]. Both the persistently enhanced mGluR-LTD and the inability of synaptic inputs to further increase protein synthesis uncovered in these studies are likely culprits in FXS, possibly involving FMRP targets such as MAP1b, eEF1A, Arc, CaMKIIα, PSD-95, SAPAP3, and APP [33, 53, 21**].
The hypothesis that overactive mGluR5 functions mediate many of the symptoms of fragile X suggests that mGluR5 antagonism may be a plausible therapeutic strategy for the disease. 2-methyl-6-(phenylethynyl)-pyridine (MPEP), a potent negative allosteric modulator of mGluR5 [54] that crosses the blood-brain barrier, can rescue behavioral and cognitive deficits in mouse models of FXS [55*, 56*]. Acute administration of MPEP reduces audiogenic seizures and the abnormal response of fmr1 KO mice in an open field test of anxiety-like phenotypes [55*]. MPEP also rescues other deficits including AMPAR internalization defects [57], prolonged epileptiform discharges in hippocampal slices [58], deficits in prepulse inhibition of startle [56*], decreased mRNA granule expression [59], excess protein synthesis in hippocampal slices [60], increased density of dendritic filopodia in hippocampal cultures [56*], and hyperactivity of glycogen synthase kinase-3 [61]. These studies have prompted development of novel therapeutic interventions; several clinical trials of drugs that target the mGluR pathway are currently underway [45**, 46**].
As a crucial validation of the mGluR theory, genetic reduction of mGluR5 in fmr1 KO mice rescues many of the disease-related phenotypes [50*, 62], but not macroorchidism [50*], and provides compelling evidence that manipulating mGluR5 signaling can reverse fragile X–related phenotypes across species. This indicates that mGluR5 is a viable target for the treatment of FXS.
MPEP can rescue several major defects of fmr1 KO mice, but not the deficit in long-term potentiation (LTP), a cellular correlate for learning and memory. While fmr1 KO mice display LTP deficits in various brain regions including the hippocampus [63*, 64*, 65], in the few brain areas surveyed thus far MPEP fails to correct the deficit in LTP [66, 67]. This indicates that not all synaptic defects may be amenable to group I mGluR-targeted intervention [46**]. Moreover, some of the proposed FMRP target proteins such as PSD95 do not show the expected basal upregulation in fmr1 KO mice [30]. Given that there are limitations of the current attempts in FXS treatment, it is important to extend our understanding of FMRP functions beyond those that involve mGluR signaling, and to explore other therapeutic avenues.
The search for additional strategies for targeted treatment
Identification of potassium channels as FMRP targets
FMRP binds ~4% of the mRNA in the mammalian brain [68*]. Over 400 putative mRNAs are found to associate with FMRP using various methods [69**, 70, 71], although fewer than 20 of these have been validated biochemically [21**]. A recent study used high-throughput sequencing of RNAs isolated through cross-linking immunoprecipitation (HITS-CLIP) has identified FMRP target mRNAs in neurons [72**], providing evidence supporting the notion that FMRP causes ribosomes to stall during the elongation phase of translation [72**]. Another approach towards an understanding of FMRP function has been the search for proteins that interact directly with FMRP or that are components of the FMRP-containing mRNP complex. Several proteins such as FXR1P, FXR2P, 82-FIP, NUFIP1, CYFIP1 and CYFIP2 have been characterized as FMRP interacting proteins to date [73]. Strikingly, these various approaches also identified several potassium channel mRNAs as well as a potassium channel protein as FMRP targets, as detailed below.
There are many different potassium channels in the nervous system including inward rectifier and leak potassium channels that control the resting membrane potential [74, 75], and voltage-gated potassium channels that regulate the action potential waveform [76]. Potassium channels have to be at the right place in the right number to endow individual neurons with their specific character. Their biophysical properties together with their spatial distribution define the signaling characteristics of a neuron.
Mouse models of FXS show defects in three kinds of potassium channels, the Na+-activated K+ channel (KNa) Slack-B [77**], and the voltage-gated K+ channels Kv3.1b [78**] and Kv4.2 [43**]. FMRP binds the mRNAs for Kv3.1b and Kv4.2, and also interacts directly with the Slack-B protein to modulate its activity in heterologous expression systems [77**, 78**, 43**] (Figure 1). We will discuss how these channels might be altered in fmr1 KO mice.
Figure 1.
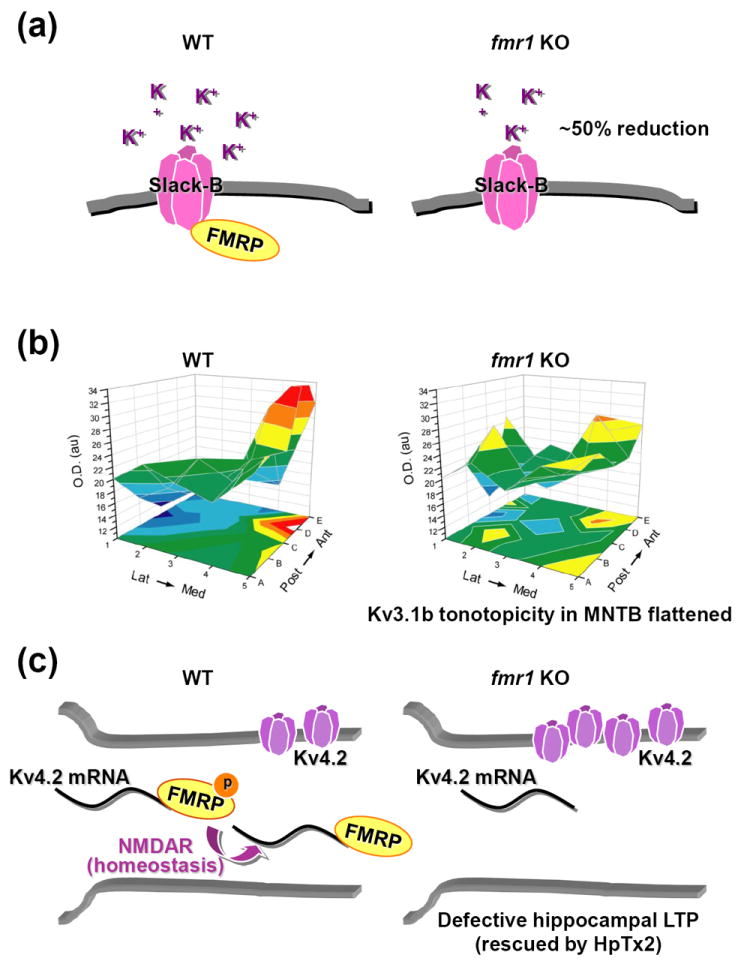
Summary of the alterations of potassium channels in fmr1 KO mice.
