Abstract
The endothelium is vital to normal vasoregulation. Although acute vasodilation associated with broad endothelial Ca2+ elevation is well-known, the control and targeting of Ca2+ dependent signals in the endothelium is poorly understood. Recent studies have revealed localized IP3-motivated Ca2+ events occurring basally along the intima that may provide the fundamental basis for various endothelial influences. Here, we provide an overview of dynamic endothelial Ca2+ signals and discuss the potential role of these signals in constant endothelial control of arterial tone and the titration of functional responses in vivo. In particular, we focus on the functional architecture contributing to the properties and ultimate impact of these signals and explore new avenues in evaluating their prevalence and specific modalities in intact tissue. Finally, we discuss spatial and temporal effector recruitment through modification of these inherent signals. It is suggested that endothelial Ca2+ signaling is a continuum in which the specific framework of store-release components and cellular targets along the endothelium allows for differential modes of Ca2+ signal expansion and distinctive profiles of effector recruitment. The precise composition and distribution of these inherent components may underlie dynamic endothelial control and specialized functions of different vascular beds.
Keywords: Calcium, endothelium, artery, dynamic, modality, analysis
INTRODUCTION
The pivotal role of the endothelium in the regulation of various aspects of vascular function and cardiovascular homeostasis is well documented and appreciated. As the luminal interface, the endothelium is a continuous hub of signaling that regulates vascular tone and permeability as well as vascular structure [13, 24, 29, 59, 62]. Ca2+ signals are integral to endothelial function, and various cellular components that control Ca2+ concentration and associated signal transduction are linked to endothelial dysfunction and cardiovascular pathology [25, 43, 50, 56]. Despite broad acceptance of the importance of Ca2+ in endothelial function, a detailed understanding of its management and impact is lacking. New findings have begun to expose the true complexity of physiologic endothelial Ca2+ signaling [19, 30, 32, 35, 37] and suggest that our entrenched view of Ca2+ dependent regulation has been grossly oversimplified.
Free intracellular Ca2+ controls multiple endothelial targets (effectors) that promote vasodilation, including the Ca2+-calmodulin-dependent proteins endothelial nitric oxide synthase (eNOS) that produces nitric oxide [5, 31] and small/intermediate conductance Ca2+-activated K+ channels, KCa2.3 and KCa3.1 [12, 18, 63], that elicit hyperpolarization of underlying vascular smooth muscle [4, 6, 24, 44, 58]. Additional vasoregulating factors, including eicosanoids derived from arachidonic acid metabolism [33] as well as reactive oxygen species such hydrogen peroxide, are also linked to changes in prevailing Ca2+ levels [22]. Precise Ca2+ targeting of endothelial effectors in vivo is still poorly understood although growing evidence suggests that dynamic control of Ca2+ signals and compartmentalized effectors may underpin true physiologic signaling. Here, we provide an overview of dynamic endothelial Ca2+ signals and explore implications on real-time vasoregulation.
Dynamic Endothelial Ca2+ signals
High-speed confocal imaging and single-excitation wavelength fluorescent indicators have dramatically improved our ability to view and record spatially and temporally discrete Ca2+ signals and have begun to highlight the integral role of dynamic Ca2+ signals in vascular biology. While spontaneous localized Ca2+ transients (e.g. Ca2+ sparks and sparklets) as well as asynchronous Ca2+ waves occurring in vascular smooth muscle have been strongly implicated in the level, coordination and feedback control of vasoconstriction [10, 42, 45, 55], basal Ca2+ transients in endothelial cells [32], including distinct spatially restricted events occurring along the intact intima [32, 37, 60], have only recently been reported and characterized. Because the endothelium is extremely fragile and is only a few microns thick, study of its function in situ is considerably challenging. Recent advances such as the introduction of the GCaMP2 transgenic mouse model that expresses an endothelial-specific Ca2+-dependent fluorescent protein [36] as well as implementation of open-artery preparations [37] have been particularly useful for live-tissue experiments, preventing spill-over indicator loading of other cell types and making broad fields of endothelia accessible to imaging [37, 46]. Intact functional endothelium can now be evaluated in its native state within the vascular wall and under controlled conditions.
