Abstract
Cognitive impairment is a core symptom in schizophrenia that has a significant impact on psychosocial function, but shows a weak response to pharmacological treatment. Consequently, a variety of cognitive remediation strategies have been evaluated to improve cognitive function in schizophrenia. The efficacy of computer-based cognitive remediation as a stand-alone intervention on general measures of neuropsychological function remains unclear. We tested the effectiveness of biweekly training using computerized cognitive remediation programs on neuropsychological and event-related potential outcome measures. Schizophrenia patients were randomly assigned to cognitive remediation training (N=17), active control (TV-watching; N=17), or treatment as usual (N=10) groups for ten weeks and run in parallel. Functional, cognitive, and ERP measures revealed no differential improvement over time in the cognitive remediation group. Practice effects might explain change over time on several cognitive measures for all groups, consistent with studies indicating task-specific improvement. Computer-assisted cognitive remediation alone may not be sufficient for robust or generalized effects on cognitive and electrophysiological measures in schizophrenia patients.
Keywords: schizophrenia, cognitive remediation, cognitive training, event-related potentials, auditory steady state potentials, intervention
1. Introduction
Schizophrenia (SZ) is a psychotic disorder characterized by cognitive deficits that occur across many domains, including attention, learning and memory, sensory processing, and executive function (Kurtz et al., 2007; Ranganath et al., 2008; Twamley et al., 2003). Cognitive deficits precede psychosis onset, are resistant to treatment with antipsychotic medication, and persist after symptoms have remitted (Medalia and Choi, 2009). Importantly, neurocognitive deficits are good predictors of psychosocial, occupational, and functional outcomes (Kurtz et al., 2007; McGurk et al., 2007; Medalia and Choi, 2009; Twamley et al., 2003). The pressing need for new therapeutic interventions is evident in SZ, where cognitive symptoms are major contributors to poor functional status and are better predictors than clinical symptoms in this regard, accounting for 20–60% of outcome variance (Green et al., 2000). Therefore, a cognitive intervention could serve as a beneficial adjunctive treatment for SZ patients.
A variety of cognitive remediation (CR) interventions has been used to improve cognitive function in SZ. Patients show improvement with practice on specific training tests, including measures of executive function and learning (Kurtz et al., 2001). Some, but not all studies have suggested that CR can improve performance on neuropsychological measures that were not part of the training set, indicative of transfer across tasks or cognitive domains. Due to variation in intervention study design, type, duration, outcome measures, and sample characteristics, metaanalytic approaches have been utilized to test for efficacy and attempt to estimate the effects of modulating factors. Recent meta-analyses have supported a small-to-moderate effect on global cognition in SZ (e.g., Grynszpan et al., 2011; McGurk et al., 2007; Wykes et al., 2011). In their recent and comprehensive meta-analysis, Wykes et al. (2011) reviewed forty studies that included outcome measures distinct from the trained tasks and tested potential moderators that might influence CR efficacy. A significant effect of CR was found for global cognition and functioning. Notably, the effect size was larger when CR was combined with other psychiatric rehabilitation and utilized a strategic training approach. Surprisingly, there was little effect of specific treatment approach or duration. McGurk et al. (2007) reviewed twenty-six randomized, controlled studies and similarly found a moderate effect size for cognitive performance (0.41).
Among CR interventions, computer-assisted CR has many attractive features, including standardized training that can adapt to performance level, immediate feedback and patient engagement by virtue of a game-like design, and cost-effectiveness. A basic question in the field, therefore, is whether computerized CR intervention has generalized effects on cognition as a stand-alone intervention. Grynszpan et al. (2011) specifically examined computer-assisted CR and found a modest effect size (0.38) on cognitive outcome measures. Grynszpan also identified methodological issues in many of these initial studies. One important interpretative issue in previous studies is whether an active control group is used. Traveling to a clinic, interacting with staff, and using a computer provide behavioral activation that may be unrelated to the computer-based remediation. Other important aspects of design have been variable as well. For example, in the Grynszpan (2011) meta-analysis of computer CR, ten of sixteen studies used a treatment-as-usual (TAU) comparison group, ten of sixteen explicitly ensured blind evaluations, and five of sixteen studies employed functional outcomes, rather than cognitive tests. Finally, attrition is another important analytic issue. Wykes et al. (2011) point out that the statistical validity of studies with a dropout rate greater than 15% is questionable, although they make up twelve of the studies they evaluated.
The present study tested outpatient computerized CR as a stand-alone intervention for outpatients with a diagnosis of SZ or schizoaffective disorder using randomized assignment to treatment, an active control arm, and later inclusion of a passive, treatment-as-usual control arm. We investigated the efficacy of a restorative, bottom-up cognitive training intervention using commercially available software developed by the PositScience Corporation (http://www.positscience.com/). Patients complete adaptive, high-intensity training exercises that target processing, attention, memory, and cognitive control to promote neural efficiency. This program incorporates errorless learning and motivating instructions that target cognitive domains as well as real-world application. Previous studies have found that SZ patients who trained for 50 or 100 hours using the PositScience auditory modules showed improved cognitive function and increased serum brain-derived neurotrophic factor (Fisher et al., 2009b, 2010; Vinogradov et al., 2009a). In contrast, two recent studies found improvement on the auditory modules but no transfer to outcome measures (Keefe et al., 2012; Murthy et al., 2012). We sought to replicate and expand previous CR findings by including a follow-up assessment, using both the auditory and visual exercises, and incorporating neurobiological outcome measures, which are rarely included in CR studies. We used a blind, multi-dimensional outcome assessment consisting of neuropsychological testing and event-related potentials (ERP) to evaluate functional, cognitive, and auditory processing outcomes. Based on the study by Fisher et al (2009a) which also used PositScience training software, we chose the primary outcome measures of global cognition (effect size (d) = 0.86 and verbal learning (d = .86). In the study design, the target N for each group was 20, yielding power of .85 to detect a treatment effect (Cohen, 1988).
