Abstract
Background
Schizophrenia has been associated with disturbances in brain connectivity, however the exact nature of these disturbances is not fully understood. Measuring temporal correlations between the activities of spatially disparate brain regions obtained during rest with functional MRI has recently emerged as a popular paradigm for estimating brain connectivity. Previous functional resting state studies in schizophrenia explored either connections related to particular clinical or cognitive symptoms (connectivity within a-priori selected networks), or connections constrained to functional networks obtained from resting state analysis. Relatively less has been done to understand global brain connectivity in schizophrenia.
Methods
Eighteen patients with chronic schizophrenia and 18 healthy volunteers underwent a resting state fMRI scan on a 3T magnet. Whole brain temporal correlations have been estimated using resting-state fMRI data and free surfer cortical parcellation, and multivariate classification method was then used to indentify brain connections that distinguish schizophrenia patients and healthy controls.
Results
The classification procedure achieved a prediction accuracy of 75% in differentiating between groups on the basis of their functional connectivity. Relative to controls, schizophrenia patients exhibited co-existing patterns of increased connectivity between parietal and frontal regions, and decreased connectivity between parietal and temporal regions, and between the temporal cortices bilaterally. The decreased parieto-temporal connectivity was associated with the severity of patients’ positive symptoms, while increased fronto-parietal connectivity was associated with patients’ negative and general symptoms.
Discussion
Our analysis revealed two co-existing patterns of functional connectivity abnormalities in schizophrenia, each related to different clinical profile. Such results provide further evidence that abnormalities in brain connectivity, characteristic of schizophrenia, are directly related to the clinical features of the disorder.
Keywords: Functional Connectivity, Schizophrenia, Positive and Negative Symptoms
2. INTRODUCTION
Schizophrenia is a devastating disorder that simultaneously affects multiple cognitive domains including language, memory, attention and executive functioning. Since each of these functions relies on efficient communication between several, often distant, brain regions, schizophrenia has been hypothesized to arise from disruptions in brain connectivity (Konrad and Winterer, 2008). Based on the clinical symptoms, lesions studies and initial in vivo MRI studies, it has been predicted that such abnormalities should affect mostly frontal, temporal and parietal regions, and their connections (Kraepelin, 1919/1971; Weinberger et al., 1992; McGuire and Frith, 1996). Since the emergence of functional MRI (fMRI), many studies have investigated functional neuroanatomy and the correlates of the cognitive dysfunctions observed in schizophrenia (Niznikiewicz et al., 2003). However, these studies are limited by several factors, one of them being the low Signal-to-Noise Ratio (SNR), typical of fMRI data derived during experiments involving cognitive tasks, forcing the interpretations of such studies to be based on aggregated data of 10 or more subjects, rather than a single individual. Another factor limiting the clinical application of fMRI is the issue of task difficulty, where participants are limited to those able to perform a given task, and hence, whose cognition is least affected by the disease (Greicius, 2008). Additional difficulty comes with using fMRI data to compare populations, where matching for cognitive performance might lead to removing the data variance due to disease related cognitive decline (Greicius, 2008).
Resting-state fMRI is a relatively new functional imaging method, with the potential to overcome most of the above limitations. I.e. since no cognitive task is involved, there is no need to correct for cognitive performance, or exclude subjects that cannot perform the task (thus biasing the sample). In this technique, functional MR data is collected in the absence of any experimental task. Rather, the subject is asked to rest quietly, either with their eyes closed or with their eyes opened and fixating on one point. Initial experiments suggest that various regions of the brain remain active during this process, expressed in low frequency BOLD fluctuations. It is believed that temporal correlations between these fluctuations reveal the intrinsic functional organization of the brain (Biswal et al., 1995; Gusnard and Raichle, 2001; Peltier et al., 2003). Univariate tests and random effects analysis are, to a great extent, the standard in population studies of functional connectivity (Greicius et al., 2007; Liang et al., 2006; Zhou et al., 2007). Using these methods several “resting state networks (RSNs)” can be robustly identified (Beckmann et al., 2005). The method has been also applied to several brain disorders, including Alzheimer’s, depression, schizophrenia, ADHD and multiple sclerosis (MS). While results of studies in Alzheimer’s are consistent and encouraging, the same is not true for schizophrenia (Greicius, 2007). Several studies describe increased connectivity within the default mode network (one of the most robust of the resting state networks (Zhou et al., 2007; Whitfield-Gabrieli et al., 2009), while others report decreased connectivity within this network (Bluhm et al., 2007; Zhou et al., 2010). Reports on changes in correlations between other RSNs are inconsistent as well (Zhou et al., 2007; Bluhm et al., 2007).
