Abstract
Numerous appetite, growth, obesity-related hormones and inflammatory factors are found in human breast-milk, but there is little evidence on their relationship with infant body composition. The purpose of the present cross-sectional pilot study was to assess the cross-sectional associations of appetite-regulating hormones and growth factors (leptin, insulin, glucose) and inflammatory factors (IL-6 and TNF-α) in human breast-milk with infant size, adiposity, and lean tissue at 1-month of age in healthy term infants. Human breast-milk was collected from nineteen exclusively breast-feeding mothers using one full breast expression between 8:00 and 10:00 am. The milk was then mixed, aliquoted, stored at −80°C and then centrifuged to remove the milk fat, prior to analyses using commercially available immunoassay kits; milk analytes were natural log transformed prior to analysis. Infant body composition was assessed using a Lunar iDXA v11-30.062 scanner (Infant whole body analysis enCore 2007 software, GE, Fairfield, CT). Maternal pre-pregnancy BMI was positively associated with milk leptin concentration (p=0.0027), and so maternal-BMI-adjusted Spearman correlations were examined between breast-milk analytes and infant growth and body composition variables. As previously reported, greater milk leptin was associated with lower BMIZ (r= −0.54, p=0.03). Glucose was positively associated with relateive weight (r = 0.6, p=0.01), and both fat and lean mass (0.43 – 0.44, p<0.10). Higher concentrations of milk insulin were associated with lower infant weight, relative weight, and lean mass (r = −0.49 – 0.58, p<0.06). Higher milk IL-6 was associated with lower relative weight, weight gain, percent fat, and fat mass (r = −0.55 – 0.70, p<0.03 for all), while higher TNF-α was associated with lower lean mass (r=−0.58, p=0.05), but not measures of adiposity. These preliminary data suggest for the first time that in the first months of life, breast-milk concentrations of insulin, glucose, IL-6 and TNF-α, in addition to leptin, may be bioactive and differentially influence the accrual of fat and lean body mass.
Keywords: growth, body composition, inflammation, breast-milk
INTRODUCTION
Child obesity risk is known to be strongly influenced by pre-natal and early post-natal exposures [1–4]. Clinical studies and animal models have identified numerous potentially modifiable maternal factors (including obesity, dietary intakes, cigarette smoking, and stress) that alter the development of the growing fetus and subsequently affect infant appetite, growth, and body composition [5–8]. Breast-milk composition is one such potentially modifiable factor [9]. Human breast-milk exhibits inter-individual variation in energy and macronutrient content [10–13], as well as intra-individual variation (e.g., diurnal and fore/hindmilk variation) [14, 15]. and also contains numerous appetite, growth, obesity-related hormones and inflammatory factors, including leptin, IGF-1, glucose, adiponectin, insulin, ghrelin, IL-6, C-reactive protein., and TNF-α [16–22]. However, whether or not these factors are bioactive in the infant and influence infant growth and body composition is still under intense investigation.
Early work suggested greater risk of obesity and glucose intolerance in breastfed offspring of diabetic mothers as compared to their counterparts fed banked breast milk[23], which was attributed to elevated insulin levels in the milk of diabetic women, although milk insulin was not directly measured in these studies. Other studies have suggested a protective association of breast-feeding on obesity in offspring of mothers with gestational diabetes [24, 25], but again, the breast-milk composition was not analyzed. To date, the strongest evidence for association of breast-milk hormones and adipokines with infant growth is for leptin, which is positively associated with maternal BMI and negatively associated with infant weight gain [26, 27], and for adiponectin [28, 29]. The roles of other growth factors and adipokines found in human milk in regulating infant growth and body composition are little known.
The purpose of the present pilot study was to assess the association of appetite-regulating hormones and growth factors (leptin, insulin, glucose) and inflammatory factors (IL-6 and TNF-α) in human breast-milk with infant size, adiposity and lean tissue at 1 -month of age.
METHODS
General study design
At 1-month (n = 19) ± 5 days mother and infant arrived at the Children’s Metabolic Research Center between 8:00–10:00 am on the campus of the University of Oklahoma Health Sciences Oklahoma City campus for testing. Upon completing informed consent and HIPAA forms, height and weight were collected in both the mother and child. A complete breast-milk sample was obtained followed by a full-body dual energy X-ray absorptiometry (DXA) scan performed in the infant.
Subjects
Nineteen mother–infant dyads who were exclusively breast-fed for 6-months (i.e. no formula supplementation) were recruited from the University Hospital at the University of Oklahoma Health Sciences Center. Prior to all testing / procedures, the appropriate approval was obtained from the Institutional Review Board. The following inclusion criteria were used: 1) maternal age between 18–45 years at the time of delivery, 2) gestation lasting ≥ 37 weeks, 3) singleton birth and 4) a postpartum hospital stay for mother and infant less than 3 days. The following exclusion criteria were used: 1) any tobacco use, 2) alcohol consumption (>1 drink per week), 3) pre and gestational diabetes and 4) presumed or known congenital birth defects. Mothers self-reported their age, parity, pre-pregnancy weight, weight gained during gestation.
