Abstract
Convergent evidence indicates that raphestriatal serotonin (5-HT) neurons can convert and release dopamine (DA) derived from exogenous administration of the pharmacotherapeutic L-3,4-dihydroxyphenyl-L-alanine(L-DOPA) as a treatment for Parkinson’s disease (PD). While aspects of such neuroplasticity may be beneficial, chronic L-DOPA may also modify native 5-HT function, precipitating the appearance prevalent non-motor PD symptoms such as anxiety and depression. To examine this, male Sprague-Dawley rats were rendered parkinsonian with bilateral medial forebrain bundle 6-OHDA infusions and treated for at least 28 days with vehicle or L-DOPA. In the first experiment, striatal, hippocampal, amygdalar, and prefrontal cortex DA and 5-HT levels were examined at various post-treatment time-points. In experiment 2, L-DOPA’s effects on DA and 5-HT cell bodies in the substantia nigra pars compacta and dorsal raphe, respectively, were examined. Finally, the effects of L-DOPA on affective behaviors were assessed in locomotor chambers, social interaction, forced swim, and elevated plus maze behavioral tests. Bilateral 6-OHDA lesion induced approximately 80% DA and 30% 5-HT depletion in the striatum compared to sham-lesioned controls, while monoamine levels remained largely unchanged in extrastriatal regions. Tissue levels of DA were increased at the expense of 5-HT levels in parkinsonian rats subjected to chronic L-DOPA injections in all regions sampled, though DA or 5-HT cell bodies were unaffected. Behaviorally, rats could only be tested 24 hours after their last L-DOPA injection due to severe dyskinesia. Despite this, prior exposure to chronic L-DOPA treatment exerted a pronounced anxiogenic phenotype. Collectively, these results suggest that chronic L-DOPA treatment may interfere with the balance of DA and 5-HT function in affect-related brain regions and could induce and/or exacerbate non-motor symptoms in PD.
Keywords: Dopamine, Serotonin, Parkinson’s disease, 6-OHDA, L-DOPA, Anxiety
Parkinson’s disease is a progressive neurodegenerative disorder that primarily affects dopamine (DA) neurons of the substantia nigra pars compacta (SNc). The loss of DA from nigral projections to the striatum leads to difficulty with movement, including bradykinesia and akinesia, rigidity, postural instability, and resting tremor. Non-motor symptoms including somnolence, present in gastric slowing, cognitive dysfunction, and changes in affect are also most patients and have significant effects on quality of life (Chaudhuri & Odin, 2010). DA replacement therapy with L-3,4-dihydroxyphenylalanine (L-DOPA) has been the gold standard for PD pharmacotherapy since the 1960s and this treatment initially imbues profound recovery in motor function (Obeso et al., 2000). However, L-DOPA offers little relief of non-motor symptoms, suggesting that the dopaminergic system is not exclusively involved in their manifestation (for review, see Eskow Jaunarajs et al., 2011).
Recently, serotonergic neurons have been shown to hyperinnervate the striatum following DA depletion in animal models of PD (Guerra et al., 1997; Rozas et al., 1998; Maeda et al.,2003, 2005). Functionally, serotonergic terminals also take up and convert exogenously administered L-DOPA to DA, releasing it into the striatum (Arai et al., 1995; Kannari et al., 2006; Yamada et al., 2007). Though 5-HT neuroplasticity may initially act as a beneficial alteration, DA released from striatal 5-HT terminals may act as a “false neurotransmitter”, unfettered by regulation via DA-sensitive autoreceptors and transporters (Carta et al., 2007). In addition, serotonergic neurons also extend prolific terminal branches to extrastriatal regions, such as the prefrontal cortex, hippocampus, and amygdala, where 5-HT serves a modulatory role in cognition, executive functions and affect (Jacobs & Azmitia. 1992).
The abundant serotonergic projections in extrastriatal sites may particularly lend themselves to excessive L-DOPA-induced swings in DA and may supersede 5-HT function following L-DOPA treatment. For example, preclinical investigations have suggested that hemi-parkinsonian rats chronically treated with L-DOPA exhibit reduced striatal and amygdalar 5-HT and 5-HIAA levels (Carta et al., 2007; Eskow Jaunarajs et al., 2010). In addition, Navailles and colleagues (2010a, 2011) recently observed reduced 5-HT and increased DA efflux in response to both acute and chronic L-DOPA treatment in the hippocampus and prefrontal cortex. Since such extrastriatal areas may lack sufficient DA regulatory systems (el Gemeyel et al., 1986) but do express DA receptors (Missale et al., 1998), supraphysiological swings in DA content also may induce functional alterations in neuronal activity. For example, L-DOPA treatment has been associated with detrimental effects on cognition (Gotham et al., 1988), impulsivity (Cools et al., 2003), and fluctuations in mood including depression and anxiety (Black et al., 2005; Richard et al., 2005; Kulisevsky et al., 2007). These findings and others ultimately challenge the traditional view of L-DOPA-induced dysfunction in PD as solely a motor phenomenon.
As such, the objective of the current research was to determine the effects of DA depletion and subsequent L-DOPA treatment on DA and 5-HT balance over time in affect-linked extrastriatal brain areas. In order to more adequately model the whole-brain modifications and behavioral changes that may occur due to these manipulations, we developed a novel bilateral rat model of PD. We expected that DA would be elevated in response to L-DOPA and would be associated with reduced 5-HT function in these areas. Due to the proposed link between psychiatric symptoms and monoamine dysfunction (Ressler & Nemeroff, 2000), the effects of DA depletion and subsequent chronic L-DOPA treatment on affective-like behaviors were also examined. We predicted that DA depletion would induce behavioral correlates of anxiety and depression, while L-DOPA treatment would further exacerbate these effects due to its modulation of DA and 5-HT function.
2. Experimental Procedure
2.1. Animals
Adult male Sprague-Dawley rats were used (225-250 g upon arrival; Taconic Farms, Hudson, NY, USA). Animals were housed in plastic cages (22 cm high, 45 cm deep and 23 cm wide) and had free access to standard lab chow (Rodent Diet 5001; Lab Diet, Brentwood, MO, USA) and water. The colony room was maintained on a 12/12 h light/dark cycle (lights on at 0700 hrs) at a temperature of 22-23° C. Animals were maintained in strict accordance with the guidelines of the Institutional Animal Care and Use Committee of Binghamton University and the “Guide for the Care and Use of Laboratory Animals” (Institute of Laboratory Animal Resources, National Academic Press 1996; NIH publication number 85-23, revised 1996).
2.2. Bilateral DA lesion surgeries
Rats (300-350 g) received bilateral sham or 6-hydroxydopamine (6-OHDA) lesions of the left medial forebrain bundle (MFB) to destroy DA neurons. Desipramine HCl (25 mg/kg, i.p.; Sigma, St. Louis, MO, USA) was given 30 min prior to the 6-OHDA injection to protect norepinephrine (NE) neurons. Rats were anesthetized with inhalant isoflurane (2-3%; Sigma) in oxygen (2.5 L/min), and then placed in a stereotaxic apparatus (David Kopf Instruments, Tujunga, CA, USA). The coordinates for 6-OHDA injections were AP: −1.8 mm, ML: ±2.0 mm, DV: −8.6 mm relative to bregma, with the incisor bar positioned 5.0 mm below the interaural line (Paxinos & Watson, 1998). Using a 10 μL Hamilton syringe attached to a 26 gauge needle, 6-OHDA (2 μg; Sigma) dissolved in 0.9% NaCl + 0.1% ascorbic acid was infused through a small burr hole in the skull at a rate of 2 μL/min for a total volume of 2 μL. The needle was withdrawn 5 min later and this process was repeated on the opposite side with a dose of 3 μg/2 μL. The side which received each concentration was counterbalanced throughout all studies. The methodology and concentration of neurotoxin used was determined by pilot studies, which revealed that a higher dose on the second side was necessary to achieve approximately equivalent DA depletion on both sides. All rats received injections of Buprenex (buprenorphine HCl; 0.03 mg/kg, i.p.; Reckitt Benckiser Pharmaceuticals Inc., Richmond, VA) as analgesic treatment. Post-surgery, rats were pair-housed and soft chow and fruit were provided as needed to facilitate recovery during the 3 weeks after surgery. Animals which were unable to feed themselves were hand-fed with chocolate nutritional supplement drink via an eye-dropper 2-3 times/day. Rats showing signs of dehydration received 10 mL saline (0.9% in distilled water, sc) 1-2 times/day. Mortality rate was approximately 13% (18/143 rats).