2. Methods and Materials
2.1. Participants
The outpatient sample was recruited through psychiatrists affiliated with the Larue Carter Hospital and the Indiana University School of Medicine. Axis-I diagnosis of schizophrenia or schizoaffective disorder (SZ) was obtained by Structured Clinical Interview for DSM-IV (SCID-I: First et al., 2001), clinical observations, and chart review. Inclusion criteria for all participants were: 1) age between 18 and 50 years; 2) no history of electroconvulsive therapy; 3) no history of neurological illness; 4) no current alcohol or drug dependence (DSM-IV criteria) as ascertained by administration of the SCID sections on substance use disorders; 5) no hearing impairments on audiometry; 6) verbal I.Q. above 70; 7) visual acuity (with correction) of 20/30 or better; 8) no alcohol use in the 24 hours prior to testing. All participants received detailed information about the study protocol and gave written and oral informed consent. The protocol was approved by the Indiana University–Purdue University Indianapolis Human Subjects Review Committee. Participants were paid for participation.
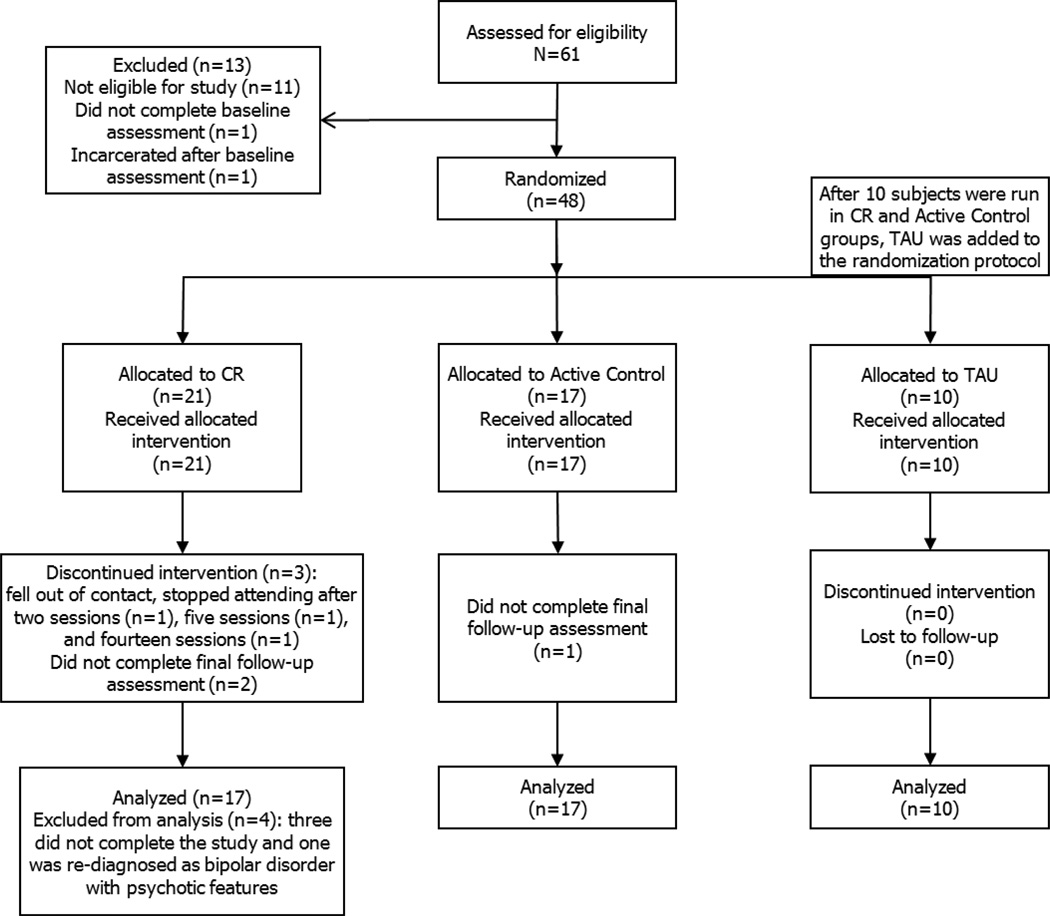
Forty-four individuals with SZ participated in the study and are described in Table 1. All but three participants were taking medications at entry (Table 2). Figure 1 is a flow diagram of progress through the phases of the trial. The attrition rate of the present study (6%) was similar to the rate found in the Wykes et al. (2011) meta-analysis (M=11%).
Table 1.
Baseline Characteristics of Participants with Schizophrenia or Schizoaffective Disorder Who Received Treatment with Computer-Assisted Cognitive Remediation or a Control Group
| CR | Active Control |
CR vs. Active Control |
TAU | All Groups | |||
|---|---|---|---|---|---|---|---|
| Variable | N=17 | N=17 | Χ2(1) | p | N=10 | Χ2(2) | p |
| Male (n) | 10 | 11 | 0.13 | 0.72 | 9 | 2.98 | 0.23 |
| Schizoaffective Disorder (n) | 9 | 11 | 0.49 | 0.49 | 2 | 5.13 | 0.08 |
| Mean (SD) | Mean | F(1,32) | P | Mean | F(2,41) | p | |
| Age (years) | 37.2 (12.5) | 45.4 (9.0) | 4.72 | 0.04 | 43.9 (8.9) | 2.78 | 0.07 |
| Illness Duration | 17.9 (11.6) | 22.6 (12.1) | 1.13 | 0.30 | 19.9 (9.3) | 0.62 | 0.55 |
| Education level | 3.2 (0.8) | 3.1 (0.8) | 0.05 | 0.83 | 3.3 (1.0) | 0.03 | 0.97 |
| WASI I.Q. | 94.8 (11.2) | 97.8 (10.0) | 0.71 | 0.41 | 91.2 (13.3) | 1.10 | 0.34 |
| PANSS Positive | 15.2 (5.6) | 16.5 (6.2) | 0.41 | 0.53 | 13.6 (3.8) | 0.90 | 0.41 |
| PANSS Negative | 12.4 (5.5) | 13.2 (5.4) | 0.17 | 0.69 | 15.0 (3.9) | 0.80 | 0.46 |
| PANSS General | 27.3 (7.9) | 30.6 (8.2) | 1.43 | 0.24 | 26.0 (3.8) | 1.49 | 0.24 |
Note. CR=cognitive remediation; TAU=treatment-as-usual. WASI=Wechsler Abbreviated Scale of Intelligence; PANSS=Positive and Negative Syndrome Scale. Four CR and three TAU participants were unable to report age of illness onset. Three CR participants had a recent (< 4 years) onset of the illness, whereas all other participants had the illness for more than 5 years (M=22, SD=11). Education level included self-report data on completion of grade school (1), junior high school (2), high school (3), some college (4), bachelor’s degree (5), master’s degree (6), and doctoral degree (7). The degrees of freedom differ for Illness Duration: F(1,28) and F(2,34).