Human breast-milk collection
Subjects typically arrived between 8:00–10:00 am, approximately 1 ½ hours after the last feeding. This was done to standardize milk collection and reduce potential diurnal variation in milk composition. Every attempt was made to ensure mothers were fasted at least 2 hours; however, it was not always confirmed. Specific human breast-milk analytes were measured in milk collected from one full breast expression. At collection, the entire contents of one breast at 1-month (mean volume 2.4 ± 0.2 oz.) were collected using an electric breast pump (Medela, Inc.) ensuring the collection of fore-, mid-, and hind-milk within each sample.
Thoroughly mixed human breast-milk was divided into ten aliquots. All aliquots were stored at −80°C until analyses. Prior to analyses, aliquots were thawed on ice and milk fat was separated from the aqueous phase by centrifugation at 3,000 × g for 10 minutes [30–32]. The resulting skimmed milk was assayed using commercially available immunoassay kits for insulin, leptin, IL-6 and TNF-α. Glucose was measured by the glucose oxidase method (2300 STAT Plus, Yellow Springs Instruments).
Growth measures
For each visit, weight and length were obtained. A nude weight was obtained in duplicate using a Seca 728 electronic infant scale (Seca, Hamburg, Germany; accuracy ± 0.1 g). Both trials had to be within 10 grams. Length was obtained in duplicate using a Seca 416 infantometer (Seca, Hamburg, Germany) with both trials having to be within 0.5 cm. In the instance that the weight and length measures were outside of the specified limits, a third measure was performed with the two closest values averaged.
Body Composition
Whole and regional body composition was obtained using a Lunar iDXA v11-30.062 (Infant whole body analysis enCore 2007 software, GE, Fairfield, CT) scanner. The iDXA has not yet been validated in infants to our knowledge, but similar DXA scanners have been successfully validated against gold-standard carcass weights in primates [33]. The scanner was calibrated daily according to the Lunar iDXA instrument manual while the day-to-day coefficient of variation (CV) for the estimation of percent fat in nine subjects in our laboratory is 1%. An advantage of iDXA is that it has superior precision compared to earlier models of the DXA (precision error is CV 0.82% for total fat mass and 0.86% for percentage fat) [34].
The infant wearing only a disposable diaper was swaddled using a light receiving blanket provided by the laboratory. Upon placement of the infant on the scanning bed, the lights were dimmed and the scan commenced while a DVD played. In the event there was excessive crying or movement, the test was immediately stopped. The same person (DAF) positioned infants and analyzed all scans.
Statistical analyses
Descriptive statistics (means and standard deviations) were calculated for demographic data, growth (weight, length and rate of weight change in grams/day from birth to 1-month), body composition (%fat, total fat mass, total lean mass, and trunk fat mass) and breast-milk analytes (glucose, IL-6, TNF-α, insulin and leptin)..
A Shapiro-Wilk normality test was performed on all growth and body composition variables and breast-milk analytes. None of the growth and body composition variables were skewed. All the breast-milk analytes were skewed except for glucose, consequently they were log transformed for subsequent analyses.
First, possible confounders were examined for bivariate association with breast milk constituents; these included maternal pre-gravid BMI, maternal gestational weight gain, maternal ethnicity, maternal household income, maternal parity, infant sex, infant age at the milk and body composition assessment, and the time since the last breast-milk feeding. Then, raw bivariate relationships among the five breast-milk constituents and between breast milk analytes and growth and body composition variables were investigated using Spearman correlation coefficient analyses. Finally, partial correlations were computed adjusting for possible confounders that were significant in the first step. All statistical analyses were conducted using SPSS 18.0. Statistical significance was set at an alpha level of p ≤ 0.05, but we present and discuss relationships at the more liberal p<0.10 as well due to the exploratory nature of this analyses.
RESULTS
Nineteen women with a wide pre-gravid BMI (26.6 ± 6.6; range of 18.5 – 42 kg/m2) and parity (2.7 ± 1.7) enrolled into the study along with their offspring (68% male). The ethnic distribution of the sample was Caucasian (72%) and African-American (11%) with the remaining comprised of Native American and Hispanics. The socioeconomic status of the population was diverse with 21% indicating an average household income of less than $30,000 while 26% indicated greater than $90,000.
Descriptive statistics of the growth, body composition and human breast-milk data at 1-month are presented in Table 1.
Table 1.
Description of study sample (n=19).
| Variable | 1-month |
|---|---|
| n = 19 | |
| Age (days) | 40 ± 3.7 |
| Weight (grams) | 4,790 ± 651 |
| Length (cm) | 55.6 ± 2.2 |
| Weight-for-length z-scorea | −0.22 ± 1.18 |
| BMI-for-age z-scorea | −0.14 ± 1.14 |
| Rate of weight change from birth to 1-month (g/day) | 31.3 ± 0.01 |
| %fat | 23.9 ± 2.9 |
| Total Fat Mass (grams) | 1,205 ± 292 |
| Total Lean Mass (grams) | 3,711 ± 508 |
| Trunk Fat Mass (grams) | 413 ± 127 |
| % male | 68% |
| % Caucasian | 72% |
| Income (<$30,000;>$90,000) | 21;26% |
| Parity | 2.7 ± 1.7 |
| Pre-gravid BMI | 26.6 ± 6.6 |
| Glucose (mg/dL) | 25.7 ± 8.6 |
| IL-6 (pg/mL) | 3.8 ± 5.3 |
| TNF-α (pg/mL) | 4.7 ± 4.9 |
| Insulin (pg/mL) | 954.9 ± 726.5 |
| Leptin (pg/mL) | 91.8 ± 47.0 |
Data presented as mean ± standard deviation.
z-scores based on WHO 2006 growth charts (http://www.who.int\childgrowth\standards\technicalreport\en\index.html.
