Abstract
Calcium-independent phospholipase A2 group VIA (iPLA2β) releases docosahexaenoic acid (DHA) from phospholipids in vitro. Mutations in the iPLA2β gene, PLA2G6, are associated with dystonia-parkinsonism and infantile neuroaxonal dystrophy. To understand the role of iPLA2β in brain, we applied our in vivo kinetic method using radiolabeled DHA in 4 to 5-month-old wild type (iPLA2β+/+) and knockout (iPLA2β−/−) mice, and measured brain DHA kinetics, lipid concentrations, and expression of PLA2, cyclooxygenase (COX), and lipoxygenase (LOX) enzymes. Compared to iPLA2β+/+ mice, iPLA2β−/− mice showed decreased rates of incorporation of unesterified DHA in plasma into brain phospholipids, reduced concentration of several fatty acid residues (including DHA) esterified in ethanolamine- and serine-glycerophospholipids, and increased lysophospholipid fatty acid concentrations. DHA turnover rates in brain phospholipids did not differ between genotypes. In iPLA2β−/− mice, brain levels of iPLA2β mRNA, protein, and activity were decreased, as was the iPLA2γ (Group VIB PLA2) mRNA level. Brain levels of secretory sPLA2-V mRNA, protein, and activity and cytosolic cPLA2-IVA mRNA were increased in iPLA2β−/− mice. Levels of COX-1 protein were decreased in brain, while COX-2 protein and mRNA were increased. Levels of 5-, 12-, and 15-LOX proteins did not differ significantly between genotypes. Thus, genetic iPLA2β deficiency in mice is associated with profound reorganization of lipid-metabolizing enzyme expression and of phospholipid fatty acid content of brain (particularly of DHA), which may be relevant to the neurologic abnormalities in humans with iPLA2β mutations.
Keywords: iPLA2, knockout, mice, docosahexaenoic acid, brain, turnover, incorporation, lipid, PLA2G6, phospholipid metabolism
INTRODUCTION
In vitro studies have demonstrated that the group VI Ca2+-independent phospholipases A2 (iPLA2, EC 3.1.1.4) hydrolyze docosahexaenoic acid (DHA) from the stereospecifically numbered (sn)-2 position of phospholipids [1, 2]. This is consistent with reduced brain DHA metabolism in unanesthetized iPLA2β-knockout mice [3]. Of known iPLA2 isoforms, iPLA2β is designated PARK14, PNPLA9, PLA2G6 or iPLA2-VIA, and iPLA2γ is designated PNPLA8 or iPLA2-VIB. Both isoforms are found post-synaptically in brain [4] and in the cytosol of resting cells [4–7], and can be activated and undergo membrane association by stimuli that induce release of Ca2+ from intracellular stores, e.g., muscarinic or serotonergic G-protein-coupled neuroreceptor signaling [3, 8–11]. iPLA2β, and to a lesser extent iPLA2γ, also can hydrolyze arachidonic acid (AA, 20:4n-6) from phospholipids [12–15].
Humans with iPLA2β mutations may show progressive regression of cognitive and motor skills, as manifest in the disorders infantile neuroaxonal dystrophy, idiopathic neurodegeneration with brain iron accumulation, dystonia-parkinsonism, and cerebellar cortical atrophy with gliosis [16–19]. In mice, mutations in iPLA2γ or iPLA2β genes cause cognitive deficits and motor abnormalities over time [14, 20, 21]. iPLA2β knockout mice display neuropathology characterized by swollen axons and vacuoles [20, 21], protein misfolding and aggregation [21], and reduced mitochondrial function [14, 22] by age 13 mo. Other studies have demonstrated a role for iPLA2β in maintaining axonal membrane stability [20] and in regulating fatty acid composition of pancreatic islet β-cell phospholipids [23].
In view of the involvement of iPLA2β in DHA hydrolysis from phospholipids [1, 2] and the reduced plasma DHA incorporation and signaling in brains of iPLA2β knockout mice [3], it is possible that neuropathology and altered behavior that arise from mutations or deficiencies in iPLA2β are related to disturbed brain DHA metabolism, Pleiotropic actions of DHA have been reported that include abilities to modulate gene transcription and membrane fluidity, to act as a signaling molecule during neurotransmission, to serve a precursor of antiinflammatory resolvins and neuroprotectins, to influence AA metabolism and rodent behavior, to act as an antioxidant, and to alter ion channel activities [1, 3, 20, 24–31].
To further characterize brain DHA metabolism in mice with genetic deficiency of iPLA2β, here we have used our in vivo kinetic infusion model [32–35] to quantify DHA incorporation and turnover in brain phospholipids and to determine the fatty acid concentration of brain phospholipids and lysophospholipids of iPLA2β−/− and wild type iPLA2β+/+ mice at age 4–5 mo. We also examined brain expression of enzymes involved in polyunsaturated fatty acid (PUFA) metabolism, including iPLA2β, iPLA2γ, cytosolic cPLA2 (Group IVA PLA2), secretory sPLA2 (Group V PLA2), cyclooxygenase (COX)-1, COX-2, 5-lipoxygenase (LOX), 12-LOX and 15-LOX,. Widespread neuropathologic changes develop by age 13 mos in iPLA2β−/− mice, and we chose to study younger mice in order to reduce the impact that such neuropathologic abnormalities might have on brain PUFA metabolism, but it should be noted that even at age 4-mo iPLA2β−/− mice exhibit tubulovesicular membranes and small vacuoles with edema in brain [14, 20–22].
METHODS AND MATERIALS
Animals
The study was conducted following the National Institutes of Health Guidelines for the Care and Use of Laboratory Animals (Publication no. 86-23) and was approved by the Animal Care and Use Committee of the Eunice Kennedy Shriver National Institute of Child Health and Human Development. Male iPLA2β−/− mice and their littermate iPLA2β+/+ controls, derived from a C57BL/6J genetic background [36], were maintained in an animal facility where the temperature, humidity, and light cycle were regulated, with free access to water and a diet (Rodent NIH-07) that contained (as percent of total fatty acid concentration), 30.6% saturated, 22.5% monounsaturated, 47.1% linoleic, 4.9% α-linolenic (α-LNA), 0.2% AA, 1.6% eicosapentaenoic (EPA), and 2.2% DHA [3]. Five mice of each genotype underwent surgical procedures, tracer infusion, and microwave fixation for determining brain DHA turnover and concentration. Six mice of each genotype were asphyxiated by CO2 inhalation and decapitated, and the brains were excised and rapidly frozen in 2-methylbutane with dry ice (at −50°C) and stored at −80°C for subsequent analyses.
Surgical Procedures and Tracer Infusion
At age 4–5 mo, mice were anesthetized with 1–3% halothane, and polyethylene catheters were inserted into a femoral artery and vein [33]. Recovery from anesthesia was allowed to occur (3 h, 25°C) with animal hindquarters loosely wrapped and taped to a wooden block. During recovery, body temperature was maintained at 37°C with a rectal probe and a heating element (Indicating Temperature Controller; Yellow Springs Instrument, Yellow Springs, OH, USA). After recovery, unanesthetized mice were infused (5 min) intravenously with HEPES buffer (130 µl, pH 7.4) containing fatty acid-free bovine serum albumin (50 mg/ml, Sigma, St. Louis, MO) and [1-14C]DHA (5 µCi, 53 mCi/mmol, 90% pure, Moravek Biochemicals, Brea, CA) at a rate of 0.0223 (1 + e –0.032t) ml/min, using a computer-controlled infusion pump (No. 22; Harvard Apparatus, South Natick, MA, USA) to achieve steady-state plasma specific activity within 1 min [37]. During infusion, timed arterial blood samples (ca. 15 µl) were collected in polyethylene-heparin lithium fluoride-coated Beckman centrifuge tubes at various intervals (0, 0.25, 0.5, 1.0, 1.5, 3.0, and 4.0 min) and a final collection (150 µl) was performed at 4.9 min. Plasma was separated by centrifugation (13,000 rpm, 1 min) and radioactivity determined by liquid scintillation counting. Unlabeled DHA concentrations of the final (4.9 min) sample were measured by gas chromatography (GC). At 5 min, was animals were anesthetized (sodium pentobarbital, 50mg/kg, i.v.) and subjected to head-focused microwave irradiation (5.5 kW, 0.9s, 75% power output; Cober Electronics, Norwalk, CT, USA) to stop metabolism [38, 39]. Brains were excised, dissected sagittally, and stored (−80°C).
Plasma and brain lipid extraction and separation
After adding heptadecanoic acid (17:0) as an internal standard, total lipids were extracted from plasma (50 µl) and from one cerebral hemisphere (~0.2 g) as reported [40]. Lipid extracts were analyzed by thin layer chromatography (TLC) on Silica Gel 60A plates (Whatman, Clifton, NJ) [41]. Neutral lipid subclasses including unesterified fatty acids were analyzed using a mixture of heptane/diethylether/glacial acetic acid (60/40/3 v/v/v), and authentic standard phospholipids, cholesterol, free fatty acids, triacylglycerols, and cholesteryl esters were analyzed in separate lanes to identify the bands. Phospholipid classes (EtnGpl, ethanolamine glycerophospholipid; ChoGpl, choline glycerophospholipid; PtdIns, phosphatidylinositol; PtdSer, phosphatidylserine) were separated in chloroform/methanol/H2O/glacial acetic acid (60/50/4/1 v/v/v) and identified by comparison with standards in separate lanes. Lysophospholipids were analyzed in chloroform/methanol/acetic acid/acetone/water (35/25/4/14/2 v/v/v/v/v). This method achieves separation of the co-migrating lysophospholipids lysophosphatidylcholine (lysoPC), lysophosphatidylinositol (lysoPI), and lysophosphatidylethanolamine (lysoPE). Plates were sprayed with 0.03% (w/v) 6-p-toluidine-2-naphthalene sulfonic acid (Acros, Fairlawn, NJ, USA) in 50 mM Tris-HCl buffer (pH 7.4), and the lipid bands were visualized with UV light. Each band was scraped from the plate, and the silica gel containing the target analyte was used to quantify radioactivity of phospholipid classes by liquid scintillation counting, to prepare fatty acid methyl esters (FAMEs) by transmethylation of neutral lipids, phospholipids, and lysophospholipids (see below), and to measure phospholipid and lysophospholipid phosphorous concentrations.
