Abstract
This work describes the production of very high specific activity 66/68Ga from natZn(p,n) and 66Zn(p,n) using proton irradiations between 7 and 16 MeV, with emphasis on 66Ga for use with common bifunctional chelates. Principle radiometallic impurities are 65Zn from (p,x) and 67Ga from (p,n). Separation of radiogallium from target material is accomplished with cation exchange chromatography in hydrochloric acid solution. Efficient recycling of Zn target material is possible using electrodeposition of Zn from its chloride form, but these measures are not necessary to achieve high specific activity or near-quantitiative radiolabeling yields from natural targets. Inductively coupled plasma mass spectroscopy (ICP-MS) measures less than 2 ppb non-radioactive gallium in the final product, and the reactivity of 66Ga with common bifunctional chelates, decay corrected to the end of irradiation, is 740 GBq/μmol (20 Ci/μmol) using natural zinc as a target material. Recycling enriched 66Zn targets increased the reactivity of 66Ga with common bifunctional chelates.
Keywords: 66Ga, 68Ga, Zn electrodeposition, PETtrace, Eclipse, cation exchange
Introduction
The radionuclide 66Ga (t1/2 = 9.3 hrs, 56.5% β+, 43.5% EC (Severin et al., 2010)) is a useful surrogate for both 67Ga and 68Ga. The former is used in single photon computed tomography (SPECT); the latter is commercially available in 68Ge/68Ga generator systems. Positron emission tomography (PET) image quality exceeds that of SPECT systems, even with high energy positrons emitted by 66Ga (E0 = 4.15 MeV). Modern preclinical PET scanners capably cope with both fast positrons and prompt gamma emissions characteristic of many nonstandard radionuclides (Laforest et al., 2002). 66Ga’s longer half-life makes it a more practical radiolabel of proteins, peptides, and antibodies, whose slower in vivo kinetics are poorly matched by 68 min 68Ga (Ugur et al., 2002). Labeling chemistry with radiogallium is well studied because of the popularity of 68Ga from 68Ge/68Ga generators (Hnatowich, 1975; Velikyan, 2009).
Several methods for the production of no-carrier-added 66Ga are reported. Lewis et al. irradiated natural and enriched Zn targets with 7 μA of 14.5 and 9.8 MeV protons, with separation accomplished by cation exchange chromatography and solvent extraction (Lewis et al., 2002). However, their use of enriched material was limited to purchased target foils, making target recycling impossible, and final specific activities were < 5 GBq/μmol, more than a factor of 2000 below theoretical limits and significantly less than those achieved for other radiometals of interest to PET (Avila-Rodriguez et al., 2007). Lewis et al.’s best results were achieved with extraction from isopropyl ether, which is challenging to automate and has been reported elsewhere (Rowshanfarzad et al., 2004). Selective precipitation and filtration has been used to separate 66Ga and 67Ga from zinc targets as well, but zinc and copper remained in final products in ppm levels (Sadeghi and Mokhtari, 2010). Thermal chromatographic distillation and acid etching have been used to rapidly separate up to 60% of radiogallium from zinc targets, but without reported use of the product in labeling chemistry (Tolmachev and Lundqvist, 1996). Electroplated zinc targets resistant to irradiation currents up to 50 μA are present in the literature (e.g., (Neirinckx, 1976). However, specific activities and enriched isotope recovery efficiencies are unreported by these studies, or commonly, copper plating substrates necessitate additional separation prior to replating (Kakavand et al., 2010; Naik et al., 2002; Rowshanfarzad et al., 2004; Sattari et al., 2006). After irradiation of zinc targets, electrodeposition from their radioactive, dissolved solutions has also been used to speed separation, post-irradiation but again, the reactivity and elemental composition of the final product in solution are unreported (Neirinckx, 1976).
Though many reports on cyclotron production of 66/67Ga exist, the effective potential of cyclotron-produced 66Ga for preclinical studies of 66Ga-labeled agents requiring tracer concentrations of the radiometal is poorly reported; perhaps, partially for this reason, PET imaging with 66Ga is scarce. Separations reported in the literature insufficiently separate contaminant Fe(III), which exhibits nearly identical coordination chemistry to that of gallium complexes (Velikyan, 2009).