Biochemical constituents were measured in human breast milk.
There were a number of associations among the five breast-milk analytes examined. Milk insulin, TNF-α and IL-6 were all negatively associated with milk glucose (p<0.06), milk insulin was positively associated with milk leptin and TNF-α (p<0.04), and TNF-α and IL-6 were positively correlated with one another (p=0.06)Table 2.
Table 2.
Correlations among measured breast milk analytes.
| Glucose | Insulin | Leptin | TNF-α | IL-6 | |
|---|---|---|---|---|---|
| Glucose | 1.00 | −0.44 (p=0.06) | 0.02 | −0.76 (p=0.0003) | −0.61 (p=0.009) |
| Insulin | 1.00 | 0.52 (p=0.03) | 0.49 (p=0.04) | 0.36 | |
| Leptin | 1.00 | −0.01 | 0.10 | ||
| TNF-α | 1.00 | 0.45 (p=0.06) | |||
| IL-6 | 1.00 |
Correlations in bold have p < 0.10
We examined many possible confounders of the breast-milk analyte – growth association, including maternal ethnicity, age, parity, pre-gravid BMI, income, gestational weight gain, and time since last feed, infant sex and age, as well as correlates of infant body composition for their association with milk glucose, insulin, leptin, TNF-alpha and IL-6 (data not shown). The only potential confounding factor found to be associated with any breast-milk variable was maternal pre-gravid BMI (Figure 1), which was positively associated with both milk leptin (r = 0.78, p<0.0001) and infant fat mass (r=0.53, p=0.01) and lean mass (r=0.52, p=0.02). Therefore, both raw and maternal pre-gravid BMI-adjusted associations between milk constituents and infant growth and body composition are reported Table 3.). In general, correlations remained the same or were strengthened by adjustment for maternal pre-gravid BMI, and so these results will be focused on here. Milk glucose was positively associated with infant WLZ (r=0.60, p=0.01), BMIZ (r=0.55, p=0.03), fat mass and lean mass (r=0.43 – 0.44, p<0.10). Milk insulin was negatively associated with infant weight (r= −0.49, p=0.06), BMIZ (r= −0.58, p=0.02), WLZ (r= −0.51, p=0.05), and lean mass (r= −0.53, p=0.03). Milk leptin was negatively associated with infant BMIZ (r = −0.54, p=0.03). Milk TNF-α was negatively associated with infant lean mass (r= −0.50, p=0.05), and IL-6 was negatively associated with infant WLZ (r= −0.62, p=0.01), BMIZ (r= −0.70, p=0.003), weight gain from birth to 1 month (r= −0.58, p=0.02), percent fat (r= −0.55, p=0.03) and fat mass (r=−0.61, p=0.009), but was not significantly associated with lean mass.
Figure 1.
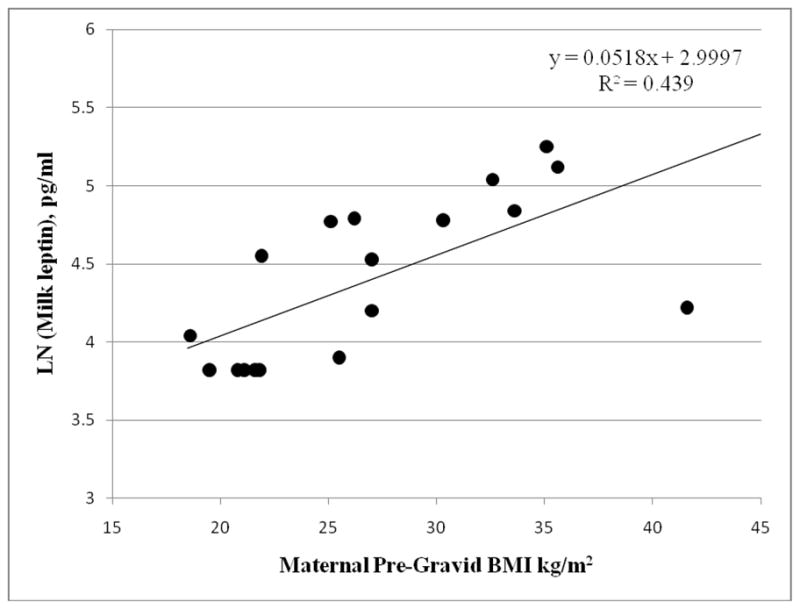
Unadjusted bivariate association between breast-milk leptin concentration measured at 1-month of age and maternal pre-pregnancy BMI.
Table 3.