2.3. Experiment 1. Effects of DA depletion and chronic L-DOPA treatment on forebrain DA and 5-HT tissue levels
In order to determine the effects of chronic L-DOPA treatment on DA and 5-HT levels in the striatum, as well as extrastriatal areas that may be involved in affect, sham or DA-lesioned rats received either vehicle (0.9% NaCl containing 0.1% ascorbic acid) or L-DOPA methyl ester (L-DOPA; 12 mg/kg, sc; Sigma) + DL-Serine 2-(2,3,4-trihydroxybenzyl) hydrazide hydrochloride (benserazide; 15 mg/kg, sc; Sigma) once daily for at least 28 days beginning 3 weeks post-surgery (n=79). L-DOPA and benserazide were dissolved in Vehicle (0.9% NaCl containing 0.1% ascorbic acid) and administered at a volume of 1.0 ml/kg. Thus, 3 main groups were formed: 1) sham-lesioned, vehicle-treated (Sham-VEH), 2) 6-OHDA-lesioned, vehicle-treated (Lesion-VEH), and 3) 6-OHDA-lesioned, L-DOPA-treated (Lesion-LD). On day 28, Sham-VEH and Lesion-VEH rats were injected with vehicle 1 h prior to decapitation. Lesion-LD rats were treated with L-DOPA (12 mg/kg + benserazide 15 mg/kg, sc) and then killed at various time points (20 min, 1 h, 4 h, and 24 h) to determine a time-course of monoaminergic changes following L-DOPA treatment. These time points were chosen since L-DOPA typically results in functional increases in striatal, prefrontal cortex, amygdalar and hippocampal DA in approximately 20 min and these levels remain elevated for up to 4 h (Navailles et al., 2010, preliminary studies). Thus, in a between-subjects design, a total of 6 subgroups were formed: 1) Sham-VEH (n=18), 2) Lesion-VEH (n=17), 3) Lesion-LD killed 20 min after L-DOPA treatment (20 min; n=11), 4) Lesion-LD killed 1 h after L-DOPA treatment (1 h; n=12), 5) Lesion-LD killed 4 h after L-DOPA treatment (4 h; n=9), and 6) Lesion-LD killed 24 h after L-DOPA treatment (24 h; n=12). Following decapitation, brains were removed and striatum, prefrontal cortex, amygdala, hippocampus and dorsal raphe nucleus were microdissected and flash-frozen to −80°C. Forebrain tissue was subsequently analyzed via high-performance liquid chromatography with electrochemical detection (HPLC-ED).
2.3.1. HPLC-ED
Reverse-phase HPLC-ED was performed on striatal, prefrontal cortex, amygdala, and hippocampus tissue obtained from all rats included in the study homogenized in 300 uL of 0.3 M perchloric acid, according to the protocol of Kilpatrick et al. (1986), a method for semi-automated catecholamine and indoleamine analysis with coulometric detection, as reported previously (Eskow et al., 2009; Eskow Jaunarajs et al., 2010). Fifty uL of each sample was taken for analysis. The limit of detection was 10−10 M for the monoamines measured. The final oxidation current values were plotted on a standard curve of known concentrations from 10−6 M to 10−9 M, adjusted to respective tissue weights and expressed as picogram (pg) of monoamine or metabolite per milligram (mg) tissue. The average tissue weight varied based on structure in the striatum (5.9 mg), prefrontal cortex (2.9 mg), amygdala (4.9 mg), and hippocampus (8.2 mg) and was dissected by a trained individual. A 5-HT/DA ratio was also tabulated by dividing 5-HT tissue levels by DA tissue levels within each structure. A higher ratio compared to control levels (Sham-VEH) indicates an imbalance favoring 5-HT, while a lower ratio indicates a DA-favoring imbalance.
2.4. Experiment 2 Effects of bilateral DA depletion and chronic L-DOPA on TH and 5-HT immunopositive cell bodies
To determine whether chronic L-DOPA treatment altered cell bodies in the SNc or dorsal raphe following DA depletion, a separate group of rats received either L-DOPA (12 mg/kg) or vehicle for at least 28 days and were then anesthetized and transcardially perfused with ice-cold 4% paraformaldehyde (PFA) in 0.1 M phosphate buffered saline. Brains were removed and immersed in PFA for 48 h, then 30% sucrose for at least 1 week, and subsequently cut with a freezing, sliding microtome into 40 micron coronal sections. Immunohistochemistry for TH was determined in SNc sections (AP: −5.20 mm to −6.00 mm from bregma) and 5-HT was determined for dorsal raphe sections (AP: −7.30 mm to −8.30 mm from bregma). Respective tissue was exposed to either 1:500 mouse anti-TH primary antibody (Millipore, Darmstadt, Germany) or 1:200 rat anti-5-HT (Millipore) primary antibody overnight at 4°C following peroxidase quenching and blocking. After washing, sections were incubated in appropriate secondary antibody (biotinylated anti-mouse for TH and biotinylated anti-rat for 5-HT, 1:1000, Vector Laboratories), followed by exposure to avidin-biotin complex. Immunoreactivity was visualized using staining with 0.5% 3,3′-diaminobenzidine (DAB, Sigma Aldrich, USA).
2.4.1. Unbiased stereology
Unbiased stereology was performed on every third nigral and dorsal raphe section (6 sections/animal, 80 microns apart) to estimate total number of TH and 5-HT positive cell bodies, respectively. An image was captured at 5X magnification using a digital camera (Zeiss Plan-NEOFLUAR) attached to a Zeiss microscope (Axioscop 2-Plus). A region of interest was traced onto the 5X image using Stereo Investigator 8.0 software (Micro Bright Field, Williston, VT, USA) based on neuroanatomical markers obtained from a rat atlas (Paxinos & Watson, 1998). TH and 5-HT immunopositive cells were counted at 40X magnification (Zeiss Plan-NEOFLUAR) using a counting frame (125 um × 125 um) within a sampling grid (170 um × 170 um) by a blinded investigator. Due to section shrinkage due to immunostaining and mounting, a guard zone of 2 um and a dissector height of 20 um was used. Estimated total cells were automatically calculated according to the following equation by Stereo Investigator 8.0 software: N=ΣQ-(1/ssf)(1/asf)(1/hsf), where N is the total cell estimate, Q is the cells counted by hand in a particular animal, ssf is the sampling fraction (every third section, 1/3), asf is the ratio between sampling grid size and counting frame size, and hsf is the height sampling fraction (24 um/40 um).
2.5. Experiment 3: Effects of DA depletion and chronic L-DOPA treatment on affect-related behaviors
In order to determine the effects of chronic L-DOPA treatment on affective behaviors in bilaterally parkinsonian rats, sham and DA-lesioned rats received either vehicle (0.9% NaCl containing 0.1% ascorbic acid) or L-DOPA methyl ester (L-DOPA; 12 mg/kg, sc; Sigma) + benserazide (15 mg/kg, sc; Sigma) once daily for 75 days beginning 3 weeks after surgery (n=26). L-DOPA and benserazide were dissolved in Vehicle (0.9% NaCl containing 0.1% ascorbic acid) and administered at a volume of 1.0 ml/kg. Thus, 3 groups were formed in a between-subjects design: 1) sham-lesioned, vehicle-treated (Sham-VEH, n=9), 2) 6-OHDA-lesioned, vehicle-treated (Lesion-VEH, n=9), and 3) 6-OHDA-lesioned, L-DOPA-treated (Lesion-LD, n=8). After day 28 of treatment, animals were tested in locomotor chambers, social interaction with a novel conspecific, forced swim test, and the elevated plus maze to determine lesion and treatment effects on movement and affect-like behaviors 12-16 h after the previous daily treatment (OFF phase) in order to ensure that L-DOPA-induced dyskinesia did not interfere with the behavioral analyses. Each behavioral test was separated by at least 1 week. One week after the last behavioral test, rats were treated with either vehicle or L-DOPA, killed and tissue was extracted and analyzed via HPLC-ED as in Experiment 1 and combined with data from Experiment 1. No neurochemical differences were observed due to timing or prior exposure to behavioral testing.
2.5.1. Locomotor chambers
Locomotor activity testing was conducted in 6 identical acrylic chambers measuring 40 cm long, 40 cm wide and 30 cm high (Accuscan Instruments, Columbus, OH, USA) in a dimmed room. Each chamber was surrounded by a 15 × 15 infrared photocell array interfaced with a computer that ran the Versamax and Versadat programs, which tabulated and processed behaviors in the test field. Chambers were cleaned with 15% ethanol/water and new shavings were applied prior to each use. For the present experiment the variables examined included locomotor measures such as movement number (frequency) and vertical movements (frequency) and anxiety measures including center whole body entries (frequency) and center vertical movements (frequency) for ten 3 min periods (total of 30 min). Reduced movement number and vertical movements are indicative of a parkinsonian effect on motor activity. Reductions in activity within the center of the test field (reduced entries, vertical movements) are traditionally considered evidence of enhanced anxiety-like behavior and are sensitive to treatment with anxiolytics (Prut & Belzung, 2003; Eskow Jaunarajs et al., 2010).
