Abstract
Objective
The prevalence of cigarette smoking among people with schizophrenia is greater than that of the general population. Because smoking and use of other drugs covary, we examined illicit drug use in current smokers not trying to quit or reduce their tobacco use. We recruited outpatient participants who had a DSM-IV diagnosis of schizophrenia or schizoaffective disorder (schizophrenia, n=70) and a control group who had no Axis I psychiatric disorders (control, n=97). During a 2-3 hour session, participants completed demographic and research questionnaires, including the Drug Use Survey (DUS).
Results
Participants with schizophrenia were older than controls (p<0.001) and smoked more cigarettes per day (p=0.01), but did not differ in degree of nicotine dependence. Ever using a drug was similar between the groups, except that significantly more participants with schizophrenia reported ever using hallucinogens (p<0.001) and inhalants (p=0.001). For alcohol, cocaine, and marijuana, fewer participants with schizophrenia were current users, but more participants with schizophrenia were past users (p’s<0.0001). Heavy smokers from the general population continued to use illicit drugs throughout their lives, while schizophrenia participants had the highest period of illicit drug use in their 20’s.
Conclusions
These data suggest that illicit drug use tends to be high in heavy cigarette smokers, regardless of a schizophrenia diagnosis. However, while illicit drug use is high across the lifespan of heavy smokers in the general population, heavy smokers with schizophrenia use illicit drugs mostly in the first decade of their illness.
Keywords: schizophrenia, smoking, cigarettes, tobacco, drug use, alcohol, marijuana
1. Introduction
Cigarette smoking is the leading preventable cause of death in the Western world (Mokdad et al, 2004). The prevalence of smoking in the United States adult population is approximately 20%, with more than 8 million people sick or disabled because of their tobacco use; Additionally, 450,000 people die from smoking related illnesses each year (Giovino, 2002; Schroeder and Warner, 2010). Those with mental illness or substance-use disorders are more susceptible to these diseases because they smoke more cigarettes per day and have more difficulty quitting (Dalack et al., 1998; de Leon 1996; de Leon et al., 2002; Hughes et al., 1986; Kelly et al., 2011).
The prevalence of cigarette smoking among people with schizophrenia is 58-90% (de Leon et al., 2002; Grant et al., 2004; Lasser et al., 2000). Although the exact causes of the high smoking prevalence in schizophrenia populations are unknown, an interaction of factors such as boredom, reward for good behavior, self-medication, and reduction of negative antipsychotic side effects is thought to be involved (Hughes et al., 1986; Simosky et al., 2002). Additionally, studies have suggested that nicotinic acetylcholine receptors (nAChRs) may contribute to the pathophysiology of schizophrenia (Leonard et al., 2000), as schizophrenia patients tend to have reduced nAChR expression compared with controls (Fonder et al., 2005; Breese et al., 2000). Schizophrenia patients tend to be heavier smokers than smokers in the general population, which is reflected in higher morbidity and mortality rates (Kelly et al., 2011; Saha et al., 2007). Among schizophrenia patients age 35-54 who smoke, the odds of cardiac death were increased 12-fold relative to nonsmokers (Kelly et al., 2011). Despite these increased risks, quit rates among people with schizophrenia remain low (de Leon, 1996; Lasser et al., 2000), especially among those patients treated with first generation antipsychotics (George et al., 2000).
A significant cause for concern in the schizophrenia population, in addition to increased morbidity and mortality rates (Goff et al., 2005; Kelly et al., 2007), is that cigarette smoking and use of other drugs often covary (SAMHSA, 2010). Comorbid schizophrenia and substance abuse has risen dramatically in recent years, from about 30% prevalence in the 1970s to approximately 60% by 1990 (el Guebaly and Hodgins, 1992; Fowler et al, 1998; Gogek, 1991; Searles et al., 1990; Westermeyer, 2006). Deinstitutionalization, psychiatric care budget reductions, and disability payments likely contribute to this rise in comorbidity (Klatte et al., 1969; Ridgely and Willenbring, 1992). Among inpatients, the prevalence of current substance abuse or dependence ranges from 8% to 42%, and lifetime abuse or dependence ranges from 22% to 70% (Kovasznay, 1991; Owen et al., 1996; Sanguineti & Samuel, 1993; Soyka et al., 1993). For outpatients, the prevalence of current abuse or dependence ranges from 6% to 27%, and lifetime abuse or dependence ranges from 51% to 60% (Chouljian et al., 1995; Drake et al., 1990; Fowler et al., 1998). The Epidemiological Catchment Area (ECA) Study found that the odds of someone having a substance abuse diagnosis who was also diagnosed with schizophrenia was 4.6 times higher than for the rest of the population (Regier et al., 1990). The combination of a psychiatric disorder with a substance use disorder greatly increases the chance that an individual will need psychological and health services (Kessler et al., 1997), be more aggressive (Angermeyer, 2000; Soyka, 2000), and be less compliant with their medication (Pristach and Smith, 1990; Swartz et al, 1998). Among schizophrenia patients, we have found that rates of using alcohol, cannabis, cocaine, heroin, and other drugs of abuse were higher in smokers than in nonsmokers (Kelly et al., 2011). Drugs of abuse can also hasten the onset of schizophrenia or exacerbate the pre-existing illness. For example, pre-onset cannabis use might elicit psychotic and prodromal symptoms sooner than if there were no cannabis use (Compton et al., 2009).
Although this issue has not been studied in people with schizophrenia, nicotine use may be a gateway to other drug use, as smoking in adolescence significantly increases the probability of drug use and drug use disorders in early adulthood (Lewinsohn et al., 1999). Sixty-eight percent of individuals diagnosed with substance abuse or dependence are current smokers (Lasser et al., 2000). Among those receiving treatment for addiction, smokers have an increased mortality risk above that of their non-smoking, addiction treatment-seeking counterparts (Hser et al., 1993, 1994; Hurt et al., 1996). Cross-tolerance or cross-sensitization to the effects of a drug can develop when there is prior use of other drugs (Agrawal et al., 2008; Hoving et al., 2007; Kalant, 1996; Patton et al., 2005). Bechtholt and Mark (2002) reported that animals given the highest dose of nicotine showed a significant increase in number of self-administered cocaine infusions by the eighth day of nicotine treatment. Similarly in humans, cocaine users are more likely to smoke cigarettes than non-users, and cigarette use increases during cocaine use (Higgins et al., 1994; Roll et al., 1996). Increased cue-induced cocaine craving following nicotine administration, and subsequent decreased cocaine craving with nicotinic antagonist administration, has also been reported in cocaine addicts (Reid et al., 1998, 1999).