FAME preparation and GC analysis
After adding appropriate quantities of internal standard (17:0/17:0-PC), FAMEs were formed from brain lipids and plasma esterified lipids in silica gel scraped from TLC plates by acid methanolysis (1% H2SO4 in methanol, 70°C, 3 h). FAMEs were then analyzed by GC (SP™-2330 fused silica capillary column, 30 m × 0.25 mm i.d., 0.25 µm film thickness; Supelco, Bellefonte, PA) and detected by flame ionization (Model 6890N detector; Agilent Technologies, Palo Alto, CA). Initial column temperature was 80°C, followed by a gradient (10°C/min) to 150°C and then a gradient (6°C/min) to 200°C, where temperature was held for 10 min, and then increased to 240°C (38 min total run time). Peaks were identified by comparison to the retention times of FAME standards (Nu-Chek-Prep, Elysian, MN, USA). Fatty acid concentration (nmol/µmol brain total phosphorous or nmol/ml plasma) was calculated by proportional comparison of GC peak areas to that of the 17:0 internal standard.
Quantification of radioactivity
Samples were placed in scintillation vials and dissolved in liquid scintillation cocktail (ReadySafe™ plus 1% glacial acetic acid), and their radioactivity was determined by liquid scintillation spectrometry (2200CA,TRI-CARB®; Packard Instruments, Meriden, CT).
Brain lipid phosphorous and plasmalogens
Phosphorous concentration of brain total lipids and phospholipid classes, separated by TLC, was quantified in phosphorous-free tubes using an assay that measures phosphate concentrations, as previously described [41]. Brain plasmenylethanolamine and plasmenylcholine concentrations were determined in EtnGpl and ChoGpl by an iodine uptake method as reported.
Brain cholesterol
Brain concentration of cholesterol was determined in the total lipid extract by GC as described previously [42]. Total lipids were concentrated to dryness and then subjected to alkaline methanolysis (1 M KOH in methanol, 1 ml, 1 h, 70°C). After adding 0.9% saline (1 ml), sterols were extracted twice into hexane (2.5 ml). The extract was dried and derivatized (0.2 ml trimethylchlorosilane,Thermo Scientific, Rockford, IL; 1 hr, 60°C). The sterol trimethylsilyl ether derivatives were concentrated under nitrogen, reconstituted in hexane (100 µl), and analyzed by GC (SP™-2330 fused silica capillary column, 30 m × 0.25 mm i.d., 0.25 µm film thickness, Supelco, Bellefonte, PA). The temperature program involved an initial temperature of 100°C (1 min) followed by a gradient (15°C/min) to 280°C, where the temperature was maintained (17 min).
Quantification of labeled and unlabeled acyl-CoA
Acyl-CoA species were extracted from the remaining microwaved half-brain samples using affinity chromatography as described with slight modifications [43]. After adding internal standard heptadecanoyl-CoA (17:0-CoA, 10 nmol) to weighed brain (~0.2 g), the sample was sonicated (20 sec) with a probe sonicator (Model W-225; Misonix, Farmingdale, NY, USA) in 25 mM potassium phosphate (2 ml). Isopropanol (2 ml) was then added to the homogenate and it was again sonicated (20 sec). Proteins were precipitated by adding saturated ammonium sulphate (0.25 ml), and the sample was mixed by manual shaking. Acetonitrile (4 ml) was then added, and the sample was vortex-mixed (30 min) before centrifugation. The supernatant was collected and diluted with 25 mM potassium phosphate (10 ml). Each sample was passed through an activated oligonucleotide purification cartridge (ABI Masterpiece™, OPC®; Applied Biosystems, Foster City, CA, USA) three times, and the cartridge was washed with 25 mM potassium phosphate (10 ml). Acyl-CoA species were eluted with 0.4 ml of isopropanol/1 mM glacial acetic acid (75:25 v/v).
Extracted acyl-CoAs were separated on a reversed phase HPLC column (Symmetry, 5 µm particle size, 4.6 mm × 250 mm, Waters-Millipore, Milford, MA), using a pump coupled with a UV/VIS detector (System Gold, Model 168, Beckman). Chromatography was performed using a linear gradient system (flow rate, 1.0 ml/min) composed of 75 mM potassium phosphate and acetonitrile. At the start, acetonitrile was 44% and held for 1 min, then increased to 49% over 25 min, increased to 68% over 10 min, held at 68% for 4 min, returned to 44% over 6 min, and held for 6 min (52 min total run time). UV absorbance was measured at 260 nm to determine acyl-CoA concentrations and at 280 nm to identify acyl-CoA species (260/280= 4:1). Acyl-CoA concentrations (nmol/mg brain) were calculated by comparison of their peak areas to that of the 17:0-CoA and were normalized to brain total lipid phosphorous. The docosahexaenoyl-CoA peak was collected in each sample, and its radioactivity was determined by liquid scintillation counter. These values were used to calculate the specific activities of docosahexaenoyl-CoA.
DHA incorporation rates and turnover
The model for determining in vivo kinetics of brain fatty acids of unanesthetized rats is described in detail elsewhere [32]. In this study, we normalized concentrations and kinetic measurements to brain lipid phosphorous rather than wet weight, because brain edema has been reported in iPLA2β−/− mice at 4–5 months of age [20, 21].
Unidirectional incorporation coefficients, (ml·s−1·mg−1 phosphorous) of DHA representing incorporation of unesterified DHA from plasma into brain lipid i and were calculated as follows:
| (Eq.1) |
nCi·(µmol phosphorous)−1 is radioactivity of brain lipid i at time T = 5 min (time of termination of experiment); t is time after starting infusion; and nCi·ml−1 is plasma concentration of labeled unesterified DHA during infusion. Integrals of plasma radioactivity were determined by trapezoidal integration. Net rates of incorporation of unlabeled unesterified DHA from plasma into brain lipid i,Jin,i(DHA), and from the brain docosahexaenoyl-CoA precursor pool, JFA,i(DHA), were calculated as follows:
| (Eq.2) |
| (Eq.3) |
cplasma(DHA) (nmol·ml−1) is the concentration of unlabeled unesterified DHA in plasma. A "dilution factor" λ is defined as the steady-state ratio during [1-14C]DHA infusion of specific activity of brain docosahexaenoyl-CoA to the specific activity of plasma unesterified DHA:
| (Eq.4) |
A steady state is reached within 1 minute after infusion starts [37]. The fractional turnover of DHA within phospholipid i, FFA,i(DHA) (%·h−1), is defined as:
| (Eq.5) |
Preparation of cytoplasmic extracts
Brain tissue was homogenized in buffer (3 vol, 10 mM HEPES, pH 7.5, with 1 mM EDTA, 0.34 M sucrose, and protease inhibitor cocktail (Roche, Indianapolis, IN)) in a glass apparatus. The homogenized sample was centrifuged (100,000 g, 1 hr, 4°C), and the supernatant was used for PLA2 enzyme activity measurements and Western blotting. Supernatants were stored at −80°C until use. Protein content was determined by the Bradford assay (Bio-Rad) [44].
Western blotting
Proteins from the cytoplasmic extracts (50µg) were analyzed on 4–20% SDS-polyacrylamide gels (PAGE) (Bio-Rad). Following SDS-PAGE, proteins were transferred electrophoretically to a polyvinylidene difluoride membrane (Bio-Rad). Protein blots were incubated (overnight, 4°C) in Tris-buffered saline containing 5% nonfat dried milk and 0.1% Tween-20 with specific primary antibodies (1:1000 dilution) directed against cPLA2-IVA, sPLA2-V, iPLA2β, COX-1, COX-2, 5-LOX, 12-LOX and 15-LOX (Santa Cruz Biotech, Santa Cruz, CA, USA). Protein blots were incubated with appropriate HRP-conjugated secondary antibodies (Cell Signaling Beverly, MA) and visualized by chemiluminescence (Pierce, Rockford, IL, USA) using BioMax X-ray film (Eastman Kodak, Rochester, NY, USA). Optical densities of immunoblot bands were measured with Alpha Innotech Software (Alpha Innotech, San Leandro, CA, USA) and were normalized to the optical density of β-actin (Sigma-Aldrich, St. Louis, MO, USA) to correct for unequal loading. All experiments were performed with 6 independent samples per group. Values are expressed as percent of control.
RNA isolation and real time RT-PCR
Total RNA was isolated from brain using commercial kits (RNeasy Lipid Tissue Kit; Qiagen, Valencia, CA). cDNA was prepared from total RNA using a high-capacity cDNA Archive Kit (Qiagen). Taqman® gene expression master mix and specific primers for real time RT-PCR were purchased from Applied Biosystems (Foster City, CA). Levels of mRNA for cPLA2-IVA, sPLA2-V, iPLA2β, iPLA2γ, COX-1 and COX-2 were measured by real time quantitative RT-PCR using the ABI PRISM 7000 sequence detection system (Applied Biosystems). The fold-change in gene expression was determined by the ΔΔCT method [45]. Data are expressed as the relative level of the target gene in the iPLA2β−/− group normalized to the endogenous control (β-globulin) and relative to the level in the iPLA2β+/+ group. All experiments were carried out in triplicate with 6 independent samples per group.
Phospholipase A2 activities
A radioisotopic method was used to measure cPLA2 type IV and calcium independent (i)PLA2 type VI activities in cytoplasmic extracts (0.3 mg protein per assay) as previously described in detail elsewhere [6, 46]. The activity of sPLA2 was measured using an sPLA2 assay kit (Cayman, Ann Arbor, MI, USA) according to the manufacturer's instructions.
Statistical analyses
Data were analyzed using SPSS 17.0 software (SPSS Inc., Chicago, IL) and are presented as mean ± SEM of 5–6 independent measurements per group. Brain fatty acid concentrations and rates of DHA incorporation and turnover are expressed per µmol lipid phosphorous [20, 21]. Breeding limitations and surgical losses limited sample size and precluded establishing normality of distribution criteria. The probability of Type II errors was mitigated by using Cohen’s d test as a measure of effect size [47], which permits qualitative interpretations of differences between means. An effect size corresponding to Cohen’s d of 0.3 is considered small, of 0.5 medium, and of 0.8 and above large [47]. We considered effect sizes greater than 0.5 to be significant.