2.5.2. Social interaction test
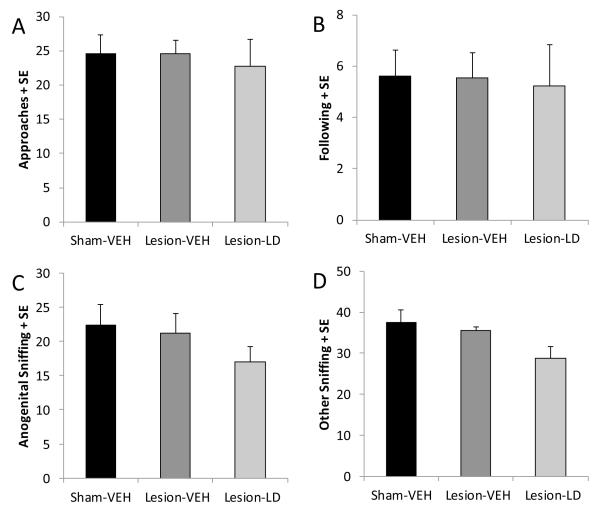
The social interaction test was used as a measure of anxiety-like behaviors in rats. The frequency of approaches and sniffing are sensitive to both anxiolytic and anxiogenic manipulations (File & Seth, 2003). Test and non-manipulated, novel conspecifics (stimulus rats) were habituated to a 25 cm × 40 cm chamber for 15 min in a darkened room. The next day, test rats were introduced to the chamber again with a stimulus rat and their interactions were recorded for 15 min via video camera onto recordable DVD. Recordings were later analyzed for frequency of approaches and anogenital or other sniffing by the test rat towards the stimulus rat. The behaviors most often associated with emotionality are approaches, following, anogenital sniffing and other sniffing of the test animal towards the stimulus animal.
2.5.3. Elevated plus maze
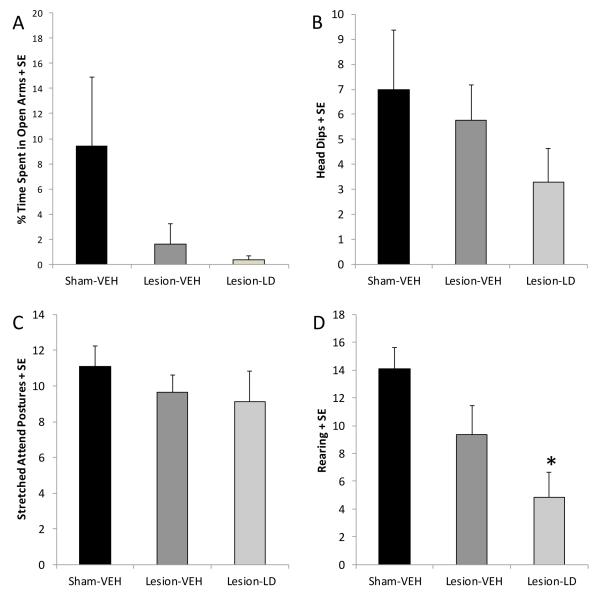
The elevated plus maze is a well-established measure of anxiety-like behaviors that is sensitive to anxiolytics and anxiogenic treatments (Hogg, 1996; Doremus et al., 2006). In the current study, an elevated plus maze (two 48.26 × 12.7 cm open arms and two 48.26 × 12.7 × 29.21 cm closed arms) with small plastic ledges (0.6 cm height) was used. The maze was located 50 cm from the floor and analyses were conducted under low light (3 lux). During each session, rats were placed into the center platform facing a closed arm. Five min sessions were videotaped by a camera mounted above the maze to allow testing without an experimenter in the room. After each session, the maze was cleaned with a 3% hydrogen peroxide solution. Videos were later analyzed for open and closed arm time, open and closed arm entries, head dips, stretched-attend postures and rearing behavior. An open and closed arm entry was considered an entry when all paws of the rat had entered the arm. Head dips were counted when the rat moved its head over the unprotected side of an open arm. Postures in which the rat completely stretched its body forward while keeping the hind legs stationary were considered to be stretched-attend postures. Rearing behavior was counted when the rat displayed a vertical movement where the front paws left the maze surface. Open arm entries, head dips, stretched-attend postures, and rearing are often considered as risky behaviors which are decreased under anxiogenic states, while closed arm entries are more often associated with overall activity (Doremus et al., 2006).
2.5.4. Modified forced swim test
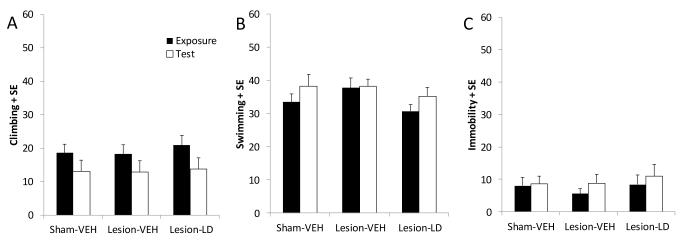
The modified forced swim test was used as a well-established measure of depressive behaviors in rats that is sensitive to both prodepressant and antidepressant medications and manipulations (Detke et al., 1995, Lucki, 1997). In the current study, rats were placed into a Plexiglass cylinder (45 cm × 20 cm) filled with 30 cm of warm water (25°C) and their responses recorded via video camera onto recordable DVD for 15 min. Twenty-four h later, rats were placed into the cylinder and their responses recorded for an additional 5 min. Recordings were later analyzed by a blinded, trained observer every 5 s for the prevailing behavior: climbing, swimming, or immobility. Climbing was defined as attempts to escape the chamber by struggling up the sides of the cylinder. Swimming was defined as mild paddling of the limbs around the cylinder and immobility as lack of limb movements (floating), except for those necessary to stay afloat. Increased incidence of immobility and reduced climbing is indicative of development of behavioral despair (Lucki, 1997).
2.6. Data analyses
Differences between groups on tissue levels of monoamines, metabolites and the 5-HT/DA ratio within each brain region were analyzed separately by between-subjects one-way ANOVAs. Post hoc comparisons were completed via Fisher LSD tests. For immunohistochemistry measures of TH and 5-HT immunoreactivity in the SNc and raphe, respectively, 2 (LESION: Sham or Lesion) × 2 (DAILY TX: VEH or L-DOPA) ANOVAs were completed and post-hoc comparisons were not necessary. Climbing, swimming and immobility in the forced swim test were analyzed via 2 (TIME: Test and Exposure, within ss) × 3 (GROUP: sham-VEH, lesion-VEH, lesion-LD, between ss) mixed model repeated measures ANOVA, as was arm time in the elevated plus maze. No post hoc comparisons were necessary for these measures. One-way between-subjects ANOVAs were also employed to analyze group differences in the frequency of behaviors in the elevated plus maze, social interaction, and locomotor chambers. Fisher LSD post-hocs were used to determine significant group differences. In all cases, alpha was set at p<0.05 and statistical analyses were conducted with Statistica 7 (Statsoft, Inc., Tulsa, OK, USA).
3. Results
3.1. Bilateral DA lesions and chronic L-DOPA treatment alter DA and 5-HT tissue levels within the striatum, amygdala, hippocampus, and prefrontal cortex
Tissue samples from distinct forebrain regions were microdissected and analyzed via HPLC-ED for levels of monoamines in order to determine the effects of bilateral 6-OHDA lesion and a time course of DA/5-HT changes following chronic L-DOPA treatment. Lesioned rats with <50% lesion of striatal DA compared to sham-lesioned rats were removed from all neurochemical analyses (n=8 out of 79 animals, ~90% success rate). Since no significant differences based on hemisphere were detected, right and left data were averaged to determine group differences and are reported in Figure 1.
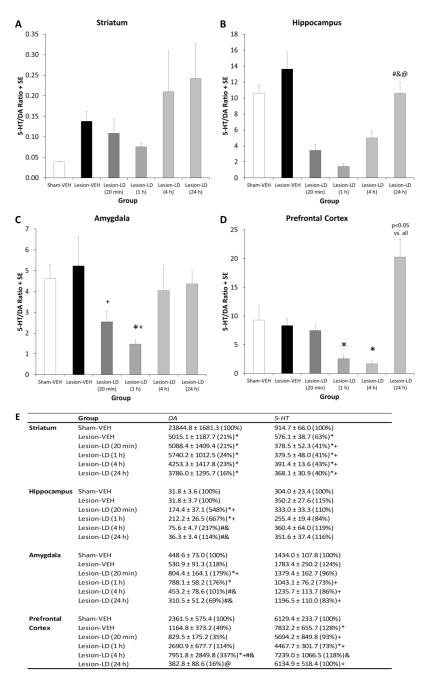
Figure 1.
Effects of bilateral sham or 6-OHDA lesion and chronic daily L-DOPA (12 mg/kg + benserazide, 15 mg/kg, sc; LD) on 5-HT/DA ratio at various time points post-L-DOPA administration. Animals were treated for 28-75 days and then killed at 20 min, 1 h, 4 h and 24 h after their respective treatments. Bars show the effects of group on tissue 5-HT/DA ratio in the (A) striatum, (B) hippocampus, (C) amygdala, and (D) prefrontal cortex. Average values from which the 5-HT/DA ratio was calculated are located in (E). Values are picogram monoamine or metabolite per milligram wet tissue weight or ratios of metabolite to monoamine (mean ± SE) with percent of Sham-VEH group in parentheses. Main effects of group were established by one-way ANOVA and subsequent Fisher LSD post hocs determined differences between groups [*p<0.05 vs. Sham-VEH, +p<0.05 vs. Lesion-VEH, #p<0.05 vs. Lesion-LD (20 min), @p<0.05 vs. Lesion-LD (1 h)].