(a) FMRP increases Slack-B channel activity by binding to the C-terminus of Slack-B. Slack potassium currents are reduced by about 50% in fmr1 KO mice.
(b) While WT animals show a tonotopic gradient of Kv3.1b expression in the MNTB, fmr1 KO mice display dramatically flattened gradients. Representative three-dimensional plots of the average Kv3.1b immunoreactivity (OD) in each of 25 stereotaxic zones. Lat, lateral; Med, medial; Post, posterior; Ant, anterior. (Adapted from [78**].)
(c) FMRP suppression of Kv4.2 levels in neuronal dendrites through its interaction with segments of Kv4.2-3’UTR. Whereas FMRP suppresses Kv4.2 in the basal condition, FMRP suppression is relieved by its dephosphorylation upon NMDAR activation, as a homeostasis mechanism to reset the neuronal activity. Without FMRP, the basal level of Kv4.2 is elevated in fmr1 KO mice. Reducing Kv4 channel activity by the specific channel blocker HpTx2 rescues the deficits of LTP induction in the hippocampus from fmr1 KO mice.
The Slack Potassium Channel as a Therapeutic Target for FXS in the Auditory System
Individuals with FXS have a range of perceptual deficits in processing auditory stimuli, and may be particularly sensitive to loud sounds [79]; they also have fluctuations in their speech pattern [80]. These symptoms interfere with brain functions such as attention, learning, language development, and social interactions. The integrity of neuronal encoding and processing of sensory inputs depends on ion channels that regulate the action potential waveform and firing pattern, such as Slack-B that has a large cytoplasmic C-terminal domain [81] and is widely expressed in the brain [81].
A recent study has found that FMRP directly binds to the C-terminus of the Slack-B channel protein and causes a several-fold activation of Slack-B channel activity by increasing channel openings (77**), thus providing the first example for direct binding of FMRP to a membrane protein (Figure 1a). Moreover, Slack potassium currents in the medial nucleus of the trapezoid body (MNTB) of the auditory brainstem are reduced by about 50% in fmr1 KO mice (Figure 1a). Slack is required for accurate timing of action potentials of these central auditory neurons in response to synaptic stimuli [82]. Defects with this potassium channel function may contribute to the difficulty of some FXS patients to adapt to the ambient auditory environment [83]. Slack activators may thus be considered as novel therapeutic agents that could act either independently or in concert with agents that affect group I mGluRs, which may in turn regulate Slack channels [84].
Alteration in Kv3.1 Levels in Auditory Brainstem Nuclei of fmr1 KO Mice
Studies have shown that fmr1 KO mice exhibit abnormal sensitivity to auditory stimuli including hyper reactivity and the induction of audiogenic seizures [50*, 55*]. While audiogenic seizures have not been reported in Fragile X patients, the onset and manifestation of autistic behavior in these individuals correlates with auditory hypersensitivity [2, 17]. Audiogenic seizures are thought to arise from increased excitation in auditory nuclei rather than an overall increase in brain excitability. Kv3.1 channels are at particularly high levels in neurons of auditory nuclei [85] and their fast gating kinetics permits neurons to fire prolonged trains of action potentials at very high frequencies with little adaptation [86].
Kv3.1 mRNA has been identified [25**] and validated [78**] as a binding target for FMRP. The physiological role of Kv3.1 channels is to allow specific types of neurons to fire at very high rates [83], which helps in decoding loud and diverse sounds. Moreover, the faithful delivery of all sound frequencies is dependent on a precise tonotopic gradient of Kv3.1b splice isoform expression within MNTB of the auditory brainstem; Kv3.1b levels are highest at the medial end, which corresponds to high auditory frequencies [83]. Disruption of this auditory space code, or map, due to the Kv3.1b mysregulation would be expected to interfere with auditory processing in auditory nuclei of the brain stem and in the auditory cortex.
To explore the regulation of the Kv3.1b by FMRP, Strumbos et al. [78**] investigated Kv3.1b immunoreactivity and potassium currents in the auditory brainstem sound localization circuit of male mice by exposing animals to high-frequency, amplitude-modulated sound stimuli, which elicit predictable and stereotyped patterns of input to the anterior ventral cochlear nucleus (AVCN) and MNTB. While wild-type (WT) animals show a tonotopic gradient with Kv3.1b expression in the MNTB, fmr1 KO mice display dramatically flattened gradients in tonotopicity as shown by the Kv3.1b immunoreactivity and K+ currents at the basal condition (Figure 1b). Moreover, after 30 min of acoustic stimulation, the levels of Kv3.1b immunoreactivity were significantly elevated in both the MNTB and AVCN of WT, but not fmr1 KO mice. This finding suggests that auditory neurons are likely to be hyperexcitable in FXS individuals and also suggests that FMRP is necessary for maintaining the gradient of Kv3.1b protein levels across the tonotopic axis of the MNTB, consistent with a role for FMRP as a repressor of protein translation. It will be important to determine the basis for the FMRP-dependent dysregulation of Kv3.1b translation in fmr1 KO mice; these future investigations may provide clues to novel approaches towards therapeutic interventions.
Rescue of LTP deficits in the hippocampus from fmr1 KO mice by a Kv4 channel blocker
As mentioned earlier, deficits in LTP have been reported in the fmr1 KO mice [63*, 64*, 65], however MPEP fails to correct this deficit in LTP [66, 67, 46**]. The levels of Kv4.2 that generate the A-type K+ currents (IA) on the dendritic membrane are critical for synaptic plasticity [87]; loss of Kv4.2 function causes enhanced induction of LTP in hippocampal CA1 pyramidal neurons [88], while increasing Kv4.2 expression abolishes the ability to induce LTP [89*].
Kv4.2 mRNA has been identified and validated as a binding target for FMRP [43**, 72**]. Kv4.2 is the most abundant isoform of dendritic voltage-gated A-type K+ channel in CA1 pyramidal neurons in the hippocampus [88]. Enriched on the spines of CA1 pyramidal neurons, Kv4.2 is under the regulation of synaptic activity and it in turn contributes to the regulation of synaptic plasticity [87, 89*]. A recent study [43**] found the dendritic localization of Kv4.2 mRNA and FMRP suppression of Kv4.2 levels through the interaction with segments of Kv4.2-3’UTR with U-rich sequences (Figure 1c). Moreover, the deficit in LTP induction can be rescued by reducing Kv4 channel activity in hippocampal slices from fmr1 KO mice [43**] (Figure 1c).