Raw and Maternal-BMI-adjusted Spearman Correlations between Breast Milk Analytes and Infant Body Composition measured at 1 month, N=19 mother-infant dyads.
| Infant Growth and Body Composition Variables | Breast Milk Analytes | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Glucose | Ln Insulin | Ln Leptin | Ln TNF-α | Ln IL-6 | ||||||
| Raw | Adjusted | Raw | Adjusted | Raw | Adjusted | Raw | Adjusted | Raw | Adjusted | |
| Weight | 0.39 (p=0.10) | 0.36 | −0.33 | −0.49 (p=0.06) | 0.13 | −0.37 | −0.40 | −0.41 | −0.43 (p=0.08) | −0.40 |
| Length | 0.02 | −0.02 | −0.03 | −0.10 | 0.27 | 0.10 | −0.14 | −0.14 | 0.07 | 0.11 |
| WHO WLZ | 0.58 (p=0.01) | 0.60 (p=0.01) | −0.30 | −0.51 (p=0.05) | −0.07 | −0.44 (p=0.08) | −0.33 | −0.35 | −0.63 (p=0.007) | −0.62 (p=0.01) |
| WHO BMIZ | 0.54 (p=0.02) | 0.55 (p=0.03) | −0.37 | −0.58 (p=0.02) | −0.04 | −0.54 (p=0.03) | −0.36 | −0.39 | −0.70 (p=0.002) | −0.70 (p=0.003) |
| Weight gain 0 – 1 month | 0.31 | 0.33 | 0.15 | 0.08 | 0.52 (p=0.03) | −0.05 | −0.20 | −0.29 | −0.51 (p=0.04) | −0.58 (p=0.02) |
| %fat | 0.128 | 0.18 | −0.04 | −0.18 | 0.19 | −0.26 | −0.08 | −0.06 | −0.56 (p=0.02) | −0.55 (p=0.03) |
| Total Fat Mass | 0.45 (p=0.05) | 0.43 (p=0.10) | −0.17 | −0.37 | 0.28 | −0.28 | −0.32 | −0.35 | −0.61 (p=0.01) | −0.61 (p=0.01) |
| Trunk Fat Mass | 0.23 | 0.14 | −0.08 | −0.24 | 0.28 | −0.37 | −0.21 | −0.22 | −0.44 (p=0.08) | −0.42 |
| Total Lean Mass | 0.44 (p=0.06) | 0.44 (p=0.09) | −0.33 | −0.53 (p=0.03) | 0.20 | −0.21 | −0.45 (p=0.06) | −0.50 (p=0.05) | −0.30 | −0.26 |
WHO WLZ (Weight-for-length z-score; z-scores based on WHO 2006 growth charts)
WHO BMIZ (BMI-for-age z-score; z-scores based on WHO 2006 growth charts)
DISCUSSION
This pilot study examined the relationships among a number of inflammatory markers and appetite-regulating hormones and growth factors in human breast-milk and their associations with infant size, adiposity and lean tissue at 1 -month of age. The findings are noteworthy because of the limited data in the literature looking at the composition of breast-milk and its role in growth and body composition in the first months of life. Novel findings from this study are that milk IL-6 concentration had pervasive negative associations with infant growth and adiposity, while milk TNF-α and insulin concentrations were associated with lower lean mass, but not fat mass. As previously found, milk leptin concentration was positively associated with maternal BMI and negatively associated with infant relative weight [26, 27]. Glucose concentration, while generally only a small contributor to the carbohydrate content of human milk [35], was positively associated with infant fat mass, relative weight, and lean mass. This is consistent with the recent finding that formula fortification with glucose polymers to increase energy content was associated with greater weight gain and fat-free mass in very low birth weight preterm infants compared to standard preterm formula[36]. To our knowledge, this is the first study that has linked inflammatory markers in breast-milk with whole and regional body composition in the first month of life. Only recently (i.e. 2010) have studies begun to appear studying in detail not only macro/micronutrient composition of breast-milk, but also low-abundant proteins [37]. Our finding that both elevated inflammatory factor concentrations (i.e. TNF-α) and insulin in milk are associated with reduced total lean mass in young infants is interesting, as it has been previously documented in cancer and other catabolic conditions that muscle wasting (cachexia) is the product of both defects in the insulin/IGF-1 signaling pathways and increases in cytokine levels (especially TNF-α) [38], and may also influence nutrient partitioning during growth [39], including the development of lean body mass stores.
Given the preliminary nature of the study, the results do not provide conclusive evidence of the independent effect of different breast milk analytes on infant body composition, but rather are hypothesis-generating. More work is needed to understand the relationship of pro-inflammatory cytokines, insulin, and glucose on infant lean and fat mass, to examine their uptake into the infant circulation and mode of action, and possible determinants of their levels in milk, such as maternal obesity and diet.
The importance of breastfeeding on future disease risk has long been appreciated with numerous observational studies suggesting that breastfeeding reduces obesity risk later in life, and it is universally accepted that it does; however, two review articles highlight the importance of more work in the area. A systematic review of the literature reported a modest reduction of obesity risk in breastfed vs. formula fed infants (odds ratio: 0.87; 95% confidence interval: 0.85–0.89) [40]. The authors concluded that early breastfeeding provided a modest protective affect against obesity risk later in life, though potential confounding factors (e.g. parity, smoking, socioeconomic status, ethnicity) need further clarification. A second review article reported a lower BMI in breastfed infants vs. formula fed infants (odds ratio: −0.04; 95% confidence interval: −0.05−0.02) [41]. The authors concluded the modest role breastfeeding played might in fact be due to publication bias and/or confounding variables. A secondary analysis in 11 studies was performed after adjusting for socioeconomic status, maternal smoking in pregnancy, and maternal BMI and the authors reported no role of breastfeeding on future BMI (odds ratio: −0.10; 95% confidence interval: −0.14−0.06 before adjustment; odds ratio:−0.01; 95% confidence interval: −0.05−0.03 after adjustment) [41].