There are few data, beyond those reported in the ECA study (Regier et al. 1990), on the correlation of cigarette smoking and drug use in schizophrenia patients and comparing the findings to a control cohort. Because of the correlation between smoking and drug use, as well as the high prevalence of cigarette smoking and comorbidity in schizophrenia populations, we investigated whether the prevalence of illicit drug use in smokers with schizophrenia was greater compared to drug use prevalence in a group of smokers without schizophrenia.
2. Study Design and Methods
2.1 Participants
We recruited smokers who were not seeking treatment for tobacco or substance dependence, were between 18-65 years old, smoked at least 5 cigarettes per day, and had a breath carbon monoxide (CO) ≥ 8 parts per million (ppm). We recruited individuals with a DSM-IV diagnosis of schizophrenia or schizoaffective disorder and those with no major Axis-I psychotic disorder (controls), as determined by the Structured Clinical Interview for Axis-I DSM-IV disorders (SCID). Schizophrenia volunteers were recruited from the Maryland Psychiatric Research Center (MPRC). Only outpatients with schizophrenia were included because of restrictions on cigarette use among inpatients. Control volunteers were recruited by the National Institute on Drug Abuse (NIDA) via print, radio, and television advertisements. Subjects were compensated for their time and travel. University of Maryland, Maryland State Department of Health and Mental Hygiene, and NIDA Institutional Review Boards approved study procedures.
2.2 Procedures
Study participation consisted of one session lasting 2-3 hours. Following consent, screening procedures took place including a breath CO measurement and SCID. At the conclusion of screening and demographic interviews, participants smoked one preferred-brand cigarette to standardize the time since last tobacco exposure. After the cigarette, study assessments began and breath CO was measured 10-15 minutes after the cigarette. Study assessments included a semi-structured interview and research questionnaires. To measure CO, participants exhaled fully, inhaled deeply, and held their breath for 20 seconds before exhaling into a portable monitor (Vitalograph, Lenexa, KS).
2.3 Measures
The Drug Use Survey (DUS; Smith et al., 1992) was used to assess lifetime and current drug use. Questions included were: a) have you ever used a substance? (alcohol, heroin, cocaine, marijuana, amphetamines, hallucinogens, inhalants); b) have you used this substance more than 5 times?; c) when was your last use? (≤ 30 days [current use] vs. > 30 days [past use]); d) at what age did you first use this substance?; e) what is your substance of choice?; and f) how much money have you spent on drugs/alcohol in the past 30 days?
The 12-item Tobacco Craving Questionnaire-Short Form (TCQ-SF) (Heishman et al., 2008) was used to assess tobacco craving in four dimensions: Emotionality (anticipation of relief from withdrawal symptoms or negative mood), Expectancy (anticipation of positive outcomes from smoking), Compulsivity (lack of control over smoking), and Purposefulness (intention and planning to smoke for positive results). The TCQ-SF was administered immediately and approximately 15 minutes after smoking the cigarette. The Fagerström Test for Nicotine Dependence (FTND) (Heatherton et al., 1991, Steinberg et al., 2005) was used to assess degree of nicotine dependence.
2.4 Statistical Analysis
Differences in demographic characteristics and history of drug use between smokers with and without schizophrenia were examined using t-tests for continuous variables and chi-square tests for categorical measures. Associations between the groups with respect to dimensions of craving on the TCQ-SF were examined with two way analysis of variance according to use of specific illicit substances.
3. Results
3.1 Population
One hundred sixty-seven smokers were enrolled in this study (N=70 schizophrenia outpatients and N=97 controls). The demographic and clinical characteristics are listed in Table 1. The schizophrenia group was older (p < 0.001) and had more Caucasians (p = 0.006). Schizophrenia participants and controls had similar FTND scores, but the schizophrenia group smoked more cigarettes per day (p = 0.01), had higher levels of expired CO (p = 0.01), and had higher TCQ-SF scores (p = 0.002). Within the schizophrenia group, there were no differences in number of cigarettes per day (p = 0.37) or FTND scores (p = 0.72) based on antipsychotic metabolism by CYP1A2.
Table 1.
Demographic and Clinical Characteristics
| Schizophrenia (N = 70) | Controls (N = 97) | Statistics | |
|---|---|---|---|
| Age (y, mean) | 46.1 ± 10.2 | 37.2 ± 10.7 | t = 5.42, df = 165, P < 0.0001 |
| Sex (male) | 51 (73%) | 63 (65%) | χ2 = 1.17, df = 1, P = 0.28 |
| Race | χ2 = 14.45, df = 4, P = 0.006 | ||
| Caucasian | 41 (59%) | 31 (32%) | |
| African American | 27 (39%) | 64 (66%) | |
| Other races | 2 (3%) | 2 (2%) | |
| Level of education (y) | 12.1 ± 2.0 | 11.8 ± 1.8 | t = 1.05, df = 162, P = 0.30. |
| Marital status | χ2 = 1.68, df = 3, P = 0.64 | ||
| Presently married | 3 (4%) | 9 (9%) | |
| Widowed | 1 (2%) | 2 (2%) | |
| Divorced/Separated | 15 (22%) | 22 (23%) | |
| Single (never married) | 49 (70%) | 63 (65%) | |
| FTND total | 5.5 ± 2.0 | 5.3 ± 1.9 | t = 0.52, df = 163, P = 0.60 |
| Number of cigarettes smoked daily | 21.3 ± 11.7 | 17.1 ± 7.9 | t = 2.6, df = 113, P = 0.01 |
| Expired CO (15 mins post-cig) | 27.7 ± 15.3 | 22.6 ± 7.9 | t = 2.5, df = 96, P = 0.01 |
| TCQ-SF total (15 mins post-cig) | 48.2 ± 19.3 | 38.9 ± 19.6 | t = 3.0, df = 165, P = 0.002 |
| Years of smoking | 28.5 ± 10.7 | 19.9 ± 11.0 | t = 5.0, df = 163, P < 0.0001 |
| CYP1A2 metabolized antipsychotic | 27 (39%) |
3.2 Drug Use Survey (DUS)
Across all drug categories, lifetime drug use was similar, except that more schizophrenia participants than controls reported ever using hallucinogens (p = 0.001) and inhalants (p = 0.001). The percentages among participants who used a drug more than 5 times were similar in smokers with and without schizophrenia for all drugs except heroin and cocaine. Schizophrenia participants were less likely than controls to use heroin (p = 0.006) or cocaine (p = 0.003) more than 5 times (see Table 2). Notably, less than 3% of schizophrenia participants reported current use of any drug other than alcohol (17% current use), whereas considerably higher percentages of controls reported current use of some drug (alcohol 69%, heroin 12%, cocaine 25%, marijuana 45%). However, more schizophrenia participants than controls reported past, but not current, use of some substances (alcohol 71% vs. 29%, cocaine 51% vs. 34%, marijuana, 84% vs. 49%) with most having not used for more than a year. Fewer schizophrenia participants than controls were current heroin users (0% vs. 12%), but the percentage of past heroin users was the same between groups (see Table 3). Controlling for age, most current substance use occurred between the ages of 20 and 30 for both groups. The rates of current use were comparable between controls and schizophrenia participants at younger ages and then became more discrepant as participants got older. The rates of current use declined for schizophrenia participants but not for controls. This trend was strongest for alcohol (p < 0.0001) and marijuana (p = 0.002; figure 1), as these groups had the highest numbers of reported current users, but a similar pattern was also seen for other drugs of abuse. When asked about past treatment history, more schizophrenia participants than controls reported seeking alcohol treatment (p = 0.009), but not drug treatment (p = 0.977).