The N-methyl-D-aspartate receptor (NMDAR)-induced total protein synthesis is defective in synaptosomes from fmr1 KO mice [90]. Given the bidirectional feedback regulation between Kv4.2 and NMDAR for the dynamic modulation of synaptic plasticity [89*] and the NMDAR-dependent regulation of dendritic Kv4.2 for branch strength potentiation [91], it is of interest to determine how the Kv4.2 protein level can recover quickly after its down regulation by NMDAR via internalization and degradation [92-94]. Lee et al. [43**] found that NMDAR activation increases Kv4.2 protein production in an FMRP-dependent process likely involving PP1-dependent dephosphorylation of FMRP, and the resulting de-repression of Kv4.2 corresponds to a homeostasis mechanism to reset the neuronal activity (Figure 1c).
Taken together, this study identifies Kv4.2 mRNA as a new target of FMRP. Whereas FMRP suppresses Kv4.2 in the basal condition, FMRP suppression is relieved by its dephosphorylation upon NMDAR activation. This derepression to increase Kv4.2 production compensates for NMDAR-mediated Kv4.2 degradation so that there is a transient down regulation of Kv4.2 – a positive feedback regulation of neuronal excitability, thereby maintaining neurons within the dynamic range of synaptic plasticity. Given that a Kv4 channel blocker (the cysteine knot venom peptide; heteropodatoxin HpTx2) rescues the LTP deficit in fmr1 KO mice, pharmacological inhibitors of Kv4.2 may be considered for potential therapeutic applications.
Therapeutic prospects
FXS symptoms likely involve multiple neuronal signaling pathways in different brain regions, including those linked to mGluR and several potassium channels that have been recently identified as novel therapeutic targets for treating FXS. A major focus of the field is to determine which pathways are crucial for the symptoms observed in patients and which of these are amenable to pharmaceutical intervention. These molecular studies of FMRP have led to the rational design of novel therapeutics. Now that the identities of many FMRP targets are known, investigators may be able to associate the dysregulation of specific target mRNAs with specific FXS phenotypes. In light of the significant variability of the disease and the limitations of mGluR drug therapy, patients with the disorder could potentially benefit from a cocktail of drugs.
Given that potassium channels (Slack, Kv3.1b and Kv4.2) show alterations in their activity or expression levels in fmr1 KO mice, modulators have therapeutic potentials in fragile X treatments. Potassium channel openers have been suggested for countering or preventing neuronal damages by interfering with different steps of the neurodegenerative cascade [95]. Thus, the discovery and development of pharmaceutical drugs targeting potassium channels is important for treating a variety of medical conditions and diseases. Recent advances in large-scale screening for molecules affecting ion channel function using optical-based and electrophysiological technologies have improved drug development in this field [96]. Moreover, methods for the discovery of peptide-based neurotoxins and other natural products have proven useful not only in the pharmacological assessment of ion channel structure and function, but also in the identification of lead molecules for drug development [96]. The extensive efforts and experience in developing pharmacological reagents targeting ion channels followed by a suitable validation process should facilitate the development of therapeutic reagents for consideration of potential FXS treatment.
Highlights.
FXS is a common form of mental disability and one of the known causes of autism.
Targeted treatments of FXS based-on the mGluR theory is a feasible therapeutic strategy.
It is also important to extend our understanding of FMRP functions beyond mGluR signaling.
Several potassium channels show alterations in mouse models of FXS.
We suggest possible therapeutic avenues for treating FXS regarding the role of potassium channels
Acknowledgments
We thank Dr. Shi-Bing Yang, David Gorczyca and Dr. Desiree Thayer for discussions, Dr. Kaczamarek for the generous permission and providing high-resolution figures for the use of Kv3.1 tonotopicity results, and the NIH grant MH065334 for support. LYJ is an HHMI investigator.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References and recommended reading
Papers of particular interest, published within the period of review, have been highlighted as:
-
*
of special interest
-
**
of outstanding interest
- 1.Coffee B, Keith K, Albizua I, Malone T, Mowrey J, Sherman SL, Warren ST. Incidence of fragile X syndrome by newborn screening for methylated FMR1 DNA. Am J Hum Genet. 2009;85:503–514. doi: 10.1016/j.ajhg.2009.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Garber KB, Visootsak J, Warren ST. Fragile X syndrome. Eur J Hum Genet. 2008;16:666–672. doi: 10.1038/ejhg.2008.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3*.Verkerk AJ, Pieretti M, Sutcliffe JS, Fu YH, Kuhl DP, Pizzuti A, Reiner O, Richards S, Victoria MF, Zhang FP. Identification of a gene (FMR-1) containing a CGG repeat coincident with a breakpoint cluster region exhibiting length variation in fragile X syndrome. Cell. 1991;65:905–914. doi: 10.1016/0092-8674(91)90397-h. Identification of the gene responsible for FXS as a trinucleotide repeat disorder. [DOI] [PubMed] [Google Scholar]
- 4.Krawczun MS, Jenkins EC, Brown WT. Analysis of the fragile-X chromosome: localization and detection of the fragile site in high resolution preparations. Hum Genet. 1985;69:209–211. doi: 10.1007/BF00293026. [DOI] [PubMed] [Google Scholar]