RESULTS
Plasma radioactivity and unesterified fatty acid concentrations
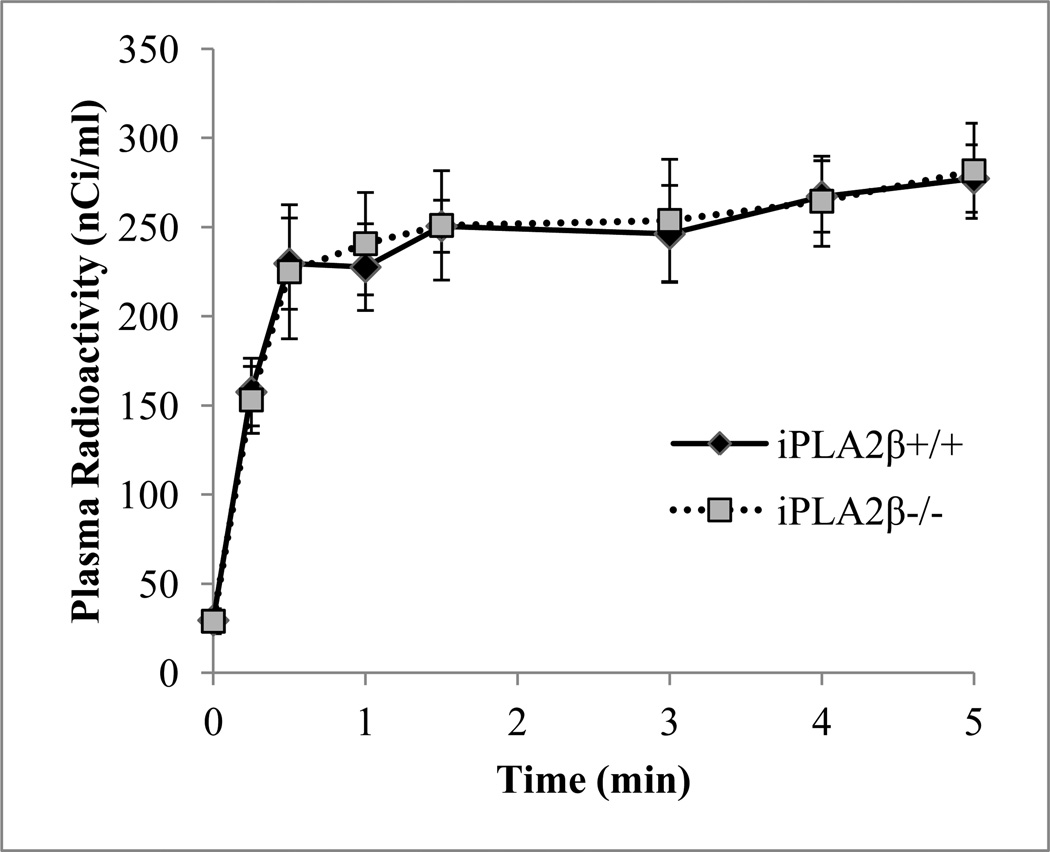
Steady-state plasma radioactivity was achieved within 1 min after initiating [1-14C]DHA infusion (Figure 1). The integral of plasma radioactivity (denominator of Eq. 1) for the 5-min infusion was 72,419 ± 13,121 nCi˙s/ml for iPLA2β+/+ mice and 73,203 ± 19,411 nCi˙s/ml for iPLA2β−/− mice (d = 0.05), which indicates no significant difference between groups.
Figure 1.
Time course of changes in arterial plasma [14C] radioactivity (nCi/ml) from brain lipid extracts of iPLA2β+/+ and iPLA2β−/− mice during intravenous infusion of 5 µCi/mouse of [1-14C]docosahexaenoic acid over 5 min at a rate of 0.0223[1+e−0.032t] ml/min. Values are mean ± SEM (n=5 / group).
Table 1 indicates that mean plasma concentrations of unesterified palmitate (16:0), palmitoleate (16:1n-7), stearate (18:0), oleate (18:1n-9), linoleate (18:2n-6) and α-linolenate (18:3n-3) were significantly (d > 0.8) higher by 18–57% in the iPLA2β−/− mice compared to wild type iPLA2β+/+ mice. Concentrations of n-3 fatty acids, including eicosapentaenoic acid (20:5n-3), n-3 docosapentaenoic acid (22:5n-3) and DHA (22:6n-3), were about 20% lower in the iPLA2β−/− mice, with medium-to-high effect sizes (d = 0.59, 0.59, and 0.64, respectively).
Table 1.
Plasma lipid concentrations in iPLA2β+/+ and iPLA2β−/− mice
| Unesterified fatty acids | Triglycerides | Phospholipids | Cholesteryl ester | |||||
|---|---|---|---|---|---|---|---|---|
| Fatty Acid |
iPLA2β+/+ | iPLA2β−/− | iPLA2β+/+ | iPLA2β−/− | iPLA2β+/+ | iPLA2β−/− | iPLA2β+/+ | iPLA2β−/− |
| nmol/ml plasma | nmol/ml plasma | nmol/ml plasma | nmol/ml plasma | |||||
| 16:0 | 167.5 ± 15.8 | 222.8 ± 15.4** | 63.7 ± 8.9 | 69.4 ± 13.2 | 759.6 ± 26.6 | 730.0 ± 69.1 | 37.2 ± 1.8 | 38.3 ± 5.3 |
| 16:1n-7 | 23.7 ± 3.0 | 37.3 ± 5.2** | 4.9 ± 0.9 | 7.3 ± 1.7** | 13.6 ± 1.7 | 17.1 ± 1.9** | 15.2 ± 2.1 | 18.3 ± 4 |
| 18:0 | 43.0 ± 4.7 | 50.8 ± 4.3* | 33.9 ± 7.5 | 25.7 ± 2.9* | 359.9 ± 9.3 | 347.3 ± 19.5 | 16.0 ± 5.6 | 13.0 ± 1.3 |
| 18:1 n-9 | 162.0 ± 17.7 | 198.2 ± 14.4** | 70.5 ± 8.6 | 73.7 ± 12.3 | 160.6 ± 4.7 | 164.6 ± 15.2 | 47.6 ± 2.3 | 46.2 ± 4.1 |
| 18:2 n-6 | 224.6 ± 24.6 | 271.9 ± 18.5** | 100.9 ± 17.2 | 103.3 ± 18.8 | 631.5 ± 1.3 | 621.5 ± 2.1 | 359.7 ± 14 | 313.7 ± 42.8* |
| 18:3 n-3 | 18.7 ± 2.0 | 24.0 ± 2.5** | 4.0 ± 0.6 | 4.6 ± 0.8 | 4.9 ± 16.6 | 4.7 ± 54.5 | 3.7 ± 0.5 | 3.7 ± 0.3 |
| 20:4 n-6 | 6.8 ± 0.9 | 6.4 ± 0.8 | 12.7 ± 1.3 | 10.5 ± 1.7* | 162.7 ± 0.2 | 149.3 ± 0.4** | 118.8 ± 24.2 | 113.3 ± 10.5 |
| 20:5 n-3 | 9.0 ± 1.9 | 7.0 ± 0.9* | 26.1 ± 1.7 | 20.5 ± 2.3** | 26.1 ± 3.9 | 31.3 ± 6.6** | 29.2 ± 4.5 | 36.5 ± 7.3* |
| 22:5 n-3 | 6.1 ± 1.0 | 4.9 ± 0.7* | ND | ND | 9.7 ± 2.4 | 9.6 ± 3.3 | ND | ND |
| 22:6 n-3 | 40.9 ± 6.7 | 33.0 ± 4.0* | 52.5 ± 4.2 | 44.9 ± 5.8* | 215 ± 2.1 | 200.1 ± 2.4 | 57.5 ± 2.1 | 45.9 ± 7.8** |
Values are means ± SEM (n=5).
0.5 ≤ d < 0.8,
d ≥ 0.8
ND, not detected
The concentration of palmitoleate (16:1n-7) esterified in plasma triglycerides and phospholipids was 26–49% higher for iPLA2β−/− than for iPLA2β+/+ mice (d > 0.5), but the concentration of esterified AA was 8–17% lower for iPLA2β−/− mice. The concentrations of stearate (18:0) and linoleate (18:2n-6) esterified in triglycerides and cholesteryl esters were 24% and 13% lower, respectively, for iPLA2β−/− than for iPLA2β+/+ mice (d > 0.5) (Table 1). Other differences included a 21% lower concentration of eicosapentaenoic acid (20:5n-3) esterified in triglycerides and a 20–25% higher concentration of 20:5n-3 esterified in phospholipids and cholesteryl esters for iPLA2β−/− compared to iPLA2β+/+ mice (d > 0.5). The concentration of DHA esterified in phospholipids and cholesteryl esters was 14–20% lower for iPLA2β−/− mice (d > 0.5).
Brain total lipid phosphorous concentration expressed in units of [(µmol P)/(g brain wet weight)] was significantly lower for iPLA2β−/− than for iPLA2β+/+ mice (59.17 ± 2.74 vs. 65.68 ± 1.20 µmol/g, d > 0.8), which may be attributable to brain edema that has been reported for iPLA2β−/− mice [20, 21] that would increase tissue water content and result in a lower measured amount of lipid per unit tissue wet weight. To correct for this, we normalized all lipid content and kinetic measurements to brain total lipid phosphorous. Table 2 summarizes the fractional concentration of individual phospholipid classes and plasmalogen species [in units of (µmol phosphorus of an individual phospholipid class)/(µmol total lipid phosphorous)] and expresses cholesterol concentration [as (µmol cholesterol)/(µmol total lipid phosphorous)] in brains from the two genotypes. Concentrations of EtnGpl and PtdIns were higher and that of lysoPC was lower for iPLA2β−/− than for iPLA2β+/+ mice (d > 0.5). Plasmenylethanolamine was increased in iPLA2β−/− mice (d = 0.5). No significant difference was seen in the plasmenylcholine concentration of ChoGpl between genotypes.
Table 2.
Brain phosphorous and cholesterol concentrations, per g wet weight or per µmol phosphorous (P) of brain total lipids, in iPLA2β+/+ and iPLA2β−/− mice
| iPLA2β+/+ | iPLA2β−/− | ||
|---|---|---|---|
| µmol/µmol P of brain total lipids | |||
| Total Phospholipid | |||
| EtnGpl | 0.30 ± 0.03 | 0.36 ± 0.04* | |
| ChoGpl | 0.38 ± 0.021 | 0.37 ± 0.010 | |
| PtdIns | 0.06 ± 0.005 | 0.07 ± 0.003** | |
| PdtSer | 0.13 ± 0.006 | 0.13 ± 0.004 | |
| Lyso PC | 0.010 ± 0.003 | 0.006 ± 0.001* | |
| LysoPE + LysoPI | 0.022 ± 0.002 | 0.020 ± 0.002 | |
| Cholesterol | 0.28 ± 0.004 | 0.27 ± 0.009 | |
| Plasmalogen | |||
| Plasmenylethanolamine | 0.14 ± 0.003 | 0.15 ± 0.003* | |
| Plasmenylcholine | 0.008 ± 0.002 | 0.006 ± 0.001 | |
Values are means ± SEM (n=5).