Basal endothelial Ca2+ events observed in mouse mesenteric arteries have been termed Ca2+ pulsars. They resemble muscle cell Ca2+ sparks although they are typically broader in spatial range and duration [37]. Also, whereas sparks originate from endoplasmic reticulum (ER) ryanodine receptors (RyR), Ca2+ pulsars release intermittently from clusters of ER inositol 1,4,5-trisphosphate receptors (IP3Rs) [37]. These endothelial events appear to be direct physiologic manifestations of previously described Ca2+ puffs, unitary localized release events stimulated by IP3 in Xenopus oocytes [26, 48, 57]. Ca2+ puffs emit from distinct densities of IP3Rs and their origination sites, frequencies and development into regional or cell-wide waves are all highly dependent on graded increases in IP3, which sensitizes Ca2+-induced Ca2+ release [26]. In this way, transients such as Ca2+ puffs and pulsars are intimately linked to and controlled by Gq-protein coupled receptor (GPCR) signaling. Importantly, Ca2+ pulsars occur basally in mesenteric arteries under resting physiological conditions, and can be blocked by inhibiting the generation of IP3 by phospholipase C (PLC) [37], suggesting these events represent a persistent mode of Ca2+ signaling that can be acutely altered by local conditions and agonists. Indeed, stimulation of the mesenteric artery endothelium with acetylcholine increases the number of pulsar-emitting sites as well as the frequencies of events at pre-existing active sites along the intima [37]. A pivotal finding with respect to function is that these events occur in very close proximity to membrane KCa channels clustered at distinct myoendothelial junction (MEJ) sites in mesenteric arteries [37]. These are sites where endothelial and smooth muscle cells form close contacts (and often heterocellular gap junctions) through holes in the internal elastic lamina (IEL) [52, 54]. Altogether, this provides a steadfast and focused mechanism for soliciting hyperpolarization and relaxation of vascular smooth muscle. The physiologic relevance of this “built-in” signaling apparatus is not yet established, but is well-supported by the known pervasive role of KCa 3.1 channels in EDH-dependent vasodilation [6, 18] and the sustained hypertension exhibited by mice lacking KCa 3.1 channels [56].
Despite advancements, analysis of Ca2+ dynamics has remained tedious as manual approaches are inherently time-consuming and prone to user-bias and error. Genuine characterization of diverse dynamics within vast cellular landscapes will ultimately require standard approaches for event detection and rigorous high-throughput analysis. We recently developed a custom autodetection and analysis algorithm that can be applied as a plug-in with ImageJ freeware [28]. This program distinguishes dynamic fluorescence signals from site-specific background/noise along two-dimensional image sequences, allowing rapid screening and comprehensive assessment of various event parameters (e.g. frequency, amplitude, duration, spatial spread and area under curve). Such automated analysis may define distinctive signaling modalities and submodalities among expansive Ca2+ event distributions. The ability to define discrete Ca2+ signaling profiles is particularly exciting considering the vast differences in endothelium dependent vasoregulation known to occur among different vascular beds and even along the series of a single vascular bed (i.e. predominance of EDH versus NO dependent vasodilation in smaller diameter arteries) [24]. Continued progress toward standardized comprehensive data processing and evaluation will be essential for defining and ultimately resolving physiologic modes of vascular Ca2+ signaling. Also, in certain vascular beds, conduits for extracellular Ca2+ entry such as transient receptor potential (TRP) channels and/or STIM/ORAI [15, 16] may initiate Ca2+ signals that superimpose on or modify existing pulsar-type signals. Future investigations should provide insight on the prevalence of basal transients and whether IP3Rs and/or other Ca2+ sources contribute to basal signals in different beds.
Ca2+ signal tuning
The occurrence and range of ongoing endothelial Ca2+ transients along the intima is limited. Because most potential Ca2+-liberating sites are untapped basally, a favorable backdrop exists for further expansion and amplification. For instance, elevation of IP3 through Gq-coupled receptor stimulation triggers Ca2+ signals at sites that were previously inactive and can concurrently amplify specific parameters of ongoing events (e.g. frequency). This suggests that endothelial stimulation elicits both binary and analog modes of recruitment by initiating new sites of activity and by adjusting the attributes of the site-specific events themselves. Such a phenomenon would be similar to that of skeletal muscle fiber recruitment in which net muscle force is increased by activating new motor neurons (spatial summation) as well as increasing the firing frequency (temporal summation) of previously active motor neurons. This paradigm is possible for IP3/IP3R signaling because IP3-sensitized Ca2+-induced Ca2+ release (and consequent inhibition of IP3Rs by high Ca2+) favors distinct thresholds for triggering and communication between groups of IP3Rs. Previous studies of Ca2+ puffs support predictable expansion of signals based on the discrete clustering of IP3Rs and proximal levels/gradients of IP3 [48]. Correspondingly, we anticipate that in the endothelium, IP3R distributions and perhaps compartmentalized or graded IP3 signals underlie incremental Ca2+ site recruitment and act to shape a broad range of event properties, both spatially and temporally.