Breast-feeding is recommended by numerous federal and professional organizations, namely the Surgeon General, the American Academy of Pediatrics, American College of Obstetricians and Gynecologists, American College of Nurse-Midwives, and the American Dietetic Association as the preferred method for infant nutriture in the first 6-months of life [42]. Results from the present study do not suggest the recommendation should be re-evaluated but rather suggests that the nursing period may be a critical window for intervention for improved metabolic outcomes in the offspring.
A unique study design sheds light on the role of breast-milk and future health outcomes [23, 44]. Offspring from both type 1 and gestational diabetic mothers’ either were allowed to nurse from their mother or receive non-diabetic banked donor breast-milk in the first 7 days of life. A positive association was observed between the volume of diabetic breast-milk consumed and increased risk of being overweight at 2 years old (odds ratio of 2.47). Results from the study showed a deleterious effect of nursing offspring from diabetic mothers with increased risk of their child being overweight early in life.
Interestingly, it has been shown that insulin concentrations in breast-milk from diabetic mothers are two times as great vs. breast-milk from normal glycemic mothers, thus potentially explaining at least some of the increased weight in the breast-fed offspring from diabetic mothers [45]. However, our study found that breast-milk insulin was negatively associated with infant body weight, and lean mass at 1-month, which is in line with its role as a regulator of food intake and energy balance. Serum insulin inhibits food intake by interaction with hypothalamic neuropeptides including NPY [46], and appears to depress the appetite-stimulating, growth-enhancing hormone ghrelin in infants [47]. Longer-term follow-up may clarify the complex, possibly age-dependent relationships between breast-milk insulin and child growth.
It might be expected that stomach enzymes released in the infant gut would digest and inactivate breast-milk hormones, and inflammatory factors, prior to entering the infant circulation. However, a number of lines of evidence suggest that the milk constituents examined in this study may be biologically active. First, gastrointestinal epithelial cell receptors for leptin, adiponectin, IGF-1 and adipokines have been found, which suggests they may enter the infant circulation [22]. Second, human milk contains antiproteases that retard proteolysis [48]. Third, developmental delays in the production of gastric acid may also favor the survival of cytokines and adipokines in the young infant [49]. Oral leptin supplementation in suckling rats has been shown to result in increased leptin concentrations in the stomach and serum acutely [50] and supplemented rats ultimately had lower food intake and lower body weight and fat mass in adulthood than un-supplemented rats[16]. This work provides proof of concept that breast-milk proteins retain physiological activity in the infant gut, but further work is required to assess these relationships for other breast-milk constituents.
The limitations of this study were; first, due to the preliminary nature of the investigation, the number of subjects enrolled was modest. The study’s sample of 19 mother-child dyads afforded 80% power to observe absolute [Pearson or Spearman] correlations that exceeded r=0.60. This estimate of a detectable effect size assumes a Type 1 error probability (alpha) of 0.05, but does not adjust for the study’s multiple comparisons. Future studies need to be adequately powered to provide robust estimates of the variance in infant growth and adiposity accounted for by constituents in breast-milk as compared to other factors. Second, we did not collect maternal or infant serum levels of these analytes, which may have confounded our results. However, at least in the case of leptin, milk concentrations have not been found to be significantly correlated with maternal or infant serum levels [51], which suggests that it is the breast-milk concentration that is of biological significance. Thirdly, we did not adjust for dietary intake of the mother or other characteristics that may be important in determining breast-milk concentrations of these factors. Fourthly, we did not collect colostrum samples nor were we able to follow the subjects past 1-month. By truncating the study in this manner, we may have missed potentially important infant growth and breast-milk associations prior to, as well as after the period of investigation. Finally, the sample population was predominantly Caucasian and therefore results from this study may not be representative of a wider spectrum of races and ethnic backgrounds.
In summary, this study found significant associations between maternal BMI and milk leptin at 1 month of age, as well as novel associations between milk glucose, insulin, IL-6, and TNF-α concentrations and infant body composition. These data suggest that in the first months of life, breast-milk concentrations of numerous appetite regulating adipokines and pro-inflammatory cytokines may play a role in the accrual of fat and lean body mass. Larger, longitudinal studies are needed to define modifiable factors that influence breast-milk composition and to clarify the different roles of individual breast-milk factors in infant body composition and future health.
Acknowledgments
Mead Johnson Nutrition provided financial support as an Independent Investigator Trial but did not have editorial control of the paper (DAF principal investigator) and EWD was supported by NIH grant HD53685. We are grateful for the dedicated mothers for completing the study with their infant. Additionally, we acknowledge Dr. Marvin Williams for help in recruiting subjects, Catherine Wolf for her work in data collection and April Teague for running breast-milk analyses.