0.5 ≤ d < 0.8,
d ≥ 0.8
Concentration of esterified fatty acids in brain phospholipids
Table 3 summarizes mean esterified fatty acid concentrations in iPLA2β+/+ and iPLA2β−/− brains expressed in units of (nmol fatty acid in an individual phospholipid class per µmol total lipid phosphorous) for EtnGpl, ChoGpl, PtdIns, PtdSer, and total phospholipids. The total fatty acid content of PtdIns was higher by 6.5 % in iPLA2β−/− mice (d = 0.66), and this reflects increased concentrations of stearate, linoleate, arachidonate and DHA. In contrast, the total fatty acid concentration in EtnGpl, ChoGpl, PtdSer, and total phospholipid was lower in iPLA2β−/− mice by 5% (d = 0.96), 3% (d = 0.78), 6% (d = 0.86), and 4% (d = 0.85), respectively, and this reflected decreased concentrations of saturated and monounsaturated fatty acid substituents in ChoGpl and PtdSer and decreased concentrations of PUFAs esterified in EtnGpl, ChoGpl and PtdSer. Similar differences were also observed for total phospholipids. The concentration of stearate esterified in ChoGpl and PtdSer was 4.7–7.5 % lower for iPLA2β−/− mice, but that for PtdIns was higher (d > 0.5). The concentration of several monounsaturated (e.g., oleate) fatty acids esterified in brain EtnGpl, ChoGpl and PtdSer was 14–20% lower for iPLA2β−/− than for iPLA2β+/+ mice, but that for PtdIns was higher (d > 0.5). The esterified concentrations of several PUFAs (e.g., AA, DHA, and 22:5n-3) in brain PtdSer and EtnGpl were up to 30% lower for iPLA2β−/− than for iPLA2β+/+ mice (d > 0.5). The concentrations of esterified oleate (18:1n-9), 20:1n-9, 22:4n-6, 22:5n-3, and DHA in total brain phospholipid were also 5–17% lower for iPLA2β−/− than for iPLA2β+/+ mice (d > 0.5).
Table 3.
Esterified fatty acid concentrations in brain phospholipids of iPLA2β+/+ and iPLA2β−/− mice, per µmol phosphorous (P) of brain total lipids
| EtnGpl | ChoGpl | PtdIns | PtdSer | Total phospholipids | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Fatty Acid | iPLA2β+/+ | iPLA2β−/− | iPLA2β+/+ | iPLA2β−/− | iPLA2β+/+ | iPLA2β−/− | iPLA2β+/+ | iPLA2β−/− | iPLA2β+/+ | iPLA2β−/− |
| nmol/µmol P of brain total lipids | nmol/µmol P of brain total lipids | nmol/µmol P of brain total lipids | nmol/µmol P of brain total lipids | nmol/µmol P of brain total lipids | ||||||
| 16:0 | 36.7 ± 0.6 | 36.3 ± 0.8 | 297.2 ± 4.5 | 291.2 ± 10.2 | 8.9 ± 0.4 | 8.0 ± 1.2 | 3.7 ± 0.2 | 4.2 ± 0.7 | 348.9 ± 5.6 | 342.9 ± 10.5 |
| 18:0 | 124.5 ± 2.6 | 126.0 ± 6.2 | 96.1 ± 1.5 | 91.6 ± 2.2** | 29.2 ± 1.3 | 31.4 ± 1.9* | 101.6 ± 2.8 | 93.9 ± 1.7** | 367.0 ± 7.8 | 357.9 ± 8.7 |
| 18:1 n-9 | 91.7 ± 1.3 | 87.9 ± 4.8 | 151.2 ± 2.4 | 143.3 ± 2.8** | 14.6 ± 1.1 | 14.8 ± 0.8 | 43.5 ± 0.8 | 41.4 ± 2.8 | 310.8 ± 5.0 | 296.4 ± 5.8** |
| 18:1 n-7 | 18.5 ± 0.6 | 21.3 ± 3.4* | 41.2 ± 0.5 | 39.7 ± 1.2* | 3.5 ± 0.2 | 3.5 ± 0.1 | ND | ND | 63.3 ± 0.8 | 64.7 ± 4.6 |
| 18:2 n-6 | 4.6 ± 0.3 | 4.6 ± 0.4 | 5.5 ± 0.2 | 5.5 ± 0.3 | 0.9 ± 0.1 | 1.4 ± 0.4** | 0.6 ± 0.0 | 0.6 ± 0.1* | 11.6 ± 0.4 | 12.3 ± 0.9 |
| 20:1n-9 | 21.4 ± 1.2 | 19.0 ± 1.4** | 7.8 ± 0.2 | 7.1 ± 0.4** | 1.9 ± 0.2 | 1.8 ± 0.0 | 3.7 ± 0.2 | 3.1 ± 0.3** | 35.6 ± 1.6 | 32.2 ± 2.2* |
| 20:4 n-6 | 72.4 ± 1.5 | 67.8 ± 2.1** | 31.4 ± 0.6 | 29.9 ± 1.8 | 27.2 ± 1.2 | 30.2 ± 2.7* | 5.6 ± 0.1 | 5.2 ± 0.2* | 136.7 ± 2.9 | 133.5 ± 6.2 |
| 22:4 n-6 | 25.2 ± 0.8 | 21.5 ± 0.7** | ND | ND | ND | ND | 5.6 ± 0.3 | 5.4 ± 0.2 | 700.0 ± 10.3 | 671.6 ± 17.5** |
| 22:5 n-3 | 2.4 ± 0.0 | 2.0 ± 0.1** | ND | ND | ND | ND | 0.7 ± 0.0 | 0.6 ± 0.0** | 3.2 ± 0.1 | 2.6 ± 0.1** |
| 22:6 n-3 | 148.1 ± 4.2 | 132.7 ± 3.6** | 31.3 ± 0.7 | 29.6 ± 1.6* | 3.1 ± 0.1 | 3.8 ± 0.3** | 65.5 ± 2.3 | 62.2 ± 1.6* | 249.4 ± 4.8 | 230.0 ± 5.5** |
| Total | 549.1 ± 10.4 | 522.5 ± 16.2** | 665.5 ± 9.7 | 642.0 ± 17.4* | 89.2 ± 4.1 | 95.0 ± 3.7* | 230.5 ± 5.4 | 216.7 ± 5.4** | 1570.0 ± 26.6 | 1510.5 ± 33.2** |
Values are means ± SEM (n=5).
0.5 ≤ d < 0.8,
d ≥ 0.8.
Esterified fatty acid concentrations of brain lysophospholipids
The fact that the esterified fatty acid concentration of brain phospholipids relative to total lipid phosphorus is reduced in iPLA2β−/− mice suggests the possibility that phosphorus-containing lipids with a relatively low fatty acid content, such as lysophospholipids, might be more abundant in iPLA2β−/− than in iPLA2β+/+ mice. Lysophospholipids have free hydroxyl groups at the sn-1 or sn-2 position and contain a single fatty acid residue per phosphorus atom, whereas diacyl-phospholipids have two. The fatty acid concentration of lysoPC (expressed as nmol fatty acid per µmol total lipid phosphorous) was increased in brains of iPLA2β−/− compared to wild type mice (d > 0.5; Table 4), and fatty acid substituents contained in lysoPC included 18:1n-9, 18:1n-7, AA, 22:4n-6 and DHA. The concentrations of 16:0, 18:2n-6, 20:1n-9 and 22:4n-6 esterified in lysoPI plus lysoPE were higher in iPLA2β−/−than for wild type mice (d > 0.5; Table 4). The total esterified fatty acid concentration in the combined lysoPI and lysoPE fraction, however, did not differ significantly between genotypes.
Table 4.
Esterified fatty acid concentrations in brain lysoPC and combined lysoPE and lysoPI fractions in iPLA2β+/+ and iPLA2β−/− mice
| LysoPC | LysoPE and LysoPI | |||
|---|---|---|---|---|
| Fatty Acid | iPLA2β+/+ | iPLA2β−/− | iPLA2β+/+ | iPLA2β−/− |
| nmol/µmol P of brain total lipids | nmol/µmol P of brain total lipids | |||
| 16:0 | 1.03 ± 0.06 | 1.06 ± 0.07 | 1.32 ± 0.18 | 2.09 ± 0.57** |
| 18:0 | 2.89 ± 0.34 | 2.63 ± 0.15 | 12.80 ± 2.00 | 12.36 ± 2.69 |
| 18:1 n-9 | 0.35 ± 0.03 | 0.40 ± 0.03* | 9.41 ± 1.20 | 8.50 ± 1.56 |
| 18:1 n-7 | 0.10 ± 0.01 | 0.13 ± 0.01* | ND | ND |
| 18:2 n-6 | ND | ND | 0.08 ± 0.01 | 0.10 ± 0.02* |
| 20:1 n-9 | 0.23 ± 0.09 | 0.29 ± 0.10 | 0.55 ± 0.17 | 0.96 ± 0.34** |
| 20:4 n-6 | 0.06 ± 0.01 | 0.09 ± 0.01** | 0.14 ± 0.02 | 0.16 ± 0.03 |
| 22:4 n-6 | 0.24 ± 0.02 | 0.34 ± 0.05** | 0.46 ± 0.08 | 0.56 ± 0.07* |
| 22:6 n-3 | 0.02 ± 0.00 | 0.03 ± 0.01** | 1.32 ± 0.60 | 1.62 ± 0.69 |
| total | 4.94 ± 0.47 | 4.97 ± 0.20 | 26.13 ± 4.12 | 26.42 ± 5.91 |
Values are means ± SEM (n=5).
0.5 ≤ d < 0.8,
d ≥ 0.8.
Brain acyl-CoA concentrations and specific radioactivity
Table 5 summarizes the mean brain concentrations of long chain fatty acyl-CoA species, the [14C] specific radioactivity of docosahexaenoyl-CoA (DHA-CoA) and mean values for λ (dilution coefficient). Brain concentrations of palmitoyl-CoA, oleaoyl-CoA, linoleoyl-CoA, arachidonoyl-CoA and DHA-CoA were higher for iPLA2β−/− than for iPLA2β+/+ mice (d > 0.5). The [14C] specific radioactivity of brain DHA-CoA also was higher for iPLA2β−/− mice (d = 0.76), but λ (Eq. 3) did not differ between genotypes (d < 0.5).
Table 5.
