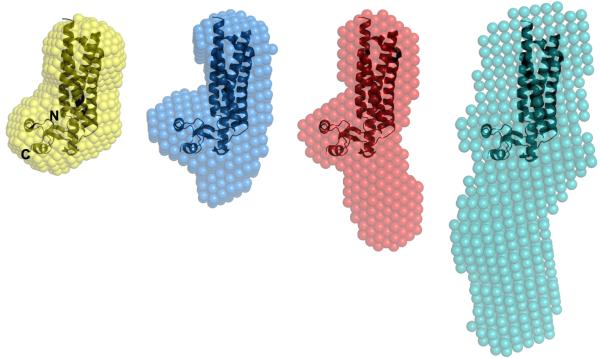
Figure 5.
Alignment of the mouse TIP47/perilipin-3 PAT-C domain crystal structure (PDB 1SZI; ribbons) spatially positioned within the consensus shape-models (spheres) derived from the SAXS data for human full-length TIP47/perilipin-3 (gray); TIP47/perilipin-3117-434 (red);TIP47/perilipin-3152-434 (blue) and; TIP47/perilipin-3187-434 (orange). The mouse homologue spatially superimposes into the molecular shape of the shortest human truncation mutant (TIP47/perilipin-3187-434) that spans the C-terminal domains of the protein. The space occupied by regions encompassing the N-terminal half of human TIP47/perilipin-3 appears to extend into solution away from the C-terminal domains.