Table 2.
| Group | |||
|---|---|---|---|
| Medication Type | CR | Active Control | TAU |
| No Medications | 3 | - | - |
| Atypical Antipsychotic | 11 | 15 | 9 |
| Conventional Antipsychotic | 2 | 3 | 2 |
| Antidepressant | 6 | 9 | 6 |
| Anticonvulsant | 4 | 3 | - |
| Possible anticholinergic | 1 | 3 | 1 |
| Definite anticholinergic | 11 | 11 | 2 |
| Other brain medication | 2 | 2 | 4 |
Note. Medications are reported for baseline assessment. CR=cognitive remediation; TAU=treatment-as-usual. Patients taking psychotropic medications typically used multiple medications. Other brain medications included benzodiazepine, muscle relaxant, opioid analgesic, and anxiolytic prescriptions.
Figure 1.
Flow diagram describing the phases of a parallel randomized trial of the cognitive remediation (CR), active control, and treatment-as-usual (TAU) groups.
2.2. Design
Participants were randomly assigned using a random number table to cognitive remediation treatment (CR) or an active control study arm. After preliminary analyses of ten participants in each group showed no systematic differences on outcome measures between the CR or active control groups, a treatment-as-usual (TAU) arm was added to the randomization procedure to evaluate potential effects of practice and social interaction. The groups were run in parallel at the Larue Carter Hospital, Indianapolis, IN. Assessments were completed at baseline, five weeks, ten-weeks, and twenty weeks follow-up by staff blind to treatment condition. TAU participants were not assessed at follow-up because no differences had been found after 10 weeks in the treatment and active control groups.
2.3. Treatment
The CR and active control participants completed assigned tasks for two hours, including breaks, two days per week for ten weeks, a treatment schedule consistent with other studies that showed positive outcomes (McGurk et al., 2007; Twamley et al., 2003; Wykes et al., 2011). The active control group watched films, cartoons, or television shows using the same schedule as the treatment arm: two-hour biweekly sessions. The TAU participants came in only for assessments. A comparison of all groups showed no differences of age, estimated I.Q., or gender (χ2=2.96, p=.23), however, the active control group was older than the CR group (Table 1).
The CR group completed a cognitive training regimen using software that applies adaptive algorithms to continuously adjust the demands of each task according to performance (Mahncke et al., 2006). Participants completed auditory exercises described previously by Fisher et al. (2009a) and visual exercises. The visual module aims to improve the speed and accuracy of visual processing to facilitate perception, improve visual memory, and reduce response time. Exercise 1 has participants locate specific birds appearing briefly on the screen and exercise 4 has participants track details of cars and road signs to enhance visual perception and field of view. Exercise 2 is designed to improve divided attention by having participants track multiple moving items. Exercise 3 aims to speed visual processing and reaction time by having participants respond to visual sweeps. Exercise 5 targets visual working memory using a task where participants must remember and match similar pictures.
2.4. Clinical and Cognitive Assessment
The Positive and Negative Syndrome Scale measured current symptom levels (PANSS; Kay et al., 1987). Vocabulary and Matrix Reasoning subtests of the Wechsler Abbreviated Scale of Intelligence (WASI) estimated premorbid intelligence (Wechsler, 1999). Verbal memory function was assessed using the Letter-Number Sequencing subtest of the Wechsler Adult Intelligence Scale (WAIS-III; Wechsler, 1997) and the Hopkins Verbal Learning Test (HVLT; Brandt and Benedict, 2001). WAIS-III Spatial Span forward and backward assessed visual working memory (Wechsler, 1997). The Brief Visuospatial Memory Test assessed non-verbal learning and memory (BVMT; Benedict, 1997). WAIS-III Digit Symbol Coding assessed information processing speed. The Trail Making Tests assessed psychomotor speed and set-shifting in working memory (Reitan and Wolfson, 1993). Letter and category fluency were assessed using a one-minute interval using the letter F and animals, respectively. The Multiple Ability Self-Report Questionnaire assessed language, visual-perceptual function, verbal memory, visual memory, and attention (MASQ; Seidenberg et al., 1994).
2.5. Electrophysiological Assessment
The Auditory Steady State Response (ASSR) is an evoked potential generated by the synchronous activity of auditory cortical neurons in response to periodic auditory stimuli (Pastor et al., 2002; Simpson et al., 2005). The ASSR is thought to reflect auditory neural circuit function (Gratton et al., 1983) and is often attenuated in SZ at the 40 Hz stimulation frequency (Brenner et al., 2009; Kwon et al., 1999; Light et al., 2006; Spencer et al., 2008; Teale et al., 2008; Vierling-Claassen et al., 2008). The P300 event-related potential (ERP) response is usually elicited by infrequent target stimuli in the background of frequent standard stimuli and elicits a large positive-going potential with a latency of approximately 300 ms after stimulus onset (Polich and Criado, 2006). P300 amplitude is usually reduced and latency is increased in SZ (Ford, 1999; O'Donnell et al., 2004).
The EEG was continuously recorded (band pass 0.1–200 Hz, sampling rate 1000 Hz) and digitized (NeuroScan SynAmps) from the scalp, using a 29-channel electrode cap with a nose reference. Electrode impedances were maintained at <10 kOhm. Epochs were corrected for ocular artifacts by algorithm (Gratton et al., 1983). Epochs with voltage exceeding ±150 µV (ASSR) and ±100 µV (P300) at any site were automatically excluded from further analyses.
2.5.1. ASSR
Eighty click trains at 80 dB were presented at the 40 Hz frequency, resulting in a stimulus duration of 475 ms and included 700-ms inter-train intervals. Time-frequency analyses were used to obtain measures of change in power from baseline (mean trial power, MTP) and phase consistency or coherence (phase locking factor, PLF) as described in previous papers (Delorme et al., 2002; Makeig et al., 2004; Rass et al., 2010; TallonBaudry et al., 1997; Vohs et al., 2010; Vohs et al., 2009). Mean values were obtained for the 100 to 500 ms interval after stimulus onset for 5 Hz below and above the 40 Hz stimulation frequency for the FCz electrode.