Brain acyl-CoA concentrations in total lipids of iPLA2β+/+ and iPLA2β−/− mice
| Acyl-CoA | iPLA2β+/+ | iPLA2β−/− | |
|---|---|---|---|
| nmol/ µmol P of brain total lipids | |||
| Mystearoyl-CoA | 14:0 | 0.009 ± 0.002 | 0.011 ± 0.003 |
| Palmitoyl-CoA | 16:0 | 0.089 ± 0.010 | 0.119 ± 0.014** |
| Stearoyl-CoA | 18:0 | 0.084 ± 0.015 | 0.094 ± 0.018 |
| Oleaoyl-CoA | 18:1 | 0.093 ± 0.011 | 0.121 ± 0.013** |
| Linoleoyl-CoA | 18:2 n6 | 0.015 ± 0.004 | 0.023 ± 0.006* |
| Arachidonoyl-CoA | 20:4 n6 | 0.015 ± 0.003 | 0.020 ± 0.005* |
| Docosahexaenoyl-CoA | 22:6 n3 | 0.015 ± 0.002 | 0.018 ± 0.004* |
| nCi/ µmol P of brain total lipids | |||
| Docosahexaenoyl-CoA | 0.011 ± 0.001 | 0.014 ± 0.002* | |
| Lambda (λ) | 0.129 ± 0.036 | 0.103 ± 0.026 | |
P, phosphorous.
Values are means ± SEM (n=5).
0.5 ≤ d < 0.8,
d ≥ 0.8
[14C]DHA incorporation into brain phospholipids
Incorporation of unesterified plasma [14C]DHA into brain lipids is characterized by an incorporation coefficient (k*) and rate (Jin), and the mean values of these parameters for various lipid classes are summarized in Table 6. The coefficient k* for [14C]DHA incorporation into PtdSer was 41% higher for iPLA2β−/− than for iPLA2β+/+mice (d = 1.84), but k* for total phospholipid or other phospholipid classes did not differ significantly between the genotypes. The rate Jin,i of DHA incorporation into phospholipid class i represents the product of k* multiplied by the plasma unesterified unlabeled DHA concentration, and this parameter was decreased for iPLA2β−/−compared to iPLA2β+/+ mice by 17% for EtnGpl (d = 0.51) and by 18% for PtdIns (d = 0.53).
Table 6.
Brain incorporation coefficients (k*), incorporation rates (Jin) of unesterified DHA from plasma, net incorporation rates from brain docosahexaenoyl-CoA (JFA) and turnover of DHA (FFA) in brain phospholipids of iPLA2β+/+ and iPLA2β−/− mice
| k* | Jin | JFA | FFA | |||||
|---|---|---|---|---|---|---|---|---|
| iPLA2β+/+ | iPLA2β−/− | iPLA2β+/+ | iPLA2β−/− | iPLA2β+/+ | iPLA2β−/− | iPLA2β+/+ | iPLA2β−/− | |
| ml/µmol P/s × 10−5 | nmol/µmol P/s × 10−4 | nmol/µmol P/s × 10−2 | % per hour | |||||
| Total Phospholipids | 0.371 ± 0.027 | 0.389 ± 0.020 | 1.51 ± 0.25 | 1.30 ± 0.18 | 0.144 ± 0.030 | 0.146 ± 0.021 | 2.08 ± 0.42 | 2.31 ± 0.37 |
| EtnGpl | 0.150 ± 0.010 | 0.152 ± 0.007 | 0.61 ± 0.11 | 0.51 ± 0.07* | 0.058 ± 0.012 | 0.057 ± 0.009 | 1.43 ± 0.30 | 1.57 ± 0.27 |
| ChoGpl | 0.130 ± 0.008 | 0.139 ± 0.010 | 0.52 ± 0.08 | 0.46 ± 0.06 | 0.051 ± 0.010 | 0.053 ± 0.008 | 5.76 ± 1.14 | 6.41 ± 1.06 |
| PtdIns | 0.075 ± 0.009 | 0.075 ± 0.004 | 0.31 ± 0.05 | 0.25 ± 0.04* | 0.029 ± 0.006 | 0.028 ± 0.003 | 33.20 ± 7.12 | 27.38 ± 4.46 |
| PtdSer | 0.017 ± 0.001 | 0.024 ± 0.002** | 0.07 ± 0.01 | 0.08 ± 0.01 | 0.006 ± 0.001 | 0.009 ± 0.002** | 0.35 ± 0.06 | 0.53 ± 0.09** |
Values are means ± SEM (n=5).
0.5 ≤ d < 0.8,
d ≥ 0.8
DHA turnover in brain phospholipids
Table 6 summarizes DHA incorporation rates from the brain precursor DHA-CoA pool (JFA) and turnover (FFA) of DHA in total phospholipid and in individual brain phospholipid classes. JFA for total phospholipids did not differ between genotypes, but JFA for brain PtdSer was increased by 33% (d = 0.87) in iPLA2β−/− compared to iPLA2β+/+- mice. DHA turnover in brain PtdSer was 52% higher (d = 1.04) for iPLA2β−/− mice, but did not differ between genotypes for any other phospholipid class or for total phospholipid.
Brain enzymatic activity and levels of mRNA and protein for sPLA2, cPLA2 and iPLA2
Compared to wild type mice, brains of iPLA2β−/− mice contained much reduced amounts of iPLA2β mRNA (> 93%) and protein (> 99.9%), and total brain iPLA2 activity was also reduced, as expected, with large effect sizes (d > 0.8; Table 7). Residual brain iPLA2 activity in the iPLA2β−/− mouse is probably attributable to iPLA2γ [48], since brain iPLA2γ mRNA was detected in both iPLA2β−/− and iPLA2β+/+ mice, but was less abundant in the latter (d > 0.8; Table 7). We did not measure iPLA2γ protein because suitable antibodies are not available at this time.
Table 7.
Enzymatic activity, protein expression and mRNA levels in the brains of iPLA2β+/+ and iPLA2β−/− mice
| iPLA2β+/+ | iPLA2β−/− | |
|---|---|---|
| Activity | pmol/mg protein/min | |
| cPLA2 | 6.6 ± 0.1 | 6.8 ± 0.4 |
| sPLA2 | 7.6 ± 0.5 | 8.4 ± 0.3* |
| iPLA2 (β+γ) | 11.9 ± 1.4 | 2.0 ± 0.3** |
| Protein | % expression | |
| cPLA2-IVA | 100 ± 20 | 117 ± 16 |
| sPLA2-V | 100 ± 25 | 129 ± 17* |
| iPLA2β | 100 ± 13 | 7 ± 0.3** |
| COX-1 | 100 ± 16 | 79 ± 6* |
| COX-2 | 100 ± 28 | 154 ± 16** |
| 5-LOX | 100 ± 16 | 111 ± 10 |
| 12-LOX | 100 ± 20 | 120 ± 24 |
| 15-LOX | 100 ± 11 | 115 ± 18 |
| mRNA | Relative fold change | |
| cPLA2-IVA | 1.0 ± 0.1 | 1.4 ± 0.2** |
| sPLA2-V | 1.0 ± 0.1 | 1.5 ± 0.2** |
| iPLA2β | 1.0 ± 0.03 | 0.01 ± 0.003** |
| iPLA2γ | 1.0 ± 0.1 | 0.6 ± 0.1** |
| COX-1 | 1.0 ± 0.1 | 1.1 ± 0.1 |
| COX-2 | 1.0 ± 0.1 | 1.2 ± 0.2* |
Values are means ± SEM (n=6).
0.5 ≤ d < 0.8,
d ≥ 0.8
Brain cPLA2-IVA mRNA was 50% higher for iPLA2β−/− than for iPLA2β+/+ mice (d = 1.05), but no significant difference between genotypes for brain levels of cPLA2-IVA protein or enzymatic activity was detected (Table 7). Levels of mRNA, protein, and enzymatic activity for sPLA2-V were higher in iPLA2β−/− than iPLA2β+/+ mice by 50% (d = 1.32), 25% (d = 0.57), and 11% (d = 0.79), respectively (Table 7).
Brain levels of COX and LOX mRNA and protein
There was no statistically significant difference in brain COX-1 mRNA level between genotypes (Table 7). Brain COX-1 protein was 21% lower (d = 0.68) and COX-2 protein was 54% higher (d = 0.97) in iPLA2β−/− than in mice (Table 7). Brain COX-2 mRNA levels were also higher by 17% in iPLA2β−/− mice (d = 0.52). No significant differences between genotypes were observed for levels of 5-LOX, 12-LOX or15-LOX proteins (Table 7).
DISCUSSION
Values for wild type mice observed here for DHA kinetic parameters and for basal levels of lipids, including free fatty acids, in plasma and in brain, are similar to published values without normalization for brain lipid phosphorous content [33, 41]. We found evidence of disturbed brain lipid metabolism in iPLA2β−/− male mice lacking the PLA2G6 gene at 4–5 months of age. Compared with wild type iPLA2β+/+ controls, iPLA2β−/− mice exhibited reduced brain consumption of DHA that is reflected by reduced incorporation rates (Jin) of unesterified DHA from plasma into several phospholipid classes and this is concordant with reported quantitative autoradiographic observations [3]. DHA in brain cannot be synthesized de novo, and conversion of the dietary precursor α-linolenic acid to DHA in brain represents less than 0.5% of the plasma DHA flux because most α-linolenic acid is rapidly oxidized in brain, as is most eicosapentaenoic acid that enters the brain [49, 50]. iPLA2β−/− mice also exhibited compensatory changes in brain expression of other brain lipid metabolizing enzymes, including Groups IVA, V, and VIB PLA2s, COX-1 and -2 isozymes, and LOX isozymes with different regiospecificities. Alterations in fatty acid concentrations in various phospholipid classes were also observed in brains of iPLA2β−/− mice, but DHA turnover in brain phospholipids did not differ between the iPLA2β−/− and iPLA2β+/+ genotypes (Table 6), even though Jin for brain EtnGpl and PtdIns was lower for iPLA2β−/− than for iPLA2β+/+ mice. Because Jin was reduced by the same proportion as the reduction in esterified DHA and because λ did not differ between genotypes, the calculated DHA turnover in brain phospholipids (Eq. 5) also did not differ between iPLA2β−/− and iPLA2β+/+ mice.
Significant reductions in net k* for DHA were demonstrated with quantitative auto-radiographic measurements in 70 of 81 brain regions examined in unanesthetized iPLA2β−/− mice compared to wild type controls [3]. Here we demonstrate by direct chemical analyses that reduced incorporation of unesterified plasma DHA into brain EtnGpl and PtdIns in iPLA2β−/− mice accounts for most of the reduction of incorporation into total phospholipids (Table 6). Although Jin for brain PtdSer increases in iPLA2β−/− mice, this is a minor contributor to the overall net change in DHA incorporation kinetics that results from iPLA2β deficiency.