Bilateral DA lesions with intra-MFB 6-OHDA infusion resulted in a >75% reduction in striatal tissue DA content compared to sham-lesioned controls, confirmed by one-way ANOVA (F5,65=34.08, p<0.05). As expected from previous findings (Carta et al., 2007; Eskow Jaunarajs et al., 2010), all DA-lesioned groups showed equivalent striatal DA depletion and were not altered at any time point by L-DOPA treatment (all p>0.05). In the hippocampus, amygdala, and prefrontal cortex, DA levels were not affected by bilateral DA lesions but were altered by L-DOPA treatment (F5,64=19.62, F5,64=3.82, F5,63=6.24, respectively; all p<0.05). Hippocampal DA was increased 548% and 667% at the 20 min and 1 h time points post-L-DOPA treatment respectively, while values approached control levels at the 4 h and 24 h time points (all p<0.05). Similar results were found in the amygdala, with DA increases induced by L-DOPA treatment approaching 200% compared to sham-lesioned rats. Interestingly, prefrontal cortex DA levels did not rise until the 4 h post-L-DOPA treatment in DA lesioned rats (p<0.05 vs. all other groups).
Bilateral DA lesions and L-DOPA treatment also appeared to influence striatal 5-HT levels as one-way ANOVA detected significant changes in striatal 5-HT (F5,65=20.11, p<0.05). Bilateral DA lesions alone reduced striatal 5-HT levels by 37% compared to sham-lesioned controls (p<0.05). L-DOPA treatment exacerbated this loss regardless of time point examined by approximately 60% (all p<0.05). While no changes in hippocampal 5-HT were observed, there were significant changes in 5-HT levels within the amygdala and prefrontal cortex based on group (F5,65=2.41 and F5,63=6.24, both p<0.05). Post hoc analyses revealed no changes based on bilateral DA lesion alone compared to sham-lesioned controls for amygdala 5-HT, though a slight upward trend was discerned. As such, decreases in amygdalar 5-HT were observed following L-DOPA treatment at all time points (all p<0.05), except 20 min post-L-DOPA when levels were comparable to Lesion-VEH. Prefrontal cortex 5-HT was enhanced due to DA lesion, but the effects of L-DOPA treatment were more complex. At the 20 min time point, 5-HT levels were attenuated by L-DOPA treatment compared to Lesion-VEH rats, with further reductions observed at the 1 h time point (all p<0.05). 5-HT levels returned to control levels after 4 and 24 h post-L-DOPA treatment.
A 5-HT/DA ratio was also calculated in order to determine changes in the balance of these neurotransmitters following lesion and/or treatment. In comparison to control levels (Sham-VEH), a larger 5-HT/DA ratio would be indicative of an imbalance which favors 5-HT, while a reduction in this ratio would signify an imbalance in favor of DA. No alteration in the striatal 5-HT/DA ratio was detected by one-way ANOVA (p>0.05; Figure 1A). However, within the hippocampus (Figure 1B; F5,62=10.70, p<0.05), imbalances in favor of DA were observed at all time points post-L-DOPA treatment, except the 24 h time point (p<0.05). A main effect of group was also determined for amygdalar 5-HT/DA ratio (F5,61=2.51, p<0.05). Again, no effect of DA lesion alone was detected, though significant reductions in the 5-HT/DA ratio were observed at 20 min with further reductions at the 1 h time point (Figure 1C; both p<0.05). In the prefrontal cortex, a significant main effect was driven by L-DOPA treatment but not DA lesion (Figure 1D; F5,55=7.22, p<0.05). Changes due to L-DOPA treatment were time-dependent: a decreased 5-HT/DA ratio was observed at 1 h and 4 h post-L-DOPA treatment, but by the 24 h time point the imbalance switched in favor of 5-HT.
3.2. L-DOPA treatment does not affect nigral DA neurons or raphe 5-HT neurons, though DA and 5-HT neurons are impaired by bilateral MFB 6-OHDA infusion
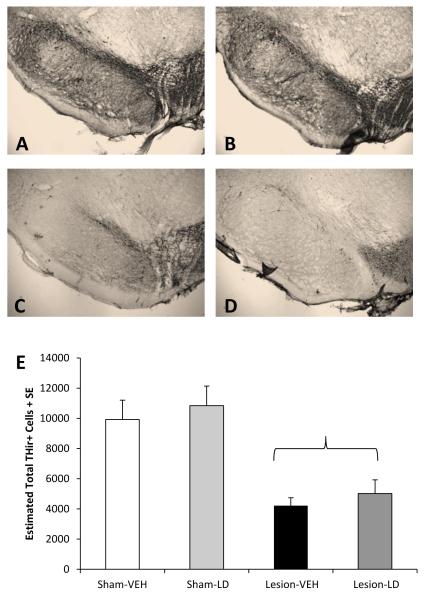
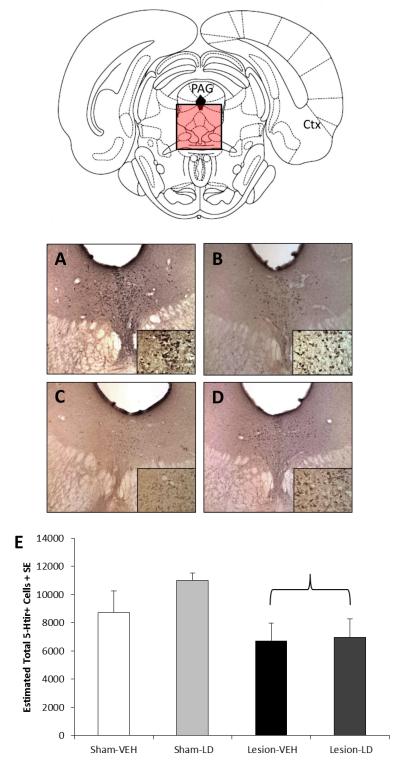
Nigral DA lesion was verified by immunohistochemistry for TH, the rate-limiting enzyme for DA synthesis. Figure 2 shows representative 5X optical zoom photographs of SNpc in each group and group means of estimated total number of THir cells as determined by non-biased stereology (mean coefficient of error=0.04). Two rats were removed from the Lesion-LD group due to low lesion severity (<10% loss of TH immunopositive neurons compared to Sham-VEH). DA lesioned rats exhibited approximately 50% fewer TH-positive neurons in the SNc compared to sham-lesioned rats (F1,19=38.85, p<0.05). Neither a daily treatment effect nor a lesion × treatment interaction was observed (all p>0.05). Bilateral MFB 6-OHDA lesions resulted in a significant reduction (22%) in number of 5-HT immunopositive neurons in the dorsal raphe nucleus compared to Sham-VEH rats (Figure 3; F1,19=11.81, p<0.05) as determined by non-biased stereology (mean coefficient of error=0.05). No additional alterations were observed as a result of daily L-DOPA treatment, nor was there an interaction between lesion and treatment (both p>0.05).
Figure 2.
Effects of bilateral sham or 6-OHDA lesion and chronic daily treatment with vehicle or L-DOPA (12 mg/kg + benserazide, 15 mg/kg, sc; LD) on THir+ cells in the SNc. Bars show the effects of group on estimated total of nigral THir+ cells as determined by unbiased stereology. Differences between groups were determined by 2-way ANOVAs for lesion and treatment. The parenthesis symbolizes a main effect of lesion (p<0.05 vs. Sham). Example photos of the SNc at 5X and 40X (inset) optical zoom for (A) Sham-VEH, (B) Sham-LD, (C) Lesion-VEH, and (D) Lesion-LD are also shown.
Figure 3.
Effects of bilateral sham or 6-OHDA lesion and chronic daily treatment with vehicle or L-DOPA (12 mg/kg + benserazide, 15 mg/kg, sc; LD) on 5-HTir+ cells in the dorsal raphe nucleus. Bars show the effects of group on estimated total of dorsal raphe 5-HTir+ cells as determined by unbiased stereology. Differences between groups were determined by 2-way ANOVAs for lesion and treatment. The parenthesis symbolizes a main effect of lesion (p<0.05 vs. Sham). Example photos at 5X and 40X (inset) optical zoom of the dorsal raphe for (A) Sham-VEH, (B) Sham-LD, (C) Lesion-VEH, and (D) Lesion-LD are also shown.