Observations in our laboratory suggest that endothelial stimulation may elicit increased Ca2+ transient frequency in one bed and increased event duration in another [27]. In fact, in swine coronary arteries, the predominant basal events are long-lasting waves (> 8 sec vs. < 0.3 sec for pulsars) that initiate locally and spread to encompass much of the cell volume. Clearly, altering the relative persistence and/or spread of a Ca2+ signal from its localized source offers a tremendous opportunity to direct effector recruitment. The pre-existing GPCR- IP3R framework may allow distinct patterning of recruitment among beds and by different stimuli. In fact, previous findings showing that different vasodilators stimulate distinct populations of endothelial cells [39] reveal phenotypic heterogeneity along the intima and support preferential tuning of recruitment and response. More recently, the possibility has surfaced that smooth muscle itself may alter or even instigate IP3R signals in the endothelium via direct communication of IP3 and/or Ca2+ across myoendothelial junctions [35, 60], providing feedback or even feed-forward regulation of endothelial vasoregulation. This is particularly relevant in the microcirculation of various beds where myoendothelial coupling is widespread and real-time endothelial control of membrane potential is crucial for blood flow regulation [14, 49]. Finally, new findings indicate that nonselective cation channels, specifically akyrin-associated transient receptor potential (TRPA1) channels, associate closely with KCa3.1 channels in the MEJs of rat cerebral arteries, and may provide an additional source of Ca2+ that promotes endothelial KCa -dependent vasodilation [20]. Whether such signals remain separate from the inherent IP3 signaling structure or interact with it (i.e. via enhanced Ca2+-induced Ca2+ release) is unknown. Finally, although we focus here on spatially restricted dynamics, it should be noted that initiating sites may spread as broad cellular and multi-cellular waves. Such signals may be amplified by endothelial stimulation, including physical stimuli such as stretch and shear [15, 30], allowing certain foci to develop into periodic oscillations or directional wave fronts along the intima [3, 47]. Correspondingly, exact distributions of endothelial receptors and channels, and controlled cell-cell communication are crucial in the ultimate physiologic response.
Effector recruitment
Investigations of endothelium dependent vasodilation have clearly established the role of Ca2+ and Ca2+-calmodulin dependent effectors. In particular, eNOS and the endothelial KCa channels, KCa 2.3 and KCa 3.1, form the primary axis for endothelium-derived relaxation and hyperpolarization of smooth muscle in a great majority of circulatory beds [21]. Nitric oxide (NO) freely diffuses to smooth muscle whereas endothelial KCa channels elicit hyperpolarization of subintimal smooth muscle [23, 64] via MEJs, either through heterocellular gap junctions [7, 8, 9, 11, 17, 40, 52] or via effluxed K+ and activation of smooth muscle inwardly rectifying K+ channels (KIR) [21, 61]. Because supraphysiological stimulation is often studied in a laboratory setting, the nuance of Ca2+ mobilization and effector recruitment has remained obscured. Moreover, cursory or global evaluations of endothelial Ca2+ have rarely addressed exactly where signals are occurring or how long they last. Given the recent appreciation for spatially and temporally dynamic Ca2+ signals, attention has begun to focus more acutely on discrete expression patterns of primary functional Ca2+ targets. As mentioned earlier, in mesenteric arteries, basally occurring Ca2+ pulsar events occur at MEJ sites where KCa3.1 channels cluster in the plasma membrane. This provides a constant impetus for smooth muscle hyperpolarization while much of the endothelial cell, including out-of-range Ca2+-dependent effectors, may remain essentially unperturbed. Moreover, stimulation increases the relative Ca2+ event frequency, allowing for amplification of this effect without necessarily engaging other Ca2+ dependent pathways cell-wide.
Distinct from KCa3.1 channels, KCa2.3 channels tend to associate with plasma membrane caveolin and distribute peripherally along endothelial cell borders [1, 53]. This general pattern suggests that whereas KCa3.1 channels are primary targets of isolated transients, KCa2.3 channels are more likely to be engaged by extended cell-wide Ca2+ events or by specific membrane-delimited signals. Endothelial NOS activity is regulated by phosphorylation as well as Ca2+-calmodulin. The later can reduce its association with membrane caveolin and further potentiate NO production [41, 51]. Interestingly, populations of eNOS are differentially distributed between the membranes of the plasmalemma and the Golgi apparatus in endothelial cells, both of which are capable of NO production [2, 38]. This relative allocation of effector may be crucial in determining accessibility to both phosphorylation and Ca2+ signals [34] of different range and duration. Moreover, differences or changes in distribution could serve as an additional means of bed-specific or dynamic regulation in vivo. The implication is that the pattern and not simply the amount of effector expression is a crucial determinant of physiologic endothelial regulation and, consequently, assessing mRNA or protein levels of specific effectors alone may lead to dubious interpretations of function.