2.5.2. P300
ERPs were elicited by 40 ms tone-pips (10 ms rise/fall time) presented at 1.2-second inter-stimulus intervals. Participants responded to infrequent (15%) high-pitched tones (1500 Hz), randomly interspersed among frequent distractor tones (1000 Hz). A 30 Hz (24 dB/octave roll-off) low-pass filter was applied to the waveform of each epoch. After averaging target epochs (−200 to 800 ms) for each participant, P300 peak latency and amplitude were obtained in the 280 to 600 ms window at the Pz channel.
2.6. Statistical Analysis
Separate mixed model Analysis of Covariance (ANCOVA) are reported for comparisons of 1) CR vs. active control and 2) CR vs. TAU to determine treatment effects on clinical, neuropsychological, and EEG measures. Baseline WASI I.Q. scores served as the covariate. The first analysis used a between-subjects factor of Group (2: CR, control) and within-subject factor of Time (3: baseline, end, follow-up). The second analysis used a between-subjects factor of Group (2: CR, TAU) and within-subject factor of Time (2: baseline, end). For all analyses, post-hoc t-tests clarified interactions revealed by ANOVA. Greenhouse-Geisser epsilon adjustments were included when appropriate. A composite score of global cognition (computed as the average across letter fluency, category fluency, spatial span, letter-number sequencing, digit symbol, HVLT total and BVMT total) was used as a primary outcome variable for comparison with studies using a similar approach (Vinogradov et al., 2009a; Vinogradov et al., 2009b). Participants with missing outcome scores at one assessment were not included in the average that time point (e.g., baseline).
3. Results
3.1. Baseline Measures
Groups did not differ by gender, illness duration, education level, IQ score, or PANSS symptom score. There were marginally fewer patients with schizoaffective disorder diagnosis in the TAU group than the other groups. The CR group was younger than the Active control (LSD p=.030).
3.2. CR vs. Active Control
Outcome measures for the CR and control groups are reported in Table 3. A 2 (Group) × 3 (Time) mixed model ANCOVA with IQ as the covariate was used to analyze treatment effects on outcome measures. Table 4 displays significant and trend results. Since the groups differed at baseline on most cognitive tests, a Group × Time interaction would indicate a treatment effect. A trend for a Group × Time interaction for BVMT Total significant revealed increases for both groups between baseline and ten weeks. From end of treatment to follow-up, the CR group continued improving, whereas the active control group decreased in perfomrnace. Active control participants had marginally greater scores at 10 weeks (t(32)=1.96-, p=.059). A main effect of Group indicated that active control participants scored higher on Letter Fluency, Letter-Number Sequencing, HVLT Total Recall, BVMT Total and Delayed Recall, and reported better visual perceptual ability and verbal memory than the CR group. No effect on P3 or ASSR was found. In summary, there was no Group × Time interactions on the primary outcome measure (global cognition) or exploratory measures to suggest a differential effect of treatment arm.
Table 3.
Scores on cognitive domains before intervention, after intervention, and following a 10-week break from intervention for the treatment and active control groups.
| PositScience Training | TV/Movie Control | |||||
|---|---|---|---|---|---|---|
| Cognitive Domain M (SD) |
Baseline | After Training |
Follow-up | Baseline | After Training |
Follow-up |
| Letter Fluency | 13.8 (3.7) | 14.8 (3.3) | 13.5 (5.0) | 16.0 (5.6) | 17.2 (5.8) | 16.6 (4.7) |
| Category Fluency | 16.4 (3.7) | 17.1 (5.4) | 18.5 (5.4) | 16.6 (4.0) | 16.1 (4.1) | 17.1 (4.0) |
| Spatial Span | 7.4 (2.8) | 7.8 (3.1) | 7.5 (3.0) | 8.5 (3.3) | 9.1 (3.3) | 8.9 (3.5) |
| Letter Number Sequencing |
8.5 (2.8) | 8.7 (2.3) | 9.0 (2.0) | 8.9 (2.3) | 8.8 (2.0) | 8.9 (3.0) |
| Digit Symbol | 6.5 (2.3) | 7.2 (2.4) | 7.1 (2.0) | 6.4 (1.3) | 7.4 (1.9) | 7.8 (1.4) |
| HVLT Total Recall | 34.1 (12.2) | 33.5 (10.2) | 39.6 (11.4) | 35.5 (8.7) | 33.2 (9.9) | 38.8 (10.8) |
| HVLT Delayed Recall | 34.8 (11.2) | 29.9 (13.5) | 36.2 (11.1) | 34.9 (10.1) | 30.7 (9.9) | 37.3 (10.4) |
| HVLT % Retention | 39.8 (14.1) | 35.9 (14.6) | 41.3 (10.7) | 38.8 (15.2) | 37.1 (14.0) | 45.0 (10.2) |
| HVLT Recognition Discrimination |
43.4 (11.4) | 39.9 (12.0) | 38.8 (11.4) | 43.3 (12.8) | 41.2 (12.5) | 44.9 (11.1) |
| BVMT Total | 31.4 (14.7) | 34.4 (12.8) | 37.5 (11.6) | 37.1 (12.5) | 43.0 (12.8) | 41.7 (11.0) |
| BVMT Learning | 52.5 (14.5) | 46.2 (8.3) | 50.8 (8.2) | 50.5 (11.0) | 49.0 (10.6) | 53.3 (6.8) |
| BVMT Delayed Recall |
32.4 (15.6) | 36.0 (16.7) | 39.4 (14.0) | 39.7 (11.5) | 46.1 (12.1) | 43.1 (11.5) |
| Global Cognition | 16.8 (4.8) | 17.6 (4.2) | 18.6 (4.2) | 18.4 (3.7) | 19.3 (4.2) | 20.0 (3.7) |
| Self-Report | ||||||
| MASQ | ||||||
| Language | 18.5 (3.8) | 19.0 (5.7) | 17.9 (6.2) | 19.5 (4.1) | 20.2 (3.2) | 19.2 (2.6) |
| Visual Perceptual Ability |
12.8 (3.6) | 12.2 (3.7) | 13.3 (4.0) | 16.2 (4.4) | 16.4 (4.1) | 16.7 (3.9) |
| Verbal Memory | 19.0 (5.4) | 18.4 (5.5) | 19.7 (5.6) | 23.4 (4.7) | 22.1 (3.8) | 22.2 (4.7) |
| Visual Spatial Memory |
16.9 (5.2) | 17.2 (6.0) | 14.9 (5.6) | 20.2 (4.8) | 20.6 (3.5) | 18.8 (6.0) |
| Attention Concentration |
19.5 (5.2) | 19.3 (6.1) | 17.8 (6.1) | 22.1 (3.9) | 20.9 (4.2) | 21.1 (5.1) |
Note. HVLT=Hopkins Verbal Learning Test; BVMT=Brief Visuospatial Memory Test; MASQ=Multiple Ability Self-Report Questionnaire.