Footnotes
The authors’ responsibility were as follows: DAF was involved in developing the concepts, conducting the study and data collection, statistical analysis, and preparing the manuscript and EWD was involved in developing the concepts, statistical analysis, and preparing the manuscript.
DAF and EWD have no conflicts of interest to report other than Mead Johnson Nutrition provided financial support for the project.
References
- 1.Kuzawa CW, Pike IL. Introduction. Fetal origins of developmental plasticity. Am J Hum Biol. 2005;17:1–4. doi: 10.1002/ajhb.20090. [DOI] [PubMed] [Google Scholar]
- 2.Bateson P, Barker D, Clutton-Brock T, et al. Developmental plasticity and human health. Nature. 2004;430:419–421. doi: 10.1038/nature02725. [DOI] [PubMed] [Google Scholar]
- 3.Gluckman PD, Hanson MA, Pinal C. The developmental origins of adult disease. Matern Child Nutr. 2005;1:130–141. doi: 10.1111/j.1740-8709.2005.00020.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Oken E, Gillman MW. Fetal origins of obesity. Obes Res. 2003;11:496–506. doi: 10.1038/oby.2003.69. [DOI] [PubMed] [Google Scholar]
- 5.Somm E, Schwitzgebel VM, Vauthay DM, et al. Prenatal nicotine exposure alters early pancreatic islet and adipose tissue development with consequences on the control of body weight and glucose metabolism later in life. Endocrinology. 2008;149:6289–6299. doi: 10.1210/en.2008-0361. [DOI] [PubMed] [Google Scholar]
- 6.Mastorci F, Vicentini M, Viltart O, et al. Long-term effects of prenatal stress: changes in adult cardiovascular regulation and sensitivity to stress. Neurosci Biobehav Rev. 2009;33:191–203. doi: 10.1016/j.neubiorev.2008.08.001. [DOI] [PubMed] [Google Scholar]
- 7.Palou M, Priego T, Sanchez J, Torrens JM, Palou A, Pico C. Moderate caloric restriction in lactating rats protects offspring against obesity and insulin resistance in later life. Endocrinology. 2010;151:1030–1041. doi: 10.1210/en.2009-0934. [DOI] [PubMed] [Google Scholar]
- 8.Rooney K, Ozanne SE. Maternal over-nutrition and offspring obesity predisposition: targets for preventative interventions. Int J Obes (Lond) 2011;35:883–890. doi: 10.1038/ijo.2011.96. [DOI] [PubMed] [Google Scholar]
- 9.Savino F, Fissore MF, Liguori SA, Oggero R. Can hormones contained in mothers’ milk account for the beneficial effect of breast-feeding on obesity in children? Clin Endocrinol (Oxf) 2009;71:757–765. doi: 10.1111/j.1365-2265.2009.03585.x. [DOI] [PubMed] [Google Scholar]
- 10.Wojcik KY, Rechtman DJ, Lee ML, Montoya A, Medo ET. Macronutrient analysis of a nationwide sample of donor breast milk. J Am Diet Assoc. 2009;109:137–140. doi: 10.1016/j.jada.2008.10.008. [DOI] [PubMed] [Google Scholar]
- 11.Jensen RG, Lammi-Keefe CJ, Ferris AM, et al. Human milk total lipid and cholesterol are dependent on interval of sampling during 24 hours. J Pediatr Gastroenterol Nutr. 1995;20:91–94. doi: 10.1097/00005176-199501000-00015. [DOI] [PubMed] [Google Scholar]
- 12.Allen JC, Keller RP, Archer P, Neville MC. Studies in human lactation: milk composition and daily secretion rates of macronutrients in the first year of lactation. Am J Clin Nutr. 1991;4:69–80. doi: 10.1093/ajcn/54.1.69. [DOI] [PubMed] [Google Scholar]
- 13.Garza C, Butte NF. Energy concentration of human milk estimated from 24-h pools and various abbreviated sampling schemes. J Pediatr Gastroenterol Nutr. 1986;5:943–948. doi: 10.1097/00005176-198611000-00022. [DOI] [PubMed] [Google Scholar]
- 14.Weber A, Loui A, Jochum F, Buhrer C, Obladen M. Breast milk from mothers of very low birthweight infants: variability in fat and protein content. Acta Paediatr. 2001;90:772–775. [PubMed] [Google Scholar]
- 15.Mitoulas LR, Kent JC, Cox DB, Owens RA, Sherriff JL, Hartmann PE. Variation in fat, lactose and protein in human milk over 24 h and throughout the first year of lactation. Br J Nutr. 2002;88:29–37. doi: 10.1079/BJNBJN2002579. [DOI] [PubMed] [Google Scholar]
- 16.Palou A, Pico C. Leptin intake during lactation prevents obesity and affects food intake and food preferences in later life. Appetite. 2009;52:249–252. doi: 10.1016/j.appet.2008.09.013. [DOI] [PubMed] [Google Scholar]