This work is an attempt to construct an economical, facile method for low energy cyclotron production of 68Ga or 66Ga from natZn or 66Zn, depending on the needs of the user, in high yields and reactivities for common bifunctional chelates. We consider a measure of reactivity, defined as the mass of chelate required to achieve near-quantitative radiolabeling yields under specified reaction conditions, to be among the most important measures of radiometals’ efficacy as PET synthons. High reactivity minimizes the mass of ligand necessary to achieve injectable radiolabeling yields and eliminates contaminant metals’ confounding effects on chemistry and the pharmacokinetics of the labeled compound.
Materials
Optima grade HCl was purchased from Fisher Scientific. natZn foils (0.25 mm thick) and natZnCl2 (both 99.999%) were purchased from Sigma Aldrich. Puratronic ammonium sulfate was purchased from VWR. Isotopically enriched 66Zn (98.58%) was purchased from Isoflex (San Francisco). The enriched zinc contained 0.76% 64Zn, 0.6% 67Zn, 0.055% 68Zn, and 0.005% 70Zn. Cation exchange resin (AG50W-X4, 100–200 mesh) was purchased from Biorad.
Methods
Zinc was electrodeposited from solutions comprised of 250–300 mg ZnCl2 in 2.5 ml 0.05 N HCl onto 0.5 mm thick gold or silver discs. A 0.1 mm diameter platinum wire anode was used to apply 4 – 9 V DC (30 – 60 mA) across 1 cm of plating solution and a ballast resistor of 100 Ω, with optimal deposition occurring at 6 V. Four hours after voltage was applied, 100 μl 0.1 N HCl was added to the solution to maintain acidity. In experiments to determine plating efficiency, reactivity, and isotope separation/recovery yields, 20 hours of plating deposited 91 ± 3% (n=6) onto an area of ~ 0.8 cm2, approximately matched to proton beam dimensions. Target thickness could be experimentally varied by altering the duration of the plating procedure; targets of different thickness were used to measure experimental target yields. Electrodeposited mass was determined by weighing a clean, vacuum-oven baked gold backing disc immediately before and after the plating process. Targets were irradiated with up to 40 μA of protons of various energies on a GE PETtrace or Siemens Eclipse HP cyclotron. Aluminum degraders lowered these accelerators’ nominal beam energies (16 and 11 MeV) to 13 and 7 MeV, respectively. Targets withstood these currents for irradiations of up to 4 hours without losing mass. When producing 66Ga, irradiated targets of natZn were left on the cyclotron overnight (13 – 16 hrs) so that short-lived products could decay, and 2.5 ml 10 N HCl dissolved the targets after removal. The solution was transferred to a column containing 7 g strong cation exchange resin preconditioned with 10 N HCl, as reported previously (Lewis et al., 2002). The column was washed with 20 ml of 10 N HCl to remove the Zn target material. An additional washing step was added to previously reported cation exchange methods, using 20 ml 7 N HCl to remove additional contaminant metal before eluting the 66Ga fraction in 5–10 ml of 4 N HCl. The eluant was collected in fractions to select the most concentrated portion of the product peak. The 20 ml fraction of 10 N HCl was saved for recycling, and the 4 N HCl fraction was evaporated under inert gas flow or in a rotoevaporator. 66Ga was recovered in 50–100 μl 0.1 N HCl for labeling use. The zinc target stock was recovered from combined 10 N HCl column wash and residual electroplating solution by evaporation with a single addition and evaporation of 0.05 N HCl.
To check product chemical purity, non-radioactive samples of dissolved targets and the various elution fractions were analyzed by inductively coupled mass spectroscopy (ICP-MS) at the University of Wisconsin Hygiene Labs and the Institute of Geology at the Universidad Autonoma in San Luis Potosi. Zinc content was also checked with produced 65Zn from irradiations above 13 MeV using a 60 cm3 high purity germanium detector (HPGe) and gamma spectroscopy (FWHM = 2.7 keV @ 1333 keV) to identify 65Zn’s characteristic 1115.5 keV gamma (Iγ = 50.0%). 66/67/68Ga yields were quantified using the same detector and characteristic 1039 keV (66Ga, Iγ = 37.0%), 300 keV (67Ga, Iγ = 16.6%), and 1077 keV (68Ga, Iγ = 3.2%) emissions. Detector energy and efficiency calibrations were performed with 137Cs, 60Co, and 152Eu sources (Oak Ridge National Lab), and data was collected using Maestro (Ortec) counting software. Errors from counting statistics (3–5%), peak fitting (2–3%) and background subtractions (2–4%) were taken as reported by the software and, combined in quadrature, resulted in an error of 4–6%. Beam current was taken as reported by cyclotron control software, with an error of 5%, and error in the measured thickness and uniformity of thick targets was conservatively estimated at 10% given the lack of thorough characterization of target morphology. The total uncertainty in reported yields (12–15%) was calculated by combining these contributing errors in quadrature. A Capintec CRC 25-R dose calibrator was used to assay 66/68Ga for chemistry yields using manufacturer-supplied calibration numbers.
