Abstract
Keap1-Nrf2-ARE pathway represents one of the most important cellular defense mechanisms against oxidative stress and xenobiotic damage. Activation of Nrf2 signaling induces the transcriptional regulation of ARE-dependent expression of various detoxifying and antioxidant defense enzymes and proteins. Keap1-Nrf2-ARE signaling has become an attractive target for the prevention and treatment of oxidative stress-related diseases and conditions including cancer, neurodegenerative, cardiovascular, metabolic and inflammatory diseases. Over the last few decades, numerous Nrf2 inducers have been developed and some of them are currently undergoing clinical trials. Recently, over-activation of Nrf2 has been implicated in cancer progression as well as in drug resistance to cancer chemotherapy. Thus, Nrf2 inhibitors could potentially be used to improve the effectiveness of cancer therapy. Herein, we review the signaling mechanism of Keap1-Nrf2-ARE pathway, its disease relevance, and currently known classes of small molecule modulators. We also discuss several aspects of Keap1-Nrf2 interaction, Nrf2-based peptide inhibitor design, and the screening assays currently used for the discovery of direct inhibitors of Keap1-Nrf2 interaction.
Keywords: Oxidative stress, Keap1 (Kelch-like ECH-associated protein 1), Nrf2 (Nuclear factor erythroid 2-related factor 2), ARE (antioxidant response element), antioxidant inflammation modulator (AIM)
1. INTRODUCTION
Human body is constantly exposed to various oxidative and electrophilic chemicals from both endogenous and exogenous sources.1 While some reactive oxygen species (ROS) and reactive nitrogen species (RNS) act as important messengers in controlling cellular redox homeostasis, sustained oxidative stress conditions can cause damage to cell structures, including lipids, proteins, and nucleic acids.2–5 This oxidative damage can lead to chronic inflammation which could subsequently turn to cancer, cardiovascular, neurodegenerative, inflammatory diseases, and aging.6–10 Cells defend against various oxidative stresses by a combination of physical, preventative, repair, and antioxidant defense mechanisms.11,12 Antioxidant defense system is the major protective mechanism and cells use antioxidants to neutralize the damaging effects of oxidants and electrophiles.13,14 Antioxidants can be classified based on source, nature and mechanism of action into endogenous (metabolic and enzymatic antioxidants) and exogenous (nutrient antioxidants) or direct antioxidants, indirect antioxidants and bifunctional antioxidants.15 Direct antioxidants are redox active and short lived and they are consumed during the process and need to be regenerated to offer further protection, whereas indirect antioxidants may or may not be redox active and exhibit their antioxidant effects through up-regulation of various cytoprotective compounds and proteins such as NAD(P)H, NAD(P)H:quinone oxidoreductase 1 (NQO1), superoxide dismutase (SOD), glutathione S-transferase (GST), glutathione peroxidase (GPx), heme oxygenase-1 (HO-1), glutamate-cysteine ligase (GCL), catalase and thioredoxin.16,17 Intriguingly, these cytoprotective proteins are referred as the “ultimate antioxidants,” as they have relatively long half-lives, are not consumed during their antioxidant actions, can catalyze a wide variety of chemical detoxification reactions, and are involved in regeneration of some direct antioxidants.15 There are three main cellular components involved in the regulation of antioxidant response; they are Kelch-like ECH-associated protein 1 (Keap1), nuclear factor erythroid 2-related factor 2 (Nrf2), and antioxidant response elements (ARE). The Keap1-Nrf2-ARE is a major signaling pathway that regulates the battery of cytoprotective proteins at transcriptional level.13,18–22 In addition to the induction of cytoprotective proteins, Keap1-Nrf2-ARE has multiple activation pathways for maintaining the cellular redox balance and metabolism.23–25 In short, The Keap1-Nrf2-ARE signaling pathway induces an adaptive response for oxidative stress which can otherwise lead to many inflammatory diseases including cancer, Alzheimer’s and Parkinson’s diseases, and diabetes.26–29 Thus, targeting the Keap1-Nrf2-ARE signaling pathway is being considered as a rational strategy to discover preventive and therapeutic agents referred to as antioxidant inflammation modulators (AIMs) for diseases and conditions involving oxidative stress and inflammation.30–37 Some of Nrf2-ARE inducing agents are already in clinical trials as chemopreventive agents for cancer or as therapeutic agents for conditions involving inflammation. For example, bardoxolone methyl, a potent inducer of the Nrf2 pathway, is currently under phase 3 clinical trials as an orally active, first-in-class AIM for the treatment of advanced chronic kidney disease (CKD) in patients with type 2 diabetes mellitus.38–43 While several reviews have published recently on Keap1-Nrf2-ARE pathway with emphasis on its biological functions,22,29,44–51 this review mainly focuses on the chemistry of currently known small molecule modulators of Keap1-Nrf2-ARE pathway and the high throughput screening strategies being devised to discover direct reversible modulators of Keap1-Nrf2 interaction as potential preventive and therapeutic agents for diseases and conditions involving oxidative stress and inflammation.
2. KEAP1-NRF2-ARE PATHWAY
A. Component structures and functions
Keap1-Nrf2-ARE pathway is an integrated redox sensitive signaling system which regulates from 1% to 10% of our genes. 49,52 Keap1 constitutively targets Nrf2 for ubiquitin-dependent proteasomal degradation under basal (reducing) conditions of cell growth.53,54 Following exposure of cells to electrophiles or oxidative stress, Nrf2 is able to escape Keap1-mediated degradation, translocate to the nucleus, and activate ARE-dependent gene expression of a series of antioxidative and cytoprotective proteins that include HO-1, NQO1, GCL, GPx, and several members of the glutathione S-transferase family.22,55,56 These proteins include phase II detoxification enzymes and regulatory and structural proteins which are essential for the metabolism, detoxification of xenobiotics, redox homeostasis and cell survival.37,45,57–59 Thereby, Keap1-Nrf2-ARE signaling system reduces the intensity of acute inflammation and induces perseverance to prevent the transformation of acute pathological conditions into chronic diseases.47,60–62
1. Kelch-like ECH-associated protein 1 (Keap1)
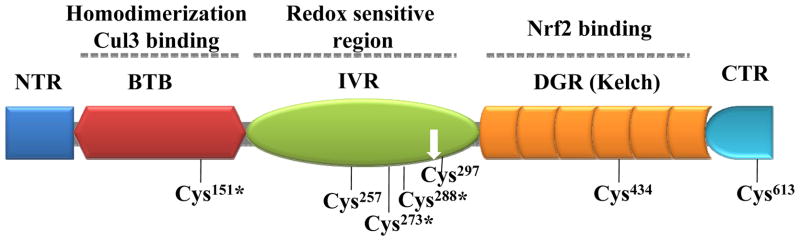
Keap1 is a 69-kDa protein that shares some homology with actin-binding Kelch protein and serves as a negative regulator of Nrf2. The human Keap1 protein sequence contains 627 amino acid residues organized into five domains as shown in Figure 1: i) the N-terminal region (NTR), ii) the Broad complex, Tramtrack, and Bric-a-Brac (BTB), iii) the linker intervening region (IVR), iv) the Kelch domain, and v) the C-terminal region (CTR). BTB domain that attached to actin-binding proteins is responsible for homodimerization and interaction with Cullin (Cul3)-based ubiquitin E3 ligase complex for Nrf2 ubiquitination. IVR containing cysteine residues are sensitive to oxidation and nuclear export signal (NES) motif. Kelch domain has six kelch repeats (KR1-KR6) and possessing multiple protein contact sites that mediate association of Keap1 with Nrf2 (the Kelch domain interacts with Neh2 domain of Nrf2) and cytoskeleton proteins actin and/or myosin.
Figure 1.
Organization and functions of structure domains in Keap1. The reactive key cysteine residues and nuclear import signaling site (white down arrow) are indicated. The redox sensitive cysteine residues are marked with (*).
Human Keap1 contains a total of 27 cysteine residues and seven of them (i.e., Cys151, Cys257, Cys273, Cys288, Cys297, Cys434, and Cys613) are highly reactive towards ROS and electrophiles and are believed to be involved in redox sensing.50,58,63–68
2. Nuclear factor erythroid 2-related factor 2 (Nrf2)
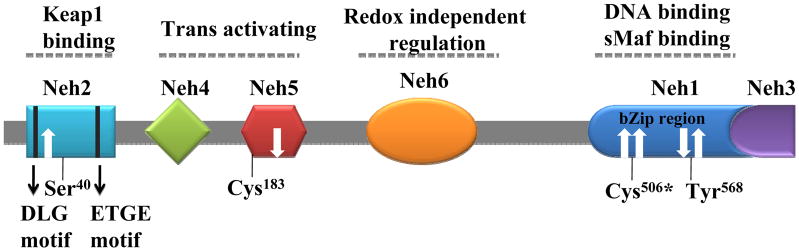
Nrf2 is a bZip (basic leucine Zipper) transcription factor and a member of CNC (cap “n” collar) family of transcription factors. Human Nrf2 has 605 amino acid residues that form six conserved domains Neh1 to Neh6 (Nrf2-ECH homology; ECH-chicken analog of Nrf2) as shown in Figure 2. Neh1 has a bZip motif responsible for heterodimerization with small Maf (musculoaponeurotic fibrosarcoma) protein, and Nrf2-Maf heterodimer then binds to ARE activating gene expression. The Neh2 domain at the N-terminus contains DLG and ETGE motifs that bind to Keap1 Kelch domain, which negatively regulates the transcriptional activity of Nrf2. Domains Neh3 at the C-terminus, Neh4, and Neh5 mediate Nrf2 transactivational activity by binding to histone acetyltransferases. Neh6 has a function of Keap1-independent negative regulation of Nrf2.69 The Nrf2 protein also contains nuclear import/localization signals (NLSs) and nuclear export signals (NESs) which regulate Nrf2 shuttling in and out of the nucleus. Human Nrf2 contain six cysteine residues, and two of the cysteines (Cys183 and Cys506) and two other key amino acid resiudes (Ser40 and Tyr568) may also regulate Nrf2 localization and transactivation of target genes through oxidation and phosphorylation, respectively. Nrf2 is truly a master regulator of the antioxidant response.19,58,69–77
Figure 2.
Organization and functions of structure domains in Nrf2. The reactive key cysteine residues, nuclear import (white down arrow), and nuclear export (white up arrow) signaling sites are indicated.