Graded expansion of Ca2+ signaling along the vascular intima
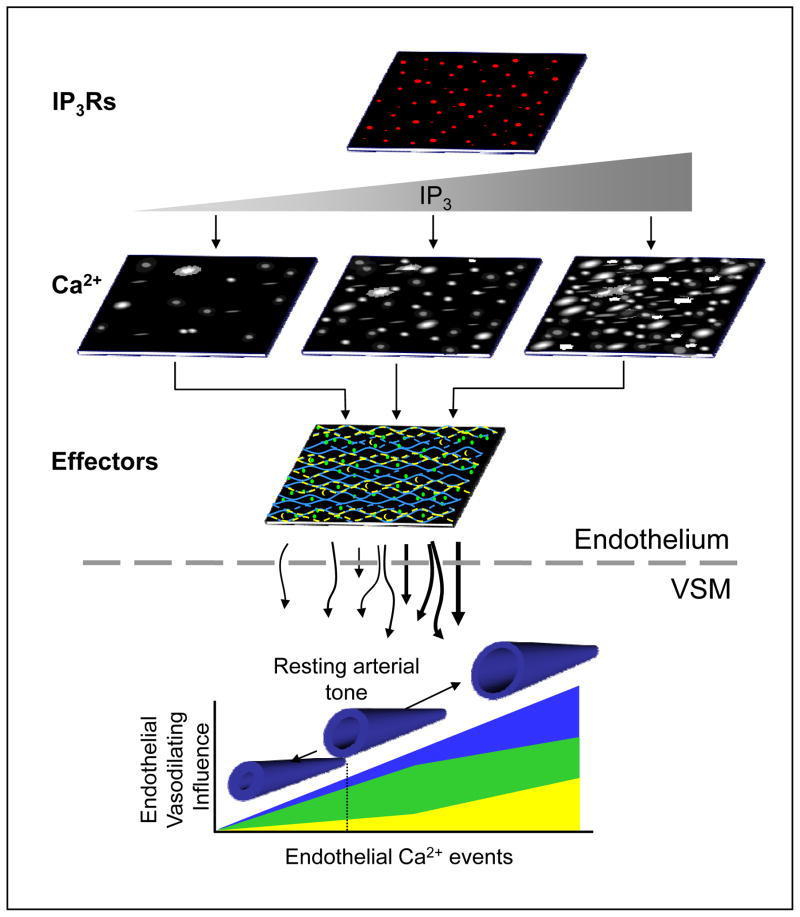
Fig. 1 depicts a general working model in which inherent endothelial Ca2+ signals constantly tune effector recruitment and vascular tone. It is suggested that the distinctive architecture of IP3Rs and Ca2+-dependent effectors within the confines of the intimal structure allow prevailing IP3 concentrations to drive predictable profiles of endothelial vasodilating influence. Specifically, IP3R distribution and intrinsic IP3 production within an endothelial field establish origination sites as well as the size and frequency of ongoing events, thereby defining a base Ca2+ signaling modality and providing the framework for spatial and temporal Ca2+ signal expansion. In this way, the endothelium may shift into specific modalities of Ca2+ signaling and hence predictable patterns of effector recruitment in response to various conditions. For instance, effectors that are closely tethered to basal Ca2+ transients (e.g. KCa3.1 channels) would be expected to exert a consistent background influence. Subsequent stimulation of additional local foci and event frequencies would increase this effect whereas stimulation that promoted widespread, long-lasting wave events would be expected to increase the activity of more peripheral components such as KCa2.3. Thus, specific effector impacts may be strictly encoded by Ca2+ event spatial spread and duration as well as site location and frequency. It follows that adjustments in the level of endothelial stimulation titrate the level and profile of effector recruitment. Notably, fluxes of Ca2+ through membrane cation channels as well as communication of IP3 through gap junction-containing MEJs may superimpose on fundamental signals to initiate new signals or further direct effector targeting. The implications of multiple convergent signals and feed-back/feed-forward regulation underscore the potential complexity of physiologic Ca2+ signaling and the need for caution and focus in future approaches.
Figure 1.
Conceptual model of dynamic Ca2+-effector coupling in the endothelium and its real-time regulation of arterial tone. It is proposed that a discrete scaffold of IP3Rs within the endoplasmic reticulum of the vascular intima establishes an intrinsic mode of dynamic Ca2+ signals. Relative shifts in IP3 production define new profiles of dynamic signaling with respect to the number of sites as well as event amplitude, frequency, duration and spatial spread. The distribution and density of specific Ca2+-dependent effectors within the endothelium, including those that are tightly coupled to local Ca2+ origination sites (blue) such as KCa3.1 channels, and those that are more peripherally or widely distributed (yellow and green) such as KCa2.3 channels and eNOS, determine the ultimate level of vasodilating influence communicated across the internal elastic lamina (IEL) to the vascular smooth muscle (VSM) as well as the predominating mechanism solicited (i.e. NO vs. hyperpolarization). This suggests a basal dynamic Ca2+ signaling modality exerts a steadfast and predictable endothelial influence on arterial tone (dotted line) that is constantly tuned by prevailing conditions.
Overall, it is suggested that the expanse and/or time course of Ca2+ signals, constantly adjust the level of vascular tone. In this respect, the endothelium may act as a constant gain control on smooth muscle contraction. For instance, by imposing a net zero gain on the rate of tone development, the endothelium might prevent progressive vasoconstriction beyond a certain point, and thereby stabilize arterial diameter over long periods of time. Clearer spatial and temporal elucidation of Ca2+ signaling patterns will help expose anomalous signal-effector coupling and target compensatory strategies against endothelial dysfunction and related cardiovascular disease. More selective experimental tools will be required to dissect the sources of dynamic endothelial Ca2+ signals as well as the relative cell-specific contributions (i.e. endothelial vs. smooth muscle). Also, detection of Ca2+ in restricted spaces (e.g. myoendothelial junctions) and in four dimensions (x,y,z with time), as well as the implementation of standard analysis tools, will provide a more complete picture of endothelial signaling in situ.