In order to more fully evaluate the utility of produced 66/68Ga for incorporation into common bifunctional chelates in the presence of contaminants not removed by our process, the reactivity of the 66/68Ga was measured by titration with the macrocycles 1,4,7,10-tetraazacyclododecane-1,4,7,10-tetraacetic acid (DOTA) (Yoo et al., 2005) and 1,4,7-triazacyclononane-1,4,7-triacetic acid (NOTA) (Li et al., 2007). Briefly, 50 μl aliquots of diluted 66/68Ga stock solution (in 0.1 N HCl) were added to vessels containing 200 μl of 0.25 M ammonium acetate buffer solution. Increasing concentrations of DOTA or NOTA solution (10−7–10−5 M in 50 μl) were added to the vessels, which were then vortexed and incubated for 30 min (80°C for DOTA, 25°C for NOTA). The percentage of the radionuclide chelated in each sample was monitored by thin layer chromatography using silica gel plates developed with 1:1 MeOH:10% NH4OAc (w/v). Evaluation of the TLC plates was performed by autoradiography on a Packard Cyclone phosphor-storage plate.
Results and Discussion
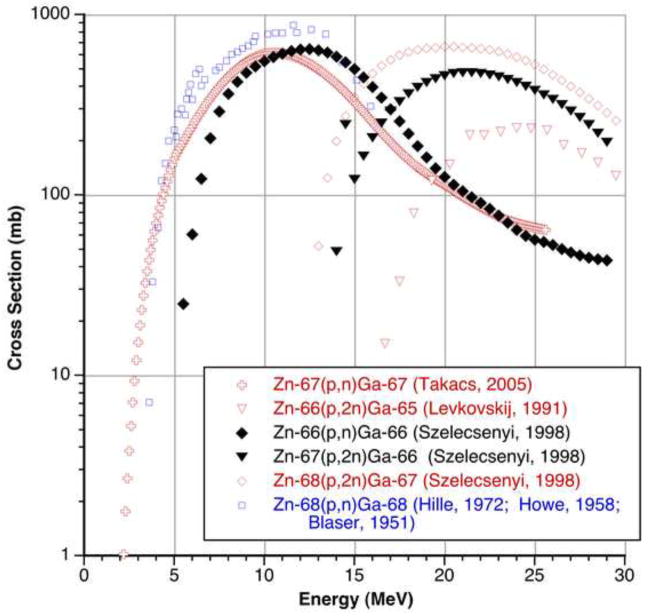
In radioisotope production for PET imaging, long-lived radioactive byproducts must be minimized. The excitation functions for the production of 65Zn, 66Ga, 67Ga, and 68Ga (summarized in Figure 1 below) are well described (Al-Saleh et al., 2007; Blaser et al., 1951; Hille et al., 1972; Howe, 1958; Levkovskij, 1991; Nortier et al., 1991; Szelecsenyi et al., 1998; Takacs et al., 2005; Tarkanyi et al., 2005). Other isotopes with shorter half-lives, such as 64Ga and 70Ga, decay rapidly enough to be of small concern for this work. Production of 65Zn by 66Zn(p,2n) and β-decay of short-lived 65Ga is open above 14 MeV, as shown in Figure 1 below, and (p,p+n) is theoretically open near 11 MeV, with a measured cross section value of 25 mb at 13.8 MeV (Levkovskij, 1991) (see also Table 1 below). In addition to expected large activities of 64/68Ga from 48.9 and 18.6% 64/68Zn, respectively, the (p,n) reaction on 4.1% 67Zn in natural targets produces significant quantities of 67Ga with a threshold below 3 MeV and a maximum near 10 MeV. 68Zn(p,2n) also produces significant 67Ga and peaks near 20 MeV. The optimal incident proton energy suggested by the literature is therefore near 13 MeV; use of enriched 66Zn targets provides an additional means of controlling radioisotopic purity.