As stated previously, schizophrenia is a multi-dimensional disease, where several separate, but interrelated cognitive domains and processes appear to be affected (Kalkstein et al., 2010), and thus clinical symptoms of schizophrenia are most likely not related to any particular brain region, or a particular brain connection, but rather appear due to instability of communication within and between networks of regions, across the spectrum of cognitive domains. Resting state fMRI data has a potential to map those interactions and their abnormalities in schizophrenia, however until now functional connectivity analysis focus on disruptions of single connections or single cognitive networks, and interactions between them are often ignored, leaving the models and clinical hypotheses that are being tested much too simplified. Specifically, most functional connectivity studies of schizophrenia use t-scores/p-values to identify the significant connections; we believe there are two issues with this approach: (1) the tests are done independently on each connection, and therefore one cannot identify networks of connections that together cause abnormalities, and (2) t-scores/p-values are not necessarily a good measure of how good the results are. Only recently have the multivariate classifier and regression approaches been used successfully in population based functional connectivity analyses (in depression (Craddock et al., 2009), and brain development (Dosenbach 2010)). In this paper, we use similar multivariate classifier approach (Random Forest classification (Breiman, 2001)). The method has been introduced, and described in detail, including its comparison to univariate tests in (Venkateraman et al., 2010). We address the above-mentioned limitations of univariate approaches by using a multi-pattern score to select the relevant features (Gini Importance), and by using prediction as the primary way to validate the results (i.e., can the model predict the diagnosis of a new subject).
Here, we applied the method to the data collected from patients with chronic schizophrenia and their matched healthy controls, intending to identify and characterize patterns of brain connetivities that can differentiate patients with schizophrenia and healthy controls. Based on schizophrenia literature as well as our previous studies, we hypothesized that the connections/networks best predicting schizophrenia diagnosis will involve fronto-temporal connections, at least partially overlapping with the default network. We further expected that the patterns of both hyper as well as hypo connectivity will be represented in schizophrenia.
3. METHODS
3.1. Subjects
Eighteen male patients diagnosed with chronic schizophrenia (SZ) (using DSM-IV criteria based on SCID-P interviews and a review of the medical records), and 18 male healthy volunteers (NC) were matched on gender, handedness, parental socio-economic status (PSES), age and premorbid IQ. All subjects gave written informed consent prior to participation in the study, and the study was approved by institutional IRB. Subjects were included in the study if they fulfilled the following criteria: right-handedness, aged between 18 and 55, no neurological illness, no alcohol or drug dependence in the last 5 years and no abuse in the past year. Healthy control subjects were additionally screened to exclude first-degree relatives with an Axis I disorder. We used the Positive and Negative Syndrome Scale (PANSS (Kay et al., 1987)) to investigate the functional role of brain connections in clinical abnormalities in schizophrenia. Demographic data is included in Table 1. At the time of the scan, majority of patients were on medication. Daily chlorpromazine equivalent antipsychotic dosage (Woods, 2003) was 356.5 ± 291.7 mg, and the content of the medication was as follows: typical antipsychotic, 7.4 %, atypical antipsychotic, 70.4 %, both, 11.1 %, and unmedicated at the scan time, 11.1 %.
Table 1.
Demographic characteristics for patients with chronic schizophrenia (SZ) and normal comparison subjects (NC)
| SZ
|
NC
|
Independent Samples t-test
|
|||
|---|---|---|---|---|---|
| (n=18) | (n=18) | df | t | P | |
| Age (yr) | 41.58 ± 9.54 | 39.21 ± 10.84 | 36 | −0.715 | 0.479 |
| Socioeconomic | |||||
| Status (SES)a | 3.37 ± 1.21 | 1.95 ± 0.71 | 36 | −4.419 | 0.001** |
| Parental SESa | 2.47 ± 1.07 | 2.11 ± 1.15 | 36 | −1.021 | 0.314 |
| Handedness | 0.71 ± 0.24 | 0.80 ± 0.17 | 36 | 1.315 | 0.197 |
| MME | 28.63 ± 2.17 | 29.36 ± 0.63 | 31 | 1.211 | 0.235 |
Mean ± SD
Higher scores indicated lower SES
3.2. Imaging Data Acquisition
Imaging was performed using a 3-T whole body MRI Echospeed system (General Electric Medical Systems, Milwaukee, WI). Two sets of data were collected: structural MRI, used for brain parcelation, and resting state fMRI, used for functional connectivity analysis. All images were collected during one imaging session with 8 Channel coil and ASSET (Array Spatial Sensitivity Encoding techniques, GE) with a SENSE-factor (speed-up) of 2. The structural MRI acquisition included two MRI pulse sequences: spoiled gradient-recalled acquisition (fastSPGR) (TR=7.4ms, TE=3ms, TI=600, 10 degree flip angle, 25.6cm2 field of view, matrix=256x256), and XETA (eXtended Echo Train Acquisition), which produced a series of contiguous T2-weighted images (TR=2500ms, TE=80ms, 25.6 cm2 field of view). Both structural acquisitions resulted in 1 mm thick slices. Resting state fMRI data was collected using an EPI BOLD sequence, containing 200 repetitions of a high resolution EPI scan (96x96 in plane, 24 cm2 field of view; 3mm thickness, TR-3000 ms, TE=30, 39 slices) acquired over 10 minutes. During this protocol, subjects were asked to close their eyes, and rest, while the magnet ran.