In summary, recent insights suggest the endothelium functions as a continuum of dynamically regulated influences that are always engaged and are constantly adjusted. Indeed, prevailing Ca2+ signaling modalities and effector distributions may underlie distinct functions of the different circulations. Further dissection of this diverse activity will allow for identification of submodalities, and potentially distinct cell phenotypes within the intima. We submit that shifts in prevailing Ca2+ dynamics necessarily impact blood pressure and flow and may predict disease. Indeed, endothelial dysfunction is an overarching feature of many cardiovascular pathologies. It is therefore imperative that future studies shift away from assumptions based on global Ca2+ changes and broad cellular protein concentrations and focus on spatially and temporally relevant aspects of real-time signaling. Continued pursuit of a definitive and predictive model of endothelial function should allow for elucidation of specific control points and therapeutic targets.
Acknowledgments
Supported by NIH (HL085887 and S10RR027535)
ACKNOWLEDGEMENTS
None
References
- 1.Absi M, Burnham MP, Weston AH, Harno E, Rogers M, Edwards G. Effects of methyl beta-cyclodextrin on EDHF responses in pig and rat arteries; association between SKCa channels and caveolin-rich domains. Br J Pharmacol. 2007;151:332–340. doi: 10.1038/sj.bjp.0707222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Andries LJ, Brutsaert DL, Sys SU. Nonuniformity of endothelial constitutive nitric oxide synthase distribution in cardiac endothelium. Circ Res. 1998;82:195–203. doi: 10.1161/01.res.82.2.195. [DOI] [PubMed] [Google Scholar]
- 3.Bagher P, Davis MJ, Segal SS. Visualizing calcium responses to acetylcholine convection along endothelium of arteriolar networks in Cx40BAC-GCaMP2 transgenic mice. Am J Physiol Heart Circ Physiol. 2011;301:H794–802. doi: 10.1152/ajpheart.00425.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Burnham MP, Bychkov R, Félétou M, Richards GR, Vanhoutte PM, Weston AH, Edwards G. Characterization of an apamin-sensitive small-conductance Ca2+-activated K+ channel in porcine coronary artery endothelium: relevance to EDHF. Br J Pharmacol. 2002;135:1133–1143. doi: 10.1038/sj.bjp.0704551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Busse R, Mulsch A. Calcium-dependent nitric oxide synthesis in endothelial cytosol is mediated by calmodulin. FEBS Lett. 1990;265:133–136. doi: 10.1016/0014-5793(90)80902-u. [DOI] [PubMed] [Google Scholar]
- 6.Bychkov R, Burnham MP, Richards GR, Edwards G, Weston AH, Félétou M, Vanhoutte PM. Characterization of a charybdotoxin-sensitive intermediate conductance Ca2+-activated K+ channel in porcine coronary endothelium: relevance to EDHF. Br J Pharmacol. 2002;137:1346–354. doi: 10.1038/sj.bjp.0705057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chaytor AT, Evans WH, Griffith TM. Central role of heterocellular gap junctional communication in endothelium-dependent relaxations of rabbit arteries. J Physiol. 1998;508:561–73. doi: 10.1111/j.1469-7793.1998.561bq.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chaytor AT, Martin PE, Edwards DH, Griffith TM. Gap junctional communication underpins EDHF type relaxations evoked by A Ch in the rat hepatic artery. Am J Physiol. 2001;280(6):H2441–50. doi: 10.1152/ajpheart.2001.280.6.H2441. [DOI] [PubMed] [Google Scholar]
- 9.Chaytor AT, Bakker LM, Edwards DH, Griffith TM. Connexin-mimetic peptides dissociate electrotonic EDHF-type signalling via myoendothelial and smooth muscle gap junctions in the rabbit iliac artery. Br J Pharmacol. 2005;144:108–114. doi: 10.1038/sj.bjp.0706046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cheng H, Lederer WJ, Cannell MB. Calcium sparks: elementary events underlying excitation-contraction coupling in heart muscle. Science. 1993;262:740–744. doi: 10.1126/science.8235594. [DOI] [PubMed] [Google Scholar]
- 11.Coleman HA, Tare M, Parkington HC. EDHF is not K+ but may be due to spread of current from the endothelium in guinea pig arterioles. Am J Physiol. 2001;280:H2478–H2483. doi: 10.1152/ajpheart.2001.280.6.H2478. [DOI] [PubMed] [Google Scholar]