Figure 1.
Published excitation functions for 67Zn(p,n)67Ga, 66Zn(p,2n)65Ga, 68Z(p,2n)67Ga, 66Zn(p,n)66Ga, 67Zn(p,2n)66Ga, and 68Zn(p,n)68Ga
Table 1.
Radioisotope product ratios of 68Ga, 67Ga, and 66Ga from 1 hour irradiations at various energies.
| Product | t1/2 | Reaction(s) | Q value (MeV) | Ratio of Product to 68Ga at EoB for 7 MeV | Ratio of Product to 66Ga 14 h after EoB at 11 MeV* | Ratio of Product to 66Ga 16 h after EoB at 13 MeV | Ratio of Product to 66Ga 16 h after 16 MeV EoB |
|---|---|---|---|---|---|---|---|
| 64Ga | 2.6 m | 64Zn(p,n) | −7.95 | -- | -- | -- | -- |
| 65Zn | 243.7 d | 66Zn(p,2n+β); 66Zn(p,pn) | −15.10; −11.06 | -- | -- | -- | 9.5x10−4 |
| 66Ga | 9.5 h | 66Zn(p,n); 67Zn(p,2n) | −5.96; −13.01 | 1.5x10−2 | 1.0 | 1.0 | 1.0 |
| 67Ga | 3.3 d | 67Zn(p,n); 68Zn(p,2n) | −1.78; −13.01 | 3.8x10−3 | 6.7x10−2 | 7.0x10−2 | 1.9x10−1 |
| 68Ga | 68 m | 68Zn(p,n) | −3.70 | 1.0 | 6.0x10−3 | 3.3x10−3 | 5.1x10−4 |
| 70Ga | 21.1 m | 70Zn(p,n) | −1.44 | -- | -- | -- | -- |
Thin target (11 → 6 MeV)
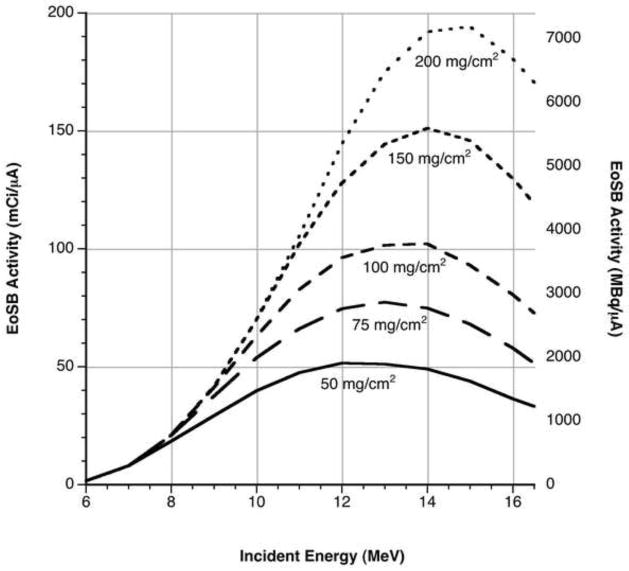
Electrodeposition of zinc targets of varying thickness affords an additional opportunity to optimize yields and radionuclidic purity. The low threshold of the 67Zn(p,n)67Ga reaction means that thin targets with proton exit energies near 6 MeV offer a reasonable compromise between 66Ga yield and 67Ga avoidance. As Figure 2 shows, the relationship between 66Ga yield and target thickness, especially when target material cost is of concern, points the cyclotron operator towards the thinnest target which can meet production demands on a given day.
Figure 2.
Calculated integral yield of 66Ga at the end of saturated bombardment using data from Szelecsenyi et al. (1998).
If enriched isotope is used, the cost of targets (~$1/mg 66Zn, ~$3/mg 68Zn) and the potential to cyclically remove transition metal contaminants suggest a recycling method with limited losses. Though highly soluble zinc salts have been reported as targets, subsequent separations with C18 media are not well characterized and the highly corrosive solutions give many cyclotron facilities cause for trepidation (Jensen and Clark, 2010). Instead, the preferred method remains electrodeposition of metal targets, which offers the prospect of high current irradiations and convenient storage.