- 17.Casabiell X, Pineiro V, Tome MA, Peino R, Dieguez C, Casanueva FF. Presence of leptin in colostrum and/or breast milk from lactating mothers: a potential role in the regulation of neonatal food intake. J Clin Endocrinol Metab. 1997;2:4270–4273. doi: 10.1210/jcem.82.12.4590. [DOI] [PubMed] [Google Scholar]
- 18.Marianna D, Katerina S, Lenka P, Vendula S, Tereza U, Jiri N. Is there any relationship between cytokine spectrum of breast milk and occurence of eosinophilic colitis? Acta Paediatr. 2010;99:1666–1670. doi: 10.1111/j.1651-2227.2010.01907.x. [DOI] [PubMed] [Google Scholar]
- 19.Zanardo V, Golin R, Amato M, et al. Cytokines in human colostrum and neonatal jaundice. Pediatr Res. 2007;62:191–194. doi: 10.1203/PDR.0b013e31809871c9. [DOI] [PubMed] [Google Scholar]
- 20.Aydin S, Ozkan Y, Kumru S. Ghrelin is present in human colostrum, transitional and mature milk. Peptides. 2006;27:878–882. doi: 10.1016/j.peptides.2005.08.006. [DOI] [PubMed] [Google Scholar]
- 21.Baxter RC, Zaltsman Z, Turtle JR. Immunoreactive somatomedin-C/insulin-like growth factor I and its binding protein in human milk. J Clin Endocrinol Metab. 1984;8:955–959. doi: 10.1210/jcem-58-6-955. [DOI] [PubMed] [Google Scholar]
- 22.Savino F, Liguori SA, Fissore MF, Oggero R. Breast milk hormones and their protective effect on obesity. Int J Pediatr Endocrinol. 2009;2009:327505. doi: 10.1155/2009/327505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Plagemann A, Harder T, Franke K, Kohlhoff R. Long-term impact of neonatal breast-feeding on body weight and glucose tolerance in children of diabetic mothers. Diabetes Care. 2002;25:16–22. doi: 10.2337/diacare.25.1.16. [DOI] [PubMed] [Google Scholar]
- 24.Mayer-Davis EJ, Dabelea D, Lamichhane AP, et al. Breast-feeding and type 2 diabetes in the youth of three ethnic groups. the SEARCh for diabetes in youth case-control study. Diabetes Care. 2008;31:470–475. doi: 10.2337/dc07-1321. [DOI] [PubMed] [Google Scholar]
- 25.Crume TL, Ogden L, Maligie M, et al. Long-term impact of neonatal breastfeeding on childhood adiposity and fat distribution among children exposed to diabetes in utero. Diabetes Care. 2011;34:641–645. doi: 10.2337/dc10-1716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Miralles O, Sanchez J, Palou A, Pico C. A physiological role of breast milk leptin in body weight control in developing infants. Obesity (Silver Spring) 2006;14:1371–1377. doi: 10.1038/oby.2006.155. [DOI] [PubMed] [Google Scholar]
- 27.Schuster S, Hechler C, Gebauer C, Kiess W, Kratzsch J. Leptin in maternal serum and breast milk: association with infants’ body weight gain in a longitudinal study over 6 months of lactation. Pediatr Res. 2011;70:633–637. doi: 10.1203/PDR.0b013e31823214ea. [DOI] [PubMed] [Google Scholar]
- 28.Woo JG, Guerrero ML, Guo F, et al. Human Milk Adiponectin Impacts Infant Weight Trajectory During The Second Year Of Life. J Pediatr Gastroenterol Nutr. 2011 doi: 10.1097/MPG.0b013e31823fde04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Martin LJ, Woo JG, Geraghty SR, et al. Adiponectin is present in human milk and is associated with maternal factors. Am J Clin Nutr. 2006;83:1106–1111. doi: 10.1093/ajcn/83.5.1106. [DOI] [PubMed] [Google Scholar]
- 30.Ermis B, Yildirim A, Tastekin A, Ors R. Influence of smoking on human milk tumor necrosis factor-alpha, interleukin-1beta, and soluble vascular cell adhesion molecule-1 levels at postpartum seventh day. Pediatr Int. 2009;51:821–824. doi: 10.1111/j.1442-200X.2009.02864.x. [DOI] [PubMed] [Google Scholar]
- 31.Aydin S, Karatas F, Geckil H. Simultaneous quantification of acylated and desacylated ghrelin in biological fluids. Biomed Chromatogr. 2008;2:1354–1359. doi: 10.1002/bmc.1065. [DOI] [PubMed] [Google Scholar]
- 32.Ilcol YO, Hizli B. Active and total ghrelin concentrations increase in breast milk during lactation. Acta Paediatr. 2007;6:1632–1639. doi: 10.1111/j.1651-2227.2007.00493.x. [DOI] [PubMed] [Google Scholar]
- 33.Black A, Tilmont EM, Baer DJ, et al. Accuracy and precision of dual-energy X-ray absorptiometry for body composition measurements in rhesus monkeys. J Med Primatol. 2001;30:94–99. doi: 10.1034/j.1600-0684.2001.300204.x. [DOI] [PubMed] [Google Scholar]
- 34.Hind K, Oldroyd B, Truscott JG. In vivo precision of the GE Lunar iDXA densitometer for the measurement of total body composition and fat distribution in adults. Eur J Clin Nutr. 2011;65:140–142. doi: 10.1038/ejcn.2010.190. [DOI] [PubMed] [Google Scholar]