3. Antioxidant response element (ARE)
ARE, which is also known as electrophile response element or EpRE, is the cis-regulatory element containing specific DNA sequences that are present in the upstream regulatory regions of genes encoding the detoxifying enzymes and cytoprotective proteins. The consensus sequence of ARE cannot be represented as a single sequence as AREs required for some genes are distinctly different for others.78 Nevertheless, the typical length of functionally active ARE is the 16 nucleotide sequence of 5′-TA/CAnnA/GTGAC/GnnnGCA/G-3′, where n is the variable that indicates any nucleotide. Under oxidative stress conditions, stabilized Nrf2 translocates to the nucleus, forms a heterodimer with Maf, and activates ARE-dependent gene expression. Bach1 (BTB and CNC homology-1) is another negative regulator of certain ARE-dependent genes; it associates with ARE and forms a dimer with Maf protein, preventing Nrf2 from binding to DNA under normal physiological conditions.74,78–83
B. Mechanisms and regulation of ARE-dependent gene expression
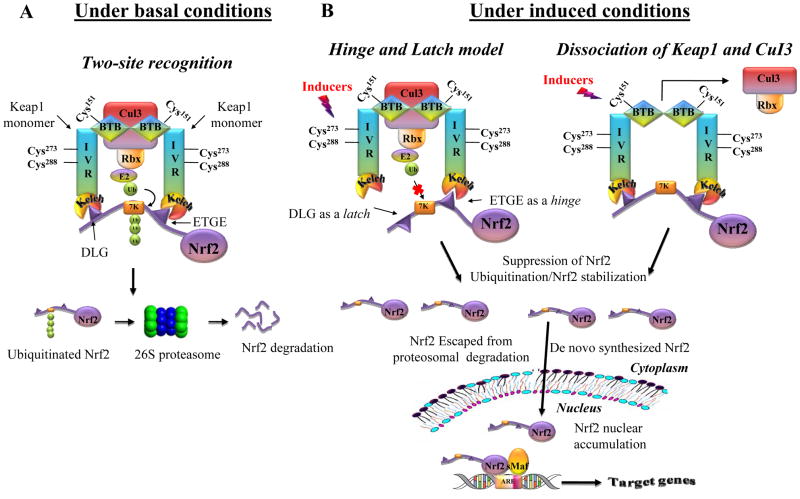
Keap1 binding to Nrf2 prevents Nrf2 from entering the nucleus; it acts as a chemical sensor that controls the steady state level of Nrf2 based on cellular redox conditions.84 Thus, Keap1 is also known as inhibitor of Nrf2 (INrf2). Under basal conditions, Nrf2 protein is rapidly degraded with a t1/2 of < 20 min; Nrf2 degradation is also mediated by Keap1 via specific ubiquitin-26S proteasomal pathway.85,86 As shown in Figure 3, Keap1 forms a homodimer via BTB domain and binds to Nrf2 with its Kelch domains. Neh2 domain of Nrf2 contains two binding motifs ETGE and DLG that bind to the Kelch domains of Keap1 with different affinities (two-site substrate recognition).87 Keap1 also associates with Cul3 and brings Nrf2 in close proximity to Cul3-based E3 ligase complex which mediates polyubiquitination of the lysine-rich α-helix between ETGE and DLG, and subsequent degradation of Nrf2. Under induced conditions, this Keap1-Nrf2-Cul3 assembly is disturbed and consequently Nrf2 can bypass the “Keap1 gate” and stabilized with an extended t1/2 of up to 200 min.54,86,88 Presently, two mechanistic models have been proposed for the Nrf2 stabilization, namely the “hinge and latch” model and the Keap1-Cul3 dissociation model.22,89
Figure 3.
The proposed regulatory mechanisms of Keap1-Nrf2-ARE pathway. (A) Under basal conditions, Nrf2 binds to Keap1 through ETGE (‘hinge’) and DLG (‘latch’) motifs (two-site recognition), and Nrf2 is constantly ubiquitinated by Keap1-Cul3-Ligase complex and degraded in the 26S proteasome (B) Under induced conditions, electrophilic modification of specific Keap1 cysteines induces a conformational change in Keap1 that disrupts the low affinity interaction with DLG (‘latch’) and consequently affects ubiquitination of Nrf2 (‘hinge’ and ‘latch’ model). Alternatively, inducers can cause the dissociation of Keap1-Cul3 complex, which prevents the ubiquitination and subsequent proteasomal degradation of Nrf2.
In the “hinge and latch” model, the high affinity binding of ETGE motif functions as a “hinge” to fix Nrf2 to Keap1 and the low affinity binding of DLG motif functions as a “latch” to lock down the Neh2 domain which facilitates the ubiquitination and constant degradation of Nrf2 in proteosomes.87,89,90 Upon exposure to oxidative stress, cysteine residues of Keap1 in its BTB and IVR domains are covalently modified, leading to conformational changes to the Keap1 dimer.54,76 This modified conformation of Keap1 could disrupt the low-affinity DLG motif interaction, but not ETGE motif interaction (~100-fold tighter than DLG motif interaction). Therefore, the DLG motif dissociates from Keap1 (latch released), whereas the ETGE motif remains associated with Keap1 (hinge attached).91 Under these circumstances, the orientation of Nrf2 is not suitable for ubiquitin ligase activity by Keap1-Cul3 complex.90 As a result, Nrf2 escapes from proteosomal degradation and accumulates in the cell along with newly de novo synthesized Nrf2, translocates to the nucleus, heterodimerizes with small Mafs, and binds to ARE, leading to transcription of ARE-dependent genes.50,54
Dissociation of Keap1 and Cul3 is another model proposed for Nrf2 stabilization.22,29 Under induced conditions, covalent modification of cysteine residue(s) in Cul3 binding BTB domain of Keap1 leading to a steric clash between Keap1 and Cul3.77 This modification does not change the conformation of Keap1 but rather disrupt Keap-Cul3 E3 ligase activity via the dissociation of Keap1-Cul3 interaction.92 Other alternative mechanisms have also been proposed for Nrf2 stabilization in response to inducers, such as nucleocytoplasmic shuttling of Keap1, ubiquitination of Keap1, and Nrf2 as a direct sensor.22
When redox balance of cell is restored, Nrf2 is transported back into cytoplasm and degraded through Keap1-Cul3-mediated ubiquitination.93 Alternatively, Nrf2 depletion is promoted by the feedback mechanism of increased expression of ARE-dependent genes participating in Nrf2 degradation and negative regulation including Bach1, Keap1, and Cul3.49,94,95 The involvement of several protein kinases, including PKC, CDK, GSK and Fyn, has been implicated in the regulation of Keap-Nrf2-ARE pathway.49 In addition, complex cross talks between the Keap1-Nrf2-ARE pathway and other pathways have also been demonstrated, which are beyond the scope of this review.96–98
As explained from the above mechanistic models, the process of covalent (oxidative/electrophilic) modification of sulfhydryl groups of cysteine residues in Keap1 is critical for Nrf2 regulation.54 Keap1 employs multiple sensing systems using different cysteine residues for different kinds of stimuli including electrophiles, ROS and heavy metals.50,99,100
Highly reactive cysteines were consistently found in the IVR region of Keap1 and two reactive cysteine residues in this region, Cys273 and Cys288 are essential for Nrf2 degradation. Within BTB domain, a cysteine residue, Cys151 is critical for inducible modifications of Keap1 function. Modification of these cysteine residues inhibits ubiquitination of Nrf2 and stabilizes Nrf2 even in the absence of stimuli (under physiological conditions).63,67,101,102 Recently, a cysteine residue of Nrf2 (Cys506) was found to be involved in the direct inducer sensing function and has been suggested as a critical residue in Keap1-Nrf2 interaction.102 Clearly, Keap1 is a key component in the regulation of Nrf2, playing major roles such as an anchor for Nrf2 protein in the cytoplasm, an adaptor for Cul3-based ubiquitin E3 ligase complex for Nrf2 ubiquitination and a sensor for stress stimuli for induction of ARE-dependent genes.
C. Diseases and Conditions Involving Keap1-Nrf2-ARE pathway
The processes of inflammation and oxidative stress are important in the pathogenesis of many diseases. Inflammation produces large amounts of ROS and RNS that can induce oxidative damage to DNA and other cellular components; our body has evolved defensive mechanisms to protect our cells in addition to repair the DNA damage.103 The Keap1-Nrf2-ARE pathway is a common defense mechanism to counteract oxidative stress and protects multiple organs and cells.103 The protective role of Nrf2 activation has also been established in many human disorders including cancer, Alzheimer’s and Parkinson’s diseases, chronic obstructive pulmonary disease (COPD), asthma, atherosclerosis, diabetes, inflammatory bowel disease, multiple sclerosis, osteoarthritis and rheumatoid arthritis. Regulation of Nrf2-ARE signaling has also been implicated in the determination of health span, longevity, and aging.104 As discussed below, the emerging role of Keap1-Nrf2-ARE pathway in oxidative stress-related pathologies offers novel therapeutic opportunities. Pharmacological interventions are being actively pursued for the discovery of modulators of this pathway as potential preventive and therapeutic agents. This section briefly summarizes the diseases and conditions that involve Keap1-Nrf2-ARE pathway and those that could potentially be prevented or treated with modulators of this pathway.
1. Cancer
The cancer chemopreventive properties have been the most intensively studied and suggested of most synthetic and natural Nrf2 inducers. Activation of Nrf2 upregulates various conjugating enzymes for the detoxication of chemical carcinogens and confers protection against carcinogenicity, mutagenicity and other forms of toxicity.36,105 Disruption of Nrf2 induction has increased the susceptibility of cells towards carcinogens and contributes to the inflammation progression, ultimately cancer formation.47 Cancer chemopreventive activities of Nrf2 activators have been demonstrated in several cancer models including colon cancer, bladder cancer, lung cancer, stomach cancer, breast cancer, skin cancer, and liver cancer.27 Indeed, several activators of Keap1-Nrf2-ARE pathway have been tested in clinic trials for the prevention of a variety of cancers.43,106–108
Nrf2 has also been shown to be over-expressed in some cancer cells, indicating a potential role in tumor cell growth and survival.48,62,109 This dual action of Nrf2 has been recognized as a “double-edged sword” with regard to the benefits or risks of Keap1-Nrf2 system in cells.33,84,110,111 Somatic mutations or single nucleotide polymorphism in Nrf2 and Keap1 genes resulted in constitutive activation of Nrf2 and promotion of tumor growth.112,113 It has been hypothesized that the cytoprotective activity of Nrf2 can be exploited by cancer cells not only to cope with the challenging tumor microenvironment, but also confer chemo- and/or radio resistance during anticancer therapies.84 Recently, it has been shown that suppression of Nrf2 activity inhibits tumor growth and enhances the efficacy of cancer chemotherapeutic agents.114,115 Thus, Nrf2 activity could be targeted for cancer treatment as well as chemoprevention though in different patient populations.
2. Chronic obstructive pulmonary disease (COPD)and other airway disorders
Oxidative stress has been implicated in the pathogenesis of various airway disorders including inflammation, cell proliferation, airway obstruction, and respiratory failure.117 Nrf2 is predominantly expressed in epithelium and alveolar macrophages and protects the lungs from oxidative stress, alveolar cell apoptosis, extracellular matrix proteolysis and chronic inflammation. The decline in Nrf2-dependent antioxidants and glutathione levels with significant decrease in Nrf2 expression was observed in COPD patient lungs.116 Activation of Nrf2 is a novel therapeutic approach for the antioxidant therapy not only in COPD but also in other airway disorders associated with oxidative stress including idiopathic pulmonary fibrosis (IPF), asthma, cystic fibrosis, bronchopulmonary dysplasia (BPD), acute lung injury (ALI)/acute respiratory distress syndrome (ARDS).117–120
3. Alzheimer’s and Parkinson’s diseases
Mitochondrial function is essential for neuronal signaling, plasticity, and transmitter release. Brain is sensitive to oxidative stress due to its high lipid content and oxygen consumption, and mitochondrial dysfunction and subsequent production of ROS are implicated in the pathogenesis of a wide variety of chronic neurodegenerative diseases including Alzheimer’s (AD) and Parkinson’s (PD) diseases.121 The protective effect of Nrf2 against oxidative stress and neurotoxicity has been demonstrated and Keap1-Nrf2-ARE Pathway has been proposed as a therapeutic target in AD and PD disease states.122–124 Several studies indicate that increasing nuclear Nrf2 function can be protective to NT2N and dopaminergic neurons.122,125–127 Activation of Nrf2 can also potentially treat other neurological disorders such as Huntington’s disease, amyotrophic lateral sclerosis (Lou Gehrig’s disease), Friedreich’s ataxia, Down syndrome, multiple sclerosis, traumatic brain injury, and cerebral hemorrhages.30,128,129
4. Atherosclerosisand heart diseases
Nrf2 is ubiquitously expressed in the cardiovascular system. Keap1-Nrf2-ARE signaling pathway is critically involved in the regulation of vascular homeostasis and also in the protection of heart against pathological cardiac hypertrophy and heart failure via the suppression of oxidative stress.130 Nrf2 pathway has evolved as a therapeutic target against cardiovascular associated diseases including atherosclerosis, a leading cause for heart attack and stroke.35,131 Although Nrf2 was shown to have antiatherogenesis and vascular protective effects,132 it was recently suggested that Nrf2 promotes the atherosclerotic plaque formation via a different mechanism.133,134 Therefore, modulation of Nrf2 may hold promise for the prevention and treatment of arteriosclerosis.