Changes in brain lipid-metabolizing enzymes in iPLA2β−/− mice include increases in cPLA2-IVA mRNA; in sPLA2-V mRNA, protein and activity; and in COX-2 mRNA and protein (Table 7). Brain COX-1 protein is also reduced in iPLA2β−/− mice. These changes reflect a profound reorganization of brain lipid metabolism and structure that result from iPLA2β−/− deficiency. Arachidonic acid can be released from phospholipids by the actions of cPLA2-IVA and sPLA2-V, and COX, LOX, and monooxygenase enzymes can convert released AA to a plethora of bioactive oxygenated metabolites, including prostaglandins, thromboxanes, and leukotrienes, inter alia [48]. The fact that the level of COX-2 protein is increased and that of COX-1 protein is reduced in brains of iPLA2β−/− mice might reflect coupling of COX-1 to iPLA2 and of COX-2 to cPLA2, as has been suggested elsewhere [51–53]. The reduced brain concentration of esterified AA in EtnGpl and PtdSer in iPLA2β−/− mice and the increased AA concentration of PtdIns may result from or reflect compensatory responses to the changes in levels of various lipid metabolizing enzymes in brains of iPLA2β−/− mice. It seems likely that the pattern of brain AA metabolism might be altered significantly in iPLA2β−/− mice and that this might result in perturbation of the generation of arachidonate oxygenation products, AA-derived endocannabinoids, and platelet activating factor, among other bioactive lipids.
Brains of iPLA2β−/− mice exhibit reduced concentrations of several fatty acid substituents esterified in EtnGpl, ChoGpl and PtdSer, which are diacyl phospholipid molecular species that contain two fatty acid residues for each phosphorus atom. In contrast, brains of iPLA2β−/− mice exhibit increased concentrations of several fatty acid substituents esterified in lysophospholipids and acyl-CoA species. The former contains a single fatty acid residue per phosphorus atom, and the latter contains three phosphorus atoms for each fatty acid residue. Lysophospholipids and long chain fatty acyl CoA molecules thus exhibit a lower fatty acid to phosphorus ratio than do diacyl phospholipids. The brain plasmenylethanolamine content is also increased for iPLA2β−/− mice, and these ether lipids also have a single mole of saponifiable fatty acid per mole of phosphate and thus also exhibit a lower fatty acid to phosphorus ratio than do diacyl phospholipids. Ether linked lysophospholipids contain no saponifiable fatty acid residues and thus contribute no signal to the fatty acid content of lysophospholipid classes.
The altered brain phospholipid concentrations of iPLA2β−/− mice may reflect disturbed membrane remodeling that occurs as a consequence of iPLA2β deficiency and compensatory changes in the expression of other PLA2 enzymes, and it is likely that this perturbs lipid metabolic homeostatic processes in brain. Tubulovesicular membranes and small vacuoles and edema are observed in brain of iPLA2β−/− mice at age 4 months, but more dramatic neuropathologic abnormalities are manifest by 13 months [20, 21]. We confirmed the presence of edema by demonstrating reduced total lipid phosphorus concentration per gram brain wet weight in the 4-month old iPLA2β−/− mice. Developmental abnormalities in fatty acid and phospholipid metabolism may contribute both to early changes and to more significant neurodegenerative and behavioral abnormalities in older mice [14, 20–22].
Brains of 4 month-old iPLA2β−/− mice exhibited lower iPLA2γ transcript levels than did brains of wild type mice, and iPLA2β−/− brain EtnGpl and PtdSer phospholipid exhibited a lower esterified DHA concentration than wild type mice. This was associated with reduced incorporation of unesterified DHA from plasma into these phospholipid classes. Both iPLA2β and iPLA2γ can hydrolyze DHA from the sn-2 position of phospholipids [1, 2]. DHA is a precursor of anti-inflammatory neuroprotectins and resolvins [26], and the reduced brain DHA concentration associated with iPLA2β deficiency may increase vulnerability to neuroinflammatory processes and other insults. Other enzymes not measured in this study that may influence DHA loss include plasmalogen-selective PLA2, but it has not yet been cloned to our knowledge [54]. Net iPLA2 activity and iPLA2β mRNA and protein also have been reported to be reduced in brains of rats deprived of dietary n-3 PUFA [52], and these animals also exhibit reduced brain DHA consumption and concentration and increased sensitivity to neuroinflammatory stress [55, 56].
Mutations in the PLA2G6 gene encoding iPLA2β have been reported in humans with infantile neuroaxonal dystrophy, idiopathic neurodegeneration with brain iron accumulation, dystonia-parkinsonism, and cerebellar cortical atrophy with gliosis [16–19]. These conditions are characterized by motor and often cognitive impairments. iPLA2β or iPLA2γ knockout mice show significant motor and cognitive deficits by 13 months of age that are associated with synaptic loss and α-synuclein accumulation in brain [20, 21]. α-Synuclein and DHA strongly interact in a manner that affects both the structure of the protein and the physical state of the lipid [57, 58]. Similar but less severe motor and cognitive behavioral abnormalities have been reported in rats that have been deprived of dietary n-3 PUFA and exhibit reduced brain DHA concentration [7, 50, 55, 56], and this is also associated with altered expression of AA and DHA metabolizing enzymes [20, 28, 52, 59]
The changes in brain DHA metabolism and metabolizing enzymes in iPLA2β−/− mice occurred despite the presence of a high (2.2%) DHA content in their diet (Rodent NIH-07) (see Methods). This dietary DHA supplementation may have slowed the evolution of neuropathology [60, 61], which was initially described in mice that were fed a diet that contained only 0.9% DHA (PicoLabA 5053, LabDiet, Purina Mills International, St. Louis, MO) [20]. Dietary deficiency of DHA or its precursors (α-LNA and EPA) could exacerbate the effects of genetic iPLA2β deficiency by further reducing plasma DHA incorporation into brain, but this remains to be tested.
This study underscores the importance of iPLA2β in brain lipid metabolism because multiple changes were found to occur in brains of mice with genetic iPLA2β deficiency, even though iPLA2γ and other enzymes that can release DHA from membrane phospholipids are expressed at normal or increased levels [2, 46, 48, 62]. The study also highlights the lack of redundancy with regard to PLA2 enzyme function in brain that is suggested by compensatory changes in expression of other enzymes and consequent changes in lipid composition that occur in brains of iPLA2β-null mice. Similar findings have been reported for mice with genetic deficiency of other lipid-metabolizing enzymes, including cPLA2 IVA, COX-2 and COX-1 [33, 41, 63–66]. It is notable in this regard that even heterozygous PLA2β+/− mice exhibit reduced plasma DHA incorporation into brain and altered DHA signaling in response to cholinergic muscarinic receptor activation [3]. Brain AA metabolism and signaling may also be disturbed in iPLA2β−/− mice in view of our findings that these mice exhibit increased activity of sPLA2 and mRNA of cPLA2-IV in brain, and the fact that both enzymes can release AA from phospholipid substrates. Brain content of esterified AA in phospholipids is also reduced in iPLA2β−/− mice.
In summary, iPLA2β−/− mice at age 4–5 mo exhibit disturbances in whole brain phospholipid composition and metabolism and in expression of enzymes involved in phospholipid metabolism. These abnormalities are associated with reduced incorporation of unesterified DHA from plasma into brain lipids and reduced esterified DHA concentrations in various lipid classes that may contribute to neuropathological and behavioral abnormalities that develop in iPLA2β−/− mice. Our observations also may be relevant to human clinical syndromes (e.g., dystonia-parkinsonism and infantile neuroaxonal dystrophy) that are associated with PLA2G6 mutations. In such conditions, DHA incorporation into brain could be imaged directly with positron emission tomography [67], and dietary n-3 PUFA supplementation and/or n-6 PUFA deprivation might be considered for therapeutic trials [51, 60, 61]. Future characterization of brain lipid changes in iPLA2β-deficient animals could elucidate mechanisms for the pathological and behavioral changes in patients with PLA2G6 mutations and might provide guidance for the design of beneficial interventions for conditions that are otherwise difficult to treat effectively.
Highlights.
The role of iPLA2β in the brain was examined by using radiolabeled DHA in iPLA2β knockout mice.
iPLA2β knockout mice exhibit disturbances in brain phospholipid composition and metabolism.
iPLA2β knockout mice reduce DHA incorporation and DHA content in brain.
Genetic iPLA2β deficiency reorganizes of lipid-metabolizing enzyme expression in brain.
These abnormalities may be relevant to neurologic defects in humans with iPLA2β mutations.
ACKNOWLEDGEMENTS
The work conducted by Y. Cheon, H-W. Kim, M. Igarashi, H. R. Modi, L. Chang, K. Ma, S. I. Rapoport and A. Y. Taha was supported by the Intramural Research Program of the National Institute on Aging and that by D. Greenstein by the Intramural Research Program of the National Institute of Mental Health, National Institutes of Health. The work conducted by M. Wohltmann and J. Turk was supported by NIH grants R37-DK34388, P41-RR00954, P60-DK20579, and P30-DK56341. We appreciate the editorial assistance of the NIH Fellows Editorial Board.