3.3. Data Analysis
Gray Matter was segmented into 77 anatomical regions of interest (ROIs) using a semi-automatic FreeSurfer software, as part of the Slicer3 environment (www.slicer.org). Parcellation into Brodmann areas by Free Surfer is a standard/default procedure, and segmentation is performed based on anatomical/atlas priors, thus no selection process is necessary. The parcellation process is described elsewhere (Fischl et al., 2004) and provides results that are similarly sensitive to disease related changes as manual tracing (Morey et al., 2009). The resting-state fMRI data was processed as follows. The first 5 scans were discarded, and the sixth was used as a target for motion correction. All remaining scans were co-registered to this scan using rigid body alignment and slicing timing correction using FSL software (Smith et al., 2004). The data was then spatially smoothed, using 6 mm Gausian filter, and temporally low-pass filtered with 0.08Hz cutoff. Finally, we removed global contributions to the time courses from the white matter, ventricles and the whole brain by using multivariate linear regression. The next step in data analysis involved non-rigid registration of the structural MRI to the fMRI space for each subject. fMRI connectivity analysis was performed as follows. For each pair of ROIs (2926 connections in total), we computed Pearson correlation coefficient between every pair of voxels in the two regions, applied a Fisher r-to-z transform to each correlation, and averaged these values. These measures served as our connectivity features for subsequent statistical analyses. We also assess the significance of prior clinical knowledge in a separate experiment by preselecting 16 brain structures (8 in each hemisphere), believed to play an important role in the pathophysiology of schizophrenia (based on literature search and previous reviews). These are the superior temporal gyrus, the rostral middle frontal gyrus (roughly corresponding to the dorsolateral prefrontal cortex), the hippocampus, the amygdala, the anterior cingulate gyrus, the posterior cingulate gyrus, the parahippocampal gyrus and the Heschl’s gyrus. Since prior results in the schizophrenia literature suggest that these regions play a role in the disease, here we focus on the associated connections. This allows us to discard potentially noisy connections between irrelevant brain regions, which may bias the results. We consider only correlations between these regions and the rest of the ROIs in our analysis (1096 connections).
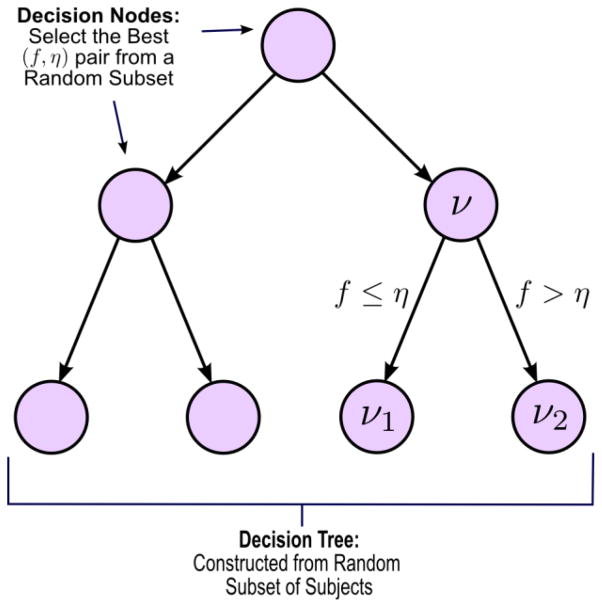
Once the correlation coefficients are estimated for each connection in each individual, we compute the Gini Importance of each connectivity feature based on a Random Forest analysis. The Random Forest is an ensemble of decision tree classifiers that incorporates multiple levels of randomization. Each tree is grown using a random subset of training data (first level of randomization); each “decision node” is constructed by searching over a random subset of features, in our case, correlation measures (second level of randomization). The Random Forest derives a score for each feature, known as Gini Importance (GI), which summarizes its discriminative power. At each decision step (node) of the tree, the algorithm selects the feature/threshold pair, from a random subset, that maximizes the separation between groups (NC vs SZ) (see Figure 1). This process is continued recursively for all nodes, until each “leaf” of the tree defines unique class (diagnosis). The final classification is obtained by a majority vote among all decision trees in the ensemble. The Random Forest algorithm was implemented in R. All other code was done in MATLAB. We use 20,000 trees in the analysis, which is roughly one order of magnitude larger than the number of features. The approach confers several advantages over univariate approaches. The randomization over subjects improves generalization accuracy, while randomization over features increases the likelihood of identifying all, rather than an uncorrelated subset, of functional connections useful for group discrimination. Finally, due to the ensemble-based learning, the Random Forest produces a nonlinear decision boundary and is able to capture significantly different patterns of functional connectivity across distributed networks in the brain. It is worth mentioning here, that the random forest algorithm does not have any conventional notion of significance, thus it does not require multiple comparison correction. In the paper introducing the method (Venkataraman et al., 2010), we demonstrate that one of the most significant advantages of our method in comparison to univariate tests when applied to this particular dataset, is that none of the connections that we found different between groups here demonstrate consistent t-scores across subsets of the data, to survive multiple comparison correction.