- 5*.The Dutch-Belgian Fragile X Consorthium. Bakker CE, Verheij C, Willemsen R, van der Helm R, Oerlemans F, Vermey M, Bygrave A, Hoogeveen AT, Oostra BA, Reyniers E, De Boule K, D’Hooge R, Cras P, van Velzen D, Nagels G, Martin J, De Deyn PP, Darby JK, Willems PJ. Fmr1 knockout mice: a model to study fragile X mental retardation. Cell. 1994;78:23–33. Report of the first fmr1 KO mouse line. [PubMed] [Google Scholar]
- 6.Fu YH, Kuhl DP, Pizzuti A, Pieretti M, Sutcliffe JS, Richards S, Verkerk AJ, Holden JJ, Fenwick RG, Warren ST. Variation of the CGG repeat at the fragile X site results in genetic instability: resolution of the Sherman paradox. Cell. 1991;67:1047–1058. doi: 10.1016/0092-8674(91)90283-5. [DOI] [PubMed] [Google Scholar]
- 7.Pieretti M, Zhang FP, Fu YH, Warren ST, Oostra BA, Caskey CT, Nelson DL. Absence of expression of the FMR-1 gene in fragile X syndrome. Cell. 1991;66:817–822. doi: 10.1016/0092-8674(91)90125-i. [DOI] [PubMed] [Google Scholar]
- 8.Verheij C, Bakker CE, de Graaff E, Keulemans J, Willemsen R, Verkerk AJ, Galjaard H, Reuser AJ, Hoogeveen AT, Oostra BA. Characterization and localization of the FMR-1 gene product associated with fragile X syndrome. Nature. 1993;363:722–724. doi: 10.1038/363722a0. [DOI] [PubMed] [Google Scholar]
- 9.Coffee B, Ikeda M, Budimirovic DB, Hjelm LN, Kaufmann WE, Warren ST. Mosaic FMR1 deletion causes fragile X syndrome and can lead to molecular misdiagnosis: a case report and review of the literature. Am J Med Genet A. 2008;146A:1358–1367. doi: 10.1002/ajmg.a.32261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Collins SC, Bray SM, Suhl JA, Cutler DJ, Coffee B, Zwick ME, Warren ST. Identification of novel FMR1 variants by massively parallel sequencing in developmentally delayed males. Am J Med Genet A. 2010;152A:2512–2520. doi: 10.1002/ajmg.a.33626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.De Boulle K, Verkerk AJ, Reyniers E, Vits L, Hendrickx J, Van Roy B, Van den Bos F, de Graaff E, Oostra BA, Willems PJ. A point mutation in the FMR-1 gene associated with fragile X mental retardation. Nat Genet. 1993;3:31–35. doi: 10.1038/ng0193-31. [DOI] [PubMed] [Google Scholar]
- 12.Greenough WT, Klintsova AY, Irwin SA, Galvez R, Bates KE, Weiler IJ. Synaptic regulation of protein synthesis and the fragile X protein. Proc Natl Acad Sci U S A. 2001;98:7101–7106. doi: 10.1073/pnas.141145998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hinton VJ, Brown WT, Wisniewski K, Rudelli RD. Analysis of neocortex in three males with the fragile X syndrome. Am J Med Genet. 1991;41:289–294. doi: 10.1002/ajmg.1320410306. [DOI] [PubMed] [Google Scholar]
- 14.Irwin SA, Patel B, Idupulapati M, Harris JB, Crisostomo RA, Larsen BP, Kooy F, Willems PJ, Cras P, Kozlowski PB, et al. Abnormal dendritic spine characteristics in the temporal and visual cortices of patients with fragile-X syndrome: a quantitative examination. Am J Med Genet. 2001;98:161–167. doi: 10.1002/1096-8628(20010115)98:2<161::aid-ajmg1025>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 15.Comery TA, Harris JB, Willems PJ, Oostra BA, Irwin SA, Weiler IJ, Greenough WT. Abnormal dendritic spines in fragile X knockout mice: maturation and pruning deficits. Proc Natl Acad Sci U S A. 1997;94:5401–5404. doi: 10.1073/pnas.94.10.5401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nimchinsky EA, Oberlander AM, Svoboda K. Abnormal development of dendritic spines in FMR1 knock-out mice. J Neurosci. 2001;21:5139–5146. doi: 10.1523/JNEUROSCI.21-14-05139.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Penagarikano O, Mulle JG, Warren ST. The pathophysiology of fragile x syndrome. Annu Rev Genomics Hum Genet. 2007;8:109–129. doi: 10.1146/annurev.genom.8.080706.092249. [DOI] [PubMed] [Google Scholar]
- 18.Devys D, Lutz Y, Rouyer N, Bellocq JP, Mandel JL. The FMR-1 protein is cytoplasmic, most abundant in neurons and appears normal in carriers of a fragile X premutation. Nat Genet. 1993;4:335–340. doi: 10.1038/ng0893-335. [DOI] [PubMed] [Google Scholar]
- 19.Hinds HL, Ashley CT, Sutcliffe JS, Nelson DL, Warren ST, Housman DE, Schalling M. Tissue specific expression of FMR-1 provides evidence for a functional role in fragile X syndrome. Nat Genet. 1993;3:36–43. doi: 10.1038/ng0193-36. [DOI] [PubMed] [Google Scholar]
- 20.Bagni C, Greenough WT. From mRNP trafficking to spine dysmorphogenesis: the roots of fragile X syndrome. Nat Rev Neurosci. 2005;6:376–387. doi: 10.1038/nrn1667. [DOI] [PubMed] [Google Scholar]
- 21**.Santoro MR, Bray SM, Warren ST. Molecular Mechanisms of Fragile X Syndrome: A Twenty-Year Perspective. Annu Rev Pathol. 2011 doi: 10.1146/annurev-pathol-011811-132457. This review of the molecular mechanism of FXS begins with the cloning of the gene for FXS in 1991 and includes recent findings to provide a twenty-year perspective on FXS. [DOI] [PubMed] [Google Scholar]
- 22.Blackwell E, Zhang X, Ceman S. Arginines of the RGG box regulate FMRP association with polyribosomes and mRNA. Hum Mol Genet. 2010;19:1314–1323. doi: 10.1093/hmg/ddq007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chen L, Yun SW, Seto J, Liu W, Toth M. The fragile X mental retardation protein binds and regulates a novel class of mRNAs containing U rich target sequences. Neuroscience. 2003;120:1005–1017. doi: 10.1016/s0306-4522(03)00406-8. [DOI] [PubMed] [Google Scholar]
- 24.Fahling M, Mrowka R, Steege A, Kirschner KM, Benko E, Forstera B, Persson PB, Thiele BJ, Meier JC, Scholz H. Translational regulation of the human achaete-scute homologue-1 by fragile X mental retardation protein. J Biol Chem. 2009;284:4255–4266. doi: 10.1074/jbc.M807354200. [DOI] [PubMed] [Google Scholar]