- 35.Emmett PM, Rogers IS. Properties of human milk and their relationship with maternal nutrition. Early Hum Dev. 1997;49(Suppl):S7–28. doi: 10.1016/s0378-3782(97)00051-0. [DOI] [PubMed] [Google Scholar]
- 36.Costa-Orvay JA, Figueras-Aloy J, Romera G, Closa-Monasterolo R, Carbonell-Estrany X. The effects of varying protein and energy intakes on the growth and body composition of very low birth weight infants. Nutr J. 2011;10:140. doi: 10.1186/1475-2891-10-140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Liao Y, Alvarado R, Phinney B, Lonnerdal B. Proteomic characterization of human milk whey proteins during a twelve-month lactation period. J Proteome Res. 2011;10:1746–1754. doi: 10.1021/pr101028k. [DOI] [PubMed] [Google Scholar]
- 38.Saini A, Al-Shanti N, Stewart CE. Waste management - cytokines, growth factors and cachexia. Cytokine Growth Factor Rev. 2006;17:475–486. doi: 10.1016/j.cytogfr.2006.09.006. [DOI] [PubMed] [Google Scholar]
- 39.Morrison JL, Duffield JA, Muhlhausler BS, Gentili S, McMillen IC. Fetal growth restriction, catch-up growth and the early origins of insulin resistance and visceral obesity. Pediatr Nephrol. 2010;5:669–677. doi: 10.1007/s00467-009-1407-3. [DOI] [PubMed] [Google Scholar]
- 40.Owen CG, Martin RM, Whincup PH, Smith GD, Cook DG. Effect of infant feeding on the risk of obesity across the life course: a quantitative review of published evidence. Pediatrics. 2005;115:1367–1377. doi: 10.1542/peds.2004-1176. [DOI] [PubMed] [Google Scholar]
- 41.Owen CG, Martin RM, Whincup PH, Davey-Smith G, Gillman MW, Cook DG. The effect of breastfeeding on mean body mass index throughout life: a quantitative review of published and unpublished observational evidence. Am J Clin Nutr. 2005;2:1298–1307. doi: 10.1093/ajcn/82.6.1298. [DOI] [PubMed] [Google Scholar]
- 42.Gartner LM, Morton J, Lawrence RA, et al. Breastfeeding and the use of human milk. Pediatrics. 2005;115:496–506. doi: 10.1542/peds.2004-2491. [DOI] [PubMed] [Google Scholar]
- 43.Executive summary: The Surgeon General’s call to action to support breastfeeding. Breastfeed Med. 2011;6:3–5. doi: 10.1089/bfm.2011.9996. [DOI] [PubMed] [Google Scholar]
- 44.Rodekamp E, Harder T, Kohlhoff R, Franke K, Dudenhausen JW, Plagemann A. Long-term impact of breast-feeding on body weight and glucose tolerance in children of diabetic mothers: role of the late neonatal period and early infancy. Diabetes Care. 2005;28:1457–1462. doi: 10.2337/diacare.28.6.1457. [DOI] [PubMed] [Google Scholar]
- 45.Jovanovic-Peterson L, Fuhrmann K, Hedden K, Walker L, Peterson CM. Maternal milk and plasma glucose and insulin levels: studies in normal and diabetic subjects. J Am Coll Nutr. 1989;8:125–131. doi: 10.1080/07315724.1989.10720287. [DOI] [PubMed] [Google Scholar]
- 46.Schwartz MW, Woods SC, Porte D, Jr, Seeley RJ, Baskin DG. Central nervous system control of food intake. NATURE. 2000;404:661–671. doi: 10.1038/35007534. [DOI] [PubMed] [Google Scholar]
- 47.Savino F, Grassino EC, Fissore MF, et al. Ghrelin, motilin, insulin concentration in healthy infants in the first months of life: relation to fasting time and anthropometry. Clin Endocrinol (Oxf) 2006;5:158–162. doi: 10.1111/j.1365-2265.2006.02561.x. [DOI] [PubMed] [Google Scholar]
- 48.Goldman AS. Modulation of the gastrointestinal tract of infants by human milk. Interfaces and interactions. An evolutionary perspective. J Nutr. 2000;30:426S–431S. doi: 10.1093/jn/130.2.426S. [DOI] [PubMed] [Google Scholar]
- 49.Goldman AS, Chheda S, Garofalo R, Schmalstieg FC. Cytokines in human milk: properties and potential effects upon the mammary gland and the neonate. J Mammary Gland Biol Neoplasia. 1996:251–258. doi: 10.1007/BF02018078. [DOI] [PubMed] [Google Scholar]
- 50.Sanchez J, Oliver P, Miralles O, Ceresi E, Pico C, Palou A. Leptin orally supplied to neonate rats is directly uptaken by the immature stomach and may regulate short-term feeding. Endocrinology. 2005;46:2575–2582. doi: 10.1210/en.2005-0112. [DOI] [PubMed] [Google Scholar]
- 51.Savino F, Liguori SA, Petrucci E, et al. Evaluation of leptin in breast milk, lactating mothers and their infants. Eur J Clin Nutr. 2010;4:972–977. doi: 10.1038/ejcn.2010.105. [DOI] [PubMed] [Google Scholar]