The principle factors affecting the reactivity of final 66/68Ga solutions for DOTA and NOTA are the purity of the feedstock material, the use of electrodeposition to prepare targets, the recycling of target material, and the quality of the chemical separation of gallium from other metals. The measured reactivity of produced 66/68Ga for DOTA or NOTA using 0.25 mm natZn foil targets does not exceed 370 MBq/μmol at end of bombardment (EoB). This is due to high Fe contamination (~50 ppm) present in the purchased product and the higher mass of target material used/needed for irradiation. However, purchased 99.999% natZnCl2 contains no such contamination, and measured reactivities for DOTA/NOTA using targets electroplated from this feedstock increase by a factor of ~102–3 over those from irradiated foils. For plated targets of both natZn and 66Zn, reactivities of 160–370 GBq/μmol for DOTA or NOTA, decay corrected to EoB, are routinely achieved and have been as high as 740 GBq/μmol depending on plating results and irradiation conditions. In order to achieve these results using enriched 66Zn, which also contains ppm quantities of iron at purchase, recycling must be employed, removing contaminant metals with iterative electroplating and chromatographic purification of the product gallium. For both natZnCl2 and recycled 66Zn targets, non-decay-corrected reactivities are approximately 70–200 GBq/μmol, and the products have been used to produce radiotracers for PET imaging (Avila-Rodriguez et al., 2011).
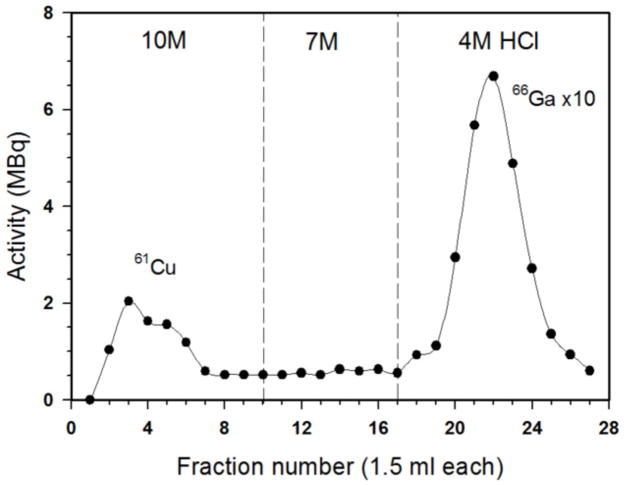
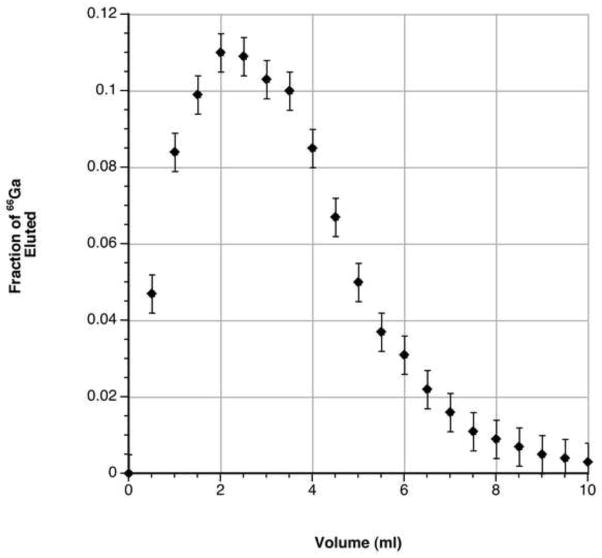
When using electroplated targets of natZnCl2 or recycled 66Zn targets, ICP-MS detects less than 2 ppb stable gallium present in the 4 N HCl final solution regardless of whether the intermediate 7 N HCl wash is employed, strongly suggesting that other contaminant radiometals are of greatest concern to reactivity measurements. ICP-MS finds approximately 50 ppm iron in the original solution of dissolved natZn foil, but only 10 ppm iron in a dissolved, electroplated target from the same foils. In the absence of the 7 N HCl wash of the resin, comparison of ICP-MS data for natZnCl2 targets from before and after plating and chemical separation gives a separation factor of 10−5 for zinc and 10−3 for iron. With the 7 N HCl wash, the separation factors for zinc and iron were improved to 10−6 and 10−4, respectively. These results were in good agreement (within 2%) with 65Zn activity quantified by HPGe γ-spectroscopy. In the same experiments, vanadium and aluminum, presumably from the cyclotron target holder, were measured by ICP-MS to exist in approximately 20 ppb quantities in the final product. A typical elution profile using cation exchange resin is shown below in Figures 3 and 4. Separation quality improves with re-use of the cation exchange resin. Typical reactivities for DOTA after packing a fresh column of resin are approximately 370 MBq/μmol; this value improves to the numbers reported above within 10 runs or with additional acid washing of the column.