Table 2.
Ever using and using more than 5 times
| Schizophrenia (N = 70) | Controls (N = 97) | Statistics | |
|---|---|---|---|
| Ever use | |||
| Alcohol (n, %) | 67 (95.7%) | 95 (97.9%) | χ2 = 0.69, df = 1, P = 0.41 |
| Heroin | 24 (34.3%) | 45 (46.4%) | χ2 = 2.46, df = 1, P = 0.12 |
| Cocaine | 37 (52.9%) | 57 (58.8%) | χ2 = 0.58, df = 1, P = 0.45 |
| Marijuana | 61 (87.1%) | 91 (93.8%) | χ2 = 2.21, df = 1, P = 0.14 |
| Amphetamines | 19 (27.1%) | 22 (22.7%) | χ2 = 0.44, df = 1, P = 0.51 |
| Hallucinogens | 34 (48.6%) | 24 (24.7%) | χ2 = 10.18, df = 1, P = 0.001 |
| Inhalants | 15 (21.4%) | 5 (5.2%) | χ2 = 10.21, df = 1, P = 0.001 |
| More than 5 times | |||
| Alcohol (n, % of lifetime users) | 66 (98.5%) | 90 (94.7%) | χ2 = 1.57, df = 1, P = 0.21 |
| Heroin | 13 (54.2%) | 38 (84.4%) | χ2 = 7.44, df = 1, P = 0.006 |
| Cocaine | 27 (73.0%) | 54 (94.7%) | χ2 = 8.92, df = 1, P = 0.003 |
| Marijuana | 52 (85.3%) | 81 (89.0%) | χ2 = 0.47, df = 1, P = 0.49 |
| Amphetamines | 12 (66.7%) | 13 (61.9%) | χ2 = 0.09, df = 1, P = 0.76 |
| Hallucinogens | 18 (52.9%) | 13 (54.2%) | χ2 = 0.008, df = 1, P = 0.93 |
| Inhalants | 12 (80.0%) | 3 (60.0%) | χ2 = 0.80, df = 1, P = 0.37 |
Table 3.
Days since reported last use
| Schizophrenia (N = 70) | Controls (N = 97) | Statistics | |||||
|---|---|---|---|---|---|---|---|
| Current use / Past usea | Current | All past | No use | Current | All past | No use | |
| Alcohol (n, %) | 17 (24.3) | 50 (71.4) | 3 (4.3) | 67 (69.1) | 28(28.9) | 2 (2.0) | χ2 = 32.7, df = 2, P < 0.0001 |
| Heroin | 0 (0) | 24 (34.3) | 46 (65.7) | 12 (12.4) | 33 (34.0) | 52 (53.6) | χ2 = 9.7, df =2, P = 0.008 |
| Cocaine | 1 (1.4) | 36 (51.4) | 33 (47.1) | 24 (24.7) | 33 (34.0) | 40 (41.3) | χ2 = 18.1, df = 2, P < 0.0001 |
| Marijuana | 2 (2.9) | 59 (84.3) | 9 (12.8) | 43 (44.8) | 47 (49.0) | 6 (6.2) | χ2 = 36.1, df = 2, P < 0.0001 |
| Amphetamines | 1 (1.5) | 17 (24.6) | 51 (73.9) | 3 (3.1) | 18 (18.8) | 75 (78.1) | χ2 = 1.2, df = 2, P = 0.55 |
| Hallucinogens | 1 (1.4) | 33 (47.2) | 36 (51.4) | 1 (1.0) | 23 (23.7) | 73 (75.3) | χ2 = 10.2, df = 2, P = 0.006 |
| Inhalants | 0 (0) | 14 (20.3) | 55 (79.7) | 0 (0) | 5 (5.2) | 92 (94.8) | χ2 = 9.1, df = 1, P = 0.0025 |
Current: < 30 days; Past: no use for ≥ 30 days
Figure 1.
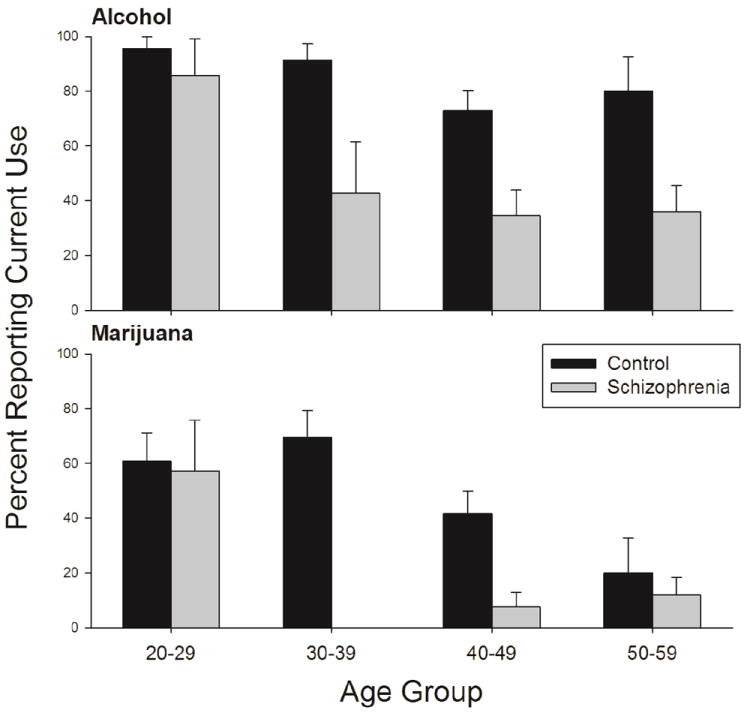
Percentage of participants who reported current alcohol (top panel) and current marijuana (bottom panel) use by age group.