- 25**.Darnell JC, Jensen KB, Jin P, Brown V, Warren ST, Darnell RB. Fragile X mental retardation protein targets G quartet mRNAs important for neuronal function. Cell. 2001;107:489–499. doi: 10.1016/s0092-8674(01)00566-9. This study identifies FMRP-associated mRNAs, including transcripts for voltage-gated potassium channels such as Kv3.1, via the SELEX method, and investigates the binding of FMRP to G–quartets that involves the RGG box but not the KH domain. [DOI] [PubMed] [Google Scholar]
- 26.Schaeffer C, Bardoni B, Mandel JL, Ehresmann B, Ehresmann C, Moine H. The fragile X mental retardation protein binds specifically to its mRNA via a purine quartet motif. EMBO J. 2001;20:4803–4813. doi: 10.1093/emboj/20.17.4803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Darnell JC, Fraser CE, Mostovetsky O, Stefani G, Jones TA, Eddy SR, Darnell RB. Kissing complex RNAs mediate interaction between the Fragile-X mental retardation protein KH2 domain and brain polyribosomes. Genes Dev. 2005;19:903–918. doi: 10.1101/gad.1276805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bechara EG, Didiot MC, Melko M, Davidovic L, Bensaid M, Martin P, Castets M, Pognonec P, Khandjian EW, Moine H, et al. A novel function for fragile X mental retardation protein in translational activation. PLoS Biol. 2009;7:e16. doi: 10.1371/journal.pbio.1000016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dictenberg JB, Swanger SA, Antar LN, Singer RH, Bassell GJ. A direct role for FMRP in activity-dependent dendritic mRNA transport links filopodial-spine morphogenesis to fragile X syndrome. Dev Cell. 2008;14:926–939. doi: 10.1016/j.devcel.2008.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zalfa F, Eleuteri B, Dickson KS, Mercaldo V, De Rubeis S, di Penta A, Tabolacci E, Chiurazzi P, Neri G, Grant SG, et al. A new function for the fragile X mental retardation protein in regulation of PSD-95 mRNA stability. Nat Neurosci. 2007;10:578–587. doi: 10.1038/nn1893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Muddashetty RS, Kelic S, Gross C, Xu M, Bassell GJ. Dysregulated metabotropic glutamate receptor-dependent translation of AMPA receptor and postsynaptic density-95 mRNAs at synapses in a mouse model of fragile X syndrome. J Neurosci. 2007;27:5338–5348. doi: 10.1523/JNEUROSCI.0937-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zalfa F, Giorgi M, Primerano B, Moro A, Di Penta A, Reis S, Oostra B, Bagni C. The fragile X syndrome protein FMRP associates with BC1 RNA and regulates the translation of specific mRNAs at synapses. Cell. 2003;112:317–327. doi: 10.1016/s0092-8674(03)00079-5. [DOI] [PubMed] [Google Scholar]
- 33.Bassell GJ, Warren ST. Fragile X syndrome: loss of local mRNA regulation alters synaptic development and function. Neuron. 2008;60:201–214. doi: 10.1016/j.neuron.2008.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Antar LN, Bassell GJ. Sunrise at the synapse: the FMRP mRNP shaping the synaptic interface. Neuron. 2003;37:555–558. doi: 10.1016/s0896-6273(03)00090-4. [DOI] [PubMed] [Google Scholar]
- 35.Li Z, Zhang Y, Ku L, Wilkinson KD, Warren ST, Feng Y. The fragile X mental retardation protein inhibits translation via interacting with mRNA. Nucleic Acids Res. 2001;29:2276–2283. doi: 10.1093/nar/29.11.2276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Laggerbauer B, Ostareck D, Keidel EM, Ostareck-Lederer A, Fischer U. Evidence that fragile X mental retardation protein is a negative regulator of translation. Hum Mol Genet. 2001;10:329–338. doi: 10.1093/hmg/10.4.329. [DOI] [PubMed] [Google Scholar]
- 37.Mazroui R, Huot ME, Tremblay S, Filion C, Labelle Y, Khandjian EW. Trapping of messenger RNA by Fragile X Mental Retardation protein into cytoplasmic granules induces translation repression. Hum Mol Genet. 2002;11:3007–3017. doi: 10.1093/hmg/11.24.3007. [DOI] [PubMed] [Google Scholar]
- 38.Lu R, Wang H, Liang Z, Ku L, O’donnell WT, Li W, Warren ST, Feng Y. The fragile X protein controls microtubule-associated protein 1B translation and microtubule stability in brain neuron development. Proc Natl Acad Sci U S A. 2004;101:15201–15206. doi: 10.1073/pnas.0404995101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ceman S, O’Donnell WT, Reed M, Patton S, Pohl J, Warren ST. Phosphorylation influences the translation state of FMRP-associated polyribosomes. Hum Mol Genet. 2003;12:3295–3305. doi: 10.1093/hmg/ddg350. [DOI] [PubMed] [Google Scholar]
- 40.Narayanan U, Nalavadi V, Nakamoto M, Pallas DC, Ceman S, Bassell GJ, Warren ST. FMRP phosphorylation reveals an immediate-early signaling pathway triggered by group I mGluR and mediated by PP2A. J Neurosci. 2007;27:14349–14357. doi: 10.1523/JNEUROSCI.2969-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Narayanan U, Nalavadi V, Nakamoto M, Thomas G, Ceman S, Bassell GJ, Warren ST. S6K1 phosphorylates and regulates fragile X mental retardation protein (FMRP) with the neuronal protein synthesis-dependent mammalian target of rapamycin (mTOR) signaling cascade. J Biol Chem. 2008;283:18478–18482. doi: 10.1074/jbc.C800055200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42*.Muddashetty RS, Nalavadi VC, Gross C, Yao X, Xing L, Laur O, Warren ST, Bassell GJ. Reversible inhibition of PSD-95 mRNA translation by miR-125a, FMRP phosphorylation, and mGluR signaling. Mol Cell. 2011;42:673–688. doi: 10.1016/j.molcel.2011.05.006. This study shows that miR-125a targeting of PSD-95 mRNA allows reversible inhibition of translation and regulation by group I mGluR signaling and regulates spine morphology. Moreover, this study reveals that the phosphorylated FMRP forms an inhibitory complex with PSD-95 mRNA, AGO2, and miR-125a, and mGluR signals the release of RISC from the FMRP/PSD-95 mRNA complex to activate translation. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43**.Lee HY, Ge WP, Huang W, He Y, Wang GX, Rowson-Baldwin A, Smith SJ, Jan YN, Jan LY. Bidirectional regulation of dendritic voltage-gated potassium channels by the fragile X mental retardation protein. Neuron. 