5. Chronic kidney diseases (CKD)and diabetes
Nrf2-mediated expression of endogenous antioxidants has been shown as a critical adaptive defense mechanism against high glucose-induced oxidative damage in diabetes.45,110,135–137 The protective role of Nrf2 in diabetic nephropathy and neuropathy has been well established, suggesting that therapeutic activation of Nrf2 could be an effective approach to prevent as well as to treat diabetes and diabetes-related metabolic disorders.138,139 For example, CDDO-Me (Bardoxolone Methyl), a potent inducer of Nrf2 pathway, is being evaluated clinically for the treatment of CKD in patients with type 2 diabetes mellitus.38
6. Inflammatory bowel diseases
Increased free radicals and impaired antioxidant defenses in the intestines have been linked to the pathogenesis of inflammatory bowel diseases.140 It was suggested that Nrf2 plays an important role in protecting intestinal integrity via regulation of pro-inflammatory cytokines and induction of phase II detoxifying enzymes.141 Therefore, Nrf2 may serve as novel target for designing therapies to prevent and treat inflammatory bowel diseases such as Crohn’s diease and ulcerative colitis.142
7. Rheumatoid arthritis and Osteoarthritis
Nrf2 regulates the innate immune response and its modulation has been involved in attenuating various immune and inflammatory responses.60,143 Disruption of Nrf2 has been found to aggravate autoimmune joint diseases such as rheumatoid arthritis and osteoarthritis.144,145 Hence, Nrf2 has been considered as a therapeutic target for arthritis and other autoimmune diseases.
3. MODULATORS OF KEAP1-NRF2-ARE PATHWAY
The Keap1-Nrf2-ARE pathway can be activated by various cellular stresses (including oxidative stress, endoplasmic reticulum stress, and shear stress) and chemical inducers from both endogenous (reactive oxygen and nitrogen species) and exogenous sources.27,61 A variety of small molecule inducers of ARE system have been identified from natural sources as well as from synthetic agents. Conversely, the Keap1-Nrf2-ARE is also induced by toxic chemicals that evoke oxidative stress.146 We can divide the small molecule modulators of Keap1-Nrf2-ARE pathway into ARE inducers and inhibitors based on their ultimate biological effect on ARE-mediated gene expression. While most known small molecule ARE inducers are indirect inhibitors of Keap1-Nrf2 interaction, efforts are underway to discover direct small molecule inhibitors of Keap1-Nrf2 interaction through high throughput screening or structure-based drug design approaches.
A. Nrf2 Inducers
Almost all currently known ARE inducers (or activators) are indirect inhibitors of Keap1-Nrf2 interaction and they are believed to form covalent adducts with the sulfhydryl groups of cysteines in Keap1 by oxidation or alkylation.43,50,100 Electrophilicity is a common property of most of the known ARE inducers. However, not all electrophiles can regulate ARE activity.86 It is well known that the biological effect of electrophiles being therapeutic to toxic depends on its hardness (‘hard’ and ‘soft’) that define the rate and selectivity of interactions with nucleophiles. Specifically, soft electrophiles will react predominantly with soft nucleophiles such as the sulfhydryl groups of cysteine, whereas hard nucleophiles target the amino and hydroxyl groups on nucleic acids and thus induce carcinogenicity and genotoxicity.48,147 Adduct formation is not only dependent on the nature of electrophile but also on the protein microenvironment of nucleophilic center which is the function of both steric and electronic factors mediated primarily through tertiary structure of the protein.48,148 Different classes of electrophilic inducers can display different reaction patterns with the cysteine residues of protein target in the Keap1-Nrf2 system which may lead to distinct biological effects.29,100 It has been speculated that the highly reactive cysteine sulfhydryl groups of Keap1 with low pKa values (due to proximal basic residues) render Keap1 as a unique cellular sensor for Nrf2 inducers.15,88,149–151 An alternative mechanism was proposed that switches ubiquitination of Nrf2 to Keap1 upon modification of Keap1 cysteine residues by electrophilic inducers leading to dissociation and nuclear translocation of Nrf2.152
The chemistry of indirect small molecule inhibitors of Keap1-Nrf2 interaction has been progressively understood. Currently, known indirect inhibitors of Keap1-Nrf2 interaction are classified into about ten chemically distinct classes based on their chemical structure and their nature of reaction/interaction with cysteine sulfhydryl groups: (1) Michael acceptors, (2) oxidizable diphenols and quinones, (3) isothiocyanates and sulfoxythiocarbamates, (4) dithiolethiones and diallyl sulfides, (5) vicinal dimercaptans, (6) trivalent arsenicals, (7) selenium-based compounds, (8) polyenes, (9) hydroperoxides, and (10) heavy metals and metal complexes.49,153–155 Representative examples are discussed here in each class.
1. Michael acceptors
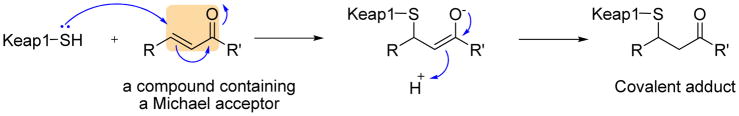
Michael acceptors (olefins or acetylenes conjugated with electron withdrawing carbonyl groups) are a prominent class of indirect inhibitors of Keap1-Nrf2 interaction. Michael acceptors are considered as soft Lewis acids and the order of ARE-inducing potency is proportional to their reactivity in the Michael addition reaction.156 As shown in Figure 4, Michael acceptors can react with the critical cysteine thiolate (soft base) groups in Keap1 and consequently suppress Nrf2 ubiquitination and induce the expression of ARE mediated antioxidative and cytoprotective enzymes.68,157 Michael acceptors usually show a bell-shaped dose-response curve, i.e. beneficial cellular response at low concentrations and unwanted cellular toxicity at high concentrations.158 Cytotoxicity at high concentrations is probably related to their “off-target” actions due to their chemical reactivity rather than through activation of Nrf2 signaling.48,51 Many structurally unrelated inducers are electrophilic Michael acceptors, typically found in various phytochemicals such as curcuminoids, cinnamic acid derivatives, coumarins, chalcones, flavonoids, and terpeniods.
Figure 4.
The proposed reaction mechanism of Michael addition reactions between Michael acceptors and cysteine sulfhydryl groups in Keap1.
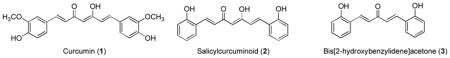
Curcumin (1), a yellow pigment from turmeric (Curcuma longa), is a principle component of Indian curry.108 Due to the presence of two phenolic functional groups and the β-diketo moeity, curcumin is known to directly scavenge radical and other reactive oxygen species.159 Curcumin contains two Michael acceptor groups that can react readily with cysteine sulfhydryl groups. It has been shown to effectively activate Keap1-Nrf2-ARE signaling pathway presumably through modification of cysteine sulfhydryl groups in Keap1.160–162 Curcumin has also shown to indirectly activate ARE system by stimulating the upstream kinase pathways.163,164 Curcumin can not only induce the upregulation of phase II and oxidative stress response enzymes but can also inhibit carcinogen-induced expression of phase I CYP450 enzymes.165 Curcumin is highly lipophilic and rapidly metabolized by glucuronidation and sulfation conjugation reactions. Curcumin has been shown to possess a number of therapeutic properties including antiinflammatory, antioxidative, antiarthritic, antiischemic and anticarcinogenic effects and has been evaluated in clinical trials for various diseases including multiple myeloma, pancreatic cancer, colon cancer, psoriasis, and Alzheimer’s disease.107,166 A synthetic analogue of curcumin, salicylcurcuminoid (2) having ortho hydroxyl substitutions relative to the α,β-unsaturated ketone system, is much more potent (~24-fold) than curcumin in inducing quinone reductase in murine hepatoma cells.160 Bis[2-hydroxybenzylidene]acetone (3) is a another synthetic analog of curcumin and was found to markedly increase the activities of NAD(P)H:quinone acceptor oxidoreductase 1 (NQO1) and glutathione reductase, and the levels of total glutathione in rapidly growing L1210 murine leukemia cells.167 The major concerns with using curcumin and analogs as preventive and therapeutic agents are their known chemical and metabolic instability, poor membrane permeability, and extremely low oral bioavailability.
Cinnamic acid esters such as caffeic acid phenethyl ester (CAPE, 4) and ferulic acid ethyl ester (EFE, 5), are α,β-unsaturated esters and were shown to induce ARE system with comparable potency to curcumin.162 CAPE (4) is an active component of propolis from honeybee hives. It is known to have antimitogenic, antiinflammatory, and immunomodulatory properties.168 Ferulic acid and its derivatives are found in many fruits and vegetables and are known to have strong protection against oxidation of protein and lipids.169,170
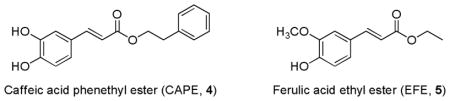
Chalcones are open-chain flavonoids found in many plants. Chemically, their backbone consists of two phenyl rings joined by a three-carbon α,β-unsaturated carbonyl system. Chalcones have been reported to have a broad spectrum of many biological properties including antiproliferation, antiinflammatory and antiinfective activities.147,171 Two important chalcones sofalcone (6)172 and isoliquiritigenin (7)173 were shown to induce cytoprotective proteins through Keap1-Nrf2-ARE pathway. Sofalcone (6) is a synthetic derivative of a sophoradin chalconoid, found in herbs of Sophora tonkinensis, which is used as traditional Chinese medicine. Isoliquiritigenin (7) is a licorice chalconoid, majorly found in the root of Glycyrrhiza glabra. Recently, synthesis of novel chalcone derivatives and their SAR on ARE induction identified 2-trifluoromethyl-2′-methoxychalone (8) as a potent activator of Nrf2.174 Some naturally occurring coumarin derivatives such as 4-methyl daphnetin (9),175 imperatorin (10),176 auraptene (11)176 and poncimarin (12)177 are reported to have ARE-mediated enzyme induction activities via Keap1-Nrf2-ARE pathway.