Table 4.
Outcome analysis for Cognitive Remediation (CR), Active Control, and Treatment-As-Usual (TAU) groups across time.
| CR vs. Active Control | CR vs. TAU | |||||
|---|---|---|---|---|---|---|
| Cognitive Domain | Group × Time |
Time | Group | Group × Time |
Time | Group |
| Letter Fluency | - | - | - |
F(1,24)= 4.30* |
- |
F(1,24)= 9.69** |
| Category Fluency | - | - | - | - |
F(1,24)= 12.68** |
- |
| Letter-Number Sequencing |
- | - | - | - | - |
F(1,23)= 3.60† |
| HVLT Total Recall | - | - | - | - | - |
F(1,24)= 3.05† |
| HVLT Recognition Discrimination |
- | - | - |
F(1,23)= 3.13† |
F(1,23)= 4.43* |
- |
| BVMT Total |
F(2,54)= 2.51† |
- | - | - | - |
F(1,24)= 11.05** |
| BVMT Delayed Recall | - | - | - | - | - |
F(1,24)= 10.06** |
| Global Cognition | - | - | - | - | - |
F(1,23)= 10.69** |
| MASQ | ||||||
| Visual Perceptual Ability |
- | - |
F(1,29)= 9.12** |
- | - |
F(1,23)= 3.03† |
| Verbal Memory | - | - |
F(1,29)= 5.21* |
- | - |
F(1,23)= 3.68† |
| Visual Spatial Memory |
- | - |
F(1,29)= 5.63* |
- | - | - |
| EEG | ||||||
| P300 Peak | - | - | - | - |
F(1,22)= 5.07* |
- |
Note. Time for CR vs. Active control included baseline, ten weeks, and follow-up assessment; time for CR vs. TAU included baseline and ten weeks assessment. For CR vs. Active Control and vs. TAU ANCOVA analyses, IQ was a significant covariate (p<.05) for the following measures: Letter Fluency, Category Fluency, Digit Symbol, Spatial Span, Letter Number sequencing, HVLT Total Recall, Delayed Recall, and Recognition Discrimination, BVMT Total Recall and Delayed Recall, and Global Cognition.
p≤.10,
p< .05,
p<.01,
p<.001.
Note. HVLT=Hopkins Verbal Learning Test; BVMT=Brief Visuospatial Memory Test; MASQ=Multiple Ability Self-Report Questionnaire.
3.3. CR vs. TAU
Outcome measures for the TAU group are reported in Table 5. A 2 (Group) × 2 (Time) mixed model ANCOVA with IQ as the covariate was used to analyze treatment effects on outcome measures. Table 4 displays significant and trend results. A Group × Time interaction for letter fluency and marginal interaction HVLT recognition discrimination did not reach significance in post-hoc comparisons. Performance on category fluency improved over time for both groups. A main effect of Group indicated significantly greater BVMT total and delay scores and marginally greater letter fluency, global cognition, MASQ visual perceptual ability, and MASQ verbal memory in the TAU group compared to the CR group at baseline and retest. A main effect of Time for revealed a decrease in P3 peak over time for both groups. No effect on ASSR was found. Again, there was no Group × Time interaction for the primary outcome measure or exploratory measures indicative of a treatment effect.
Table 5.
Scores on cognitive domains at baseline and after ten weeks for the Treatment as Usual Group.
| Cognitive Domain M (SD) | Baseline | After 10 Weeks |
|---|---|---|
| Letter Fluency | 18.1 (4.9) | 15.6 (5.5) |
| Category Fluency | 17.9 (3.8) | 18.3 (4.7) |
| Spatial Span | 8.7 (2.5) | 8.7 (3.7) |
| Letter Number Sequencing | 9.1 (1.5) | 10.0 (1.8) |
| Digit Symbol | 7.1 (2.2) | 8.1 (3.0) |
| HVLT | ||
| Total Recall | 39.0 (13.5) | 39.5 (15.1) |
| Delayed Recall | 34.8 (13.6) | 37.1 (15.1) |
| % Retention | 34.8 (13.3) | 38.7 (15.4) |
| Recognition Discrimination | 37.1 (15.1) | 43.1 (14.7) |
| BVMT | ||
| Total | 40.3 (12.2) | 48.8 (16.1) |
| Learning | 51.2 (10.6) | 48.0 (9.8) |
| Delayed Recall | 46.0 (16.7) | 45.7 (15.0) |
| Global Cognition | 20.1 (4.3) | 21.3 (5.7) |
| MASQ | ||
| Language | 21.0 (4.3) | 19.6 (4.7) |
| Visual Perceptual Ability | 15.3 (3.4) | 14.6 (4.9) |
| Verbal Memory | 22.6 (4.6) | 22.2 (5.0) |
| Visual Spatial Memory | 18.9 (5.7) | 18.9 (5.7) |
| Attention Concentration | 20.7 (3.6) | 21.6 (6.7) |
Note. HVLT=Hopkins Verbal Learning Test; BVMT=Brief Visuospatial Memory Test; MASQ=Multiple Ability Self-Report Questionnaire.