3.3. Bilateral DA lesions and chronic L-DOPA treatment induce anxiety but not depression-like behavioral changes
3.3.1. Locomotor chambers
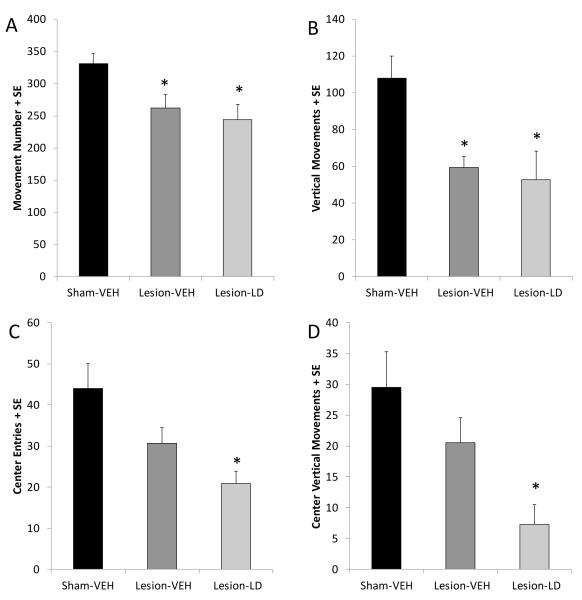
Due to the development of mild to severe dyskinesias, rats were tested in the OFF state 16-24 h after their last L-DOPA treatment to ensure their ability to perform behavioral tasks. In the current study, locomotor chambers were used to inform about the effect of lesion and prior exposure to L-DOPA treatment on overall movement and anxiety-like symptoms. As expected, a main effect of group in movement number and vertical movements was observed (Figure 4A and B, respectively; F2,22=6.00 and F2,22=7.09, both p<0.05). Post hoc analyses revealed that bilateral 6-OHDA lesion significantly reduced the frequency of movement bouts and vertical movements compared to sham lesions (p<0.05) by approximately 50%. Importantly, there was no difference on either measure between Lesion-VEH and Lesion-LD groups in this measure (both p>0.05). There was a main effect of group on center entries (Figure 4C; F2,21=6.00, p<0.05) and center vertical movements (Figure 4D; F2,21=5.69, p<0.05). Post hoc tests revealed that only DA-lesioned rats receiving chronic L-DOPA showed reductions in center entries (p<0.05). Lesion-L-DOPA rats also displayed an attenuation in center vertical movements compared to sham-lesioned groups (p<0.05), with a strong trend towards reduced center vertical movements compared to Lesion-VEH rats (p=0.06). These reductions cannot be explained by less overall motor activity as there was no difference between DA-lesioned groups in movement number or vertical movements. Thus, changes in center activity are likely attributable to the anxiogenic effect of chronic L-DOPA treatment.
Figure 4.
Effects of bilateral MFB 6-OHDA and subsequent chronic L-DOPA (12 mg/kg, + benserazide, 15 mg/kg, sc; LD) on motor and anxiety-like behaviors in the locomotor chambers. Bars denote the effect of group on (A) movement number, (B) overall vertical movement number, (C) center entries, and (D) center vertical movements. Group effects were determined by one-way ANOVAs and Fisher LSD post hocs were used to analyze group differences (*p<0.05 vs. Sham-VEH).
3.3.2. Social interaction
Reduced interaction with a novel conspecific is also indicative of an anxiogenic effect of treatment and/or experimental manipulation (File & Seth, 2003). In the current study, there were no significant effects of either bilateral DA lesions or chronic L-DOPA treatment in DA lesioned rats on any measure (Figure 5A-D; all p>0.05).
Figure 5.
Effects of bilateral MFB 6-OHDA and subsequent chronic L-DOPA (12 mg/kg, + benserazide, 15 mg/kg, sc; LD) on anxiety-like behaviors in the social interaction test with a novel conspecific. Bars denote the effect of group on (A) approaches, (B) following, (C) anogenital sniffing, and (D) other sniffing of the test rat towards the stimulus rat. Group effects were determined by one-way ANOVAs.
3.3.3. Elevated plus maze
As shown in Figure 6A, rats in the current study showed no differences in overall activity as closed arm entries were equivalent between groups (F2,22=1.35, ns). However, rats in all groups spent the 5 min test period almost exclusively in the closed arms of the elevated plus maze (F1,22=261.95, p<0.05). Thus, no group differences were observed in time spent in either the closed arms or open arms (F2,22=0.28, ns). Though no differences between groups in head dips (Figure 6B) and stretched-attend postures (Figure 6C) were detected, the frequency of rearing behavior in the maze was reduced by chronic L-DOPA treatment in DA-lesioned rats compared to sham-lesioned rats (Figure 6D; p<0.05). There was also a trend signifying a reduction in rears in Lesion-LD rats compared to Lesion-VEH rats (p=0.07), suggesting a treatment dependent alteration in some measures of anxiety-like behaviors in the elevated plus maze.
Figure 6.
Effects of bilateral MFB 6-OHDA and subsequent chronic L-DOPA (12 mg/kg, + benserazide, 15 mg/kg, sc; LD) on anxiety-like behaviors in the elevated plus maze. (A) Time spent in the open versus closed arms are denoted by black and white bars, respectively. Bars denote the effect of group on frequency of (B) head dips, (C) stretched-attend postures, and (D) rearing within the maze. Group effects were determined by one-way ANOVAs and Fisher LSD post-hocs were used to analyze specific group differences (*p<0.05 vs. Sham-VEH).
3.3.4. Forced swim test
The forced swim test was employed in the current study as a behavioral measure sensitive to both antidepressant and depressogenic treatments (Lucki, 1997). Depicted in Figure 7A, a two-way ANOVA revealed an effect of test day (Exposure vs. Test) on climbing behavior (F1,23=18.84, p<0.05), but no effect of group and no interaction. For swimming and immobility behaviors, no significant differences were observed based on group or test day, nor were any interactions detected (Figure 7B and 7C, respectively; ns). Collectively, results of the forced swim test showed no depressogenic effects of either bilateral DA depletion or chronic L-DOPA treatment.
Figure 7.
Effects of bilateral MFB 6-OHDA and subsequent chronic L-DOPA (12 mg/kg, + benserazide, 15 mg/kg, sc; LD) on depressive-like behaviors in the forced swim test. Frequency of (A) climbing, (B) mild swimming, and (C) immobility were determined during the first 5 min of exposure and the 5 min of testing 24 h later as shown by the black and white bars, respectively. Group effects were determined by two-way repeated measures ANOVAs.
4. Discussion
The current results provide insight into the consequences of DA depletion and subsequent L-DOPA therapy on both neurochemistry and behavior associated with motor and affective symptoms in a bilateral MFB 6-OHDA rat model of PD. We determined that chronic L-DOPA treatment increased DA at the expense of 5-HT levels in forebrain areas. These serotonergic deficits persisted even 24 h after L-DOPA administration in parkinsonian rats, leading to an imbalance in 5-HT and DA levels in the hippocampus, prefrontal cortex, and amgydala. These changes were not due to any effect of chronic L-DOPA treatment on the neuronal viability of SNpc or dorsal raphe monoaminergic neurons. In addition, a long-term anxiogenic effect of chronic L-DOPA treatment was observed in parkinsonian rats. The present findings show novel evidence of a potential link between DA/5-HT dysfunction and anxiety-like symptoms in a bilateral rat model of PD, which may contribute to the high prevalence of affective disorders in PD patients.
Bilateral MFB 6-OHDA lesions have been an elusive goal for the PD research field. While bilateral intracerebroventricular, intranigral, and striatal 6-OHDA lesions have been well-established (Deumens et al., 2002), development of viable MFB lesions has been hindered by high subject mortality and lesion variability. Such inconsistency appears to be due to the small volume and concentration of the injection (Paille et al., 2007). Indeed, even slight alterations in concentration have been found to profoundly impact both lesion severity and survival rate (personal observations; Smith et al., 2002; Truong et al., 2006). While the current lesion protocol also resulted in somewhat variable lesion severity (ranging from 55-99% depletion from sham-lesioned controls), approximately 70% of DA-lesioned rats had over 70% striatal DA depletion. Such levels are typically indicative of a moderate DA lesion comparable to the early stages of symptomatic PD, as patients often do not present with clinical symptoms until over 70% of DA neurons are lost (Fearnley & Lees, 1991).
In the current study, bilateral DA lesions led to reductions in both striatal DA content (Figure 1) and SNc TH immunoreactivity (Figure 2). However, DA levels in the hippocampus, amygdala, and prefrontal cortex were no different than sham-lesioned controls and may reflect differential innervation from SNc versus the ventral tegmental area (VTA), which is typically less affected by MFB 6-OHDA lesions and PD neuropathology (Kish et al., 1988; Fearnley & Lees, 1991). Mild, but significant deficits in striatal 5-HT (Figure 1) and dorsal raphe 5-HT immunoreactivity (Figure 3) were also observed due to bilateral MFB DA lesion. The pronounced DA loss and mild 5-HT depletion observed is a unique feature of this lesion protocol and may more accurately model the human condition than existing neurotoxin models (Deumens et al., 2002; Kish et al., 2008). The 5-HT reduction could be driven via indirect effects on serotonergic function as a result of loss of excitatory dopaminergic influences (Di Giovanni et al., 2008; Guiard et al., 2008) or damage during 6-OHDA injection since serotonergic fibers project via the MFB to innervate forebrain areas, including the striatum (Wallman et al., 2011). However, serotonergic projections to the amgydala, hippocampus, and prefrontal cortex also travel through the MFB and no serotonergic deficit was observed in these areas (Figure 1). In fact, elevations in 5-HT content were found in some extrastriatal regions. Increased 5-HT in affect-related brain areas in response to DA cell loss may signal compensatory hyperinnervation, as has been shown in the striatum following 6-OHDA lesions, though this remains speculative (Maeda et al., 2005).