3.3 Relationship of Cigarette Smoking Variables to Drug Use
We examined the relationship between current cigarette smoking, tobacco craving, and drug use. Cocaine use was associated with greater TCQ-SF scores in schizophrenia participants, but not in controls (p = 0.0072; see Table 4). We also examined the relationship between the number of cigarettes smoked, expired CO, FTND scores, current tobacco craving, and each substance of abuse. No drug of abuse was related to any measure of cigarette smoking.
Table 4.
TCQ total scores by diagnosis and cocaine use
| Cocaine | Schizophrenia | Controls | Statistics | |||||
|---|---|---|---|---|---|---|---|---|
| n | Mean | SD | n | Mean | SD | |||
| Total score | Yes | 37 | 50.6 | 18.3 | 57 | 39.4 | 18.1 | t = -2.72, df = 163, P = 0.0072 |
| No | 33 | 45.6 | 20.2 | 40 | 38.4 | 21.6 | t = -1.57, df = 163, P = 0.1191 | |
| Emotionality | Yes | 37 | 12.2 | 5.3 | 57 | 7.9 | 4.9 | t = -3.88, df = 163, P = 0.0001 |
| No | 33 | 9.5 | 5.0 | 40 | 8.5 | 6.1 | t = -0.78, df = 163, P = 0.4352 | |
| Expectancy | Yes | 37 | 13.2 | 5.6 | 57 | 12.0 | 6.0 | t = -0.94, df = 163, P = 0.3492 |
| No | 33 | 12.2 | 6.3 | 40 | 11.2 | 6.9 | t = -0.72, df = 163, P = 0.4746 | |
| Compulsivity | Yes | 37 | 11.4 | 5.6 | 57 | 8.1 | 5.1 | t = -2.86, df = 163, P = 0.0047 |
| No | 33 | 10.8 | 6.2 | 40 | 8.0 | 5.5 | t = -2.19, df = 163, P = 0.0299 | |
| Purposefulness | Yes | 37 | 13.7 | 4.9 | 57 | 11.4 | 5.2 | t = -1.99, df = 163, P = 0.0484 |
| No | 33 | 13.0 | 5.7 | 40 | 10.7 | 6.0 | t = -1.80, df = 163, P = 0.0741 | |
3.4 Drug of Choice and Illicit Drug Spending
The drugs of choice reported most frequently by controls were cigarettes and marijuana (31% for both substances). The drug of choice reported most frequently by schizophrenia participants was cigarettes (64%). More schizophrenia participants than controls chose alcohol and cigarettes as their drugs of choice (p < 0.0001). Schizophrenia participants also reported spending less money on illicit drugs and/or alcohol in the past 30 days (mean = $119) than controls (mean = $384; p = 0.01), as well as a smaller percentage of their monthly income (13.6% vs. 19.8%; p = 0.01).
4. Discussion
The co-occurrence of schizophrenia, tobacco dependence, and drug use remains a serious health problem and a barrier to improving this population’s health and quality of life. We hypothesized that the higher prevalence of cigarette use in the schizophrenia population would be correlated with greater drug use. However, we found that a smaller percentage of schizophrenia participants were current users of illicit substances than control smokers. We also found that the number of current drug users with schizophrenia declined with increasing age compared to controls. This suggests that cigarette smoking is not associated with increased illicit drug use in smokers with schizophrenia compared with controls who had a similar smoking history. However, schizophrenia participants reported more past substance use than controls, specifically for alcohol, cocaine, marijuana, hallucinogens, and inhalants. Although we do not know specifically when the drug use occurred, this is a concern, as early-onset drug use may increase an already vulnerable individual’s chance of developing psychosis. For example, Caspi et al. (2005) found that individuals who possess the Val allele (Val/Val or Val/Met) on the COMT gene and had early-onset cannabis use were at an increased risk of developing psychotic symptoms compared with their adult-onset cannabis use and Met/Met counterparts.
There were also unique patterns of drug use within each of our populations. Although schizophrenia participants used illicit drugs less than controls, they reported higher lifetime rates of inhalant and hallucinogen use. Higher lifetime prevalence of hallucinogen use has been reported among patients with schizophrenia compared with controls or other psychiatric patients (Tsuang et al., 1982; McLellan and Druley, 1977; Breakey at al., 1974). In a South Pacific island population, Daniels and Latcham (1984) also found that patients with schizophrenia have higher lifetime prevalence of gasoline inhalation than controls. In this study, we found that controls reported more heroin and cocaine use after they had established a history of past use. This could be due, in part, to regional influences, as Baltimore has high rates of heroin and cocaine use in the general population (ONDCP, 2006). This could also be explained by the fact that cocaine and heroin are expensive illicit drugs that require coordination of effort and intact cognitive function to obtain. Inhalants may be more readily available and less expensive. This is an interesting and troubling finding, as emergency department mentions of inhalant use are on the rise (SAMHSA, 2003) and chronic inhalant use has the potential to produce persistent psychotic symptoms in individuals at risk for psychosis (Byrne et al., 1991).
Consistent with smoking prevalence rates in the schizophrenia population (de Leon et al., 2002; Lasser et al., 2000; Grant, et al., 2004), we found that the mean number of cigarettes smoked per day was higher in schizophrenia participants than in controls. We also observed that schizophrenia participants had higher levels of breath CO 15 minutes post-cigarette, which is consistent with Tidey et al. (2005) who reported that schizophrenia patients obtained greater CO boosts from smoking than controls. In the current study, schizophrenia participants also reported higher TCQ-SF scores 15 minutes after a cigarette compared with controls (Lo et al., 2011). Interestingly, this tobacco craving was more robust in the schizophrenia participants with a past history of cocaine use. People with schizophrenia are known to have alterations in dopamine in the reward-mediated circuitry (Davis et al., 1991; Howes and Kapur, 2009; Goldstein and Deutch, 1992; Seeman et al., 1987). Administration of cocaine has been show to increase dopamine levels in this reward pathway (Venton et al., 2006; Daws et al., 2002). The fact that past cocaine use in schizophrenia participants, but not in controls, was associated with current elevated tobacco craving may be due, in part, to this altered reward pathway. Smoking cessation attempts in smokers with schizophrenia with past cocaine use may present a treatment challenge.