4. Discussion
Schizophrenia patients were randomly assigned to forty hours of cognitive remediation (CR), an active control group of TV/Movie watching, or Treatment-as-Usual (TAU). Neuropsychological and electrophysiological outcome measures evaluated the efficacy of treatment on cognitive performance outside of trained tasks. Patients in all groups improved on measures of information processing, verbal memory, and visuospatial memory during the ten-week intervention. It is important to note that baseline performance differences, which occurred despite randomized assignment, might limit possible treatment effect size. For example, the active and TAU control groups had generally better baseline scores on cognitive measures. Change over time on some tests likely reflected practice effects or behavioral activation across groups. While meta-analyses (Grynszpan et al., 2011) indicate a range of effect sizes for computer CR in the moderate range, study design has been quite variable. The present results are similar to studies finding that improvement on training tasks did not generalize to cognitive or functional outcome measures (Dickinson et al, 2012; Keefe et al., 2012; Murthy et al., 2012). Similarly, Field and colleagues (1997) attributed any performance improvement to practice effects. Although a recent study found a trend toward normalization of the M100 auditory cortex response in SZ patients following 50 hours of PositScience auditory training, we were unable to find improved auditory cortical function using EEG measures (Adcock et al., 2009). The present controlled trial of computer-assisted CR suggests that cognitive training alone may not be sufficient to produce effects that generalize to functional, cognitive, and electrophysiological outcomes.
Several factors may contribute to the absence of differential improvement in the CR group compared to either control group. Large effects (d = .86) were predicted for the primary outcome measures based on the Fisher et al (2009b) study. The small sample size may not have produced sufficient power to find the small-to-moderate effect sizes of training found in previous studies (Wykes et al., 2011). However, since change scores for measures of interest showed little systematic direction between treatment and comparison groups, it is questionable whether increasing the sample size would detect a significant or clinically meaningful treatment effect. Incorporating complementary rehabilitation strategies, like vocational and social training, may produce a larger effect size by promoting transfer of cognitive training effects as suggested by the Wykes et al. (2011) meta-analysis. Other factors might play a role in our null findings, including an anticholinergic burden from medications, a training regimen lacking the necessary intensity, and illness severity and chronicity (Vinogradov et al., 2009b). These factors might account for the absence of expected practice-related changes in neural activation (Holcomb, 2004; Kelly et al., 2006; Wexler et al., 2000). Future studies are necessary to determine the most effective framework for computer CR while incorporating individual differences, such as disorder length and onset.
Acknowledgments
We thank Colleen Merrill and Mallory Klaunig for their assistance with collecting the data presented in this report.
Role of Funding Source
We are grateful for support from NIMH RO1 MH62150 (BFO), NIMH R21 MH091774, and IUSM/CTR, NIH/NCRR Grant Number RR025761 to BFO; NIMH R01 MH074983 to WPH; NARSAD (ARB) and NIDA T32 DA024628-01 (OR). These institutions had no role in study design, analysis and interpretation of data, writing of the report, or in the decision to submit the report for publication. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the NIH.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributors
Olga Rass (rasso@indiana.edu) was the primary author of this manuscript.
Jennifer Forsyth (jenforsyth@gmail.com) assisted with the patient recruitment and assessment, database management, literature searches, questionnaire and WAIS scoring, statistical analyses, and writing the manuscript.
William P. Hetrick (whetrick@indiana.edu) and Brian F. O’Donnell (bodonnel@indiana.edu) were responsible for the design of this study and supervised subject recruitment, diagnostic procedures, symptom assessment, neuropsychological testing; and contributed to the interpretation of the statistical analysis.
Alan Breier (abreier@iupui.edu) assisted in design, recruitment, diagnosis, and interpretation of the data.
Amanda R Bolbecker (arhoskin@indiana.edu) assisted with training and supervision of the research technicians, assisted with diagnostic procedures, carried out initial data analysis and contributed to the interpretation of the statistical analysis.
Paul H. Lysaker (plysaker@iupui.edu) assisted in the design and interpretation of the study.
All authors contributed to and have approved the final manuscript.
Conflict of Interest.
There are no conflicts of interest for any of the authors of this article. No author has any possible financial gain for the findings presented here.
References
- Adcock RA, Dale C, Fisher M, Aldebot S, Genevsky A, Simpson GV, Nagarajan S, Vinogradov S. When top-down meets bottom-up: auditory training enhances verbal memory in schizophrenia. Schizophr Bull. 2009;35(6):1132–1141. doi: 10.1093/schbul/sbp068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benedict RHB. Brief Visuospatial Memory Test-Revised: Professional Manual. Lutz, FL: Psychological Assessment Resources, Inc.; 1997. [Google Scholar]
- Birchwood M, Smith J, Cochrane R, Wetton S, Copestake S. The Social Functioning Scale. The development and validation of a new scale of social adjustment for use in family intervention programmes with schizophrenic patients. Br J Psychiatry. 1990;157:853–859. doi: 10.1192/bjp.157.6.853. [DOI] [PubMed] [Google Scholar]