Fig 1.
Implementation of a single decision tree in the Random Forest algorithm. ν is a given decision node. (f,η) are the feature and threshold pair used to create child nodes ν1, and ν2.
4. RESULTS
4.1. Schizophrenia group abnormalities
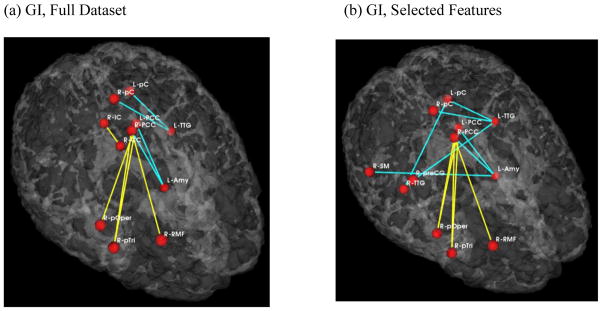
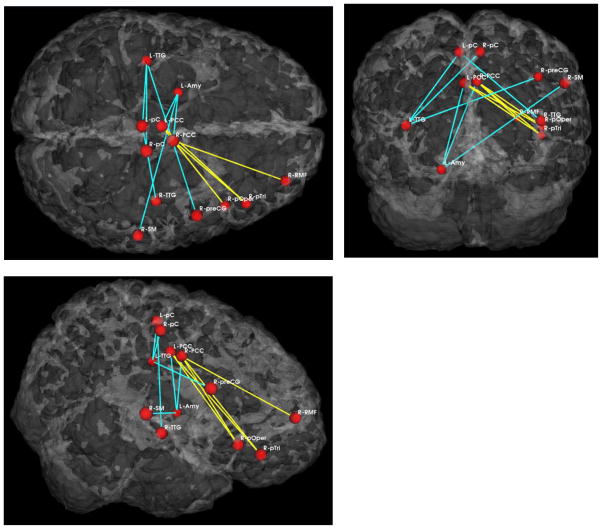
Schizophrenia patients exhibited increased functional connectivity between the medial parietal region, including the posterior cingulate gyrus, and the frontal lobe (pars triangularis and opercularis of the inferior frontal gyrus, and dorsolateral prefrontal cortex). This was true for both the full dataset analysis and for the analysis based on the pre-selected brain regions. Along with the increased functional connectivity, abnormal schizophrenia connectivity pattern also included reduced functional connectivity between the same medial parietal region and the left temporal lobe (inferior temporal gyrus and amygdala). Again, same pattern and features were highlighted using both the full dataset and the selected features. In addition, the interhemispheric connectivity between the left and the right temporal regions expressed reduced functional connectivity in the selected features analysis only. Results of the GI analysis are demonstrated on figures 3 and 4. Finally, when using GI scores and preselected group of features (regions) for predicting group membership, we achieved as high as 75% prediction accuracy in distinguishing between controls and schizophrenia subjects.
Fig 3.
Abnormal functional connections in schizophrenia. View from the top right side. Blue lines indicate higher connectivity in the control group; yellow lines indicate higher connectivity in the schizophrenia population.
Fig 4.
Results of GI with selected features in three views (from the top, back and the right side).
4.2. Clinical Correlations
In addition to group comparison, in order to understand clinical implications of functional connectivity disruptions in schizophrenia, we also calculated correlation coefficients between clinical symptoms obtained from PANSS (Positive and Negative Symptom Scale (Kay et al., 1987)) and individual functional connectivities that revealed group differences in our analysis. The functional connections that exhibited hyper-connectivity in the schizophrenia group (i.e., between the medial parietal and inferior frontal and dorsolateral prefrontal regions) were negatively correlated with patients’ scores on the Negative (social withdrawal rho=−0.47, P=0.041) and General (anxiety rho=−0.50, P=0.26, retardation rho=−0.49, P=0.030 and attention −0.49, P=0.029) subscales of the PANSS. Furthermore, the functional connections that exhibited hypo-connectivity in the schizophrenia group (i.e., between the medial parietal and temporal regions) were positively correlated with patients’ scores on the Positive subscales of the PANSS (delusions rho=0.50, P=0.028 and hallucinations rho=0.54, P=0.016).