2011;72:630–642. doi: 10.1016/j.neuron.2011.09.033. This study reports dendritic localization of Kv4.2 mRNA and FMRP suppression of local translation of Kv4.2 in dendrites, and shows the 3’UTR of Kv4.2 binds the RGG domain of FMRP and is involved in FMRP suppression of Kv4.2 in hippocampal neuronal dendrites. This suppression may be relieved by NMDA receptor-mediated dephosphorylation of FMRP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cheever A, Ceman S. Phosphorylation of FMRP inhibits association with Dicer. RNA. 2009;15:362–366. doi: 10.1261/rna.1500809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45**.Berry-Kravis E, Knox A, Hervey C. Targeted treatments for fragile X syndrome. J Neurodev Disord. 2011;3:193–210. doi: 10.1007/s11689-011-9074-7. This review describes targeted treatments developed for FXS and subgroups of the autism spectrum disorders. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46**.Hagerman R, Lauterborn J, Au J, Berry-Kravis E. Fragile X syndrome and targeted treatment trials. Results Probl Cell Differ. 2012;54:297–335. doi: 10.1007/978-3-642-21649-7_17. This review describes the use of mGluR blockers and gamma amino-butyric acid (GABA) agonists in mouse and fly models and in clinical trials for treating FXS. Proposed new pharmacological treatments and educational interventions are also discussed for treating FXS with possible translational applications to other neurodevelopmental disorders including autism. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47**.Huber KM, Gallagher SM, Warren ST, Bear MF. Altered synaptic plasticity in a mouse model of fragile X mental retardation. Proc Natl Acad Sci U S A. 2002;99:7746–7750. doi: 10.1073/pnas.122205699. This report shows that mGluR-LTD is significantly augmented in the hippocampus of fmr1-KO mice. Therefore, the authors proposed that exaggerated LTD and/or mGluR function are responsible for aspects of the behavioral phenotype in FXS, and that antagonists of group 1 mGluRs should be considered as possible therapeutic agents. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Nosyreva ED, Huber KM. Metabotropic receptor-dependent long-term depression persists in the absence of protein synthesis in the mouse model of fragile X syndrome. J Neurophysiol. 2006;95:3291–3295. doi: 10.1152/jn.01316.2005. [DOI] [PubMed] [Google Scholar]
- 49.Qin M, Kang J, Burlin TV, Jiang C, Smith CB. Postadolescent changes in regional cerebral protein synthesis: an in vivo study in the FMR1 null mouse. J Neurosci. 2005;25:5087–5095. doi: 10.1523/JNEUROSCI.0093-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50*.Dolen G, Osterweil E, Rao BS, Smith GB, Auerbach BD, Chattarji S, Bear MF. Correction of fragile X syndrome in mice. Neuron. 2007;56:955–962. doi: 10.1016/j.neuron.2007.12.001. This paper presents genetic evidence that reduction of mGluR5 levels rescues FXS phenotypes in mice. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51**.Bear MF, Huber KM, Warren ST. The mGluR theory of fragile X mental retardation. Trends Neurosci. 2004;27:370–377. doi: 10.1016/j.tins.2004.04.009. This review proposes the mGluR theory of FXS. [DOI] [PubMed] [Google Scholar]
- 52**.Krueger DD, Bear MF. Toward fulfilling the promise of molecular medicine in fragile X syndrome. Annu Rev Med. 2011;62:411–429. doi: 10.1146/annurev-med-061109-134644. This review describes recent progress in the study of FXS and discusses the implications of these findings for autism and other related neurodevelopmental disorders. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Waung MW, Huber KM. Protein translation in synaptic plasticity: mGluR-LTD, Fragile X. Curr Opin Neurobiol. 2009;19:319–326. doi: 10.1016/j.conb.2009.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Gasparini F, Lingenhohl K, Stoehr N, Flor PJ, Heinrich M, Vranesic I, Biollaz M, Allgeier H, Heckendorn R, Urwyler S, et al. 2-Methyl-6-(phenylethynyl)-pyridine (MPEP), a potent, selective and systemically active mGlu5 receptor antagonist. Neuropharmacology. 1999;38:1493–1503. doi: 10.1016/s0028-3908(99)00082-9. [DOI] [PubMed] [Google Scholar]
- 55*.Yan QJ, Rammal M, Tranfaglia M, Bauchwitz RP. Suppression of two major Fragile X Syndrome mouse model phenotypes by the mGluR5 antagonist MPEP. Neuropharmacology. 2005;49:1053–1066. doi: 10.1016/j.neuropharm.2005.06.004. This is the first reported study to examine the effect of MPEP on the FXS mouse model. MPEP could rescue audiogenic seizures and open field activity, two of the major phenotypes of fmr1 KO mice. [DOI] [PubMed] [Google Scholar]
- 56*.de Vrij FM, Levenga J, van der Linde HC, Koekkoek SK, De Zeeuw CI, Nelson DL, Oostra BA, Willemsen R. Rescue of behavioral phenotype and neuronal protrusion morphology in Fmr1 KO mice. Neurobiol Dis. 2008;31:127–132. doi: 10.1016/j.nbd.2008.04.002. This study demonstrates a clear defect in prepulse inhibition of startle in fmr1 KO mice that could be rescued by MPEP. Moreover, it showed for the first time a structural rescue of Fragile X related protrusion morphology with two independent mGluR5 antagonists. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Nakamoto M, Nalavadi V, Epstein MP, Narayanan U, Bassell GJ, Warren ST. Fragile X mental retardation protein deficiency leads to excessive mGluR5-dependent internalization of AMPA receptors. Proc Natl Acad Sci U S A. 2007;104:15537–15542. doi: 10.1073/pnas.0707484104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Chuang SC, Zhao W, Bauchwitz R, Yan Q, Bianchi R, Wong RK. Prolonged epileptiform discharges induced by altered group I metabotropic glutamate receptor-mediated synaptic responses in hippocampal slices of a fragile X mouse model. J Neurosci. 