The effects of chronic L-DOPA on striatal DA and 5-HT have been characterized previously in hemiparkinsonian rats (Carta et al., 2007; Eskow et al., 2007; Zeng et al., 2010; Rylander et al., 2010). However, investigations of the extrastriatal changes in DA and 5-HT balance have been limited and have not been investigated in bilateral models. In the current study, chronic L-DOPA did not appear to promote any significant organizational changes on DA tone within any structure nor did it alter striatal DA at any time point post-injection (Carta et al., 2007; Eskow et al., 2007, 2009), likely due to inherent dopaminergic metabolic activity within this DA-rich brain region. In contrast, DA levels were time-dependently increased by an acute injection of L-DOPA in non-motor regions. Surges in DA content were observed in the hippocampus, amygdala, and prefrontal cortex compared to both sham and 6-OHDA-lesioned controls as soon as 20 min after L-DOPA treatment. DA tissue levels remained elevated in all structures for several hours. “Overdosing” of limbic structures after L-DOPA treatment has been suggested to occur in the prefrontal cortex and amygdala in human PD patients (Cools, 2006; Delaveau et al., 2009) and could stem from largely intact mesolimbic DA projections. However, it is intriguing that the hippocampus region, known for scant DA concentrations stemming from the VTA and SNc (Gasbarri et al., 1997), showed the largest and most prolonged increase in tissue DA levels following L-DOPA treatment. Of note in this context, these areas do have resplendent serotonergic innervation. In support, Navailles and colleagues (2010b) observed that L-DOPA-induced increases in DA in microdialysate from the hippocampus and prefrontal cortex of unilaterally DA-lesioned rats were blocked by serotonergic lesions, suggesting a raphe-dependent mechanism. As such, it is likely that the extensive serotonergic inputs to extrastriatal structures were responsible for the L-DOPA-induced rise in DA levels.
In conjunction with increases in DA, chronic L-DOPA treatment in bilaterally DA-lesioned rats induced deficits in 5-HT content in the striatum, amygdala, and prefrontal cortex (Figure 1). While no changes in 5-HT tissue levels were observed in the hippocampus, a significant reduction in the 5-HT metabolite, 5-HIAA was found in rats with chronic L-DOPA treatment (data not shown). The reduced 5-HT function found even 24 h after the previous L-DOPA injection could reflect a perseverative deficit in 5-HT function. The current findings also corroborate observations from other groups. In intact rats, chronic L-DOPA treatment induced increases in DA and consequent decreases in 5-HT tissue content in widespread brain regions, including the hippocampus and raphe nuclei (Borah & Mohanakumar, 2007). L-DOPA treatment has been shown to exert a growth-promoting effect on 5-HT axon terminals in rat models and non-human primate models of LID and could be a compensatory change due to L-DOPA-induced 5-HT dysfunction (Rylander et al., 2010; Zeng et al., 2010). Navailles and colleagues (2010a,b, 2011) recently presented a series of studies determining that 5-HT neurons release DA as a “false neurotransmitter” and that L-DOPA treatment impairs 5-HT levels and extracellular release in the hippocampus and prefrontal cortex of hemiparkinsonian rats. A mechanism whereby L-DOPA-derived DA displaces 5-HT from its vesicles could explain the decrease in 5-HT content after chronic L-DOPA treatment (Carta et al., 2007). In addition, Kuhn and Arthur (1998, 1999) found that in vitro exposure of the rate limiting enzyme for 5-HT synthesis, tryptophan hydroxylase, to L-DOPA-quinones results in inactivation of the catalytic core of the TPH enzyme, substantially reducing 5-HT synthesis. Interestingly, raphe tryptophan hydroxylase levels an in vivo model of PD were also shown to be impaired by L-DOPA treatment (Eskow Jaunarajs et al., 2011) and may be one mechanism whereby L-DOPA inhibits 5-HT function. Studies addressing the mechanisms and outcomes of such imbalances in DA/5-HT extrastriatal areas have been limited, despite the reality that monoaminergic imbalances have been implicated in the development of deleterious changes in affect which may be manifested in PD.
Investigations into DA lesion-induced anxiety and depression-like behaviors have provided variable results (Branchi et al., 2008; Tadaiesky et al., 2008; Winter et al., 2007). Our laboratory recently established that severe (>95%) unilateral 6-OHDA lesions increased anxiety and depression-like symptoms (Eskow Jaunarajs et al., 2010), supporting a role for DA loss in the onset of affective disorders in PD. Such results may be dependent upon extent of DA lesion, as no effects on affective behaviors were observed in rats with more moderate DA depletion (~85%) in the current study. Due to the development of severe dyskinesias in approximately 50% of animals, the current studies were not able to determine the effects of L-DOPA treatment during its ON phase. Though some research suggests that L-DOPA treatment during the ON phase may improve anxiety and depression symptoms in animal models (Winter et al., 2007), no lasting benefit of L-DOPA was found here during the OFF phase and corroborates previous findings in unilateral, 6-OHDA-lesioned rats (Eskow Jaunarajs et al., 2010). More specifically, chronic L-DOPA treatment appeared to exacerbate anxiety-like behaviors (Figures 4-7). Such changes were observed despite no differences in overall locomotor activity between parkinsonian rats with daily vehicle or L-DOPA treatment (Figure 4A and 4B). In corroboration, L-DOPA treatment in PD patients can induce detrimental fluctuations in mood and anxiety in the OFF phase that do not necessarily correlate with motor fluctuations (Richard et al., 2005; Kulisevsky et al., 2007). As such, these results suggest that chronic L-DOPA may exert an anxiogenic effect that is not explained solely by DA loss and could be linked to the imbalance in DA/5-HT induced by chronic L-DOPA treatment.
Repeated oscillations in DA and 5-HT levels in forebrain areas due to daily L-DOPA treatment in PD undoubtedly change brain neurocircuitry and reactivity to stressful situations. For example, DA and 5-HT exert neuromodulatory influences on the prefrontal cortex-amygdala loop which has been implicated in the development of appropriate behavioral responses to stress and fear-inducing stimuli. It is thought that the amygdala is responsible for incurring anxiogenic behavioral responses and that the frontal cortex provides an inhibitory influence on amygdalar output (Grace & Rosenkranz, 2002). Rosenkrantz and Grace (2001) established a particular role for DA in this effect and other findings have shown that enhanced DA receptor activation of both D1 and D2 receptors in the amygdala may underlie anxiety (de la Mora et al., 2010; Benanej et al., 2011). In addition, serotonergic receptors have been implicated in the manifestation of anxiety-like behaviors in rats (Campbell & Merchant, 2003; Christianson et al., 2010), presumably by exerting tonic inhibitory activity on dopaminergic neurons (for review see Millan, 2005). This and the long-established understanding that alterations in 5-HT function are linked to the development of affective symptoms (Ressler & Nemeroff, 2000), collectively suggest a primary role for both DA and 5-HT in the expression of anxiety.
The current set of experiments established the neurochemical and behavioral effects of chronic L-DOPA treatment in a novel bilateral rat model of PD. Profound and long-lasting imbalances of 5-HT and DA levels were found in extrastriatal brain areas following chronic L-DOPA treatment. Though the mechanism whereby L-DOPA treatment alters serotonergic function remains unknown, it is clear that these changes likely have important implications for PD patients. Due to the nature of L-DOPA treatment in PD patients whereby dosages are taken at 4-6 h intervals throughout the day, 5-HT and DA levels may never return to baseline levels and could result in a persistent hyperdopaminergic/hyposerotonergic state. In the current study, these changes corresponded to an exacerbation of anxiety-like behaviors in parkinsonian rats. The potential link between DA/5-HT imbalance and increased anxiety-like behavior in the current study may aid in the discovery of effective treatments for anxiety and depression in PD, ultimately providing clues towards the determination of the underlying neurobiology of affective disorders in the general population.
Highlights.
A novel bilateral rat model of Parkinson’s disease resulted in loss of approximately 80% striatal DA
Chtonic L-DOPA induced time-dependent imbalances in DA/5-HT levels in extrastriatal brain areas
Prior exposure to L-DOPA did not damage DA and 5-HT neurons
Anziogenic effects of chronic L-DOPA were observed in bilateral-lesioned rats during the OFF phase.