- 12.Crane GJ, Gallagher N, Dora KA, Garland CJ. Small- and intermediate-conductance calciumactivated K+ channels provide different facets of endothelium-dependent hyperpolarization in rat mesenteric artery. J Physiol. 2003;553(1):183–9. doi: 10.1113/jphysiol.2003.051896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Davis GE, Stratman AN, Sacharidou A, Koh W. Molecular basis for endothelial lumen formation and tubulogenesis during vasculogenesis and angiogenic sprouting. Int Rev Cell Mol Biol. 2011;288:101–65. doi: 10.1016/B978-0-12-386041-5.00003-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.de Wit C, Wölfle SE. EDHF and gap junctions: important regulators of vascular tone within the microcirculation. Curr Pharm Biotechnol. 2007;8(1):11–25. doi: 10.2174/138920107779941462. [DOI] [PubMed] [Google Scholar]
- 15.Deng X, Wang Y, Zhou Y, Soboloff J, Gill DL. STIM and Orai: dynamic intermembrane coupling to control cellular calcium signals. J Biol Chem. 2009;284(34):22501–5. doi: 10.1074/jbc.R109.018655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dietrich A, Kalwa H, Gudermann T. TRPC channels in vascular cell function. Thromb Haemost. 2010;103(2):262–70. doi: 10.1160/TH09-08-0517. [DOI] [PubMed] [Google Scholar]
- 17.Dora KA, Sandow SL, Gallagher NT, Takano H, Rummery NM, Hill CE, Garland CJ. Myoendothelial gap junctions may provide the pathway for EDHF in mouse mesenteric artery. J Vasc Res. 2003;40(5):480–90. doi: 10.1159/000074549. [DOI] [PubMed] [Google Scholar]
- 18.Dora KA, Gallagher NT, McNeish A, Garland CJ. Modulation of endothelial cell KCa3.1 channels during endothelium-derived hyperpolarizing factor signaling in mesenteric resistance arteries. Circ Res. 2008;102(10):1247–55. doi: 10.1161/CIRCRESAHA.108.172379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Duza T, Sarelius IH. Localized transient increases in endothelial cell Ca2+ in arterioles in situ: Implications for coordination of vascular function. Am J Physiol. 2004;286(6):H2322–31. doi: 10.1152/ajpheart.00006.2004. [DOI] [PubMed] [Google Scholar]
- 20.Earley S, Gonzales AL, Crnich R. Endothelium-dependent cerebral artery dilation mediated by TRPA1 and Ca2+-Activated K+ channels. Circ Res. 2009;104(8):987–94. doi: 10.1161/CIRCRESAHA.108.189530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Edwards G, Dora KA, Gardener MJ, Garland CJ, Weston AH. K+ is an endothelium-derived hyperpolarizing factor in rat arteries. Nature. 1998;396:269–272. doi: 10.1038/24388. [DOI] [PubMed] [Google Scholar]
- 22.Edwards DH, Li Y, Griffith TM. Hydrogen peroxide potentiates the EDHF phenomenon by promoting endothelial Ca2+ mobilization. Arterioscler Thromb Vasc Biol. 2008;28(10):1774–81. doi: 10.1161/ATVBAHA.108.172692. [DOI] [PubMed] [Google Scholar]
- 23.Emerson GG, Segal SS. Electrical Coupling Between Endothelial Cells and Smooth Muscle Cells in Hamster Feed Arteries Role in Vasomotor Control. Circ Res. 2000;87:474–479. doi: 10.1161/01.res.87.6.474. [DOI] [PubMed] [Google Scholar]
- 24.Félétou M, Vanhoutte PM. Endothelium-dependent hyperpolarization of vascular smooth muscle cells. Acta Pharmacol Sin. 2000;21:1–18. [PubMed] [Google Scholar]
- 25.Félétou M, Köhler R, Vanhoutte PM. Endothelium-derived vasoactive factors and hypertension: possible roles in pathogenesis and as treatment targets. Curr Hypertens Rep. 2010;12(4):267–75. doi: 10.1007/s11906-010-0118-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Foskett JK, White C, Cheung KH, Mak DO. Inositol trisphosphate receptor Ca2+ release channels. Physiol Rev. 2007;87:593–658. doi: 10.1152/physrev.00035.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Francis M, Solodushko V, Taylor MS. Endothelial modulation of swine coronary artery tone through coupling of basal IR3R-dependent Ca2+ signals to endothelial SK and IK channels. FASEB J. 2009;23:1032.10. (Abstr) [Google Scholar]
- 28.Francis M, Solodushko V, Taylor MS. High Throughput Analysis of Spontaneous Endothelial Calcium Dynamics in Porcine Coronary Arteries. FASEB J. 2011;25:820.20. (Abstr) [Google Scholar]
- 29.Furchgott RF, Zawadzki JV. The obligatory role of endothelial cells in the relaxation of arterial smooth muscle by acetylcholine. Nature. 1980;288(5789):373–6. doi: 10.1038/288373a0. [DOI] [PubMed] [Google Scholar]
- 30.Geiger RV, Berk BC, Alexander RW, Nerem RM. Flow-induced calcium transients in single endothelial cells: spatial and temporal analysis. Am J Physiol. 1992;262(6 Pt 1):C1411–7. doi: 10.1152/ajpcell.1992.262.6.C1411. [DOI] [PubMed] [Google Scholar]