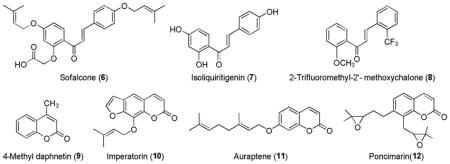
Flavonoids are regularly consumed in our human diet and are present at high concentrations in fruits, vegetables, and other plant-derived foods, such as teas and other beverages. Structurally, they are derivatives of 2-phenylchromen-4-one (2-phenyl-1,4-benzopyrone), which contain α,β-unsaturated ketone. A number of flavonoids have been reported to have ARE gene-induction properties; these include synthetic flavonoids like 4-bromo flavone (13)178 and β-naphthoflavone (14),32 a natural isoflavone sappanone A (15),175 and flavonoids-like benzofuran-containing substituted aurones (16)177 and their corresponding indole derivatives, benzylidene-indolin-2-ones (17).179
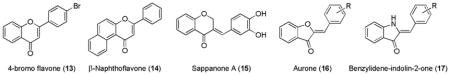
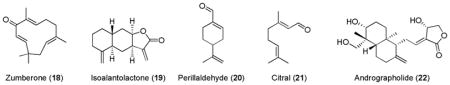
Sesquiterpenes such as zumberone (18)180 and isoalantolactone (19),181 and terpenoids containing α,β-unsaturated fragrant aldehydes182 such as perillaldehyde (20) and citral (21) were shown to induce Nrf2-mediated expression of detoxifying enzymes. Andrographolide (22), a bitter diterpenoid lactone extracted from the stem and leaves of the Andrographis paniculata, was identified as an ARE inducer through Keap1-Nrf2-ARE pathway.183
The known antitumor and antiinflammatory activities of a natural triterpenoid oleanolic acid were explored to develop potent inducers of Keap1-Nrf2-ARE system.184 The introduction of highly activated Michael acceptors (α,β-unsaturated α-cyano ketone) to the A and C rings of oleanolic acid scaffold resulted in potent Nrf2 inducers CDDO (23) and its methyl ester (24, also known as bardoxolone methyl or CDDO-Me).185–187 Another modification of -COOMe in 24 to CN group led to TP-255 (25) with even higher ARE-inducing potency.185 Further simplification of pentacyclic system to tricylic system (TBE-31, 26) did not affect the ARE-inducing activity. Investigation of ARE-inducing activities of monocyclic systems such as MCE-1 (27) and MCE-5 (28) demonstrated that 27 was ~2 fold less potent than 26, but ~10 fold more potent than 28.153,188,189 This observation indicates that two electron-withdrawing groups in the Michael acceptor may be beneficial for the covalent modification with Keap1 cysteine residues.22 Other triterpenoid natural products such as avicinD (29)190 and gedunin (30)175 were also shown to increase the expression of certain cytoprotective enzymes via Nrf2 activation.
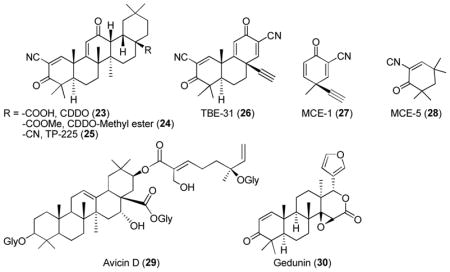
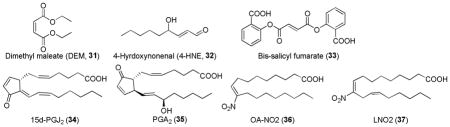
Other various compounds with Michael acceptors such as diethyl maleate (DEM, 31),100 4-hyrdoxynonenal (4-HNE, 32)100 and bis-salicyl fumarate (33)175 and some endogenous prostaglandins (15d-PGJ2 [34] and PGA2 [35])191 and nitro fatty acids (OA-NO2 [36] and LNO2 [37])192,193 were also shown to activate Nrf2.
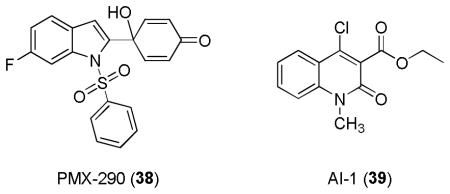
Recently, synthetic compounds like heteroaromatic 4-arylquinol (PMX-290, 38),194 a compound with known antiproliferative activity in vitro and antitumor activity in vivo in tumor xenografts, and AI-1 (39)195, a quinolinone derivative identified in a cell-based high throughput screening assay of chemical libraries, were shown to induce Nrf2. Their structures all contain Michael acceptors that can react with sulfhydryl groups.
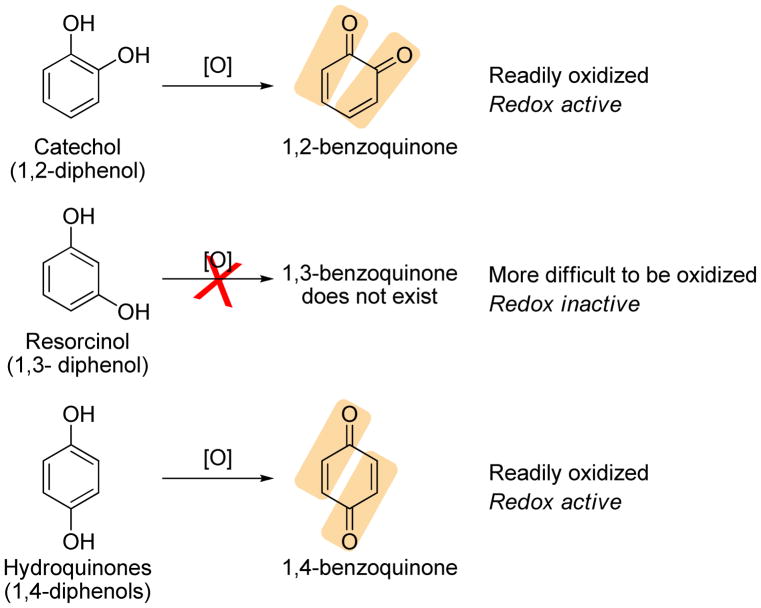
2. Oxidizable phenols and quinones
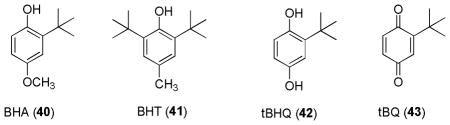
Polyphenolic compounds are able to directly quench free radical species and were used as antioxidants, even before Keap1-Nrf2-ARE signaling pathway was indentified.196 In late 1970s, a prototypic phenolic antioxidant BHA (40) was found to induce the expression of cytoprotective enzymes.197,198 Structural modifications of BHA including BHT (41) and tBHQ (42) revealed that structural changes had very little impact on ARE-inducing activity.199 After realization of the role of Michael acceptors played in the induction of ARE-genes, it was hypothesized that phenolic ARE-inducers may similarly exert their ARE-induction effect after oxidation to their corresponding electrophilic quinones which contain Michael acceptors.22,157 This hypothesis was supported by the oxidation potential and determination of ARE-inducing activities of three isomeric diphenols, catechol (1,2-diphenol), resorcinol (1,3-diphenol), and hydroquinone (1,4-diphenol). Catechols and hydroquinones were found to be active as ARE-inducers, whereas resorcinols were inactive; this is consistent with the varying oxidation potentials of the three diphenols as shown in Figure 5.200,201 In presence of oxygen and transition metals, tBHQ (42) was oxidized to tBQ (43) and rapidly undergo Michael addition with Keap1 protein and activate ARE-dependent transcription. It was also suggested that diphenols undergo cytochrome P450-mediated oxidation in vivo to form quinones as the ultimate inducers.202 Endogenous p- and o-hydroquinones, such as catechol estrogens (44), dopamine (45) and L-DOPA (46), also have been shown to induce ARE-dependent defensive responses.202 (S)-(+)-Apomorphine (47),203,204 a catechol-containing tetrahydroquinoline derivative and a non-selective dopamine agonist, has also been shown to activate Nrf2–mediated gene expression.
Figure 5.
Diphenols and their oxidation products that contain electrophilic Michael acceptors.
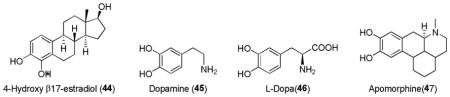
Quercetin (48) is a plant-derived polyphenolic flavonoid antioxidant found in many fruits, vegetables, leaves and grains. Quercetin has been promoted as being effective against a wide variety of diseases, including cancer.205 The cancer-preventive effects of quercetin have been attributed to its pharmacological properties including antiproliferative, antioxidative and antiinflammatory activities. Quercetin has been shown to increase cellular Nrf2 level not only by inhibiting Nrf2 degradation but also by increasing the level of Nrf2 mRNA.206 Other natural flavonoids such as fisetin (49),207 koparin (50),175 and genistein (51)208 were found to induce the expression of ARE-controlled antioxidant genes209.
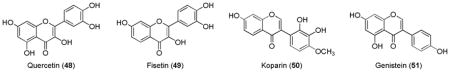
Resveratrol (52) is a stilbenoid and nonflavonoid polyphenolic compound mainly found in the skin of red grapes and in other fruits. It has been shown to extend lifespan and retard aging parameters.210 Resveratrol was found to induce Nrf2-mediated ARE genes in a similar fashion to quercetin.211,212
Green tea contains catechin polyphenols, the most abundant of which is epigallocatechin gallate (EGCG, 53) which is believed to be responsible for most of the antioxidant and chemopreventive activities of green tea.213 It has been shown that EGCG increases the Nrf2 level in nucleus and also induces the ARE-luciferase reporter gene transactivation.214,215
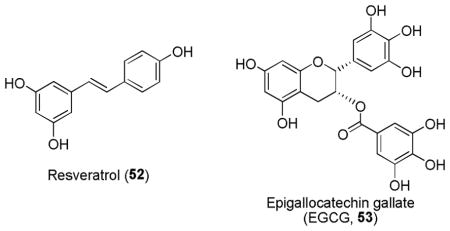
Carnosol (54) and its acid derivative, carnosic acid (55) are catechol-type abietane diterpenes from Rosmarinus officinalis (rosemary). They are used as a preservative (for their antimicrobial effect) or antioxidant in food products and were shown to increase Nrf2 levels and reduce lipid peroxidation and ROS generation.216 Carnosic acid (55) exhibited a superior protection than carnosol (54), probably due to its increased solubility or decreased toxicity.217
Other catechol-type antioxidant compounds such as purpurogallin carboxylate175 (56), nordihydroguaiaretic acid175,218 (57),160,203 1,2-naphthoquinone (58)219 and its natural diterpene derivative tanshinone IIA (59),175 were also shown to activate Nrf2 and upregulate some of the Nrf2’s downstream genes.
3. Isothiocyanates and sulfoxythiocarbamates
Natural isothiocyanates (ITCs) occur as inert glucosinolate precursors that are abundant in cruciferous vegetables such as broccoli and cabbage.220 Upon ingestion, glucosinolates are converted to ITCs by the action of gastrointestinal microbial flora. The central carbon atom of the isothiocyanate (–N=C=S) group is highly electrophilic which can readily react with sulfhydryl group to form a dithiocarbamate.221 This reaction of ITCs with sulfhydryl groups of cysteine residues in Keap1 is believed to result in the disruption of Keap1-Nrf2 interactions and the ultimate induction of ARE genes.