- Brandt J, Benedict RHB. Hopkins Verbal Learning Test—Revised. Professional manual. Lutz, FL: Psychological Assessment Resources, Inc.; 2001. [Google Scholar]
- Brenner CA, Krishnan GP, Vohs JL, Ahn WY, Hetrick WP, Morzorati SL, O'Donnell BF. Steady state responses: electrophysiological assessment of sensory function in schizophrenia. Schizophr Bull. 2009;35(6):1065–1077. doi: 10.1093/schbul/sbp091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delorme A, Makeig S, Fabre-Thorpe M, Sejnowski T. From single-trial EEG to brain area dynamics. Neurocomputing. 2002;44:1057–1064. [Google Scholar]
- Dickinson D, Tenhula W, Morris S, Brown C, Peer J, Spencer K, Li L, Gold JM, Bellack AS. A randomized, controlled trial of computer-assisted cognitive remediation for schizophrenia. Am J Psychiatry. 2010;167(2):170–180. doi: 10.1176/appi.ajp.2009.09020264. [DOI] [PubMed] [Google Scholar]
- Field CD, Galletly C, Anderson D, Walker P. Computer-aided cognitive rehabilitation: possible application to the attentional deficit of schizophrenia, a report of negative results. Percept Mot Skills. 1997;85(3 Pt 1):995–1002. doi: 10.2466/pms.1997.85.3.995. [DOI] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JBW. Biometrics Research Department. New York: New York State Psychiatric Institute; 2001. Structured Clinical Interview for DSM-IV-TR Axis I Disorders - Patient Edition (SCID-I/P, 2/2001 revision) [Google Scholar]
- Fisher M, Holland C, Merzenich MM, Vinogradov S. Using neuroplasticity-based auditory training to improve verbal memory in schizophrenia. Am J Psychiatry. 2009a;166(7):805–811. doi: 10.1176/appi.ajp.2009.08050757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher M, Holland C, Subramaniam K, Vinogradov S. Neuroplasticity-Based Cognitive Training in Schizophrenia: An Interim Report on the Effects 6 Months Later. Schizophr Bull. 2009b;36(4):869–879. doi: 10.1093/schbul/sbn170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher M, Holland C, Subramaniam K, Vinogradov S. Neuroplasticity-based cognitive training in schizophrenia: an interim report on the effects 6 months later. Schizophr Bull. 2010;36(4):869–879. doi: 10.1093/schbul/sbn170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ford JM. Schizophrenia: the broken P300 and beyond. Psychophysiology. 1999;36(6):667–682. [PubMed] [Google Scholar]
- Gratton G, Coles MG, Donchin E. A new method for off-line removal of ocular artifact. Electroencephalogr Clin Neurophysiol. 1983;55(4):468–484. doi: 10.1016/0013-4694(83)90135-9. [DOI] [PubMed] [Google Scholar]
- Green MF, Kern RS, Braff DL, Mintz J. Neurocognitive deficits and functional outcome in schizophrenia: are we measuring the "right stuff"? Schizophr Bull. 2000;26(1):119–136. doi: 10.1093/oxfordjournals.schbul.a033430. [DOI] [PubMed] [Google Scholar]
- Grynszpan O, Perbal S, Pelissolo A, Fossati P, Jouvent R, Dubal S, Perez-Diaz F. Efficacy and specificity of computer-assisted cognitive remediation in schizophrenia: a meta-analytical study. Psychological medicine. 2011;41(1):163–173. doi: 10.1017/S0033291710000607. [DOI] [PubMed] [Google Scholar]
- Holcomb HH. Practice, learning, and the likelihood of making an error: how task experience shapes physiological response in patients with schizophrenia. Psychopharmacology (Berl) 2004;174(1):136–142. doi: 10.1007/s00213-004-1834-6. [DOI] [PubMed] [Google Scholar]
- Kay S, Fiszbein A, Opler L. The positive and negative syndrome scale (PANSS) for schizophrenia. Schizophr Bull. 1987;13(2):261. doi: 10.1093/schbul/13.2.261. [DOI] [PubMed] [Google Scholar]
- Keefe RSE, Vinogradov S, Medalia A, Buckley PF, Caroff SN, D’Souza DC, Harvey PD, Graham KA, Hamer RM, Marder SM, Miller DD, Olson SJ, Patel JK, Velligan D, Walker TM, Haim AJ, Stroup TS. Feasibility and Pilot Efficacy Results From the Multisite Cognitive Remediation in the Schizophrenia Trials Network (CRSTN) Study: A Randomized Controlled Trial. J Clin Psychiatry. 2012 doi: 10.4088/JCP.11m07100. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly C, Foxe JJ, Garavan H. Patterns of normal human brain plasticity after practice and their implications for neurorehabilitation. Arch Phys Med Rehabil. 2006;87(12 Suppl 2):S20–S29. doi: 10.1016/j.apmr.2006.08.333. [DOI] [PubMed] [Google Scholar]
- Kurtz MM, Seltzer JC, Shagan DS, Thime WR, Wexler BE. Computer-assisted cognitive remediation in schizophrenia: what is the active ingredient? Schizophr Res. 2007;89(1–3):251–260. doi: 10.1016/j.schres.2006.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwon JS, O'Donnell BF, Wallenstein GV, Greene RW, Hirayasu Y, Nestor PG, Hasselmo ME, Potts GF, Shenton ME, McCarley RW. Gamma frequency-range abnormalities to auditory stimulation in schizophrenia. Arch Gen Psychiatry. 1999;56(11):1001–1005. doi: 10.1001/archpsyc.56.11.1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Light GA, Hsu JL, Hsieh MH, Meyer-Gomes K, Sprock J, Swerdlow NR, Braff DL. Gamma band oscillations reveal neural network cortical coherence dysfunction in schizophrenia patients. Biol Psychiatry. 2006;60(11):1231–1240. doi: 10.1016/j.biopsych.2006.03.055. [DOI] [PubMed] [Google Scholar]
- Mahncke HW, Connor BB, Appelman J, Ahsanuddin ON, Hardy JL, Wood RA, Joyce NM, Boniske T, Atkins SM, Merzenich MM. Memory enhancement in healthy older adults using a brain plasticity-based training program: a randomized, controlled study. Proc Natl Acad Sci U S A. 2006;103(33):12523–12528. doi: 10.1073/pnas.0605194103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makeig S, Debener S, Onton J, Delorme A. Mining event-related brain dynamics. Trends in Cognitive Sciences. 2004;8(5):204–210. doi: 10.1016/j.tics.2004.03.008. [DOI] [PubMed] [Google Scholar]
- McGurk SR, Twamley EW, Sitzer DI, McHugo GJ, Mueser KT. A meta-analysis of cognitive remediation in schizophrenia. Am J Psychiatry. 2007;164(12):1791–1802. doi: 10.1176/appi.ajp.2007.07060906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medalia A, Choi J. Cognitive remediation in schizophrenia. Neuropsychol Rev. 2009;19(3):353–364. doi: 10.1007/s11065-009-9097-y. [DOI] [PubMed] [Google Scholar]