- 31.Ignarro LJ, Buga GM, Wood KS, Byrns RE, Chaudhuri G. Endothelium-derived relaxing factor produced and released from artery and vein is nitric oxide. Proc Natl Acad Sci U S A. 1987;84:9265–9269. doi: 10.1073/pnas.84.24.9265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Isshiki M, Mutoh A, Fujita T. Subcortical Ca2+ waves sneaking under the plasma membrane in endothelial cells. Circ Res. 2004;95:e11–e21. doi: 10.1161/01.RES.0000138447.81133.98. [DOI] [PubMed] [Google Scholar]
- 33.Jaffe EA, Grulich J, Weksler BB, Hampel G, Watanabe K. Correlation between thrombin-induced prostacyclin production and inositol trisphosphate and cytosolic free calcium levels in cultured human endothelial cells. J Biol Chem. 1987;262:8557–8565. [PubMed] [Google Scholar]
- 34.Jagnandan D, Sessa WC, Fulton D. Intracellular location regulates calcium-calmodulin-dependent activation of organelle-restricted eNOS. Am J Physiol. 2005;289:C1024–C1033. doi: 10.1152/ajpcell.00162.2005. [DOI] [PubMed] [Google Scholar]
- 35.Kansui Y, Garland CJ, Dora KA. Enhanced spontaneous Ca2+ events in endothelial cells reflect signalling through myoendothelial gap junctions in pressurized mesenteric arteries. Cell Calcium. 2008;44(2):135–46. doi: 10.1016/j.ceca.2007.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kotlikoff MI. Genetically encoded Ca2+ indicators: Using genetics and molecular design to understand complex physiology. J Physiol. 2007;578:55–67. doi: 10.1113/jphysiol.2006.120212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ledoux J, Taylor MS, Bonev AD, Hannah RM, Solodushko V, Shui B, Tallini Y, Kotlikoff MI, Nelson MT. Functional architecture of inositol 1,4,5-trisphosphate signaling in restricted spaces of myoendothelial projections. Proc Natl Acad Sci U S A. 2008;105:9627–32. doi: 10.1073/pnas.0801963105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Liu J, Hughes TE, Sessa WC. The first 35 amino acids and fatty acylation sites determine the molecular targeting of endothelial nitric oxide synthase into the Golgi region of cells: a green fluorescent protein study. J Cell Biol. 1997;137:1525–35. doi: 10.1083/jcb.137.7.1525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Marie I, Bény JL. Calcium imaging of murine thoracic aorta endothelium by confocal microscopy reveals inhomogeneous distribution of endothelial cells responding to vasodilator agents. J Vasc Res. 2002;39(3):260–7. doi: 10.1159/000063691. [DOI] [PubMed] [Google Scholar]
- 40.Mather S, Dora KA, Sandow SL, Winter P, Garland CJ. Rapid endothelial cell-selective loading of connexin 40 antibody blocks endothelium-derived hyperpolarizing factor dilation in rat small mesenteric arteries. Circ Res. 2005;97:399–407. doi: 10.1161/01.RES.0000178008.46759.d0. [DOI] [PubMed] [Google Scholar]
- 41.Michel JB, Feron O, Sacks D, Michel T. Reciprocal regulation of endothelial nitric-oxide synthase by Ca2+-calmodulin and caveolin. J Biol Chem. 1997;272:15583–86. doi: 10.1074/jbc.272.25.15583. [DOI] [PubMed] [Google Scholar]
- 42.Mufti RE, Brett SE, Tran CH, Abd El-Rahman R, Anfinogenova Y, El-Yazbi A, Cole WC, Jones PP, Chen SR, Welsh DG. Intravascular pressure augments cerebral arterial constriction by inducing voltage-insensitive Ca2+ waves. J Physiol. 2010;588:3983–4005. doi: 10.1113/jphysiol.2010.193300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Munaron L, Fiorio Pla A. Endothelial calcium machinery and angiogenesis: understanding physiology to interfere with pathology. Curr Med Chem. 2009;16(35):4691–703. doi: 10.2174/092986709789878210. [DOI] [PubMed] [Google Scholar]
- 44.Murphy ME, Brayden JE. Apamin-sensitive K+ channels mediate an endothelium-dependent hyperpolarization in rabbit mesenteric arteries. J Physiol. 1995;489:723–734. doi: 10.1113/jphysiol.1995.sp021086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nelson MT, Cheng H, Rubart M, Santana LF, Bonev AD, Knot HJ, Lederer WJ. Relaxation of arterial smooth muscle by calcium sparks. Science. 1995;270(5236):633–7. doi: 10.1126/science.270.5236.633. [DOI] [PubMed] [Google Scholar]
- 46.Nelson M, Ledoux J, Taylor M, Bonev A, Hannah R, Solodushko V, Shui B, Tallini Y, Kotlikoff M. Spinning disk confocal microscopy of calcium signaling in blood vessel wall. Microscopy and Analysis. 2010;24(2):5–8. [PMC free article] [PubMed] [Google Scholar]
- 47.Neylon CB, Irvine RF. Synchronized repetitive spikes in cytoplasmic calcium in confluent monolayers of human umbilical vein endothelial cells. FEBS Lett. 1990;275:173–176. doi: 10.1016/0014-5793(90)81465-z. [DOI] [PubMed] [Google Scholar]