Figure 3.
Elution profile of Zn-Cu-Ga separation using cation exchange resin AG50W-X4 (7 g, 1.5 cm diameter column), 14 h after 11 MeV irradiation of natZn target. Zinc is co-eluted with 61Cu. Activity was measured by ionization chamber and activity of fractions 17–27 was divided by 10.
Figure 4.
Detailed elution profile of 66Ga in 500 μl aliquots of 4 N HCl from AG50W cation exchange resin. Error bars are the standard deviation of three consecutive measurements. Reactivity measurements are made using fractions 4 – 8 to minimize tailing metal impurities from the 7 N HCl column wash.
65Zn is below the limits of gamma spectroscopy detection in the dissolved product of 13 MeV thick target irradiations of any target used. Measured yields of 66Ga, 67Ga, and 68Ga, the only gallium radioisotopes present 10 hours after the end of bombardment (EoB), establish that without limits on 67Ga contamination shorter irradiations maximize the ratio of 66Ga to 67Ga.
Table 1 below summarizes the activity ratios for irradiation conditions chosen for the production of 66/68Ga. For preclinical studies, the 300 keV gamma emission and longer-lived 67Ga from natZn targets may relax the timing of biodistribution experiments involving resection in animal subjects. It is also possible to produce radionuclidically clean 68Ga at EoB using low energy (7 MeV) irradiations for immediate use. In this work, 68Ga had a measured thick target yield of 200 ± 30 MBq/μA from natZn, extrapolated to the end of saturated bombardment (EoSB; Fig. 5). The 18.5% isotopic abundance of the primary target, 68Zn, and a lower threshold energy than the reactions which produce 66Ga (see Table 1 below) make this production route a viable choice using natural targets. However, 68Ga productions for any clinical purpose would require irradiation of enriched 68Zn, because the value of this production lies in the initially large ratio of 68Ga to contaminating, longer-lived 67Ga and 66Ga.
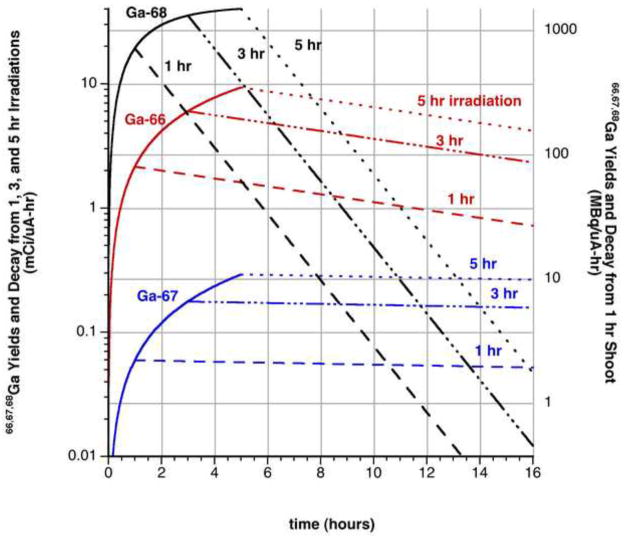
Figure 5.
Calculated extrapolation from measured thick target EoB production yields and decay of 66Ga, 67Ga, and 68Ga for 1, 3, and 5 hr proton irradiations of natZn at 13 MeV.
Irradiations with higher beam energies produce higher relative amounts of 66Ga and 67Ga, and are hence more suitable to the production of the longer-lived 66Ga. Irradiating natZn targets at 13 MeV, measured EoSB thick target yields for 66Ga, 67Ga, and 68Ga were 1150 ± 130, 260 ± 40, and 1550 ± 170 MBq/μA, respectively, while a 160 mg/cm2 target yielded 880 ± 100 MBq/μA of 66Ga at 11 MeV. Irradiating a 130 mg/cm2 66Zn target produced EoSB 66Ga and 67Ga yields of 2800 ± 310 and 70 ± 10 MBq/μA, respectively; a 152 mg/cm2 66Zn target produced EoSB yields of 3810 ± 460 and 110 ± 20 MBq/μA. In both cases, 67Ga represented less than 0.1% of activity produced for hour-long irradiations.