Some limitations of this study should be mentioned. Our sample was mostly comprised of heavy smokers who were not married. This may be due to some important behavioral differences between those who smoke and use drugs and those who do not, and this may limit the generalizability of our results. Drug use data were collected retrospectively, and recall bias could have resulted in underestimation or overestimation of drug use. We also did not report past and current illicit drug use by antipsychotic medication in the schizophrenia group because of the wide variety of medication regimens (e.g., only two participants were on aripiprazole). Stuyt et al. (2006) reported that, among dual-diagnosis patients, more subjects on second generation antipsychotics completed a drug treatment program compared with those on first generation antipsychotics. Additionally, within the participants on second generation antipsychotics, those on risperidone and ziprasidone had better treatment outcomes than those on olanzapine, highlighting the mechanism of CYP1A2 metabolism. In the current study, we did not report past and current illicit drug use by antipsychotic metabolism, but we also did not find any differences in smoking characteristics related to antipsychotic metabolism. Thus, we do not think that this is an adequate explanation for the differential drug use observed. The schizophrenia participants in the present study averaged 9 years older than controls, which might account for the 9 year difference in years smoked between the two groups, but we do not think that years of smoking significantly altered our drug use or nicotine dependence data, as drug use rates were comparable between the two groups at the younger ages. However, the older age in the schizophrenia group might have affected their tendency to use drugs, as drug use generally decreases with age (SAMHSA, 2010). Also, we did not include nonsmokers in this study, which would have allowed for a better appreciation of how drug use, cigarette smoking, and schizophrenia interact. Because of the high rates of tobacco use in the schizophrenia population, and the resulting difficulties that would accompany recruiting schizophrenia patients who do not smoke, we only included smokers in this study. We also did not assess cognitive functioning, depressive symptoms, or negative symptoms, which are likely to influence ease of access to drugs. However, our patients were outpatients, so we think that any clinical symptomatology would not have been severe enough to create significant differences in drug use patterns between the two groups. A strength of this study is that we were able to recruit large numbers of participants for both the schizophrenia and control groups, so our analyses yielded valid comparisons. We also studied outpatients with schizophrenia who were not constrained by institutional rules or smoke-free campuses, which increased the external validity of our data.
The prevalence of schizophrenia patients with comorbid substance abuse is increasing (Westermeyer, 2006; Searles et al., 1990; Fowler et al, 1998; el Guebaly and Hodgins, 1992; Gogek, 1991). Although our data suggest lower levels of current substance use in smokers with schizophrenia compared to controls, we found a substantial history of past substance use among the schizophrenia participants, especially those between the ages of 20 and 30. Enhanced efforts to treat tobacco dependence in people with schizophrenia are needed, not only for the obvious health benefits, but to help protect against initiation of drug use. Clinicians should also be vigilant when treating schizophrenia patients who are in the early stages of their illness, as prodromal and first-episode patients may be more vulnerable to drug use, as we observed more past drug use than current use in our schizophrenia participants.
Supplementary Material
Acknowledgments
We thank Suzanne Lo and Heather Raley for assistance in conduct of the study. Preliminary results of this manuscript were presented at the Society for Research on Nicotine and Tobacco (SRNT), Toronto, ON, February 2011 and the American Psychological Association meeting (APA), Washington, DC, August 2011.
Funding
This research was supported by the Intramural Research Program of the NIH, National Institute on Drug Abuse (NIDA), and NIDA Residential Research Support Services Contract HHSN271200599091CADB, NO-1DA-5-9909 (PI: Deanna L. Kelly). Both NIDA funds and personnel supported the design, study methods, and analysis of this study.
Footnotes
Contributors
Ms. Mackowick managed the literature searches, aided in data cleaning, and prepared the manuscript.
Dr. Heishman assisted in the development of the protocol and the manuscript.
Dr. Wehring assisted in the study data analysis plan and manuscript preparation.
Ms. Liu coordinated study data collection, cleaning and management, and ran the statistical testing and reporting of results.
Dr. McMahon assisted in protocol development and statistical testing, and assisted in manuscript preparation.
Dr. Kelly designed and wrote the study protocol and assisted in writing the manuscript. She supervised the study procedures, regulatory compliance, and data dissemination plan.
All authors reviewed, edited, contributed to, and have approved the final manuscript.
Conflict of Interest
Kristen M. Mackowick has nothing to disclose.
Stephen J. Heishman has nothing to disclose.
Heidi J. Wehring has nothing to disclose.
Fang Liu has nothing to disclose.
Robert P. McMahon is currently a consultant to Amgen, Inc.
Deanna L. Kelly has received grant support from Janssen Pharmaceutica and Bristol Myers Squibb.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Stephen J. Heishman, Email: Heishman@nih.gov.
Heidi J. Wehring, Email: hwehring@mprc.umaryland.edu.
Fang Liu, Email: fliu@mprc.umaryland.edu.
Robert P. McMahon, Email: rmcmahon@mprc.umaryland.edu.
Deanna L. Kelly, Email: dkelly@mprc.umaryland.edu.