2005;25:8048–8055. doi: 10.1523/JNEUROSCI.1777-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Aschrafi A, Cunningham BA, Edelman GM, Vanderklish PW. The fragile X mental retardation protein and group I metabotropic glutamate receptors regulate levels of mRNA granules in brain. Proc Natl Acad Sci U S A. 2005;102:2180–2185. doi: 10.1073/pnas.0409803102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Osterweil EK, Krueger DD, Reinhold K, Bear MF. Hypersensitivity to mGluR5 and ERK1/2 leads to excessive protein synthesis in the hippocampus of a mouse model of fragile X syndrome. J Neurosci. 2010;30:15616–15627. doi: 10.1523/JNEUROSCI.3888-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Min WW, Yuskaitis CJ, Yan Q, Sikorski C, Chen S, Jope RS, Bauchwitz RP. Elevated glycogen synthase kinase-3 activity in Fragile X mice: key metabolic regulator with evidence for treatment potential. Neuropharmacology. 2009;56:463–472. doi: 10.1016/j.neuropharm.2008.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Bassell GJ, Gross C. Reducing glutamate signaling pays off in fragile X. Nat Med. 2008;14:249–250. doi: 10.1038/nm0308-249. [DOI] [PubMed] [Google Scholar]
- 63*.Lauterborn JC, Rex CS, Kramar E, Chen LY, Pandyarajan V, Lynch G, Gall CM. Brain-derived neurotrophic factor rescues synaptic plasticity in a mouse model of fragile X syndrome. J Neurosci. 2007;27:10685–10694. doi: 10.1523/JNEUROSCI.2624-07.2007. This study finds that LTP elicited by threshold levels of theta burst stimulation (TBS) is severely impaired in the hippocampal field CA1 of young adult fmr1 KO mice. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64*.Shang Y, Wang H, Mercaldo V, Li X, Chen T, Zhuo M. Fragile X mental retardation protein is required for chemically-induced long-term potentiation of the hippocampus in adult mice. J Neurochem. 2009;111:635–646. doi: 10.1111/j.1471-4159.2009.06314.x. This report demonstrate that FMRP is required for glycine-induced LTP (Gly-LTP) in the CA1 of hippocampus. [DOI] [PubMed] [Google Scholar]
- 65.Chen LY, Rex CS, Babayan AH, Kramar EA, Lynch G, Gall CM, Lauterborn JC. Physiological activation of synaptic Rac>PAK (p-21 activated kinase) signaling is defective in a mouse model of fragile X syndrome. J Neurosci. 2010;30:10977–10984. doi: 10.1523/JNEUROSCI.1077-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wilson BM, Cox CL. Absence of metabotropic glutamate receptor-mediated plasticity in the neocortex of fragile X mice. Proc Natl Acad Sci U S A. 2007;104:2454–2459. doi: 10.1073/pnas.0610875104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Suvrathan A, Hoeffer CA, Wong H, Klann E, Chattarji S. Characterization and reversal of synaptic defects in the amygdala in a mouse model of fragile X syndrome. Proc Natl Acad Sci U S A. 2010;107:11591–11596. doi: 10.1073/pnas.1002262107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68*.Ashley CT, Wilkinson KD, Reines D, Warren ST. FMR1 protein: conserved RNP family domains and selective RNA binding. Science. 1993;262:563–566. doi: 10.1126/science.7692601. This paper reports the discovery that FMRP is an RNA-binding protein. [DOI] [PubMed] [Google Scholar]
- 69**.Brown V, Jin P, Ceman S, Darnell JC, O’Donnell WT, Tenenbaum SA, Jin X, Feng Y, Wilkinson KD, Keene JD, et al. Microarray identification of FMRP-associated brain mRNAs and altered mRNA translational profiles in fragile X syndrome. Cell. 2001;107:477–487. doi: 10.1016/s0092-8674(01)00568-2. This study identifies putative FMRP-associated brain mRNAs. [DOI] [PubMed] [Google Scholar]
- 70.Miyashiro KY, Beckel-Mitchener A, Purk TP, Becker KG, Barret T, Liu L, Carbonetto S, Weiler IJ, Greenough WT, Eberwine J. RNA cargoes associating with FMRP reveal deficits in cellular functioning in Fmr1 null mice. Neuron. 2003;37:417–431. doi: 10.1016/s0896-6273(03)00034-5. [DOI] [PubMed] [Google Scholar]
- 71.Zou K, Liu J, Zhu N, Lin J, Liang Q, Brown WT, Shen Y, Zhong N. Identification of FMRP-associated mRNAs using yeast three-hybrid system. Am J Med Genet B Neuropsychiatr Genet. 2008;147B:769–777. doi: 10.1002/ajmg.b.30678. [DOI] [PubMed] [Google Scholar]
- 72**.Darnell JC, Van Driesche SJ, Zhang C, Hung KY, Mele A, Fraser CE, Stone EF, Chen C, Fak JJ, Chi SW, et al. FMRP stalls ribosomal translocation on mRNAs linked to synaptic function and autism. Cell. 2011;146:247–261. doi: 10.1016/j.cell.2011.06.013. This study uses high-throughput sequencing of RNAs isolated through cross-linking immunoprecipitation (HITS-CLIP) to identify FMRP target mRNAs in neurons, revealing that FMRP stalls ribosomes during elongation. The FMRP targets identified include transcripts for several potassium channels such as Kv4.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Bardoni B, Castets M, Huot ME, Schenck A, Adinolfi S, Corbin F, Pastore A, Khandjian EW, Mandel JL. 82-FIP, a novel FMRP (fragile X mental retardation protein) interacting protein, shows a cell cycle-dependent intracellular localization. Hum Mol Genet. 2003;12:1689–1698. doi: 10.1093/hmg/ddg181. [DOI] [PubMed] [Google Scholar]
- 74.Nichols CG, Lopatin AN. Inward rectifier potassium channels. Annu Rev Physiol. 1997;59:171–191. doi: 10.1146/annurev.physiol.59.1.171. [DOI] [PubMed] [Google Scholar]
- 75.Enyedi P, Czirjak G. Molecular background of leak K+ currents: two-pore domain potassium channels. Physiol Rev. 2010;90:559–605. doi: 10.1152/physrev.00029.2009. [DOI] [PubMed] [Google Scholar]
- 76.Lai HC, Jan LY. The distribution and targeting of neuronal voltage-gated ion channels. Nat Rev Neurosci. 2006;7:548–562. doi: 10.1038/nrn1938. [DOI] [PubMed] [Google Scholar]