5. DISCUSSION
Our results of whole brain, multivariate analysis of functional connectivity in schizophrenia indicate that when compared to healthy controls, patients with schizophrenia exhibit two distinct patterns of differences. Rather than showing uniformly increased or decreased connectivity, schizophrenia patients, when compared to controls, exhibit abnormally increased connectivity between the medial parietal and frontal lobes, and decreased connectivity between the medial parietal and temporal regions and between the temporal cortex bilaterally. Furthermore, these connectivity abnormalities show a differential relationship with patients’ clinical symptoms, in that the networks associated with sub-normal connectivity in the patient group are associated with the severity of patients’ positive symptoms, while networks associated with supra-normal connectivity in the patient group shows associations with the severity of patients’ negative and general symptoms. Taken together, these results suggest that abnormal patterns of functional connectivity are associated with schizophrenia clinical symptoms.
As discussed previously, the most frequently studied feature (network) identified in resting state fMRI data is the default mode network (DMN). According to various sources, it includes medial frontal (including anterior cingulate), parietal (including posterior cingulate, precuneus and inferior parietal) and medial temporal (including hippocampus) areas of the brain (Raichle et al., 2001; Greicius et al., 2003). The activation pattern (low-frequency fluctuations) within all the elements of this network seem to strongly correlate with each other, and network as a whole has been associated with spontaneous and task independent, internally generated thought processes (Fox et al., 2005). It is further believed that the activation within this network is actively suppressed during the cognitive tasks, and that the degree of this suppression correlates with task performance (semantic recognition and semantic priming) (Jeong and Kubicki, 2010). In schizophrenia, few similar observations have been made in relationship to the task performance, i.e. Whitfield-Gabrieli et al., (2009) report anticorrelations between default-mode network and working memory performance, while Jeong and Kubicki (2010) report anticorrelations between default-mode network and semantic processes in schizophrenia. Both those studies suggest that the decreased activation and poorer cognitive performance in schizophrenia might be partially related to increased activation/decreased suppression within the default-mode network.
Both increased (Zhou et al., 2010) and decreased connectivity (Liang et al., 2006; Bluhm et al., 2007) in the default-mode network has been reported previously in patients with schizophrenia. The results of our study show that those previous results do not necessarily contradict each other. Instead, schizophrenia might, even at the level of each functional network, be associated with distinct patterns of functional connectivity abnormalities, in which certain connections (i.e., parietal-temporal and the temporal cortices bilaterally) have subnormal levels of functional connectivity while others (i.e., parietal-frontal) show supra-normal connectivity.
Increased levels of activation within the anterior part of the default network in schizophrenia (which also overlap with the brain regions involved in executive function and attention) could potentially interact with the posterior connections (i.e., temporo-parietal), thereby decreasing their effective connectivity and potentially affecting important cognitive processes that would rely on such connectivity, such as early auditory (Javitt et al., 2003), or semantic (Nestor et al., 2003; Saykin et al., 1991) processes. Furthermore, since the posterior and inferior temporal-parietal regions have been consistently implicated in the clinical symptoms of schizophrenia (specifically, in hallucinations and thought disorder (Woodruff et al., 1997), it is possible that the functional connectivity disruptions between these regions, such as observed in the present study, as well as their correlations with hallucinations and delusions also observed here, directly reflect anatomical abnormalities reported in the literature, such as volume decreases in the STG, Heschl’s gyrus (associated with auditory hallucinations Bartha et al., 1990), and the amygdala-hippocampus complex (associated with thought disorder Shenton et al., 2001), (for the review also see Buckley 2005). The second possibility is that decreased anatomical connectivity between temporal and posterior parietal regions (implicated by anatomical DTI studies reporting abnormalities in white matter integrity in the cingulum bundle and arcuate fasciculus (for the review, see (Kubicki et al., 2007)), might decrease the inhibitory, “task related” input into the medial parietal region. This would further reflect in hyperactivation and hyperconnectivity within the frontal connections and attention deficits that are subserved by this connection, and are quite frequent in schizophrenia (as well as reflected by correlations with general and negative symptoms reported here). In addition to parieto-frontal and parieto-temporal connectivity abnormalities observed in our sample, our abnormal connectivity pattern also involved interhemispheric connections between temporal regions. Such abnormalities, even though not sufficiently understood, have been also suggested in multiple theories involving neurodevelopment and neurodegeneration in schizophrenia (Crow et al., 2007), and further suggest necessity of involving connections between left and right hemisphere in all experimental models of schizophrenia.