References
- Agrawal A, Madden PAF, Bucholz KK, Heath AC, Lynskey MT. Transitions to regular smoking and to nicotine dependence in women using cannabis. Drug Alcohol Depend. 2008;95(1-2):107–114. doi: 10.1016/j.drugalcdep.2007.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angermeyer MC. Schizophrenia and violence. Acta Psychiat Scand Supl. 2000;407:63–67. doi: 10.1034/j.1600-0447.2000.00012.x. [DOI] [PubMed] [Google Scholar]
- Bechtholt AJ, Mark GP. Enchancement of cocaine-seeking beheavior by repeated nicotine exposure in rats. Psychopharmacology. 2002;162(2):178–185. doi: 10.1007/s00213-002-1079-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breakey WR, Goodell H, Lorenz PC, McHugh PR. Hallucinogenic drugs as precipitants of schizophrenia. Psychol Med. 1974;4(3):255–261. doi: 10.1017/s0033291700042938. [DOI] [PubMed] [Google Scholar]
- Breese CR, Lee MJ, Adams CE, et al. Abnormal regulation of high affinity nicotinic receptors in subjects with schizophrenia. Neuropsychopharmacology. 2000;23(4):351–364. doi: 10.1016/S0893-133X(00)00121-4. [DOI] [PubMed] [Google Scholar]
- Byrne A, Kirby B, Zibin T, et al. Psychiatric and neurological effects of chronic inhalant abuse. Can J Psychiatry. 1991;36:735–738. doi: 10.1177/070674379103601008. [DOI] [PubMed] [Google Scholar]
- Caspi A, Moffitt TE, Cannon M, et al. Moderation of the effect of adolescent-onset cannabis use in adult psychosis by a functional polymorphism in the catechol-o-methyltransferase gene: Longitudinal evidence of a gene X environment interaction. Biol Psychiatry. 2005;57:1117–1127. doi: 10.1016/j.biopsych.2005.01.026. [DOI] [PubMed] [Google Scholar]
- Chouljian TL, Shumway M, Balancio E, Dwyer EV, Surber R, Jacobs M. Substance use among schizophrenic outpatients: Prevalence, course, and relation to functional status. Ann Clin Psychiatry. 1995;7(1):19–24. doi: 10.3109/10401239509149020. [DOI] [PubMed] [Google Scholar]
- Compton MT, Kelley ME, Ramsay CE, et al. Association of pre-onset cannabis, alcohol, and tobacco use with age at onset of prodrome and age at onset of psychosis in first-episode patients. Am J Psychiatry. 2009;166(11):1251–1257. doi: 10.1176/appi.ajp.2009.09030311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalack GW, Healy DJ, Meador-Woodruff JH. Nicotine dependence in schizophrenia: Clinical phenomenology and laboratory findings. Am J Psychiatry. 1998;155(11):1490–1501. doi: 10.1176/ajp.155.11.1490. [DOI] [PubMed] [Google Scholar]
- Daniels AM, Latcham RW. Petrol sniffing and schizophrenia in a Pacific island paradise (Letter) Lancet. 1984;1(8373):389. doi: 10.1016/s0140-6736(84)90432-x. [DOI] [PubMed] [Google Scholar]
- Davis KL, Kahn RS, Ko G, Davidson M. Dopamine in schizophrenia: A review and reconceptualization. Am J Psychiatry. 1991;148(11):1474–1486. doi: 10.1176/ajp.148.11.1474. [DOI] [PubMed] [Google Scholar]
- Daws LC, Callaghan PD, Morón JA, et al. Cocaine increases dopamine uptake and cell surface expression of dopamine transporters. Biochem Biophys Res Commun. 2002;290(5):1545–1550. doi: 10.1006/bbrc.2002.6384. [DOI] [PubMed] [Google Scholar]
- de Leon J. Smoking and vulnerability for schizophrenia. Schizophrenia Bull. 1996;22(3):405–409. doi: 10.1093/schbul/22.3.405. [DOI] [PubMed] [Google Scholar]
- de Leon J, Becona E, Gurpegui M, Conzalea-Pinto A, Diaz FJ. The association between high nicotine dependence and severe mental illness may be consistent across countries. J Clin Psychiatry. 2002;63(9):812–816. doi: 10.4088/jcp.v63n0911. [DOI] [PubMed] [Google Scholar]
- Drake RE, Osher FC, Noordsy DL, Hurlbut SC, Teague GB, Beaudett MS. Diagnosis of alcohol use disorders in schizophrenia. Schizophrenia Bull. 1990;16(1):55–67. doi: 10.1093/schbul/16.1.57. [DOI] [PubMed] [Google Scholar]
- el Guebaly N, Hodgins DC. Schizophrenia and substance abuse: Prevalence issues. Can J Psychiatry. 1992;37(10):704–710. doi: 10.1177/070674379203701006. [DOI] [PubMed] [Google Scholar]
- Fonder MA, Sacco KA, Termine A, et al. Smoking cue reactivity in schizophrenia: Effects of a nicotinic receptor agonist. Biol Psychiatry. 2005;57(7):802–808. doi: 10.1016/j.biopsych.2004.12.027. [DOI] [PubMed] [Google Scholar]
- Fowler IL, Carr VJ, Carter NT, Lewin TJ. Patterns of current and lifetime substance abuse in schizophrenia. Schizophrenia Bull. 1998;24(3):443–445. doi: 10.1093/oxfordjournals.schbul.a033339. [DOI] [PubMed] [Google Scholar]
- George TP, Ziedonis DM, Feingold A, et al. Nicotine transdermal patch and atypical antipsychotic medications for smoking cessation in schizophrenia. Am J Psychiatry. 2000;157(11):1835–1842. doi: 10.1176/appi.ajp.157.11.1835. [DOI] [PubMed] [Google Scholar]
- Giovino GA. Epidemiology of tobacco use in the United States. Oncogene. 2002;21(48):7326–7340. doi: 10.1038/sj.onc.1205808. [DOI] [PubMed] [Google Scholar]
- Goff DC, Cather C, Evins AE, et al. Medical morbidity and mortality in schizophrenia: Guidelines for psychiatrists. J Clin Psychiatry. 2005;66(2):183–194. doi: 10.4088/jcp.v66n0205. [DOI] [PubMed] [Google Scholar]
- Gogek EB. Prevalence of substance abuse in psychiatric patients. Am J Psychiatry. 1991;148(8):1086. doi: 10.1176/ajp.148.8.1086a. [DOI] [PubMed] [Google Scholar]
- Goldstein M, Deutch AY. Dopaminergic mechanisms in the pathogenesis of schizophrenia. FASEB J. 1992;6(7):2413–2421. [PubMed] [Google Scholar]
- Grant BF, Hasin DS, Chou SP, Stinson FS, Dawson DA. Nicotine dependence and psychiatric disorders in the United States: Results from the National Epidemiologic Survey on Alcohol and Related Conditions. Arch Gen Psychiatry. 2004;61(11):1107–1115. doi: 10.1001/archpsyc.61.11.1107. [DOI] [PubMed] [Google Scholar]