Natural isothiocyanates sulforaphane (SFN, 60) and phenethyl isothiocyanate (PEITC, 61) are the most well studied in this group and many synthetic isothiocyanates (including a derivative with C=O replacement of S=O, 62) were evaluated for their ARE-inducing activities.157,222–227 Recently, a new series of sulfoxythiocarbamate sulforaphane analogs were synthesized, while retaining the structural features important for ARE-inducing activity.224 Sulfoxythiocarbamate analogs are considerably less reactive electrophiles than sulforaphane, unlike isothiocyanates that form reversible conjugates with thiols; the reaction with sulfoxythiocarbamates is essentially irreversible. An analogue, S-ethyl (4-hex-5-ynyloxy-benzyl)-(5-oxohexyl)-thiocarbamate sulfoxide (63) was found to be a useful probe for further investigation of Nrf2 activation.224
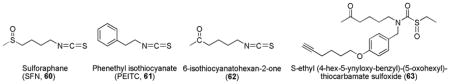
4. Dithiolethiones and Diallyl sulfides
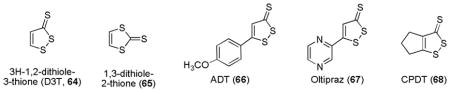
Dithiolethiones are five-membered cyclic sulfur-containing compounds known to have cytoprotective properties against cancinogens.108 3H-1,2-Dithiole-3-thione (D3T, 64) is the simplest dithiolethione isolated from cruciferous vegetables such as cabbage and brussel sprouts. It contains within the five-membered ring a disulfide bond that is known to undergo thiol-disulfide exchange with sulfhydryl groups.228 Moreover, its regioisomer 1,3-dithiole-2-thione (65) was ineffective even at much higher concentrations, indicating the importance of 1,2-disulfide structure.229 D3T and other synthetic substituted derivatives such as ADT (66) and oltipraz (67) have been shown to induce the expression of cytoprotective genes through the Keap1-Nrf2 signaling pathway.230,231 Oltipraz was originally developed as an antischistosomal agent and is currently under clinical trials as cancer chemopreventive agents. Recently, a cyclopentane analogue CPDT (68) has emerged as a promising ARE-inducer in this class.232
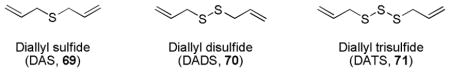
Allyl sulfides are a class of organosulfur compounds found in Allium vegetables (including garlic and onion) and have been reported to suppress the growth of multiple cancer cells.233 Some lipophilic thioethers such as diallyl sulfide (DAS, 69), diallyl disulfide (DADS, 70), and diallyl trisulfide (DATS, 71) have been shown to upregulate the expression of detoxifying enzymes with inducing strength in the order of DATS > DADS > DAS; but, the mechanism underlying ARE-inducing activity is poorly understood.234,235
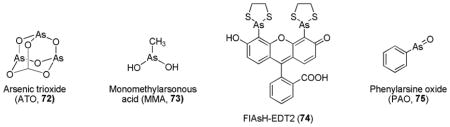
5. Trivalent arsenicals
The high affinity of arsenic (III) for thiols is believed to be one of the causes for both acute and chronic toxicity of arsenic compounds.236 Interestingly, it has been shown that low doses of arsenic exposure can decrease the incidence of cancer.237 Arsenic compounds such as sodium arsenite (NaAsO2), Arsenic trioxide (ATO, As2O3, 72) and monomethylarsonous acid (CH3As[OH]2, MMA, 73) were shown to activate Nrf2 via the modulation of Keap1-Cul3 E3 ubiquitin ligase complex.146 Recently, the fluorescein-based biarsenical thiol labeling reagent (FlAsH-EDT2, 74) and phenylarsine oxide (PAO, 75) were found to produce Keap1-dependent activation of Nrf2.102
6. Vicinal dimercaptans
Vicinal dithiol groups (dimercaptans) can be transformed in vivo into the electrophilic disulfide bonds. Dimercaptans like (±)-2,3-dimercapto-1-propanol (British antilewisite or BAL) (76),32,49 1,2-ethanedithiol (77)49 and 2,3-dimercaptosuccinic acid (78)175 were shown to activate Nrf2. Another dimercaptan variant, (R)-Lipoic acid or Kα-Lipoic acid (79), is derived from octanoic acid with 6,8-dithiol forming a 5-membered ring via a disulfide bond. (R)-Lipoic acid (79) is found in almost all foods, but slightly more in spinach and broccoli, and it is also produced endogenously. (R)-Lipoic acid (79) is an orthomolecular nutrient, originally claimed as an antioxidant and it has been shown to induce various antioxidant enzymes through the activation of Nrf2.238,239

7. Selenium-based compounds
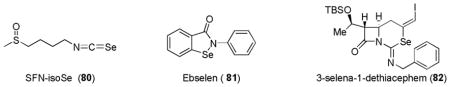
Mechanistically, thiol-selenide or selenol-sulfide exchange is well known due to the similar electronegativity of sulfur and selenium.239 Selenites and various organoselenium compounds are reported to have cytoprotective activities.240 Interestingly, SFN-isoSe (80), a selenium isostere of sulforaphane (SFN, 60) was found to have better Nrf2-inducing activity than sulforaphane.241 Ebselen (81), a potent multifunctional antioxidant and antiinflammatory agent, was shown to regulate the Keap1-Nrf2-mediated expression of detoxification enzyme genes.242,243 Recently, an organoselenium compound, 3-selena-1-dethiacephem (82) was identified as an activator of Nrf2-ARE and as a direct ROS scavenger.244
8. Polyenes
Polyenes are poly-unsaturated organic compounds that contain one or more sequences of alternating double and single carbon-carbon bonds. Due to the high degree of unsaturation, these compounds may readily undergo biotransformtion to electrophilic metabolites that can react with free sulfhydryl groups. Carotenoids, a class of polyene have been shown to have cancer preventive properties.245 It has been demonstrated that carotenoids stimulate the ARE transcription system and induce antioxidative and cytoprotective enzymes. A recent study investigated the ARE-inducing activity of lycopene (83), a red carotenoid pigment mainly found in tomatoes, and its potential oxidation metabolite, 10,10′-diapocarotene-10,10′-dial (84).246 It was found that 84 is a more potent ARE-inducer than the intact lycopene, but is about equally potent as compared to an oxidized preparation of lycopene. It was suggested that caroteinoids like lycopene may be metabolized in vivo to reactive electrophilic metabolites like 84 which contains Michael acceptors that covalently modify Keap1 resulting in activation of Nrf2 and elevated expression of ARE genes.246
![]()
9. Hydroperoxides
Oxidative stress is caused by the over-production of free radicals and peroxides that could overwhelm the biological system’s ability to detoxify the reactive species or to repair the resulting damage.247 The O-O bond in peroxides can easily break and release oxygen free radicals that readily react with sulfhydryl groups to form sulfenates (RSO−). Low levels of H2O2 and organic peroxides like tert-butyl hydroperoxide (85) and cumol hydroperoxide (86) can act as inducers of ARE genes through the oxidation of sulfhydryl groups in Keap1 leading to the activation of Nrf2 and ARE genes.49,150

10. Heavy metals and metal complexes
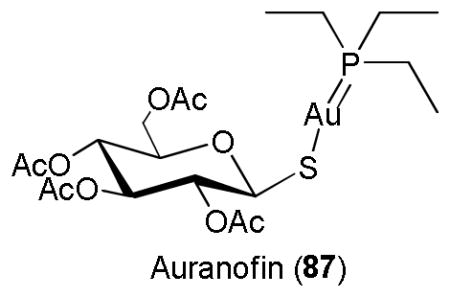
Humans require varying amounts of heavy metals such as iron, cobalt, copper, and zinc, but excessive heavy metals can be dangerous, and some heavy metals such as mercury, cadmium, gold, and lead can be very toxic.247 A number of the heavy metals are known to induce the expression of ARE genes in the order of Hg > Cd > Zn.32 Cadmium chloride (CdCl2) is a known human carcinogen and it induces cancer by multiple mechanisms and the most important among them are aberrant gene expression, inhibition of DNA damage repair, induction of oxidative stress, and inhibition of apoptosis.248 Cd also found to induce ARE genes, which can be viewed as a biological defense mechanism to counter-act the effects of Cd-induced oxidative stress.248,249 Auranofin (87) is an organogold complex and antirheumatic drug, and it was also shown to have antiinflammatory effects through the activation of Keap1-Nrf2-ARE signaling pathway.250
11. Miscellaneous inducers
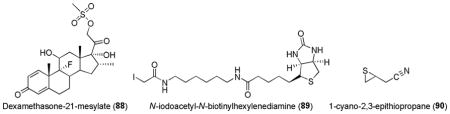
Other ARE-inducers that have been shown to covalently modify Keap1 include those with a good leaving group that can react with sulfhydryl groups in a SN2 fashion such as dexamethasone-21-mesylate (88) and N-iodoacetyl-N-biotinylhexylenediamine (89).67,86 Recently, 1-cyano-2,3-epithiopropane (90), a thiirane degradation product of glucosinolates found in cabbage, was shown to induce ARE genes and confer protection of rodent hepatocytes against the genotoxicity of acrolein.251
B. Nrf2 Inhibitors
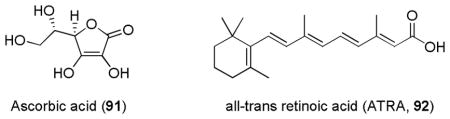
In contrast to Nrf2 inducers, the identification of Nrf2 inhibitors or inactivators has not gained much attention until the last few years. With the recent understanding of the molecular mechanism of Nrf2 inactivation and its potential medical application, several small molecules have been found to suppress the Nrf2 pathway.48,51,84,109 Ascorbic acid (Vitamin C, 91), an antioxidant, was found to reduce peroxide levels and suppress the levels of Nrf2/DNA complex at the ARE of the GCL gene promoter. Treatment of imatinib-resistant BCR/ABL+ CML cell line KCL22/SR with ascorbic acid resulted in reduced GSH level and enhanced sensitivity to imatinib. The restoration of imatinib sensitivity in KCL22/SR was at least partially due to ascorbic acid’s inhibition of Nrf2 activity.252 EGCG (53) was also shown to suppress Nrf2 activity and reduce HO-1 expression in A549 cells, although the concentrations of EGCG required for its inhibitory effect were high (> 200 μM).253 All-trans-retinoic acid (ATRA, 92) significantly reduced both in vitro and in vivo the ability of Nrf2 to mediate the induction of ARE-driven genes by known ARE-inducing agents including tert-butylhydroquinone (tBHQ). ATRA inhibition of ARE gene expression was not the result of decreased nuclear accumulation of Nrf2 but due to the reduced binding of Nrf2 to ARE; in the presence of ATRA, Nrf2 forms a complex with retinoic acid receptor alpha (RARα) and the Nrf2:RARα-containing complexes do not bind to ARE.254
A quassinoid compound purified from the extract of plant Brucea javanica, brusatol (93), was found to enhance the degradation of Nrf2 in a Keap1-dependent manner and inhibit the Nrf2-mediated stress response and tumor growth.114 However, brusatol is a well known cytotoxic, its specificity and precise mechanism in Nrf2 degradation is not yet understood.255 Using a cell-based ARE-reporter assay, luteolin (94) was shown to be a potent Nrf2 inhibitor. Luteolin is a flavonoid that inhibited ARE-driven gene expression in a redox independent manner.115 In non-small-cell lung cancer A549 cells, which possess constitutively active Nrf2, luteolin elicited a dramatic reduction in Nrf2 at both the mRNA and the protein levels, leading to decreased Nrf2 binding to AREs, down-regulation of ARE-driven genes, and depletion of reduced glutathione. The luteolin’s activity appears to be related to its role in accelerating the turnover of Nrf2 mRNA.115 However, in PC12 cells, luteolin was shown to have opposite effect of preventing apoptosis, increasing the expression of heme oxygenase-1 (HO-1) mRNA and protein levels, and enhancing the binding of Nrf2 to ARE.256 Since luteolin is a polyphenolic flavonoid, it is not surprising that luteolin possess some of the similar ARE-inducing activities in certain cell lines as other flavonoids.