It is worth mentioning that while our analysis exhibits a distinct prediction power, unlike PCA approaches, the results do not imply that the important connections belong to the same functional network. Since not all schizophrenia patients share the same clinical manifestations, functional signal fluctuations should be more variable within this group than within healthy controls. Our method is designed to find connections that consistently and together differentiate patients and controls. Accordingly, in our previous, methodological publication (Venkataraman et al., 2010), we demonstrated that Gini Importance, as opposed to univariate scores, remains consistent across cross-validation iterations, and significant connectivity features have reasonable predictive power in distinguishing those populations. We notice, however, that in current experiment, a small subset of subjects is still consistently misclassified. This suggests that functional connectivity differences between two populations are quite subtle. Additionally, since resting functional connectivity is not a well-understood phenomenon, the results may be confounded by external factors, which include anatomical variability in “white matter connectivity”, age, medication levels, etc. Once fully understood, accounting for these factors might vastly improve analytic power of functional connectivity experiments. It is worth recalling, however, that not only is schizophrenia a clinically inhomogeneous disease, but various anatomical and/or physiological disturbances might lead to the same clinical manifestation. This renders the search for a schizophrenia phenotype even more complex and difficult.
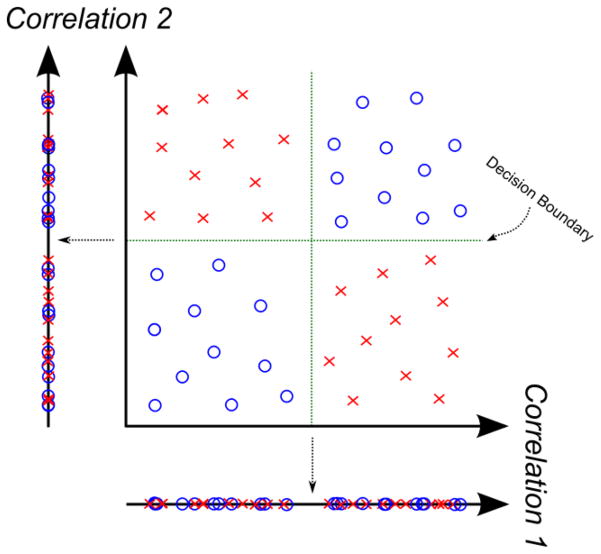
Fig 2.
Importance of multivariate analysis. Consider the toy example illustrated here. The data points are two-dimensional; for example, each one may correspond to a different functional correlation. The red X’s and blue circles denote the two classes (i.e., control vs. clinical subjects). Visually, we can separate the data into quadrants. However, individually, neither dimension (correlation value) can distinguish between the populations. Rather, we need the nonlinear decision boundary in green, which is defined jointly over both dimensions.
Acknowledgments
Funding Sources
This study was supported, in part, by the National Alliance for Medical Image Computing (NA-MIC), supported through the National Institutes of Health Roadmap for Medical Research, Grant U54 EB005149 (MK, CFW, PG); National Institute of Health (R01 M074794 to CFW and MK). This work was also supported by grant from the Medical Research Council of Australia (Overseas-Based Biomedical Training Fellowship (NHMRC 520627), through the Melbourne Neuro-Psychiatry Centre at the University of Melbourne, to TJW).
Biography
Mrs Venkataraman designed the study and run the analyses. Dr Kubicki provided fMRI data. Drs Kubicki, Golland and Westin supervised various aspects of the study. Dr Whitford undertook clinical statistical analyses, Drs Kubicki and Whitford helped interpreting clinical findings, Mrs Venkataraman wrote the first draft of the manuscript. All authors contributed to and have approved the final manuscript.
Footnotes
Authors report no conflict of interests
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Beckmann CF, DeLuca M, Devlin JT, Smith SM. Investigations into resting-state connectivity using independent component analysis. Philos Trans R Soc Lond B Biol Sci. 2005 May 29;360(1457):1001–13. doi: 10.1098/rstb.2005.1634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biswal B, Yetkin FZ, Haughton VM, Hyde JS. Functional connectivity in the motor cortex of resting human brain using echo-planar MRI. Magn Reson Med. 1995 Oct;34(4):537–41. doi: 10.1002/mrm.1910340409. [DOI] [PubMed] [Google Scholar]
- Bluhm RL, Miller J, Lanius RA, Osuch EA, Boksman K, Neufeld RW, et al. Spontaneous low-frequency fluctuations in the BOLD signal in schizophrenic patients: anomalies in the default network. Schizophr Bull. 2007 Jul;33(4):1004–12. doi: 10.1093/schbul/sbm052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breiman L. Random Forests. Machine Learning. 2001;45:5–32. [Google Scholar]