- 77**.Brown MR, Kronengold J, Gazula VR, Chen Y, Strumbos JG, Sigworth FJ, Navaratnam D, Kaczmarek LK. Fragile X mental retardation protein controls gating of the sodium-activated potassium channel Slack. Nat Neurosci. 2010;13:819–821. doi: 10.1038/nn.2563. Using biochemical and electrophysiological studies, these authors found that FMRP binds to the C-terminus of the Slack to activate the channel in mice. These findings suggest that Slack activity provides a link between patterns of neuronal firing and changes in potassium channel functions. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78**.Strumbos JG, Brown MR, Kronengold J, Polley DB, Kaczmarek LK. Fragile X mental retardation protein is required for rapid experience-dependent regulation of the potassium channel Kv3.1b. J Neurosci. 2010;30:10263–10271. doi: 10.1523/JNEUROSCI.1125-10.2010. This report shows that fmr1 KO mice display dramatically flattened tonotopicity in Kv3.1b immunoreactivity and K+ currents as compared to WT controls. Moreover, after 30 min of acoustic stimulation, the levels of Kv3.1b immunoreactivity were significantly elevated in both the MNTB and AVCN of WT, but not fmr1 KO mice. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Miller LJ, McIntosh DN, McGrath J, Shyu V, Lampe M, Taylor AK, Tassone F, Neitzel K, Stackhouse T, Hagerman RJ. Electrodermal responses to sensory stimuli in individuals with fragile X syndrome: a preliminary report. Am J Med Genet. 1999;83:268–279. [PubMed] [Google Scholar]
- 80.Hanson DM, Jackson AW, Hagerman RJ. Speech disturbances (cluttering) in mildly impaired males with the Martin-Bell/fragile X syndrome. Am J Med Genet. 1986;23:195–206. doi: 10.1002/ajmg.1320230114. [DOI] [PubMed] [Google Scholar]
- 81.Bhattacharjee A, Joiner WJ, Wu M, Yang Y, Sigworth FJ, Kaczmarek LK. Slick (Slo2.1), a rapidly-gating sodium-activated potassium channel inhibited by ATP. J Neurosci. 2003;23:11681–11691. doi: 10.1523/JNEUROSCI.23-37-11681.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Yang B, Desai R, Kaczmarek LK. Slack and Slick K(Na) channels regulate the accuracy of timing of auditory neurons. J Neurosci. 2007;27:2617–2627. doi: 10.1523/JNEUROSCI.5308-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Kaczmarek LK, Bhattacharjee A, Desai R, Gan L, Song P, von Hehn CA, Whim MD, Yang B. Regulation of the timing of MNTB neurons by short-term and long-term modulation of potassium channels. Hear Res. 2005;206:133–145. doi: 10.1016/j.heares.2004.11.023. [DOI] [PubMed] [Google Scholar]
- 84.Nanou E, El Manira A. Mechanisms of modulation of AMPA-induced Na+-activated K+ current by mGluR1. J Neurophysiol. 2010;103:441–445. doi: 10.1152/jn.00584.2009. [DOI] [PubMed] [Google Scholar]
- 85.Wang LY, Gan L, Forsythe ID, Kaczmarek LK. Contribution of the Kv3.1 potassium channel to high-frequency firing in mouse auditory neurones. J Physiol. 1998;509(Pt 1):183–194. doi: 10.1111/j.1469-7793.1998.183bo.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Rudy B, McBain CJ. Kv3 channels: voltage-gated K+ channels designed for high-frequency repetitive firing. Trends Neurosci. 2001;24:517–526. doi: 10.1016/s0166-2236(00)01892-0. [DOI] [PubMed] [Google Scholar]
- 87.Kim J, Hoffman DA. Potassium channels: newly found players in synaptic plasticity. Neuroscientist. 2008;14:276–286. doi: 10.1177/1073858408315041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Chen X, Yuan LL, Zhao C, Birnbaum SG, Frick A, Jung WE, Schwarz TL, Sweatt JD, Johnston D. Deletion of Kv4.2 gene eliminates dendritic A-type K+ current and enhances induction of long-term potentiation in hippocampal CA1 pyramidal neurons. J Neurosci. 2006;26:12143–12151. doi: 10.1523/JNEUROSCI.2667-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89*.Jung SC, Kim J, Hoffman DA. Rapid, bidirectional remodeling of synaptic NMDA receptor subunit composition by A-type K+ channel activity in hippocampal CA1 pyramidal neurons. Neuron. 2008;60:657–671. doi: 10.1016/j.neuron.2008.08.029. Authors show that altering functional Kv4.2 expression level leads to a rapid, bidirectional remodeling of CA1 synapses. Neurons exhibiting enhanced IA show a decrease in relative synaptic NR2B/NR2A subunit composition and do not exhibit LTP. Conversely, reducing IA by expression of a Kv4.2 dominant-negative or through genomic knockout of Kv4.2 led to an increased fraction of synaptic NR2B/NR2A and enhanced LTP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Pilpel Y, Kolleker A, Berberich S, Ginger M, Frick A, Mientjes E, Oostra BA, Seeburg PH. Synaptic ionotropic glutamate receptors and plasticity are developmentally altered in the CA1 field of Fmr1 knockout mice. J Physiol. 2009;587:787–804. doi: 10.1113/jphysiol.2008.160929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Losonczy A, Makara JK, Magee JC. Compartmentalized dendritic plasticity and input feature storage in neurons. Nature. 2008;452:436–441. doi: 10.1038/nature06725. [DOI] [PubMed] [Google Scholar]
- 92.Kim J, Jung SC, Clemens AM, Petralia RS, Hoffman DA. Regulation of dendritic excitability by activity-dependent trafficking of the A-type K+ channel subunit Kv4.2 in hippocampal neurons. Neuron. 2007;54:933–947. doi: 10.1016/j.neuron.2007.05.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Lei Z, Deng P, Xu ZC. Regulation of Kv4.2 channels by glutamate in cultured hippocampal neurons. J Neurochem. 2008;106:182–192. doi: 10.1111/j.1471-4159.2008.05356.x. [DOI] [PubMed] [Google Scholar]
- 94.Lei Z, Deng P, Li Y, Xu ZC. Downregulation of Kv4.2 channels mediated by NR2B-containing NMDA receptors in cultured hippocampal neurons. Neuroscience. 2010;165:350–362. doi: 10.1016/j.neuroscience.2009.10.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Rundfeldt C. Potassium Channels and Neurodegenerative Diseases. Drug News Perspect. 1999;12:99–104. [Google Scholar]
- 96.Thayer DA, Jan LY. Mechanisms of distribution and targeting of neuronal ion channels. Curr Opin Drug Discov Devel. 2010;13:559–567. [PubMed] [Google Scholar]