Ochratoxin A (OTA, 95) is produced by Aspergillus and Penicillium subspecies and frequently present in feedstuffs. OTA’s nephrotoxicity and carcinogenicity could be related to its inhibition of Nrf2.257 Reduction by OTA in Nrf2-dependent gene expression was observed in the kidney and the OTA-mediated effects could be prevented by pretreatment with inducers of Nrf2 activity. In another study, OTA significantly decreased the mRNA levels of Nrf2 as well as Nrf2-dependent genes including γ-glutamylcysteinyl synthetase and glutathione-S-transferase in LLC-PK1 cells, which was accompanied by a lowered nuclear translocation and transactivation of Nrf2.258
C. Towards the discovery of direct inhibitors of Keap1-Nrf2 interaction
As discussed above, most of the known inducers activate ARE system through electrophilic attack on the cysteine sulfhydryl group of Keap1, thereby disrupting Keap1-Cul3 and/or Keap1-Nrf2 interactions. Modulation of ARE activation via direct inhibition of these Keap1 associated protein-protein interactions could present novel opportunities for the discovery of novel small molecule modulators of the Keap1-Nrf2-ARE pathway potentially with many advantages over indirect covalent modulators of Keap1 protein.259
Association between Keap1 and Cul3 has been proven to be important for Nrf2 ubiquitination, but the molecular details of their interaction remains poorly understood.77 Another concern is that disruption of Keap1 BTB domain binding to Cul3 may have limited benefits since many other BTB-Kelch substrate adaptor proteins have similarly conserved BTB domain for their association.260 Only further crystallographic structural and functional studies can shed light on molecular aspects of Keap1-Cul3 interaction and its potential for inhibitor design. Gratefully, high resolution structural data is available for the interaction between Keap1-Nrf2, which can be exploited for rational design of compounds targeting Nrf2 binding site of Keap1 with greater selectivity and affinity.261
1. Keap1-Nrf2 interaction-Structural insights forinhibitor design
The high resolution three dimensional structures of the Kelch domain of Keap1 with and without Nrf2 derived peptides were determined by x-ray crystallography.261–265 The Kelch domain forms a highly symmetric six-bladed β-propeller structure with each blade consisting of four β-sheets and highly conserved kelch repeats, such as the glycine doublet and Tyr/Trp pair. All six blades of the β-propeller structure contribute to the binding of Neh2 domain ETGE motif of Nrf2.
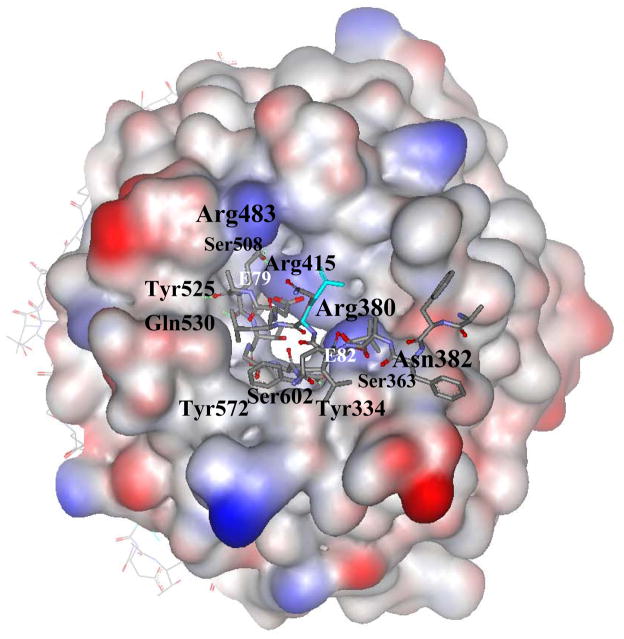
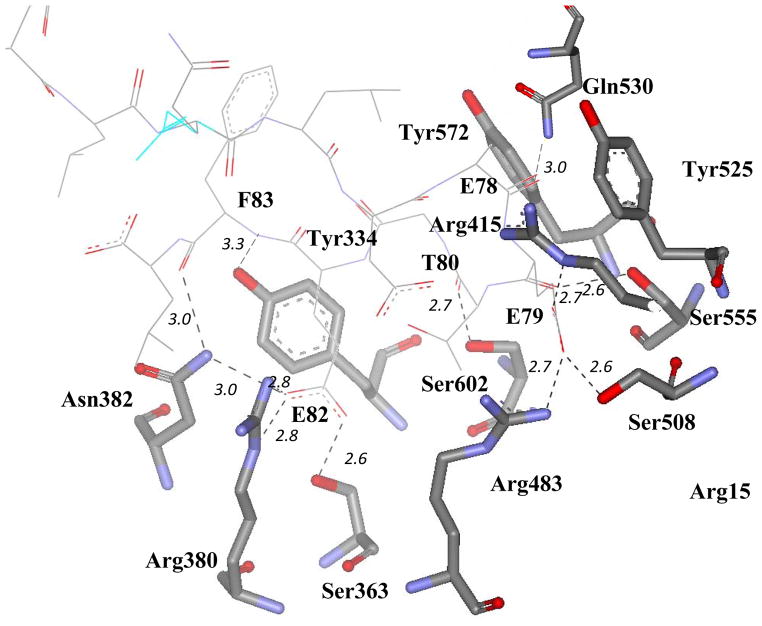
Nrf2-derived peptide of 16 residues corresponding to Nrf2 amino acids 69AFFAQLQLDEETGEFL84 binds to a shallow pocket at the top face of the Kelch domain as shown in Figure 6.261 The 16mer Nrf2 peptide has two antiparallel β-strands connected by two tight overlapping type-1 β-turns with the highly conserved sequence DxETGE motif comprising the tip of the hairpin when bound to Keap1. The turn region is stabilized by hydrogen bonds involving the side chains of D77 and T80 and the peptide backbone. The peptide backbone makes five contacts with the Kelch domain, four from the carbonyl oxygen atoms of E78, E79, T80, and F83, and one from a backbone amide group of F83. The peptide binding site is positively charged region due to highly conserved Arg residues. Only the side chains of peptide glutamate residues E79 and E82 make multiple interactions with the binding site. The carboxylate group of E79 interacts with the side chains of Arg415, Arg483, and Ser508; whereas the carboxylate group atoms of E82 make contacts with the side chains of Ser363, Asn382, and Arg380 as shown in Figure 7. Further, there are plenty of functional and structural evidences that strongly support the DxETGE motif as the principal Keap1 binding site in Nrf2, which is consistent with the hinge and latch model of Nrf2 activation. The lysine-rich α-helix required for ubiquitination is located at a distance of 10–30 amino acids away and the second low affinity Keap1 binding motif of LxxQDxDLG is positioned at a distance of approximately 50 amino acids away on the N-terminal side of the DxETGE motif. This structural information suggested that structure-based design of small molecules that disrupt the high affinity interaction of DxETGE motif with Kelch domain of Keap1 that interfere with ubiquitination of Nrf2 and ultimately become target-directed and selective reversible inducers of Nrf2-ARE system.
Figure 6.
The binding mode of 16mer Nrf2 peptide in the Kelch domain of Keap1 (PDB Code: 2FLU). The bound protein residues (three letter code) involved in the interaction are depicted on the surface. Nrf2 peptide is displayed as stick models and interacting amino acid E79 and E82 residues are indicated.
Figure 7.
The contacts between the 16mer Nrf2 peptide and the Kelch domain of Keap1 (PDB Code: 2FLU). Keap1 residues are displayed as stick models and Nrf2 peptide resides are displayed as line. Intermolecular hydrogen bonds are given with their distances (Å). Residues are not involved in binding are omitted for clarity.
2. Nrf2-based peptide inhibitors of Keap1-Nrf2 interaction
Kelch domain of Keap1 was initially shown to bind to the fusion protein of GST-Nrf2 Neh2 domain immobilized on a sensor chip surface coated with anti-GST antibody in a concentration-dependent manner with a calculated KDsurface of 580 nM.19 More recently, binding affinities between Keap1 and Nrf2 in solution were characterized to be in the single digit nanomolar ranger by isothermal titration calorimetry (ITC).91,112,152 The ETGE motif binds to Keap1 with a much higher binding affinity than the DLG motif does (KD= 5×10−9 vs 1.0×10−6 M).89,90
Several ETGE-containing Nrf2 peptides have been reported to disrupt the interaction between Keap1 and Nrf2.73 The 16mer Nrf2 peptide 69AFFAQLQLDEETGEFL84 and the 14mer Nrf2 peptide 74LQLDEETGEFLPIQ87 were able to displace Nrf2 from Keap1-Nrf2 complex effectively with comparable KD’s of 20 nM as obtained using ITC.261 The shorter 10mer Nrf2 peptide 76LDEETGEFLP85, however, was shown to be less effective for the displacement. To better understand the interaction between Keap1 and Nrf2, and to systematically determine the minimal Nrf2 peptide sequence required for disrupting Keap1-Nrf2 interaction, we designed and synthesized a series of Nrf2 peptides containing the ETGE motif and determined their binding affinities to the Kelch domain of Keap1 in solution using a surface plasmon resonance (SPR)-based solution competition assay.266 The binding affinity of Nrf2 peptides to Keap1 Kelch domain was not lost until after the deletion of eight residues from the N-terminus of the 16mer Nrf2 peptide. The 9mer Nrf2 peptide has a moderate KDsolution of 352 nM, which was decreased to a KDsolution of 21 nM upon removal of the positive charge at the N-terminus by acetylation. These results suggest that the minimal Nrf2 peptide sequence required disrupt the interaction between Keap1 and Nrf2 is the 9mer Nrf2 peptide with the sequence of 76LDEETGEFL84.
Since Nrf2 can elevate the expression of multiple cytoprotective proteins to protect cells following insults such as stroke and traumatic brain injury,267–269 Hybrid peptides were constructed by incorporating ETGE-containing Nrf2 peptide and cell permeating domain of HIV-TAT protein and tested in vivo by intracerebroventricular (i.c.v.) infusion into brain injured mice. The TAT-Cal-DEETGE hybrid peptide with a calpain cleavable peptide (PLFAER) inserted in between the 10mer Nrf2 peptide (76LDEETGEFLP85) and TAT peptide (YGRKKRRQRRR) was shown to increase the mRNA level of several Nrf2-regulated genes and also attenuate blood brain barrier (BBB) compromise associated with brain injury when infused before or after the brain injury.270,271 It is worthwhile to note that the uninjured mice brain was not affected by the injection of the same peptide, which suggested selectivity and therapeutic potential of such an approach.