Abbreviations
- PD
Parkinson’s disease
- DA
dopamine
- 5-HT
serotonin
- L-DOPA
L-3,4-dihydroxyphenylalanine
- SNpc
substantia nigra pars compacta
- 6-OHDA
6-hydroxydopamine
- MFB
medial forebrain bundle
- NE
norepinephrine
- VEH
vehicle
- LD
L-DOPA
- HPLC-ED
high-performance liquid chromatography with electrochemical detection
- pg
picogram
- mg
milligram
- PFA
paraformaldehyde
- TH
tyrosine hydroxylase
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Arai R, Karasawa N, Geffard M, Nagatsu I. L-DOPA is converted to dopamine in serotonergic fibers of the striatum of the rat: a double-labeling immunofluorescence study. Neurosci Lett. 1995;195:195–198. doi: 10.1016/0304-3940(95)11817-g. [DOI] [PubMed] [Google Scholar]
- Black KJ, Hershey T, Hartlein JM, Carl JL, Perlmutter JS. Levodopa challenge neuroimaging of levodopa-related mood fluctuations in Parkinson’s disease. Neuropsychopharmacol. 2005;30:590–601. doi: 10.1038/sj.npp.1300632. [DOI] [PubMed] [Google Scholar]
- Benanej M, Karimi-Sori A, Zarrindast MR, Ahmadi S. D1 and D2 dopaminergic systems in the rat basolateral amygdala are involved in anxiogenic-like effects induced by histamine. J Psychopharmacol. 2011 doi: 10.1177/0269881111405556. in press. [DOI] [PubMed] [Google Scholar]
- Borah A, Mohanakumar KP. Long-term L-DOPA treatment causes indiscriminate increase in dopamine levels at the cost of serotonin synthesis in discrete brain regions of rats. Cell Mol Neurobiol. 2007;27:985–996. doi: 10.1007/s10571-007-9213-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Branchi I, D’Andrea I, Armida M, Cassano T, Pezzola A, Potenza RL, Morgese MG, Popoli P, Alleva E. Nonmotor symptoms in Parkinson’s disease: investigating early-phase onset of behavioral dysfunction in the 6-hydroxydopamine-lesioned rat model. J Neurosci Res. 2008;86:2050–2061. doi: 10.1002/jnr.21642. [DOI] [PubMed] [Google Scholar]
- Campbell BM, Merchant KM. Serotonin 2C receptors within the basolateral amygdala induce acute fear-like responses in an open-field environment. Brain Res. 2003;993:1–9. doi: 10.1016/s0006-8993(03)03384-5. [DOI] [PubMed] [Google Scholar]
- Carta M, Carlsson T, Kirik D, Bjorklund A. Dopamine released from 5-HT terminals is the cause of L-DOPA-induced dyskinesia in parkinsonian rats. Brain. 2007;130:1819–1833. doi: 10.1093/brain/awm082. [DOI] [PubMed] [Google Scholar]
- Chaudhuri KR, Odin P. The challenge of non-motor symptoms in Parkinson’s disease. Prog Brain Res. 2010;184:325–341. doi: 10.1016/S0079-6123(10)84017-8. [DOI] [PubMed] [Google Scholar]
- Christianson JP, Ragole T, Amat J, Greenwood BN, Strong PV, Paul ED, Fleshner M, Watkins LR, Maier SF. 5-hydroxytryptamine 2C receptors in the basolateral amygdala are involved in the expression of anxiety after uncontrollable traumatic stress. Biol Psychiatry. 2010;67:339–345. doi: 10.1016/j.biopsych.2009.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cools R, Barker RA, Sahakian BJ, Robbins TW. L-Dopa medication remediates cognitive inflexibility, but increases impulsivity in patients with Parkinson’s disease. Neuropsychologia. 2003;41:1431–1441. doi: 10.1016/s0028-3932(03)00117-9. [DOI] [PubMed] [Google Scholar]
- Cools R. Dopaminergic modulation of cognitive function-implications for L-DOPA treatment in Parkinson’s disease. Neurosci Biobehav Rev. 2006;30:1–23. doi: 10.1016/j.neubiorev.2005.03.024. [DOI] [PubMed] [Google Scholar]
- de la Mora MP, Gallegos-Cari A, Arizmendi-Garcia Y, Marcellino D, Fuxe K. Role of dopamine receptor mechanisms in the amygdaloid modulation of fear and anxiety: Structural and functional analysis. Prog Neurobiol. 2010;90:198–216. doi: 10.1016/j.pneurobio.2009.10.010. [DOI] [PubMed] [Google Scholar]
- Delaveau P, Salgado-Pineda P, Witjas T, Micallef-Roll J, Fakra E, Azulay JP, Blin O. Dopaminergic modulation of amygdala activity during emotion recognition in patients with Parkinson disease. J Clin Psychopharm. 2009;29:548–554. doi: 10.1097/JCP.0b013e3181bf1c5f. [DOI] [PubMed] [Google Scholar]
- Detke MJ, Rickels M, Lucki I. Active behaviors in the rat forced swimming test differentially produced by serotonergic and noradrenergic terminals. Psychopharm (Berlin) 1995;121:66–72. doi: 10.1007/BF02245592. [DOI] [PubMed] [Google Scholar]
- Deumens R, Blokland A, Prickaerts J. Modeling Parkinson’s disease in rats: an evaluation of 6-OHDA lesions of the nigrostriatal pathway. Exp Neurol. 2002;175:303–317. doi: 10.1006/exnr.2002.7891. [DOI] [PubMed] [Google Scholar]
- Di Giovanni G, Di Matteo V, Pierucci M, Esposito E. Serotonin-dopamine interaction: electrophysiological evidence. Prog Brain Res. 2008;172:45–71. doi: 10.1016/S0079-6123(08)00903-5. [DOI] [PubMed] [Google Scholar]
- Doremus TL, Varlinskaya EI, Spear LP. Factor analysis of elevated plus-maze behavior in adolescent and adult rats. Pharmacol Biochem Behav. 2006;83:570–577. doi: 10.1016/j.pbb.2006.03.019. [DOI] [PubMed] [Google Scholar]
- el Gameyel G, Trouvin JH, Prioux-Guyonneau M, Jacquot C, Cohen Y. Dopaminergic metabolism in various rat brain areas after L-DOPA loading. J Pharm Pharmacol. 1986;38:691–694. doi: 10.1111/j.2042-7158.1986.tb03112.x. [DOI] [PubMed] [Google Scholar]
- Eskow Jaunarajs KL, Angoa-Perez M, Kuhn DM, Bishop C. Potential mechanisms underlying anxiety and depression in Parkinson’s disease: consequences of l-DOPA treatment. Neurosci Biobehav Rev. 2011;35:556–564. doi: 10.1016/j.neubiorev.2010.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eskow Jaunarajs KL, Dupre KB, Ostock CY, Button T, Bishop C. Behavioral and neurochemical effects of chronic L-DOPA treatment on non-motor sequelae in the hemiparkinsonian rat. Behav Pharmacol. 2010;21:627–637. doi: 10.1097/FBP.0b013e32833e7e80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eskow KL, Dupre KB, Barnum CJ, Dickinson SO, Park JY, Bishop C. The role of the dorsal raphe nucleus in the development, expression, and treatment of L-DOPA-induced dyskinesia in hemiparkinsonian rats. Synapse. 2009;63:610–620. doi: 10.1002/syn.20630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eskow KL, Gupta V, Alam S, Park JY, Bishop C. The partial 5-HT(1A) agonist buspirone reduces the expression and development of l-DOPA-induced dyskinesia in rats and improves l-DOPA efficacy. Pharmacol Biochem Behav. 2007;87:306–314. doi: 10.1016/j.pbb.2007.05.002. [DOI] [PubMed] [Google Scholar]
- Fearnley JM, Lees AJ. Ageing and Parkinson’s disease: substantia nigra regional selectivity. Brain. 1991;114:2283–2301. doi: 10.1093/brain/114.5.2283. [DOI] [PubMed] [Google Scholar]
- File SE, Seth P. A review of 25 years of the social interaction test. Eur J Pharmacol. 2003;463:35–53. doi: 10.1016/s0014-2999(03)01273-1. [DOI] [PubMed] [Google Scholar]
- Gasbarri A, Sulli A, Packard MG. The dopaminergic mesencephalic projections to the hippocampal formation in the rat. Prog Neuropsychopharmacol Biol Psychiatry. 1997;21:1–22. doi: 10.1016/s0278-5846(96)00157-1. [DOI] [PubMed] [Google Scholar]
- Gotham AM, Brown RG, Marsden CD. Frontal’ cognitive function in patients with Parkinson’s disease ‘on’ and ‘off’ levodopa. Brain. 1988;111:299–321. doi: 10.1093/brain/111.2.299. [DOI] [PubMed] [Google Scholar]
- Grace AA, Rosenkranz JA. Regulation of conditioned responses of basolateral amygdala neurons. Physiol Behav. 2002;77:489–493. doi: 10.1016/s0031-9384(02)00909-5. [DOI] [PubMed] [Google Scholar]
- Guerra MJ, Liste I, Labandeira-Garcia JL. Effects of lesions of the nigrostriatal pathway and of nigral grafts on striatal serotonergic innervation in adult rats. Neuroreport. 1997;8:3485–3488. doi: 10.1097/00001756-199711100-00014. [DOI] [PubMed] [Google Scholar]
- Guiard BP, El Mansari M, Merali Z, Blier P. Functional interactions between dopamine, serotonin and norepinephrine neurons: an in-vivo electrophysiological study in rats with monoaminergic lesions. Int J Neuropsychopharmacol. 2008;11:625–639. doi: 10.1017/S1461145707008383. [DOI] [PubMed] [Google Scholar]
- Hogg S. A review of the validity and variability of the elevated plus-maze as an animal model of anxiety. Pharmacol Biochem Behav. 1996;54:21–30. doi: 10.1016/0091-3057(95)02126-4. [DOI] [PubMed] [Google Scholar]