Conclusions
66Ga has been produced in very high specific activities (<2 ppb stable gallium) from natZn and 66Zn targets. Reactivities for common bifunctional chelates of 740 GBq/μmol, or 6% of carrier free specific activity (12.2 TBq/μmol) have been achieved. Radionuclidic purity of the product is >95% between 2 and 5 half-lives of 66Ga following irradiation and exceeds 99.9% when the 66Zn target is used. 68Ga can also be produced with high radioisotopic purity using this method, using either targets of natZn or 68Zn. The separation is simple and the principal cost incurred, even using enriched targets, results from the use of ultra-pure reagents.
Highlights.
Cyclotron-produced 66/68Ga with AG50W separation has high S.A. (<2 ppb cold Ga).
Product 66/68Ga has a reactivity for NOTA of >700 GBq/μmol (20 Ci/μmol).
natZn and 66Zn targets are economical and produce similar radiochemical purities.
Acknowledgments
We are grateful to A. Flores-Moreno and A. Zarate-Morales for irradiations on the Eclipse cyclotron and acknowledge the financial support of CONACYT Grant 121652 and International Atomic Energy Agency Grant RC16467. JWE and GWS gratefully acknowledge the support of NIH Radiological Sciences Training Grant T32 CA009206
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Al-Saleh FS, Mungren KSA, Azzam A. Excitation function measurements and integral yields estimation for natZn(p,x) reactions at low energies. Applied Radiation and Isotopes. 2007;65:1101–1107. doi: 10.1016/j.apradiso.2007.05.004. [DOI] [PubMed] [Google Scholar]
- Avila-Rodriguez MA, Ferro-Flores G, Pedraza-Lopez M, Murphy CAd. Preparation and evaluation of 68Ga-DOTA-Glu-[cyclo(Arg-Gly-Asp-D-Phe-Lys)]2 labeled with cyclotron produced Ga-68. Journal of Labeled Compounds and Radiopharmaceuticals Program Summary. 2011;54:S353. [Google Scholar]
- Avila-Rodriguez MA, Nye JA, Nickles RJ. Simultaneous production of high specific activity 64Cu and 61Co with 11.4 MeV protons on enriched 64Ni. Applied Radiation and Isotopes. 2007;65:1115–1120. doi: 10.1016/j.apradiso.2007.05.012. [DOI] [PubMed] [Google Scholar]
- Blaser JP, Boehm F, Marmier P, Peaslee DC. Fonctions d’excitation de la reaction (p,n) Helvetica Physica Acta. 1951;24:3. [Google Scholar]
- Hille M, Hille P, Uhl M, Weisz W. Excitation function of (p,n) and (alpha,n) reactions on Ni, Cu, and Zn. Nuclear Physics A. 1972;198:625. [Google Scholar]
- Hnatowich N. A method for the preparation and quality control of 68Ga radiopharmaceuticals. Journal of Nuclear Medicine. 1975;16:764–768. [PubMed] [Google Scholar]
- Howe HA. (p,n) cross sections of copper and zinc. Physical Review. 1958;109:2083. [Google Scholar]
- Jensen M, Clark J. Direct production of Ga-68 from proton bombardment of concentrated aqueous solutions of [Zn-68] zinc chloride. World Conference on Targetry and Target Chemistry; Risoe National Lab, Denmark. 2010. p. 052. [Google Scholar]
- Kakavand T, Sadeghi M, Mokhtari L, Majdabadi A. Zinc electrodeposition on copper substrate using cyanide bath for the production of 66,67,68Ga. Journal of Radioanalytical and Nuclear Chemistry. 2010;283:197–201. [Google Scholar]
- Laforest R, Rowland DJ, Welch MJ. MicroPET imaging with nonconventional isotopes. IEEE Transactions on Nuclear Science. 2002;49:2119–2126. [Google Scholar]
- Levkovskij VN. Activation cross section nuclides of average masses (A=40–100) by protons and alpha-particles with average energies (E=10–50 MeV) Institute Yadernoi Fiziki; Almaty, Kazakhstan, Moscow: 1991. [Google Scholar]