- Murthy NV, Mahncke H, Wexler BE, Maruff P, Inamdar A, Zucchetto M, Lund J, Shabbir S, Shergill S, Keshavan M, Kapur S, Laruelle M, Alexander R. Computerized cognitive remediation training for schizophrenia: An open label, multi-site, multinational methodology study. Schizophr Res. 2012 doi: 10.1016/j.schres.2012.01.042. (in press) [DOI] [PubMed] [Google Scholar]
- O'Donnell BF, Vohs JL, Hetrick WP, Carroll CA, Shekhar A. Auditory event-related potential abnormalities in bipolar disorder and schizophrenia. Int J Psychophysiol. 2004;53(1):45–55. doi: 10.1016/j.ijpsycho.2004.02.001. [DOI] [PubMed] [Google Scholar]
- Pastor MA, Artieda J, Arbizu J, Marti-Climent JM, Penuelas I, Masdeu JC. Activation of human cerebral and cerebellar cortex by auditory stimulation at 40 Hz. J Neurosci. 2002;22(23):10501–10506. doi: 10.1523/JNEUROSCI.22-23-10501.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polich J, Criado JR. Neuropsychology and neuropharmacology of P3a and P3b. Int J Psychophysiol. 2006;60(2):172–185. doi: 10.1016/j.ijpsycho.2005.12.012. [DOI] [PubMed] [Google Scholar]
- Ranganath C, Minzenberg MJ, Ragland JD. The cognitive neuroscience of memory function and dysfunction in schizophrenia. Biol Psychiatry. 2008;64(1):18–25. doi: 10.1016/j.biopsych.2008.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rass O, Krishnan G, Brenner CA, Hetrick WP, Merrill CC, Shekhar A, O'Donnell BF. Auditory steady state response in bipolar disorder: relation to clinical state, cognitive performance, medication status, and substance disorders. Bipolar Disord. 2010;12(8):793–803. doi: 10.1111/j.1399-5618.2010.00871.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reitan R, Wolfson D. The Halstead-Reitan neuropsychological test battery: Theory and clinical interpretation. Tucson, AZ: Neuropsychology Press; 1993. [Google Scholar]
- Seidenberg M, Haltiner A, Taylor MA, Hermann BB, Wyler A. Development and validation of a Multiple Ability Self-Report Questionnaire. J Clin Exp Neuropsychol. 1994;16(1):93–104. doi: 10.1080/01688639408402620. [DOI] [PubMed] [Google Scholar]
- Simpson MI, Hadjipapas A, Barnes GR, Furlong PL, Witton C. Imaging the dynamics of the auditory steady-state evoked response. Neurosci Lett. 2005;385(3):195–197. doi: 10.1016/j.neulet.2005.05.043. [DOI] [PubMed] [Google Scholar]
- Spencer KM, Salisbury DF, Shenton ME, McCarley RW. Gamma-band auditory steady-state responses are impaired in first episode psychosis. Biol Psychiatry. 2008;64(5):369–375. doi: 10.1016/j.biopsych.2008.02.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TallonBaudry C, Bertrand O, Delpuech C, Pernier J. Oscillatory gamma-band (30–70 Hz) activity induced by a visual search task in humans. Journal of Neuroscience. 1997;17(2):722–734. doi: 10.1523/JNEUROSCI.17-02-00722.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teale P, Collins D, Maharajh K, Rojas DC, Kronberg E, Reite M. Cortical source estimates of gamma band amplitude and phase are different in schizophrenia. Neuroimage. 2008;42(4):1481–1489. doi: 10.1016/j.neuroimage.2008.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Twamley EW, Jeste DV, Bellack AS. A review of cognitive training in schizophrenia. Schizophr Bull. 2003;29(2):359–382. doi: 10.1093/oxfordjournals.schbul.a007011. [DOI] [PubMed] [Google Scholar]
- Vierling-Claassen D, Siekmeier P, Stufflebeam S, Kopell N. Modeling GABA alterations in schizophrenia: a link between impaired inhibition and altered gamma and beta range auditory entrainment. J Neurophysiol. 2008;99(5):2656–2671. doi: 10.1152/jn.00870.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vinogradov S, Fisher M, Holland C, Shelly W, Wolkowitz O, Mellon SH. Is serum brain-derived neurotrophic factor a biomarker for cognitive enhancement in schizophrenia? Biol Psychiatry. 2009a;66(6):549–553. doi: 10.1016/j.biopsych.2009.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vinogradov S, Fisher M, Warm H, Holland C, Kirshner MA, Pollock BG. The Cognitive Cost of Anticholinergic Burden: Decreased Response to Cognitive Training in Schizophrenia. Am J Psychiatry. 2009b;166(9):1055–1062. doi: 10.1176/appi.ajp.2009.09010017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vohs JL, Chambers RA, Krishnan GP, O'Donnell BF, Berg S, Morzorati SL. GABAergic modulation of the 40 Hz auditory steady-state response in a rat model of schizophrenia. Int J Neuropsychopharmacol. 2010;13(4):487–497. doi: 10.1017/S1461145709990307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vohs JL, Chambers RA, Krishnan GP, O'Donnell BF, Hetrick WP, Kaiser ST, Berg S, Morzorati SL. Auditory sensory gating in the neonatal ventral hippocampal lesion model of schizophrenia. Neuropsychobiology. 2009;60(1):12–22. doi: 10.1159/000234813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wechsler D. WAIS-III: Administration and Scoring Manual: Wechsler Adult Intelligence Scale, Psychological Corporation. 3 ed. San Antonio, TX: 1997. [Google Scholar]
- Wechsler D. Wechsler Abbreviated Scale of Intelligence, Psychological Corporation. 3 ed. San Antonio, TX: 1999. [Google Scholar]
- Wexler BE, Anderson M, Fulbright RK, Gore JC. Preliminary evidence of improved verbal working memory performance and normalization of task-related frontal lobe activation in schizophrenia following cognitive exercises. Am J Psychiatry. 2000;157(10):1694–1697. doi: 10.1176/appi.ajp.157.10.1694. [DOI] [PubMed] [Google Scholar]
- Winterer G, Egan MF, Raedler T, Sanchez C, Jones DW, Coppola R, Weinberger DR. P300 and genetic risk for schizophrenia. Arch Gen Psychiatry. 2003;60(11):1158–1167. doi: 10.1001/archpsyc.60.11.1158. [DOI] [PubMed] [Google Scholar]
- Wykes T, Huddy V, Cellard C, McGurk SR, Czobor P. A meta-analysis of cognitive remediation for schizophrenia: methodology and effect sizes. Am J Psychiatry. 2011;168(5):472–485. doi: 10.1176/appi.ajp.2010.10060855. [DOI] [PubMed] [Google Scholar]