- 48.Parker I, Choi J, Yao Y. Elementary events of InsP3-induced Ca2+ liberation in Xenopus oocytes: hot spots, puffs and blips. Cell Calcium. 1996;20(2):105–21. doi: 10.1016/s0143-4160(96)90100-1. [DOI] [PubMed] [Google Scholar]
- 49.Parkington HC, Chow JA, Evans RG, Coleman HA, Tare M. Role of endothelium derived hyperpolarizing factor in vascular tone in rat mesenteric and hindlimb circulations in vivo. J Physiol. 2002;542(Pt 3):929–37. doi: 10.1113/jphysiol.2002.021030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Quyyumi AA. Prognostic value of endothelial function. Am J Cardiol. 2003;91(12A):19H–24H. doi: 10.1016/s0002-9149(03)00430-2. [DOI] [PubMed] [Google Scholar]
- 51.Rath G, Dessy C, Feron O. Caveolae, caveolin and control of vascular tone: nitric oxide (NO) and endothelium derived hyperpolarizing factor (EDHF) regulation. J Physiol Pharmacol. 2009;60:105–109. [PubMed] [Google Scholar]
- 52.Sandow SL, Tare M, Coleman HA, Hill CE, Parkington HC. Involvement of myoendothelial gap junction in the actions of endothelium-derived hyperpolarizing factor. Circ Res. 2002;90:1108–1113. doi: 10.1161/01.res.0000019756.88731.83. [DOI] [PubMed] [Google Scholar]
- 53.Sandow SL, Neylon CB, Chen MX, Garland CJ. Spatial separation of endothelial small- and intermediate-conductance calcium-activated potassium channels (KCa) and connexins: possible relationship to vasodilator function? J Anat. 2006;209:689–698. doi: 10.1111/j.1469-7580.2006.00647.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sandow SL, Haddock RE, Hill CE, Chadha PS, Kerr PM, Welsh DG, Plane F. What’s where and why at a vascular myoendothelial microdomain signalling complex. Clin Exp Pharmacol Physiol. 2008;36(1):67–76. doi: 10.1111/j.1440-1681.2008.05076.x. [DOI] [PubMed] [Google Scholar]
- 55.Santana LF, Navedo MF, Amberg GC, Nieves-Cintrón M, Votaw VS, Ufret-Vincenty CA. Calcium sparklets in arterial smooth muscle. Clin Exp Pharmacol Physiol. 2008;35(9):1121–6. doi: 10.1111/j.1440-1681.2007.04867.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Si H, Heyken WT, Wölfle SE, Tysiac M, Schubert R, Grgic I, Vilianovich L, Giebing G, Maier T, Gross V, Bader M, de Wit C, Hoyer J, Köhler R. Impaired endothelium-derived hyperpolarizing factor-mediated dilations and increased blood pressure in mice deficient of the intermediate-conductance Ca2+-activated K+ channel. Circ Res. 2006;99:537–544. doi: 10.1161/01.RES.0000238377.08219.0c. [DOI] [PubMed] [Google Scholar]
- 57.Sun XP, Callamaras N, Marchant JS, Parker I. A continuum of InsP3-mediated elementary Ca2+ signalling events in Xenopus oocytes. J Physiol. 1998;509:67–80. doi: 10.1111/j.1469-7793.1998.067bo.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Taylor MS, Bonev AD, Gross TP, Eckman DM, Brayden JE, Bond CT, Adelman JP, Nelson MT. Altered expression of small-conductance Ca2+-activated K+ (SK3) channels modulates arterial tone and blood pressure. Circ Res. 2003;93:124–131. doi: 10.1161/01.RES.0000081980.63146.69. [DOI] [PubMed] [Google Scholar]
- 59.Taylor SG, Weston AH. Endothelium-derived hyperpolarizing factor: a new endogenous inhibitor from the vascular endothelium. Trends Pharmacol Sci. 1988;9(8):272–274. doi: 10.1016/0165-6147(88)90003-x. [DOI] [PubMed] [Google Scholar]
- 60.Tran CH, Taylor MS, Plane F, Nagaraja S, Tsoukias NM, Solodushko V, Vigmond EJ, Furstenhaupt T, Brighan M, Welsh DG. Endothelial Ca2+ wavelets and the induction of myoendothelial feedback. Am J Physiol Cell Physiol. 2012 doi: 10.1152/ajpcell.00418.2011. (In press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ulusoy HB, Kaya MG. Potassium induced dilation in bovine coronary artery involves both inward rectifier potassium channels and Na+/K+ ATPase. Acta Physiol Hung. 2009;96:427–436. doi: 10.1556/APhysiol.96.2009.4.3. [DOI] [PubMed] [Google Scholar]
- 62.Vandenbroucke E, Mehta D, Minshall R, Malik AB. Regulation of Endothelial Junctional Permeability. Ann NY Acad Sci. 2008;1123:134–145. doi: 10.1196/annals.1420.016. [DOI] [PubMed] [Google Scholar]
- 63.Xia XM, Fakler B, Rivard A, Wayman G, Johnson-Pais T, Keen JE, Ishii T, Hirschberg B, Bond CT, Lutsenko S, Maylie J, Adelman JP. Mechanism of calcium gating in small-conductance calcium activated potassium channels. Nature. 1998;395(6701):503–7. doi: 10.1038/26758. [DOI] [PubMed] [Google Scholar]
- 64.Yashiro Y, Duling BR. Integrated Ca2+ signaling between smooth muscle and endothelium of resistance vessels. Circ Res. 2000;87(11):1048–54. doi: 10.1161/01.res.87.11.1048. [DOI] [PubMed] [Google Scholar]