- Heatherton TF, Kozlowski LT, Frecker RC, Fagerström KO. The Fagerström test for nicotine dependence: A revision of the Fagerström Tolerance Questionnaire. Br J Addictions. 1991;86(9):1119–1127. doi: 10.1111/j.1360-0443.1991.tb01879.x. [DOI] [PubMed] [Google Scholar]
- Heishman SJ, Singleton EG, Pickworth WB. Reliability and validity of a short version of the Tobacco Craving Questionnaire. Nicotine Tob Res. 2008;10(4):643–651. doi: 10.1080/14622200801908174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins ST, Budney AJ, Hughes JR, Bickel WK, Lynn M, Mortensen A. Influence of cocaine use on cigarette smoking. J Am Med Assoc. 1994;272(22):1724. [PubMed] [Google Scholar]
- Hoving C, Reubaset A, deVries H. Predictors of smoking stage transitions for adolescent boys and girls. Prev Med. 2007;44(6):485–489. doi: 10.1016/j.ypmed.2007.02.011. [DOI] [PubMed] [Google Scholar]
- Howes OD, Kapur S. The dopamine hypothesis of schizophrenia: Version III – the final common pathway. Schizophrenia Bull. 2009;35(3):549–562. doi: 10.1093/schbul/sbp006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hser YI, Anglin MD, Powers K. A 24-year follow-up of California narcotic addicts. Arch Gen Psychiatry. 1993;50(7):577–584. doi: 10.1001/archpsyc.1993.01820190079008. [DOI] [PubMed] [Google Scholar]
- Hser YI, McCarthy WJ, Anglin MD. Tobacco use as a distal predictor of mortality among long-term narcotic addicts. Prev Med. 1994;23(1):61–69. doi: 10.1006/pmed.1994.1009. [DOI] [PubMed] [Google Scholar]
- Hughes JR, Hatsukami DK, Mitchell JE, Dahlgren LA. Prevalence of smoking among psychiatric outpatients. Am J Psychiatry. 1986;143(8):993–997. doi: 10.1176/ajp.143.8.993. [DOI] [PubMed] [Google Scholar]
- Hurt RD, Offord KP, Croghan IT, et al. Mortality following inpatient addictions treatment: Role of tobacco use in a community-based cohort. J Am Med Assoc. 1996;275(14):1097–1103. doi: 10.1001/jama.275.14.1097. [DOI] [PubMed] [Google Scholar]
- Kalant H. Current state of knowledge about the mechanisms of alcohol tolerance. Addict Biol. 1996;1(2):133–141. doi: 10.1080/1355621961000124756. [DOI] [PubMed] [Google Scholar]
- Kelly DL, Boggs DL, Conley RR. Reaching for wellness in schizophrenia. Psychiatr Clin North Am. 2007;30(3):453–479. doi: 10.1016/j.psc.2007.04.003. [DOI] [PubMed] [Google Scholar]
- Kelly DL, McMahon RP, Wehring HJ, et al. Cigarette smoking and mortality risk in people with schizophrenia. Schizophrenia Bull. 2011;37(4):832–838. doi: 10.1093/schbul/sbp152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler RC, Crum RM, Warner LA, Nelson CB, Schulenberg J, Anthony JC. Lifetime co-occurrence of DSM-III-R alcohol abuse and dependence with other psychiatric disorders in the National Comorbidity Survey. Arch Gen Psychiatry. 1997;54(4):313–321. doi: 10.1001/archpsyc.1997.01830160031005. [DOI] [PubMed] [Google Scholar]
- Klatte EW, Lipscomb WR, Rozynko VV, Pugh LA. Changing the legal status of mental hospital patients. Hosp Community Psych. 1969;20(7):199–202. doi: 10.1176/ps.20.7.199. [DOI] [PubMed] [Google Scholar]
- Kovasznay B. Substance abuse among veterans with a diagnosis of schizophrenia. Hosp Community Psych. 1991;42(9):948–949. doi: 10.1176/ps.42.9.948. [DOI] [PubMed] [Google Scholar]
- Leonard S, Breese C, Adams C, et al. Smoking and schizophrenia: Abnormal nicotinic receptor expression. Eur J Pharmacol. 2000;393(1-3):237–242. doi: 10.1016/s0014-2999(00)00035-2. [DOI] [PubMed] [Google Scholar]
- Lasser K, Boyd JW, Woolhandler S, Himmelstein DU, McCormick D, Bor DH. Smoking and mental illness: A population-based prevalence study. J Amer Med Assoc. 2000;284(20):2606–2610. doi: 10.1001/jama.284.20.2606. [DOI] [PubMed] [Google Scholar]
- Lewinsohn PM, Rohde P, Brown RA. Level of current and past adolescent cigarette smoking as predictors of future substance use disorders in young adulthood. Addiction. 1999;94(6):913–921. doi: 10.1046/j.1360-0443.1999.94691313.x. [DOI] [PubMed] [Google Scholar]
- Lo S, Heishman SJ, Raley H, et al. Tobacco craving in smokers with and without schizophrenia. Schizophr Res. 2011;127(1-3):241–245. doi: 10.1016/j.schres.2010.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLellan AT, Druley KA. Non-random relation between drugs of abuse and psychiatric diagnosis. J Psychiatry Res. 1977;13(3):179–184. doi: 10.1016/0022-3956(77)90007-3. [DOI] [PubMed] [Google Scholar]
- Mokdad AH, Marks JS, Stroup DF, et al. Actual causes of death in the United States, 2000. J Amer Med Assoc. 2004;291(10):1238–1245. doi: 10.1001/jama.291.10.1238. [DOI] [PubMed] [Google Scholar]
- Office of National Drug Control Policy. Baltimore, MD Profile of Drug Indicators: December 2006. Rockville, MD: White House Office of National Drug Control Policy (ONDCP) Drug Policy Information Clearinghouse; 2006. [Google Scholar]
- Owen RR, Fischer EP, Booth BM, Cuffel BJ. Medication noncompliance and substance abuse among patients with schizophrenia. Psychiatr Serv. 1996;47(8):853–858. doi: 10.1176/ps.47.8.853. [DOI] [PubMed] [Google Scholar]
- Patton GC, Coffey C, Carlin JB, Sawyer SM, Lynskey M. Reverse gateways? Frequent cannabis use as a predictor of tobacco initiation and nicotine dependence. Addiction. 2005;100(10):1518–1525. doi: 10.1111/j.1360-0443.2005.01220.x. [DOI] [PubMed] [Google Scholar]
- Pristach CA, Smith CM. Medication compliance and substance abuse among schizophrenia patients. Hosp Community Psych. 1990;41(12):1345–1348. doi: 10.1176/ps.41.12.1345. [DOI] [PubMed] [Google Scholar]