Abbreviations
- AA
arachidonic acid
- ChoGpl
choline glycerophospholipid
- COX
cyclooxygenase
- cPLA2
cytosolic phospholipase A2 (Group IVA PLA2)
- DHA
docosahexaenoic acid
- DHA-CoA
docosahexaenoyl-CoA
- EtnGpl
ethanolamine glycerophospholipid
- FAME
fatty acid methyl esters
- GC
gas chromatography
- iPLA2
Ca2+-independent phospholipase A2 (Group VIA PLA2)
- LOX
lipoxygenase
- PUFA
polyunsaturated fatty acid
- PtdIns
phosphatidylinositol
- PtdSer
phosphatidylserine
- sPLA2
secretory phospholipase A2
- sn
stereospecifically numbered
- TLC
thin layer chromatography
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited anuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Strokin M, Sergeeva M, Reiser G. Docosahexaenoic acid and arachidonic acid release in rat brain astrocytes is mediated by two separate isoforms of phospholipase A2 and is differently regulated by cyclic AMP and Ca2+ British journal of pharmacology. 2003;139:1014–1022. doi: 10.1038/sj.bjp.0705326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Strokin M, Sergeeva M, Reiser G. Prostaglandin synthesis in rat brain astrocytes is under the control of the n-3 docosahexaenoic acid, released by group VIB calcium-independent phospholipase A2. Journal of neurochemistry. 2007;102:1771–1782. doi: 10.1111/j.1471-4159.2007.04663.x. [DOI] [PubMed] [Google Scholar]
- 3.Basselin M, Rosa AO, Ramadan E, Cheon Y, Chang L, Chen M, Greenstein D, Wohltmann M, Turk J, Rapoport SI. Imaging decreased brain docosahexaenoic acid metabolism and signaling in iPLA(2)beta (VIA)-deficient mice. J Lipid Res. 2010;51:3166–3173. doi: 10.1194/jlr.M008334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ong WY, Yeo JF, Ling SF, Farooqui AA. Distribution of calcium-independent phospholipase A2 (iPLA2) in monkey brain. Journal of neurocytology. 2005;34:447–458. doi: 10.1007/s11068-006-8730-4. [DOI] [PubMed] [Google Scholar]
- 5.Schaloske RH, Dennis EA. The phospholipase A2 superfamily and its group numbering system. Biochim Biophys Acta. 2006;1761:1246–1259. doi: 10.1016/j.bbalip.2006.07.011. [DOI] [PubMed] [Google Scholar]
- 6.Yang HC, Mosior M, Johnson CA, Chen Y, Dennis EA. Group-specific assays that distinguish between the four major types of mammalian phospholipase A2. Anal Biochem. 1999;269:278–288. doi: 10.1006/abio.1999.4053. [DOI] [PubMed] [Google Scholar]
- 7.Ackermann EJ, Kempner ES, Dennis EA. Ca(2+)-independent cytosolic phospholipase A2 from macrophage-like P388D1 cells. Isolation and characterization. J Biol Chem. 1994;269:9227–9233. [PubMed] [Google Scholar]
- 8.Jones CR, Arai T, Rapoport SI. Evidence for the involvement of docosahexaenoic acid in cholinergic stimulated signal transduction at the synapse. Neurochemical research. 1997;22:663–670. doi: 10.1023/a:1027341707837. [DOI] [PubMed] [Google Scholar]
- 9.Garcia MC, Kim HY. Mobilization of arachidonate and docosahexaenoate by stimulation of the 5-HT2A receptor in rat C6 glioma cells. Brain research. 1997;768:43–48. doi: 10.1016/s0006-8993(97)00583-0. [DOI] [PubMed] [Google Scholar]
- 10.DeGeorge JJ, Nariai T, Yamazaki S, Williams WM, Rapoport SI. Arecoline-stimulated brain incorporation of intravenously administered fatty acids in unanesthetized rats. Journal of neurochemistry. 1991;56:352–355. doi: 10.1111/j.1471-4159.1991.tb02603.x. [DOI] [PubMed] [Google Scholar]
- 11.Rosa AO, Rapoport SI. Intracellular- and extracellular-derived Ca2+ influence phospholipase A2-mediated fatty acid release from brain phospholipids. Biochim Biophys Acta. 2009;1791:697–705. doi: 10.1016/j.bbalip.2009.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jenkins CM, Wolf MJ, Mancuso DJ, Gross RW. Identification of the calmodulin-binding domain of recombinant calcium-independent phospholipase A2beta. implications for structure and function. J Biol Chem. 2001;276:7129–7135. doi: 10.1074/jbc.M010439200. [DOI] [PubMed] [Google Scholar]
- 13.Sharma J, Turk J, McHowat J. Endothelial cell prostaglandin I(2) and platelet-activating factor production are markedly attenuated in the calcium-independent phospholipase A(2)beta knockout mouse. Biochemistry. 2010;49:5473–5481. doi: 10.1021/bi100752u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mancuso DJ, Kotzbauer P, Wozniak DF, Sims HF, Jenkins CM, Guan S, Han X, Yang K, Sun G, Malik I, Conyers S, Green KG, Schmidt RE, Gross RW. Genetic ablation of calcium-independent phospholipase A2{gamma} leads to alterations in hippocampal cardiolipin content and molecular species distribution, mitochondrial degeneration, autophagy and cognitive dysfunction. J Biol Chem. 2009;284:35632–35644. doi: 10.1074/jbc.M109.055194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Meyer MC, Kell PJ, Creer MH, McHowat J. Calcium-independent phospholipase A2 is regulated by a novel protein kinase C in human coronary artery endothelial cells. Am J Physiol Cell Physiol. 2005;288:C475–C482. doi: 10.1152/ajpcell.00306.2004. [DOI] [PubMed] [Google Scholar]
- 16.Gregory A, Westaway SK, Holm IE, Kotzbauer PT, Hogarth P, Sonek S, Coryell JC, Nguyen TM, Nardocci N, Zorzi G, Rodriguez D, Desguerre I, Bertini E, Simonati A, Levinson B, Dias C, Barbot C, Carrilho I, Santos M, Malik I, Gitschier J, Hayflick SJ. Neurodegeneration associated with genetic defects in phospholipase A(2) Neurology. 2008;71:1402–1409. doi: 10.1212/01.wnl.0000327094.67726.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Khateeb S, Flusser H, Ofir R, Shelef I, Narkis G, Vardi G, Shorer Z, Levy R, Galil A, Elbedour K, Birk OS. PLA2G6 mutation underlies infantile neuroaxonal dystrophy. Am J Hum Genet. 2006;79:942–948. doi: 10.1086/508572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Morgan NV, Westaway SK, Morton JE, Gregory A, Gissen P, Sonek S, Cangul H, Coryell J, Canham N, Nardocci N, Zorzi G, Pasha S, Rodriguez D, Desguerre I, Mubaidin A, Bertini E, Trembath RC, Simonati A, Schanen C, Johnson CA, Levinson B, Woods CG, Wilmot B, Kramer P, Gitschier J, Maher ER, Hayflick SJ. PLA2G6, encoding a phospholipase A2, is mutated in neurodegenerative disorders with high brain iron. Nat Genet. 2006;38:752–754. doi: 10.1038/ng1826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yoshino H, Tomiyama H, Tachibana N, Ogaki K, Li Y, Funayama M, Hashimoto T, Takashima S, Hattori N. Phenotypic spectrum of patients with PLA2G6 mutation and PARK14-linked parkinsonism. Neurology. 2010;75:1356–1361. doi: 10.1212/WNL.0b013e3181f73649. [DOI] [PubMed] [Google Scholar]
- 20.Malik I, Turk J, Mancuso DJ, Montier L, Wohltmann M, Wozniak DF, Schmidt RE, Gross RW, Kotzbauer PT. Disrupted membrane homeostasis and accumulation of ubiquitinated proteins in a mouse model of infantile neuroaxonal dystrophy caused by PLA2G6 mutations. Am J Pathol. 2008;172:406–416. doi: 10.2353/ajpath.2008.070823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shinzawa K, Sumi H, Ikawa M, Matsuoka Y, Okabe M, Sakoda S, Tsujimoto Y. Neuroaxonal dystrophy caused by group VIA phospholipase A2 deficiency in mice: a model of human neurodegenerative disease. J Neurosci. 2008;28:2212–2220. doi: 10.1523/JNEUROSCI.4354-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mancuso DJ, Sims HF, Han X, Jenkins CM, Guan SP, Yang K, Moon SH, Pietka T, Abumrad NA, Schlesinger PH, Gross RW. Genetic ablation of calcium-independent phospholipase A2gamma leads to alterations in mitochondrial lipid metabolism and function resulting in a deficient mitochondrial bioenergetic phenotype. J Biol Chem. 2007;282:34611–34622. doi: 10.1074/jbc.M707795200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bao S, Bohrer A, Ramanadham S, Jin W, Zhang S, Turk J. Effects of stable suppression of Group VIA phospholipase A2 expression on phospholipid content and composition, insulin secretion, and proliferation of INS-1 insulinoma cells. J Biol Chem. 2006;281:187–198. doi: 10.1074/jbc.M509105200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Horrocks LA, Farooqui AA. Docosahexaenoic acid in the diet: its importance in maintenance and restoration of neural membrane function. Prostaglandins Leukot Essent Fatty Acids. 2004;70:361–372. doi: 10.1016/j.plefa.2003.12.011. [DOI] [PubMed] [Google Scholar]
- 25.de Urquiza AM, Liu S, Sjoberg M, Zetterstrom RH, Griffiths W, Sjovall J, Perlmann T. Docosahexaenoic acid, a ligand for the retinoid X receptor in mouse brain. Science. 2000;290:2140–2144. doi: 10.1126/science.290.5499.2140. [DOI] [PubMed] [Google Scholar]
- 26.Hong S, Gronert K, Devchand PR, Moussignac RL, Serhan CN. Novel docosatrienes and 17S-resolvins generated from docosahexaenoic acid in murine brain, human blood, and glial cells. Autacoids in anti-inflammation. J Biol Chem. 2003;278:14677–14687. doi: 10.1074/jbc.M300218200. [DOI] [PubMed] [Google Scholar]
- 27.Vreugdenhil M, Bruehl C, Voskuyl RA, Kang JX, Leaf A, Wadman WJ. Polyunsaturated fatty acids modulate sodium and calcium currents in CA1 neurons. Proc Natl Acad Sci U S A. 1996;93:12559–12563. doi: 10.1073/pnas.93.22.12559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.DeMar JC, Jr, Ma K, Bell JM, Igarashi M, Greenstein D, Rapoport SI. One generation of n-3 polyunsaturated fatty acid deprivation increases depression and aggression test scores in rats. J Lipid Res. 2006;47:172–180. doi: 10.1194/jlr.M500362-JLR200. [DOI] [PubMed] [Google Scholar]
- 29.Contreras MA, Rapoport SI. Recent studies on interactions between n-3 and n-6 polyunsaturated fatty acids in brain and other tissues. Curr. Opin. Lipidol. 2002;13:267–272. doi: 10.1097/00041433-200206000-00006. [DOI] [PubMed] [Google Scholar]
- 30.Strokin M, Sergeeva M, Reiser G. Role of Ca2+-independent phospholipase A2 and n-3 polyunsaturated fatty acid docosahexaenoic acid in prostanoid production in brain: perspectives for protection in neuroinflammation. Int J Dev Neurosci. 2004;22:551–557. doi: 10.1016/j.ijdevneu.2004.07.002. [DOI] [PubMed] [Google Scholar]