- Craddock RC, Holtzheimer PE, 3rd, Hu XP, Mayberg HS. Disease state prediction from resting state functional connectivity. Magn Reson Med. 2009 Dec;62(6):1619–28. doi: 10.1002/mrm.22159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crow TJ, Paez P, Chance SA. Callosal misconnectivity and the sex difference in psychosis. Int Rev Psych. 2007 Aug 19;4:449–57. doi: 10.1080/09540260701486282. [DOI] [PubMed] [Google Scholar]
- Dosenbach NF, Nardos B, Cohen AL, Fair DA, Power JD, Church JA, et al. Prediction of Individual Brain Maturity Using fMRI. Science. 2010;329 (5997):1358–1361. doi: 10.1126/science.1194144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischl B, van der Kouwe A, Destrieux C, Halgren E, Segonne F, Salat DH, et al. Automatically parcellating the human cerebral cortex. Cereb Cortex. 2004 Jan;14(1):11–22. doi: 10.1093/cercor/bhg087. [DOI] [PubMed] [Google Scholar]
- Fox MD, Snyder AZ, Vincent JL, Corbetta M, Van Essen DC, Raichle ME. The human brain is intrinsically organized into dynamic, anticorrelated functional networks. Proc Natl Acad Sci U S A. 2005 Jul 5;102(27):9673–8. doi: 10.1073/pnas.0504136102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gusnard DA, Raichle ME. Searching for a baseline: functional imaging and the resting human brain. Nat Rev Neurosci. 2001 Oct;2(10):685–94. doi: 10.1038/35094500. [DOI] [PubMed] [Google Scholar]
- Greicius MD, Krasnow B, Reiss AL, Menon V. Functional connectivity in the resting brain: a network analysis of the default mode hypothesis. Proc Natl Acad Sci U S A. 2003 Jan 7;100(1):253–8. doi: 10.1073/pnas.0135058100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greicius MD, Flores BH, Menon V, Glover GH, Solvason HB, Kenna H, et al. Resting-state functional connectivity in major depression: abnormally increased contributions from subgenual cingulate cortex and thalamus. Biol Psychiatry. 2007 Sep 1;62(5):429–37. doi: 10.1016/j.biopsych.2006.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greicius M. Resting-state functional connectivity in neuropsychiatric disorders. Curr Opin Neurol. 2008 Aug;21(4):424–30. doi: 10.1097/WCO.0b013e328306f2c5. [DOI] [PubMed] [Google Scholar]
- Javitt DC, Doneshka P, Zylberman I, Ritter W, Vaughan HG., Jr Impairment of early cortical processing in schizophrenia: an event-related potential confirmation study. Biol Psychiatry. 1993 Apr 1;33(7):513–9. doi: 10.1016/0006-3223(93)90005-x. [DOI] [PubMed] [Google Scholar]
- Jeong BS, Kubicki M. Reduced Task-related Suppression during Semantic Repetition Priming in Schizophrenia. Psychiatry Research Neuroimaging. 2010;181(2):114–120. doi: 10.1016/j.pscychresns.2009.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kay SR, Fiszbein A, Opler LA. The positive and negative syndrome scale (PANSS) for schizophrenia. Schizophr Bull. 1987;13(2):261–76. doi: 10.1093/schbul/13.2.261. [DOI] [PubMed] [Google Scholar]
- Kalkstein S, Hurford I, Gur RC. Neurocognition in schizophrenia. Curr Top Behav Neurosci. 2010;4:373–90. doi: 10.1007/7854_2010_42. [DOI] [PubMed] [Google Scholar]
- Konrad A, Winterer G. Disturbed structural connectivity in schizophrenia primary factor in pathology or epiphenomenon? Schizophr Bull Jan. 2008;34(1):72–92. doi: 10.1093/schbul/sbm034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraepelin E. In: Dementia Praecox, E. Barclay SB, editor. New York: Churchill Livingstone Inc; 1919/1971. [Google Scholar]
- Kubicki M, McCarley R, Westin CF, Park HJ, Maier S, Kikinis R, et al. A review of diffusion tensor imaging studies in schizophrenia. Journal of Psychiatric Research. 2007 Jan-Feb;41(1–2):15–30. doi: 10.1016/j.jpsychires.2005.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang M, Zhou Y, Jiang T, Liu Z, Tian L, Liu H, et al. Widespread functional disconnectivity in schizophrenia with resting-state functional magnetic resonance imaging. Neuroreport. 2006 Feb 6;17(2):209–13. doi: 10.1097/01.wnr.0000198434.06518.b8. [DOI] [PubMed] [Google Scholar]
- McGuire PK, Frith CD. Disordered functional connectivity in schizophrenia [editorial] Psychol Med. 1996;26(4):663–7. doi: 10.1017/s0033291700037673. [DOI] [PubMed] [Google Scholar]