3. Screening assays for the discovery of small molecule modulators of Keap1-Nrf2-ARE pathway
Several assays including HTS assays have been developed for the screening and identification of small molecule modulators of Keap1-Nrf2-ARE pathway.272–274 Instead of the widely used reporter gene assay for monitoring the transcriptional activation of ARE genes, this section focuses on the screening assays recently developed to directly measure the modulation of Keap1-Nrf2 interaction.
a. Cell-based Neh2-Luciferase assay
Neh2 domain as one of six Nrf2 domains is reported to specifically interact with Keap1 Kelch domain and is responsible for the subsequent ubiquitin conjugation. Based on the findings that Neh2 domain is critical for Keap1 binding and sufficient for recognition and degradation of Neh2-containing protein, 275 a neh2-luciferase reporter system is constructed with Neh2 domain fused to luciferase gene as a tool for real-time monitoring the Nrf2 activation.276 The overexpressed Neh2-lusciferase fusion protein competes with endogenous Nrf2 for Keap1 binding and subsequent ubiquitination and proteasomal degradation, whereas Nrf2 activators disrupt Neh2-luc-Keap1-Cul3 complex and prevent the Neh2-luciferase protein from degradation. Different from the ARE-luciferase assay, the increase luciferase activity could be a direct measure of the ability of a particular compound on disrupting Keap1-Nrf2 interaction and/or Keap1-Cul3 interaction, i.e. the effect on the step controlling Nrf2 stability. In contrast to the ARE reporter gene assay which has a delayed response, the Neh2-luciferase assay has the advantage of immediate response upon addition of Nrf2 activators. Such real-time monitoring enables differentiation of various Nrf2 activators by following their kinetics of reporter activation. Neh2-luciferase reporter system has been shown to be suitable for HTS with Z′-value of >0.7. However, as a cell-based assay the constant reading will rely on the stability of the reporter cell line.
b. SPR-based solution competition assay
To screen for selective Nrf2 activators that directly inhibit Keap1-Nrf2 interaction, we developed an SPR-based solution competition assay by allowing the Kelch Domain of Keap1 to flow in solution over an SPR sensor chip surface with the 16mer Nrf2 peptide immobilized.266 Instead of immobilizing the unstable Nrf2 or Keap1 protein onto the sensor chip surface, a biotin-labeled 16mer Nrf2 peptide was immobilized as the ligand on a streptavidin sensor chip, which was demonstrated to be the optimal immobilization method that provided sensitive and stable surfaces for both kinetic analysis of Keap1-Nrf2 peptide interaction and detection of the free Keap1 Kelch domain protein concentration in solution competition assays. The assay is capable of measuring direct inhibition of Keap1-Nrf2 interaction and determining the minimal Nrf2 peptide sequence required to bind to Keap1. The 9mer Nrf2 peptide with the sequence of LDEETGEFL was found to be the minimal sequence required for Keap1 binding using the SPR-based solution competition assay. One limitation of SPR-based assay is its limited throughput, which prevents it from being used as the primary assay in high throughput applications.
c. Fluorescence polarization assay
In the effort to identify small molecule inhibitors of Keap1-Nrf2 interaction, we developed a fluorescence polarization assay that have adapted to the high throughput screening of large chemical libraries.277 Fluorescently labeled Nrf2 peptides containing the ETGE motif were designed and synthesized as tracers to detect the direct inhibitors of Keap1-Nrf2 interaction. The tracers were optimized to increase the dynamic range of the assay and their binding affinity to Keap1 Kelch domain. FITC labeled Nrf2 9mer peptide amide was shown to have the largest assay window due to the reduced “propeller effect” and a strong binding affinity that rivals the longer 16mer Nrf2 peptide. The solution competition binding assay with FITC-9mer Nrf2 amide as probe was able to rank the binding affinity of 7mer to 16mer Nrf2 peptide inhibitors that were consistent with those measured using other biochemical methods such as ITC and SPR.264,266 The FP assay exhibits considerable tolerance towards DMSO and produced Z′-factors as high as 0.9 in 384-well format and can be easily adapted to 1534-well format in high throughput screening. This FP assay has been successfully applied for the screening of the NCI Diversity Set II of 1364 compounds and the NIH Clinical Collection of 446 compounds and used by the Broad Institute in the screening of MLPCN library of 330,000 compounds for the discovery of small molecule inhibitors of Keap1-Nrf2 interaction (PubChem Assay ID: 504523, 504540). Further optimization of the probe led to cyanine-labeled 9mer Nrf2 peptide amide, which can be used along with the FITC-9mer Nrf2 peptide amide in a HTS screening assay to discover small molecule inhibitors of Keap1-Nrf2 interaction as Nrf2 activators.
4. CONCLUSION
In summary, the Keap1-Nrf2-ARE signaling pathway is an important antioxidant defense mechanism which activates cellular adaptive response against oxidative and other types of stress. Keap1 is the negative regulator of Nrf2 and Nrf2 is a master effector of ARE system. Activation of ARE system by negatively controlling the Keap1 protein can induce a battery of antioxidant response genes critical for preventing oxidative damage, inflammation and tumourigenesis. Thus, pharmacological activation of Keap1-Nrf2-ARE system holds a great promise for the development of novel class of antioxidant, antiinflammatory and anticancer agents. A number of small molecules from different classes of natural products and synthetics have been identified as ARE inducers. Several potent inducers such as sulforaphane, bardoxolone methyl (CDDO-me), oltipraz, and ebselen are currently undergoing clinical trials for a variety of diseases and conditions such as breast and prostate cancer, cystic fibrosis, asthma, COPD, chronic kidney disease in type 2 diabetes, and non-alcoholic fatty liver disease. Most of the known inhibitors share a common feature of the electrophilicity, presumably modify the cysteine sulfhydryl groups of Keap1 for ARE activation. A risk of “off-target” toxic effects has also been associated with these ARE inducers due to their potential to react with other cysteine-containing proteins and enzymes. Optimum activation of Nrf2 by these ARE inducers at low sub-toxic dose level is highly desirable. Intriguingly, Keap1-Nrf2 interface has evolved as a direct molecular target of ARE activation and opens up a new direction for the design of reversible ARE inducers with high specificity and potency. In a different perspective, aberrant Nrf2 activation promotes tumor formation and contributes to the drug resistance during cancer chemotherapy, and specific inhibitors of Nrf2 can be developed to improve the effectiveness of cancer therapy using chemotherapeutic agents. Therefore, modulation of Keap1-Nrf2-ARE pathway with pharmacological agents can lead to innovative therapeutic strategies for many diseases and conditions.
Acknowledgments
We gratefully acknowledge the financial support of grants CA133791, CA125868, and MH093197 from the National Institutes of Health.
Footnotes
This articleis dedicated to Professor Lester A. Mitscher on the occasion of his 80th birthday.
References
- 1.Finkel T, Holbrook NJ. Oxidants, oxidative stress and the biology of ageing. Nature. 2000;408(6809):239–247. doi: 10.1038/35041687. [DOI] [PubMed] [Google Scholar]
- 2.Leeuwenburgh C, Heinecke JW. Oxidative stress and antioxidants in exercise. Curr Med Chem. 2001;8(7):829–838. doi: 10.2174/0929867013372896. [DOI] [PubMed] [Google Scholar]
- 3.Ozben T. Oxidative stress and apoptosis: impact on cancer therapy. J Pharm Sci. 2007;96(9):2181–2196. doi: 10.1002/jps.20874. [DOI] [PubMed] [Google Scholar]
- 4.Devasagayam TP, Tilak JC, Boloor KK, Sane KS, Ghaskadbi SS, Lele RD. Free radicals and antioxidants in human health: current status and future prospects. J Assoc Physicians India. 2004;52:794–804. [PubMed] [Google Scholar]
- 5.Wojcik M, Burzynska-Pedziwiatr I, Wozniak LA. A review of natural and synthetic antioxidants important for health and longevity. Curr Med Chem. 2010;17(28):3262–3288. doi: 10.2174/092986710792231950. [DOI] [PubMed] [Google Scholar]
- 6.Barnham KJ, Masters CL, Bush AI. Neurodegenerative diseases and oxidative stress. Nat Rev Drug Discov. 2004;3(3):205–214. doi: 10.1038/nrd1330. [DOI] [PubMed] [Google Scholar]
- 7.Benz CC, Yau C. Ageing, oxidative stress and cancer: paradigms in parallax. Nat Rev Cancer. 2008;8(11):875–879. doi: 10.1038/nrc2522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Khansari N, Shakiba Y, Mahmoudi M. Chronic inflammation and oxidative stress as a major cause of age-related diseases and cancer. Recent Pat Inflamm Allergy Drug Discov. 2009;3(1):73–80. doi: 10.2174/187221309787158371. [DOI] [PubMed] [Google Scholar]
- 9.Federico A, Morgillo F, Tuccillo C, Ciardiello F, Loguercio C. Chronic inflammation and oxidative stress in human carcinogenesis. Int J Cancer. 2007;121(11):2381–2386. doi: 10.1002/ijc.23192. [DOI] [PubMed] [Google Scholar]
- 10.Sayre LM, Perry G, Smith MA. Oxidative stress and neurotoxicity. Chem Res Toxicol. 2008;21(1):172–188. doi: 10.1021/tx700210j. [DOI] [PubMed] [Google Scholar]
- 11.Sen S, Chakraborty R, Sridhar C, Reddy YSR, De B. Free Radicals, Antioxidants, Diseases and Phytomedicines: Current Status and Future Prospect. Int J Pharm Sci Rev Res. 2010;3(1):91–100. [Google Scholar]
- 12.Jacob R. The integrated antioxidant system. Nutr Res. 1995;15(5):755–766. [Google Scholar]
- 13.Lyakhovich VV, Vavilin VA, Zenkov NK, Menshchikova EB. Active defense under oxidative stress. The antioxidant responsive element. Biochemistry (Mosc) 2006;71(9):962–974. doi: 10.1134/s0006297906090033. [DOI] [PubMed] [Google Scholar]
- 14.Urso ML, Clarkson PM. Oxidative stress, exercise, and antioxidant supplementation. Toxicology. 2003;189(1–2):41–54. doi: 10.1016/s0300-483x(03)00151-3. [DOI] [PubMed] [Google Scholar]