- 31.Cole GM, Frautschy SA. Docosahexaenoic acid protects from amyloid and dendritic pathology in an Alzheimer's disease mouse model. Nutr Health. 2006;18:249–259. doi: 10.1177/026010600601800307. [DOI] [PubMed] [Google Scholar]
- 32.Robinson PJ, Noronha J, DeGeorge JJ, Freed LM, Nariai T, Rapoport SI. A quantitative method for measuring regional in vivo fatty-acid incorporation into and turnover within brain phospholipids: review and critical analysis. Brain Res Brain Res Rev. 1992;17:187–214. doi: 10.1016/0165-0173(92)90016-f. [DOI] [PubMed] [Google Scholar]
- 33.Rosenberger TA, Villacreses NE, Contreras MA, Bonventre JV, Rapoport SI. Brain lipid metabolism in the cPLA2 knockout mouse. J Lipid Res. 2003;44:109–117. doi: 10.1194/jlr.m200298-jlr200. [DOI] [PubMed] [Google Scholar]
- 34.Rapoport SI. In vivo fatty acid incorporation into brain phosholipids in relation to plasma availability, signal transduction and membrane remodeling. J Mol Neurosci. 2001;16:243–261. doi: 10.1385/JMN:16:2-3:243. discussion 279–284. [DOI] [PubMed] [Google Scholar]
- 35.Chang MC, Bell JM, Purdon AD, Chikhale EG, Grange E. Dynamics of docosahexaenoic acid metabolism in the central nervous system: lack of effect of chronic lithium treatment. Neurochemical research. 1999;24:399–406. doi: 10.1023/a:1020989701330. [DOI] [PubMed] [Google Scholar]
- 36.Bao S, Miller DJ, Ma Z, Wohltmann M, Eng G, Ramanadham S, Moley K, Turk J. Male mice that do not express group VIA phospholipase A2 produce spermatozoa with impaired motility and have greatly reduced fertility. J Biol Chem. 2004;279:38194–38200. doi: 10.1074/jbc.M406489200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Washizaki K, Smith QR, Rapoport SI, Purdon AD. Brain arachidonic acid incorporation and precursor pool specific activity during intravenous infusion of unesterified [3H]arachidonate in the anesthetized rat. Journal of neurochemistry. 1994;63:727–736. doi: 10.1046/j.1471-4159.1994.63020727.x. [DOI] [PubMed] [Google Scholar]
- 38.Deutsch J, Rapoport SI, Purdon AD. Relation between free fatty acid and acyl-CoA concentrations in rat brain following decapitation. Neurochemical research. 1997;22:759–765. doi: 10.1023/a:1022030306359. [DOI] [PubMed] [Google Scholar]
- 39.Bazinet RP, Lee HJ, Felder CC, Porter AC, Rapoport SI, Rosenberger TA. Rapid high-energy microwave fixation is required to determine the anandamide (N-arachidonoylethanolamine) concentration of rat brain. Neurochemical research. 2005;30:597–601. doi: 10.1007/s11064-005-2746-5. [DOI] [PubMed] [Google Scholar]
- 40.Folch J, Lees M, Sloane Stanley GH. A simple method for the isolation and purification of total lipides from animal tissues. J Biol Chem. 1957;226:497–509. [PubMed] [Google Scholar]
- 41.Ma K, Langenbach R, Rapoport SI, Basselin M. Altered brain lipid composition in cyclooxygenase (COX)-2 knockout mouse. J Lipid Res. 2007 doi: 10.1194/jlr.M600400-JLR200. [DOI] [PubMed] [Google Scholar]
- 42.Adams ML, Sullivan DM, Smith RL, Richter EF. Evaluation of direct saponification method for determination of cholesterol in meats. J Assoc Off Anal Chem. 1986;69:844–846. [PubMed] [Google Scholar]
- 43.Deutsch J, Grange E, Rapoport SI, Purdon AD. Isolation and quantitation of long-chain acyl-coenzyme A esters in brain tissue by solid-phase extraction. Anal Biochem. 1994;220:321–323. doi: 10.1006/abio.1994.1344. [DOI] [PubMed] [Google Scholar]
- 44.Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 45.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(−Delta Delta C(T)) Method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 46.Lucas KK, Dennis EA. Distinguishing phospholipase A2 types in biological samples by employing group-specific assays in the presence of inhibitors. Prostaglandins Other Lipid Mediat. 2005;77:235–248. doi: 10.1016/j.prostaglandins.2005.02.004. [DOI] [PubMed] [Google Scholar]
- 47.Cohen J. A power primer. Psychol Bull. 1992;112:155–159. doi: 10.1037//0033-2909.112.1.155. [DOI] [PubMed] [Google Scholar]
- 48.Six DA, Dennis EA. The expanding superfamily of phospholipase A2 enzymes: classification and characterization. Biochim Biophys Acta. 2000;1488:1–19. doi: 10.1016/s1388-1981(00)00105-0. [DOI] [PubMed] [Google Scholar]
- 49.Chen CT, Liu Z, Ouellet M, Calon F, Bazinet RP. Rapid beta-oxidation of eicosapentaenoic acid in mouse brain: an in situ study. Prostaglandins Leukot Essent Fatty Acids. 2009;80:157–163. doi: 10.1016/j.plefa.2009.01.005. [DOI] [PubMed] [Google Scholar]
- 50.Igarashi M, DeMar JC, Jr, Ma K, Chang L, Bell JM, Rapoport SI. Docosahexaenoic acid synthesis from alpha-linolenic acid by rat brain is unaffected by dietary n-3 PUFA deprivation. J Lipid Res. 2007;48:1150–1158. doi: 10.1194/jlr.M600549-JLR200. [DOI] [PubMed] [Google Scholar]
- 51.Kim HW, Rao JS, Rapoport SI, Igarashi M. Dietary n-6 PUFA deprivation downregulates arachidonate but upregulates docosahexaenoate metabolizing enzymes in rat brain. Biochim Biophys Acta. 2011;1811:111–117. doi: 10.1016/j.bbalip.2010.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Rao JS, Ertley RN, DeMar JC, Jr, Rapoport SI, Bazinet RP, Lee HJ. Dietary n-3 PUFA deprivation alters expression of enzymes of the arachidonic and docosahexaenoic acid cascades in rat frontal cortex. Mol Psychiatry. 2007;12:151–157. doi: 10.1038/sj.mp.4001887. [DOI] [PubMed] [Google Scholar]
- 53.Murakami M, Kambe T, Shimbara S, Kudo I. Functional coupling between various phospholipase A2s and cyclooxygenases in immediate and delayed prostanoid biosynthetic pathways. J Biol Chem. 1999;274:3103–3115. doi: 10.1074/jbc.274.5.3103. [DOI] [PubMed] [Google Scholar]
- 54.Ong WY, Farooqui T, Farooqui AA. Involvement of cytosolic phospholipase A(2), calcium independent phospholipase A(2) and plasmalogen selective phospholipase A(2) in neurodegenerative and neuropsychiatric conditions. Curr Med Chem. 2010;17:2746–2763. doi: 10.2174/092986710791859289. [DOI] [PubMed] [Google Scholar]
- 55.Contreras MA, Greiner RS, Chang MC, Myers CS, Salem N, Jr, Rapoport SI. Nutritional deprivation of alpha-linolenic acid decreases but does not abolish turnover and availability of unacylated docosahexaenoic acid and docosahexaenoyl-CoA in rat brain. J Neurochem. 2000;75:2392–2400. doi: 10.1046/j.1471-4159.2000.0752392.x. [DOI] [PubMed] [Google Scholar]
- 56.DeMar JC, Jr, Ma K, Bell JM, Rapoport SI. Half-lives of docosahexaenoic acid in rat brain phospholipids are prolonged by 15 weeks of nutritional deprivation of n-3 polyunsaturated fatty acids. J Neurochem. 2004;91:1125–1137. doi: 10.1111/j.1471-4159.2004.02789.x. [DOI] [PubMed] [Google Scholar]
- 57.Golovko MY, Rosenberger TA, Feddersen S, Faergeman NJ, Murphy EJ. Alpha-synuclein gene ablation increases docosahexaenoic acid incorporation and turnover in brain phospholipids. J Neurochem. 2007;101:201–211. doi: 10.1111/j.1471-4159.2006.04357.x. [DOI] [PubMed] [Google Scholar]
- 58.De Franceschi G, Frare E, Bubacco L, Mammi S, Fontana A, de Laureto PP. Molecular insights into the interaction between alpha-synuclein and docosahexaenoic acid. Journal of molecular biology. 2009;394:94–107. doi: 10.1016/j.jmb.2009.09.008. [DOI] [PubMed] [Google Scholar]
- 59.Fedorova I, Hussein N, Di Martino C, Moriguchi T, Hoshiba J, Majchrzak S, Salem N., Jr An n-3 fatty acid deficient diet affects mouse spatial learning in the Barnes circular maze. Prostaglandins Leukot Essent Fatty Acids. 2007;77:269–277. doi: 10.1016/j.plefa.2007.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Calon F, Lim GP, Yang F, Morihara T, Teter B, Ubeda O, Rostaing P, Triller A, Salem N, Jr, Ashe KH, Frautschy SA, Cole GM. Docosahexaenoic acid protects from dendritic pathology in an Alzheimer's disease mouse model. Neuron. 2004;43:633–645. doi: 10.1016/j.neuron.2004.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Igarashi M, Gao F, Kim HW, Ma K, Bell JM, Rapoport SI. Dietary n-6 PUFA deprivation for 15 weeks reduces arachidonic acid concentrations while increasing n-3 PUFA concentrations in organs of post-weaning male rats. Biochim Biophys Acta. 2009;1791:132–139. doi: 10.1016/j.bbalip.2008.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ma Z, Turk J. The molecular biology of the group VIA Ca2+-independent phospholipase A2. Prog Nucleic Acid Res Mol Biol. 2001;67:1–33. doi: 10.1016/s0079-6603(01)67023-5. [DOI] [PubMed] [Google Scholar]
- 63.Bosetti F, Weerasinghe GR. The expression of brain cyclooxygenase-2 is down-regulated in the cytosolic phospholipase A2 knockout mouse. J Neurochem. 2003;87:1471–1477. doi: 10.1046/j.1471-4159.2003.02118.x. [DOI] [PubMed] [Google Scholar]
- 64.Bosetti F, Langenbach R, Weerasinghe GR. Prostaglandin E2 and microsomal prostaglandin E synthase-2 expression are decreased in the cyclooxygenase-2-deficient mouse brain despite compensatory induction of cyclooxygenase-1 and Ca2+-dependent phospholipase A2. J Neurochem. 2004;91:1389–1397. doi: 10.1111/j.1471-4159.2004.02829.x. [DOI] [PubMed] [Google Scholar]
- 65.Choi SH, Langenbach R, Bosetti F. Cyclooxygenase-1 and -2 enzymes differentially regulate the brain upstream NF-kappaB pathway and downstream enzymes involved in prostaglandin biosynthesis. J Neurochem. 2006;98:801–811. doi: 10.1111/j.1471-4159.2006.03926.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Basselin M, Villacreses NE, Langenbach R, Ma K, Bell JM, Rapoport SI. Resting and arecoline-stimulated brain metabolism and signaling involving arachidonic acid are altered in the cyclooxygenase-2 knockout mouse. J Neurochem. 2006;96:669–679. doi: 10.1111/j.1471-4159.2005.03612.x. [DOI] [PubMed] [Google Scholar]
- 67.Umhau JC, Zhou W, Carson RE, Rapoport SI, Polozova A, Demar J, Hussein N, Bhattacharjee AK, Ma K, Esposito G, Majchrzak S, Herscovitch P, Eckelman WC, Kurdziel KA, Salem N., Jr Imaging incorporation of circulating docosahexaenoic acid into the human brain using positron emission tomography. J Lipid Res. 2009;50:1259–1268. doi: 10.1194/jlr.M800530-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]