- Lewis MR, Reichert DE, Laforest R, Margenau WH, Shefer RE, Klinkowstein RE, Hughey BJ, Welch MJ. Production and purification of gallium-66 for preparation of tumor-targeting radiopharmaceuticals. Nuclear Medicine and Biology. 2002;29:701–706. doi: 10.1016/s0969-8051(02)00330-x. [DOI] [PubMed] [Google Scholar]
- Li ZB, Chen K, Chen X. 68Ga-labeled multimeric RGD peptides for microPET imaging of integrin avB3 expression. European Journal of Nuclear Medicine and Molecular Imaging. 2007;35:1100–1108. doi: 10.1007/s00259-007-0692-y. [DOI] [PubMed] [Google Scholar]
- Naik YA, Venkatesha TV, Nayak PV. Electrodeposition of zinc from chloride solution. Turkish Journal of Chemistry. 2002;26:725–733. [Google Scholar]
- Neirinckx RD. A high-yield production method for gallium-67 using an electroplated natural zinc or enriched Zn-66 target. International Journal of Applied Radiation and Isotopes. 1976;27:1–4. [Google Scholar]
- Nortier FM, Mills SJ, Steyn GF. Excitation functions and yields of relevance to the production of 67Ga by proton bombardment of natZn and natGe up to 100 MeV. International Journal of Radiation Applications and Instrumentation. Part A. Applied Radiation and Isotopes. 1991;42:353–359. doi: 10.1016/0883-2889(91)90139-r. [DOI] [PubMed] [Google Scholar]
- Rowshanfarzad P, Jalilian AR, Sabet M, Akhlaghi M. Production and quality control of 66Ga as a PET radioisotope. Iranian Journal of Radiation Research. 2004;2:149–158. [Google Scholar]
- Sadeghi M, Mokhtari L. Rapid separation of 67,68Ga from 68Zn target using precipitation technique. Journal of Radioanalytical and Nuclear Chemistry. 2010;284:471–473. [Google Scholar]
- Sattari A, Shadanpoor N, Aslani G, Rahininejad A. Production of Ga-66 from natural zinc. ALASBIMN Journal. 2006;8:31–35. [Google Scholar]
- Severin GW, Knutson LD, Voytas PA, George EA. Half-life of ^{66}Ga. Physical Review C. 2010;82:067301. [Google Scholar]
- Szelecsenyi F, Boothe TE, Takacs S, Tarkanyi F, Tavano E. Evaluated cross section and thick target yield databases of Zn+p processes for practical applications. Applied Radiation and Isotopes. 1998;49:1005–1032. [Google Scholar]
- Takacs S, Tarkanyi F, Hermanne A. Validation and upgrade of the recommended cross section data of charged particle reactions to produce gamma emitter radioisotopes. Nuclear Instruments and Methods in Physics Research B. 2005;240:790–802. [Google Scholar]
- Tarkanyi F, Ditroi F, Csikai J, Takacs S, Uddin MS, Hagiwara M, Baba M, Shubin YN, Dityuk AI. Activation cross-sections of long-lived products of proton-induced nuclear reactions on zinc. Applied Radiation and Isotopes. 2005;62:73–81. doi: 10.1016/j.apradiso.2004.06.008. [DOI] [PubMed] [Google Scholar]
- Tolmachev V, Lundqvist H. Rapid separation of gallium from zinc targets by thermal diffusion. Applied Radiation and Isotopes. 1996;47:297–299. [Google Scholar]
- Ugur O, Kothari PJ, Finn RD, Zanzonico P, Ruan S, Guenther I, Maecke HR, Larson SM. Ga-66 labeled somatostatin analogue DOTA-DPhe-Tyr-octreotide as a potential agent for positron emission tomography imaging and receptor mediated internal radiotherapy of somatostatin receptor positive tumors. Nuclear Medicine and Biology. 2002;29:147–157. doi: 10.1016/s0969-8051(01)00290-6. [DOI] [PubMed] [Google Scholar]
- Velikyan I. Vdm Verlag. 2009. Gallium-68 for the labeling of peptides and oligonucleotides. [Google Scholar]
- Yoo J, Tang L, Perkins TA, Rowland DJ, Laforest R, Lewis JS, Welch MJ. Preparation of high specific activity 86Y using a small biomedical cyclotron. Nuclear Medicine and Biology. 2005;32:891–897. doi: 10.1016/j.nucmedbio.2005.06.007. [DOI] [PubMed] [Google Scholar]