- Morey RA, Petty CM, Xu Y, Hayes JP, Wagner HR, 2nd, Lewis DV, et al. A comparison of automated segmentation and manual tracing for quantifying hippocampal and amygdala volumes. Neuroimage. 2009 Apr 15;45(3):855–66. doi: 10.1016/j.neuroimage.2008.12.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nestor PG, Shenton ME, McCarley RW, Haimson J, Smith RS, O’Donnell B, et al. Neuropsychological correlates of MRI temporal lobe abnormalities in schizophrenia. Am J Psychiatry. 1993 Dec;150(12):1849–55. doi: 10.1176/ajp.150.12.1849. [DOI] [PubMed] [Google Scholar]
- Niznikiewicz MK, Kubicki M, Shenton ME. Recent structural and functional imaging findings in schizophrenia. Curr Opin Psychiatry. 2003;16:123–47. [Google Scholar]
- Peltier SJ, Polk TA, Noll DC. Detecting low-frequency functional connectivity in fMRI using a self-organizing map (SOM) algorithm. Hum Brain Mapp. 2003 Dec;20(4):220–6. doi: 10.1002/hbm.10144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raichle ME, MacLeod AM, Snyder AZ, Powers WJ, Gusnard DA, Shulman GL. A default mode of brain function. Proc Natl Acad Sci U S A. 2001 Jan 16;98(2):676–82. doi: 10.1073/pnas.98.2.676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saykin AJ, Gur RC, Gur RE, Mozley PD, Mozley LH, Resnick SM, et al. Neuropsychological function in schizophrenia. Selective impairment in memory and learning. Arch Gen Psychiatry. 1991 Jul;48(7):618–24. doi: 10.1001/archpsyc.1991.01810310036007. [DOI] [PubMed] [Google Scholar]
- Shenton ME, Dickey CC, Frumin M, McCarley RW. A review of MRI findings in schizophrenia. Schizophr Res. 2001;49(1–2):1–52. doi: 10.1016/s0920-9964(01)00163-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SM, Jenkinson M, Woolrich MW, Beckmann CF, Behrens TE, Johansen-Berg H, et al. Advances in functional and structural MR image analysis and implementation as FSL. Neuroimage. 2004;23(Suppl 1):S208–19. doi: 10.1016/j.neuroimage.2004.07.051. [DOI] [PubMed] [Google Scholar]
- Stephane M, Barton S, Boutros NN. Auditory verbal hallucinations and dysfunction of the neural substrates of speech. Schizophr Res. 2001 May;30;50(1–2):61–78. doi: 10.1016/s0920-9964(00)00150-x. [DOI] [PubMed] [Google Scholar]
- Venkataraman A, Kubicki M, Westn CF, Golland P. Robust Feature Selection in Resting-State fMRI Connectivity Based on Population Studies. Proc. MMBIA: IEEE Computer Society Workshop on Mathematical Methods in Biomedical Image Analysis; 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinberger DR, Berman KF, Suddath R, Torrey EF. Evidence of dysfunction of a prefrontal-limbic network in schizophrenia: a magnetic resonance imaging and regional cerebral blood flow study of discordant monozygotic twins. Am J Psychiatry. 1992;149(7):890–7. doi: 10.1176/ajp.149.7.890. [DOI] [PubMed] [Google Scholar]
- Whitfield-Gabrieli S, Thermenos HW, Milanovic S, Tsuang MT, Faraone SV, McCarley RW, et al. Hyperactivity and hyperconnectivity of the default network in schizophrenia and in first-degree relatives of persons with schizophrenia. Proc Natl Acad Sci U S A. 2009 Jan 27;106(4):1279–84. doi: 10.1073/pnas.0809141106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitford TJ, Kubicki M, Schneiderman JS, O’Donnell LJ, King R, Alvarado JL, Khan U, Markant D, Nestor PG, Niznikiewicz M, McCarley RW, Westin C-F, Shenton ME. Corpus callosum abnormalities and their association with psychotic symptoms in patients with schizophrenia. Biological Psychiatry. 2010;68:70–77. doi: 10.1016/j.biopsych.2010.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodruff PW, Wright IC, Shuriquie N, Russouw H, Rushe T, Howard RJ, et al. Structural brain abnormalities in male schizophrenics reflect fronto-temporal dissociation. Psychol Med. 1997;27(6):1257–66. doi: 10.1017/s0033291797005229. [DOI] [PubMed] [Google Scholar]
- Woods SW. Chlorpromazine equivalent doses for the newer atypical antipsychotics. J Clin Psychiatry. 2003 Jun;64(6):663–7. doi: 10.4088/jcp.v64n0607. [DOI] [PubMed] [Google Scholar]
- Zhou Y, Liang M, Jiang T, Tian L, Liu Y, Liu Z, et al. Functional dysconnectivity of the dorsolateral prefrontal cortex in first-episode schizophrenia using resting-state fMRI. Neurosci Lett. 2007 May 7;417(3):297–302. doi: 10.1016/j.neulet.2007.02.081. [DOI] [PubMed] [Google Scholar]
- Zhou J, Greicius MD, Gennatas ED, Growdon ME, Jang JY, Rabinovici GD, et al. Divergent network connectivity changes in behavioural variant frontotemporal dementia and Alzheimer’s disease. Brain. 2010 May;133(Pt 5):1352–67. doi: 10.1093/brain/awq075. [DOI] [PMC free article] [PubMed] [Google Scholar]