- Regier DA, Farmer ME, Rae DS, et al. Comorbidity of mental disorders with alcohol and other drug abuse. J Amer Med Assoc. 1990;264(19):2511–2518. [PubMed] [Google Scholar]
- Reid MS, Mickalian JD, Delucchi KL, Hall SM, Berger SP. An acute dose of nicotine enhances cue-induced cocaine craving. Drug Alcohol Depend. 1998;49(2):95–104. doi: 10.1016/s0376-8716(97)00144-0. [DOI] [PubMed] [Google Scholar]
- Reid MS, Mickalian JD, Delucchi KL, Berger SP. A nicotine antagonist, mecamylamine, reduces cue-induced cocaine craving in cocaine-dependent subjects. Neuropsychopharmacology. 1999;20(3):297–307. doi: 10.1016/S0893-133X(98)00076-1. [DOI] [PubMed] [Google Scholar]
- Ridgely SM, Willenbring ML. Application of case management to drug abuse treatment: Overview of models and research issues. In: Ashery R, editor. Progress and Issues in Case Management. NIDA Research Monograph 127, DHHS Publ No (ADM)92-1946. Washington, DC: Supt. of Documents, US Government Printing Office; 1992. [PubMed] [Google Scholar]
- Roll JM, Higgins ST, Budney AJ, Bickel WK, Badger GJ. A comparison of cocaine-dependent cigarette smokers and non-smokers on demographic, drug use, and other characteristics. Drug Alcohol Depend. 1996;40(3):195–201. doi: 10.1016/0376-8716(96)01219-7. [DOI] [PubMed] [Google Scholar]
- Saha S, Chant D, McGrath J. A systematic review of mortality in schizophrenia: A record lineage study. Arch Gen Psychiatry. 2007;64:1123–1131. doi: 10.1001/archpsyc.64.10.1123. [DOI] [PubMed] [Google Scholar]
- Sanguineti VR, Samuel SE. Comorbid substance abuse and recovery from acute psychiatric relapse. Hosp Community Psychiatry. 1993;44:1073–1076. doi: 10.1176/ps.44.11.1073. [DOI] [PubMed] [Google Scholar]
- Schroeder SA, Warner KE. Don’t forget tobacco. New Engl J Med. 2010;363(3):201–204. doi: 10.1056/NEJMp1003883. [DOI] [PubMed] [Google Scholar]
- Searles JS, Alterman AI, Purtill JJ. The detection of alcoholism in hospitalized schizophrenics: A comparison of the MAST and the MAC. Alcohol Clin Exp Res. 1990;14(4):557–560. doi: 10.1111/j.1530-0277.1990.tb01199.x. [DOI] [PubMed] [Google Scholar]
- Seeman P, Bzowej NH, Guan HC, et al. Human brain D1 and D2 dopamine receptors in schizophrenia, Alzheimer’s, Parkinson’s, and Huntington’s diseases. Neuropsychopharmacology. 1987;1(1):5–15. doi: 10.1016/0893-133x(87)90004-2. [DOI] [PubMed] [Google Scholar]
- Simosky JK, Stevens KE, Freedman R. Nicotinic agonists and psychosis. Curr Drug Targets CNS Neurol Disord. 2002;1(2):149–162. doi: 10.2174/1568007024606168. [DOI] [PubMed] [Google Scholar]
- Smith SS, O’Hara BF, Persico AM, et al. Genetic vulnerability to drug abuse. The D2 dopamine receptor Taq I B1 restriction fragment length polymorphism appears more frequently in polysubstance abusers. Arch Gen Psychiatry. 1992;49(9):723–727. doi: 10.1001/archpsyc.1992.01820090051009. [DOI] [PubMed] [Google Scholar]
- Soyka M. Substance misuse, psychiatric disorder, and violent and disturbed behaviour. Brit J Psychiatry. 2000;176:345–350. doi: 10.1192/bjp.176.4.345. [DOI] [PubMed] [Google Scholar]
- Soyka M, Albus M, Kathman N, et al. Prevalence of alcohol and drug abuse in schizophrenic inpatients. Eur Arch Psychiatry Clin Neurosci. 1993;242:362–372. doi: 10.1007/BF02190250. [DOI] [PubMed] [Google Scholar]
- Steinberg ML, Williams JM, Steinberg HR, Krejci JA, Ziedonis DM. Applicability of the Fagerström test for nicotine dependence in smokers with schizophrenia. Addict Behav. 2005;30(1):49–59. doi: 10.1016/j.addbeh.2004.04.012. [DOI] [PubMed] [Google Scholar]
- Stuyt EB, Sajbel TA, Allen MH. Differing effects of antipsychotic medications on substance abuse treatment patients with co-occurring psychotic and substance abuse disorders. Am J Addiction. 2006;15(2):166–173. doi: 10.1080/10550490500528613. [DOI] [PubMed] [Google Scholar]
- Substance Abuse and Mental Health Services Administration. Office of Applied Studies, NSDUH Series H-38A, HHS Publication No SMA 10-4856Findings. I. Rockville, MD: 2010. Results from the 2009 national survey on drug use and health: Summary of national findings. [Google Scholar]
- Substance Abuse and Mental Health Services Administration, Office of Applied Studies. DAWN Series: D-24, DHHS Publication No (SMA) 03-3780. Rockville, MD: 2003. Emergency Department Trends From the Drug Abuse Warning Network, Final Estimates 1995-2002. [Google Scholar]
- Swartz MS, Swanson JW, Hiday VA, Borum R, Wagner HR, Burns BJ. Violence and severe mental illness: The effects of substance abuse and non-adherence to medication. Am J Psychiatry. 1998;155(2):226–231. doi: 10.1176/ajp.155.2.226. [DOI] [PubMed] [Google Scholar]
- Tidey JW, Rohsenow DJ, Kaplan GB, Swift RM. Cigarette smoking topography in smokers with schizophrenia and matched non-psychiatric controls. Drug Alcohol Depen. 2005;80(2):259–265. doi: 10.1016/j.drugalcdep.2005.04.002. [DOI] [PubMed] [Google Scholar]
- Tsuang MT, Simpson JC, Kronfol Z. Subtypes of drug abuse with psychosis. Demographic characteristics, clinical features, and family history. Arch Gen Psychiatry. 1982;39(2):141–147. doi: 10.1001/archpsyc.1982.04290020013003. [DOI] [PubMed] [Google Scholar]
- Venton BJ, Seipel AT, Phillips PE, et al. Cocaine increases dopamine release by mobilization of a synapsin-dependent reserve pool. J Neurosci. 2006;2(12):3206–3209. doi: 10.1523/JNEUROSCI.4901-04.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westermeyer J. Comorbid schizophrenia and substance abuse: A review of epidemiology and course. Am J Addiction. 2006;15(5):345–355. doi: 10.1080/10550490600860114. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