- Jacobs BL, Azmitia EC. Structure and function of the brain serotonin system. Physiological Rev. 1992;72:165–229. doi: 10.1152/physrev.1992.72.1.165. [DOI] [PubMed] [Google Scholar]
- Kannari K, Shen H, Arai A, Tomiyama M, Baba M. Reuptake of L-DOPA-derived extracellular dopamine in the striatum with dopaminergic denervation via serotonin transporters. Neurosci Lett. 2006;402:62–65. doi: 10.1016/j.neulet.2006.03.059. [DOI] [PubMed] [Google Scholar]
- Kilpatrick IC, Jones MW, Phillipson OT. A semiautomated analysis method for catecholamines, indoleamines, and some prominent metabolites in microdissected regions of the nervous system: an isocratic HPLC technique employing coulometric detection and minimal sample preparation. J Neurochem. 1986;46:1865–1876. doi: 10.1111/j.1471-4159.1986.tb08506.x. [DOI] [PubMed] [Google Scholar]
- Kish SJ, Shannak K, Hornykiewicz O. Uneven pattern of dopamine loss in the striatum of patients with idiopathic Parkinson’s disease. Pathophysiologic and clinical implications. NEJM. 1988;318:876–880. doi: 10.1056/NEJM198804073181402. [DOI] [PubMed] [Google Scholar]
- Kish SJ, Tong J, Hornykiewicz O, Rajput A, Chang LJ, Guttman M, Furukawa Y. Preferential loss of serotonin markers in caudate versus putamen in Parkinson’s disease. Brain. 2008;131:120–131. doi: 10.1093/brain/awm239. [DOI] [PubMed] [Google Scholar]
- Kuhn DM, Arthur R., Jr Dopamine inactivates tryptophan hydroxylase and forms a redox-cycling quinoprotein: possible endogenous toxin to serotonin neurons. J Neurosci. 1998;18:7111–7117. doi: 10.1523/JNEUROSCI.18-18-07111.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhn DM, Arthur RE., Jr. L-DOPA-quinone inactivates tryptophan hydroxylase and converts the enzyme to a redox-cycling quinoprotein. Brain Res Mol Brain Res. 1999;73:78–84. doi: 10.1016/s0169-328x(99)00238-7. [DOI] [PubMed] [Google Scholar]
- Kulisevsky J, Pascual-Sedano B, Barbanoj M, Gironell A, Pagonabarraga J, García-Sánchez C. Acute effects of immediate and controlled-release levodopa on mood in Parkinson’s disease: A double-blind study. Mov Disord. 2007;22:62–67. doi: 10.1002/mds.21205. [DOI] [PubMed] [Google Scholar]
- Lucki I. The forced swimming test as a model for core and componenet behavioral effects of antidepressant drugs. Behav Pharmacol. 1997;8:523–532. doi: 10.1097/00008877-199711000-00010. [DOI] [PubMed] [Google Scholar]
- Maeda T, Kannari K, Shen H, Arai A, Tomiyama M, Matsunaga M, Suda T. Rapid induction of serotonergic hyperinnervation in the adult rat striatum with extensive dopaminergic denervation. Neurosci Lett. 2003;343:17–20. doi: 10.1016/s0304-3940(03)00295-7. [DOI] [PubMed] [Google Scholar]
- Maeda T, Nagata K, Yoshida Y, Kannari K. Serotonergic hyperinnervation into the dopaminergic denervated striatum compensates for dopamine conversion from exogenously administered L-DOPA. Brain Res. 2005;1046:230–233. doi: 10.1016/j.brainres.2005.04.019. [DOI] [PubMed] [Google Scholar]
- Millan MJ. Serotonin 5-HT2C receptors as a target for the treatment of anxious and depressive states: focus on novel therapeutic strategies. Therapie. 2005;60:441–460. doi: 10.2515/therapie:2005065. [DOI] [PubMed] [Google Scholar]
- Missale C, Nash SR, Robinson SW, Jaber M, Caron MG. Dopamine receptors: from structure to function. Physiological Rev. 1998;78:189–225. doi: 10.1152/physrev.1998.78.1.189. [DOI] [PubMed] [Google Scholar]
- Navailles S, Banzzouz A, Bioulac B, Gross C, De Deurwaerdere P. High-frequency stimulation of the subthalamic nucleus and L-3,4-dihydroxyphenylalanine inhibit in vivo serotonin release in the prefrontal cortex and hippocampus in a rat model of Parkinson’s disease. J Neurosci. 2010a;30:2356–2364. doi: 10.1523/JNEUROSCI.5031-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navailles S, Bioulac B, Gross C, De Deurwaerdere P. Serotonergic neurons mediate ectopic release of dopamine induced by L-DOPA in a rat model of Parkinson’s disease. Neurobiol Dis. 2010b;38:136–143. doi: 10.1016/j.nbd.2010.01.012. [DOI] [PubMed] [Google Scholar]
- Navailles S, Bioulac B, Gross C, De Deurwaerdere P. Chronic L-DOPA therapy alters central serotonergic function and L-DOPA-induced dopamine release in a region-dependent manner in a rat model of Parkinson’s disease. Neurobiol Dis. 2011;41:585–590. doi: 10.1016/j.nbd.2010.11.007. [DOI] [PubMed] [Google Scholar]
- Obeso JA, Rodriguez-Oroz MC, Chana P, Lera G, Rodriguez M, Olanow CW. The evolution and origin of motor complications in Parkinson’s disease. Neurology. 2000;55:S13–20. [PubMed] [Google Scholar]
- Paille V, Henry V, Lescaudron L, Brachet P, Damier P. Rat model of Parkinson’s disease with bilateral motor abnormalities, reversible with levodopa, and dyskinesias. Mov Disord. 2007;22:533–539. doi: 10.1002/mds.21308. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. 4th edn Academic Press; Orlando: 1998. [Google Scholar]
- Prut L, Belzung C. The open field as a paradigm to measure the effects of drugs on anxiety-like behaviors: a review. Eur J Pharmacol. 2003;463:3–33. doi: 10.1016/s0014-2999(03)01272-x. [DOI] [PubMed] [Google Scholar]
- Ressler KJ, Nemeroff CB. Role of serotonergic and noradrenergic systems in the pathophysiology of depression and anxiety disorders. Dep Anx. 2000;12:S2–19. doi: 10.1002/1520-6394(2000)12:1+<2::AID-DA2>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- Richard IH, Frank S, McDermott MP, Wang H, Justus AW, LaDonna KA, Kurlan R. The ups and downs of Parkinson disease: a prospective study of mood and anxiety fluctuations. Cogn Behav Neurol. 2005;17:201–207. [PubMed] [Google Scholar]
- Rosenkranz JA, Grace AA. Dopamine attenuates prefrontal cortical suppression of sensory inputs to the basolateral amygdala of rats. J Neurosci. 2001;21:4090–4103. doi: 10.1523/JNEUROSCI.21-11-04090.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rozas G, Liste I, Guerra MJ, Labandeira-Garcia JL. Sprouting of the serotonergic afferents into striatum after selective lesion of the dopaminergic system by MPTP in adult mice. Neurosci Lett. 1998;245:151–154. doi: 10.1016/s0304-3940(98)00198-0. [DOI] [PubMed] [Google Scholar]
- Rylander D, Parent M, O’Sullivan SS, Dovero S, Lees AJ, Bezard E, Descarries L, Cenci MA. Maladaptive plasticity of serotonin axon terminals in levodopa-induced dyskinesia. Ann Neurol. 2010;68:619–628. doi: 10.1002/ana.22097. [DOI] [PubMed] [Google Scholar]
- Smith AD, Almalric M, Koob GF, Zigmond MJ. Effect of bilateral 6-OHDA lesions of the medial forebrain bundle on reaction time. Neuropsychopharmacology. 2002;26:756–764. doi: 10.1016/S0893-133X(01)00420-1. [DOI] [PubMed] [Google Scholar]
- Tadaiesky MT, Dombrowski PA, Figueiredo CP, Cargnin-Ferreira E, Da Cunha C, Takahashi RN. Emotional, cognitive, and neurochemical alterations in a premotor stage model of Parkinson’s disease. Neuroscience. 2008;156:830–840. doi: 10.1016/j.neuroscience.2008.08.035. [DOI] [PubMed] [Google Scholar]
- Truong L, Allbutt H, Kassiou M, Henderson JM. Developing a preclinical model of Parkinson’s disease: a study of behavior in rats with graded 6-OHDA lesions. Behav Brain Res. 2006;169:1–9. doi: 10.1016/j.bbr.2005.11.026. [DOI] [PubMed] [Google Scholar]
- Wallman MJ, Gagnon D, Parent M. Serotonin innervation of human basal ganglia. Eur J Neurosci. 2011;33:1519–1532. doi: 10.1111/j.1460-9568.2011.07621.x. [DOI] [PubMed] [Google Scholar]
- Winter C, von Rumohr A, Mundt A, Petrus D, Klein J, Lee T, Morgenstern R, Kupsch A, Juckel G. Lesions of dopaminergic neurons in the substantia nigra pars compacta and in the ventral tegmental area enhance depressive-like behavior in rats. Behav Brain Res. 2007;184:133–141. doi: 10.1016/j.bbr.2007.07.002. [DOI] [PubMed] [Google Scholar]
- Yamada H, Aimi Y, Nagatsu I, Taki K, Kudo M, Arai R. Immunohistochemical detection of L-DOPA-derived dopamine within serotonergic fibers in the striatum and substantia nigra pars reticulate in Parkinsonian model rats. Neurosci Res. 2007;59:1–7. doi: 10.1016/j.neures.2007.05.002. [DOI] [PubMed] [Google Scholar]
- Zeng BY, Iravani MM, Jackson MJ, Rose S, Parent A, Jenner P. Morphological changes in serotoninergic neurites in the striatum and globus pallidus in levodopa primed MPTP treated common marmosets with dyskinesia. Neurobiol Dis. 2010;40:599–607. doi: 10.1016/j.nbd.2010.08.004. [DOI] [PubMed] [Google Scholar]