- 15.Dinkova-Kostova AT, Talalay P. Direct and indirect antioxidant properties of inducers of cytoprotective proteins. Mol Nutr Food Res. 2008;52 (Suppl 1):S128–138. doi: 10.1002/mnfr.200700195. [DOI] [PubMed] [Google Scholar]
- 16.Talalay P. Chemoprotection against cancer by induction of phase 2 enzymes. Biofactors. 2000;12(1–4):5–11. doi: 10.1002/biof.5520120102. [DOI] [PubMed] [Google Scholar]
- 17.Holtzclaw WD, Dinkova-Kostova AT, Talalay P. Protection against electrophile and oxidative stress by induction of phase 2 genes: the quest for the elusive sensor that responds to inducers. Adv Enzyme Regul. 2004;44:335–367. doi: 10.1016/j.advenzreg.2003.11.013. [DOI] [PubMed] [Google Scholar]
- 18.Itoh K, Chiba T, Takahashi S, Ishii T, Igarashi K, Katoh Y, Oyake T, Hayashi N, Satoh K, Hatayama I, Yamamoto M, Nabeshima Y. An Nrf2/small Maf heterodimer mediates the induction of phase II detoxifying enzyme genes through antioxidant response elements. Biochem Biophys Res Commun. 1997;236(2):313–322. doi: 10.1006/bbrc.1997.6943. [DOI] [PubMed] [Google Scholar]
- 19.Itoh K, Wakabayashi N, Katoh Y, Ishii T, Igarashi K, Engel JD, Yamamoto M. Keap1 represses nuclear activation of antioxidant responsive elements by Nrf2 through binding to the amino-terminal Neh2 domain. Genes Dev. 1999;13(1):76–86. doi: 10.1101/gad.13.1.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nguyen T, Yang CS, Pickett CB. The pathways and molecular mechanisms regulating Nrf2 activation in response to chemical stress. Free Radic Biol Med. 2004;37(4):433–441. doi: 10.1016/j.freeradbiomed.2004.04.033. [DOI] [PubMed] [Google Scholar]
- 21.Stewart JD, Hengstler JG, Bolt HM. Control of oxidative stress by the Keap1-Nrf2 pathway. Arch Toxicol. 2011;85(4):239. doi: 10.1007/s00204-011-0694-1. [DOI] [PubMed] [Google Scholar]
- 22.Baird L, Dinkova-Kostova AT. The cytoprotective role of the Keap1-Nrf2 pathway. Arch Toxicol. 2011;85(4):241–272. doi: 10.1007/s00204-011-0674-5. [DOI] [PubMed] [Google Scholar]
- 23.Hochmuth CE, Biteau B, Bohmann D, Jasper H. Redox regulation by Keap1 and Nrf2 controls intestinal stem cell proliferation in Drosophila. Cell Stem Cell. 2011;8(2):188–199. doi: 10.1016/j.stem.2010.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Surh YJ, Kundu JK, Na HK. Nrf2 as a master redox switch in turning on the cellular signaling involved in the induction of cytoprotective genes by some chemopreventive phytochemicals. Planta Med. 2008;74(13):1526–1539. doi: 10.1055/s-0028-1088302. [DOI] [PubMed] [Google Scholar]
- 25.Brigelius-Flohe R, Flohe L. Basic Principles and Emerging Concepts in the Redox Control of Transcription Factors. Antioxid Redox Signal. 2011 doi: 10.1089/ars.2010.3534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hayes JD, McMahon M. NRF2 and KEAP1 mutations: permanent activation of an adaptive response in cancer. Trends Biochem Sci. 2009;34(4):176–188. doi: 10.1016/j.tibs.2008.12.008. [DOI] [PubMed] [Google Scholar]
- 27.Kensler TW, Wakabayashi N, Biswal S. Cell survival responses to environmental stresses via the Keap1-Nrf2-ARE pathway. Annu Rev Pharmacol Toxicol. 2007;47:89–116. doi: 10.1146/annurev.pharmtox.46.120604.141046. [DOI] [PubMed] [Google Scholar]
- 28.Lee JM, Johnson JA. An important role of Nrf2-ARE pathway in the cellular defense mechanism. J Biochem Mol Biol. 2004;37(2):139–143. doi: 10.5483/bmbrep.2004.37.2.139. [DOI] [PubMed] [Google Scholar]
- 29.Taguchi K, Motohashi H, Yamamoto M. Molecular mechanisms of the Keap1-Nrf2 pathway in stress response and cancer evolution. Genes Cells. 2011;16(2):123–140. doi: 10.1111/j.1365-2443.2010.01473.x. [DOI] [PubMed] [Google Scholar]
- 30.Calkins MJ, Johnson DA, Townsend JA, Vargas MR, Dowell JA, Williamson TP, Kraft AD, Lee JM, Li J, Johnson JA. The Nrf2/ARE pathway as a potential therapeutic target in neurodegenerative disease. Antioxid Redox Signal. 2009;11(3):497–508. doi: 10.1089/ars.2008.2242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jeong WS, Jun M, Kong AN. Nrf2: a potential molecular target for cancer chemoprevention by natural compounds. Antioxid Redox Signal. 2006;8(1–2):99–106. doi: 10.1089/ars.2006.8.99. [DOI] [PubMed] [Google Scholar]
- 32.Giudice A, Montella M. Activation of the Nrf2-ARE signaling pathway: a promising strategy in cancer prevention. Bioessays. 2006;28(2):169–181. doi: 10.1002/bies.20359. [DOI] [PubMed] [Google Scholar]
- 33.Lau A, Villeneuve NF, Sun Z, Wong PK, Zhang DD. Dual roles of Nrf2 in cancer. Pharmacol Res. 2008;58(5–6):262–270. doi: 10.1016/j.phrs.2008.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yates MS, Kensler TW. Chemopreventive promise of targeting the Nrf2 pathway. Drug News Perspect. 2007;20(2):109–117. doi: 10.1358/dnp.2007.20.2.108343. [DOI] [PubMed] [Google Scholar]
- 35.Li J, Ichikawa T, Janicki JS, Cui T. Targeting the Nrf2 pathway against cardiovascular disease. Expert Opin Ther Targets. 2009;13(7):785–794. doi: 10.1517/14728220903025762. [DOI] [PubMed] [Google Scholar]
- 36.Yu X, Kensler T. Nrf2 as a target for cancer chemoprevention. Mutat Res. 2005;591(1–2):93–102. doi: 10.1016/j.mrfmmm.2005.04.017. [DOI] [PubMed] [Google Scholar]
- 37.Jung KA, Kwak MK. The Nrf2 system as a potential target for the development of indirect antioxidants. Molecules. 2010;15(10):7266–7291. doi: 10.3390/molecules15107266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pergola PE, Krauth M, Huff JW, Ferguson DA, Ruiz S, Meyer CJ, Warnock DG. Effect of bardoxolone methyl on kidney function in patients with T2D and Stage 3b-4 CKD. Am J Nephrol. 2011;33(5):469–476. doi: 10.1159/000327599. [DOI] [PubMed] [Google Scholar]
- 39.Pergola PE, Raskin P, Toto RD, Meyer CJ, Huff JW, Grossman EB, Krauth M, Ruiz S, Audhya P, Christ-Schmidt H, Wittes J, Warnock DG. Bardoxolone methyl and kidney function in CKD with type 2 diabetes. N Engl J Med. 2011;365(4):327–336. doi: 10.1056/NEJMoa1105351. [DOI] [PubMed] [Google Scholar]
- 40.Nagaraj S, Youn JI, Weber H, Iclozan C, Lu L, Cotter MJ, Meyer C, Becerra CR, Fishman M, Antonia S, Sporn MB, Liby KT, Rawal B, Lee JH, Gabrilovich DI. Anti-inflammatory triterpenoid blocks immune suppressive function of MDSCs and improves immune response in cancer. Clin Cancer Res. 2010;16(6):1812–1823. doi: 10.1158/1078-0432.CCR-09-3272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kim SG, Kim YM, Choi JY, Han JY, Jang JW, Cho SH, Um SH, Chon CY, Lee DH, Jang JJ, Yu E, Lee YS. Oltipraz therapy in patients with liver fibrosis or cirrhosis: a randomized, double-blind, placebo-controlled phase II trial. J Pharm Pharmacol. 2011;63(5):627–635. doi: 10.1111/j.2042-7158.2011.01259.x. [DOI] [PubMed] [Google Scholar]
- 42.Egner PA, Chen JG, Wang JB, Wu Y, Sun Y, Lu JH, Zhu J, Zhang YH, Chen YS, Friesen MD, Jacobson LP, Munoz A, Ng D, Qian GS, Zhu YR, Chen TY, Botting NP, Zhang Q, Fahey JW, Talalay P, Groopman JD, Kensler TW. Bioavailability of Sulforaphane from two broccoli sprout beverages: results of a short-term, cross-over clinical trial in Qidong, China. Cancer Prev Res (Phila) 2011;4(3):384–395. doi: 10.1158/1940-6207.CAPR-10-0296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Shapiro TA, Fahey JW, Dinkova-Kostova AT, Holtzclaw WD, Stephenson KK, Wade KL, Ye L, Talalay P. Safety, tolerance, and metabolism of broccoli sprout glucosinolates and isothiocyanates: a clinical phase I study. Nutr Cancer. 2006;55(1):53–62. doi: 10.1207/s15327914nc5501_7. [DOI] [PubMed] [Google Scholar]
- 44.Hur W, Gray NS. Small molecule modulators of antioxidant response pathway. Curr Opin Chem Biol. 2011;15(1):162–173. doi: 10.1016/j.cbpa.2010.12.009. [DOI] [PubMed] [Google Scholar]
- 45.Sykiotis GP, Habeos IG, Samuelson AV, Bohmann D. The role of the antioxidant and longevity-promoting Nrf2 pathway in metabolic regulation. Curr Opin Clin Nutr Metab Care. 2011;14(1):41–48. doi: 10.1097/MCO.0b013e32834136f2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Martin-Montalvo A, Villalba JM, Navas P, de Cabo R. NRF2, cancer and calorie restriction. Oncogene. 2011;30(5):505–520. doi: 10.1038/onc.2010.492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Slocum SL, Kensler TW. Nrf2: control of sensitivity to carcinogens. Arch Toxicol. 2011;85(4):273–284. doi: 10.1007/s00204-011-0675-4. [DOI] [PubMed] [Google Scholar]
- 48.Kensler TW, Wakabayashi N. Nrf2: friend or foe for chemoprevention? Carcinogenesis. 2010;31(1):90–99. doi: 10.1093/carcin/bgp231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tkachev VO, Menshchikova EB, Zenkov NK. Mechanism of the Nrf2/Keap1/ARE signaling system. Biochemistry (Mosc) 2011;76(4):407–422. doi: 10.1134/s0006297911040031. [DOI] [PubMed] [Google Scholar]
- 50.Uruno A, Motohashi H. The Keap1-Nrf2 system as an in vivo sensor for electrophiles. Nitric Oxide. 2011;25(2):153–160. doi: 10.1016/j.niox.2011.02.007. [DOI] [PubMed] [Google Scholar]
- 51.Maher J, Yamamoto M. The rise of antioxidant signaling--the evolution and hormetic actions of Nrf2. Toxicol Appl Pharmacol. 2010;244(1):4–15. doi: 10.1016/j.taap.2010.01.011. [DOI] [PubMed] [Google Scholar]
- 52.Li J, Lee JM, Johnson JA. Microarray analysis reveals an antioxidant responsive element-driven gene set involved in conferring protection from an oxidative stress-induced apoptosis in IMR-32 cells. J Biol Chem. 2002;277(1):388–394. doi: 10.1074/jbc.M109380200. [DOI] [PubMed] [Google Scholar]
- 53.Villeneuve NF, Lau A, Zhang DD. Regulation of the Nrf2-Keap1 antioxidant response by the ubiquitin proteasome system: an insight into cullin-ring ubiquitin ligases. Antioxid Redox Signal. 2010;13(11):1699–1712. doi: 10.1089/ars.2010.3211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kobayashi A, Kang MI, Watai Y, Tong KI, Shibata T, Uchida K, Yamamoto M. Oxidative and electrophilic stresses activate Nrf2 through inhibition of ubiquitination activity of Keap1. Mol Cell Biol. 2006;26(1):221–229. doi: 10.1128/MCB.26.1.221-229.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kobayashi M, Yamamoto M. Molecular mechanisms activating the Nrf2-Keap1 pathway of antioxidant gene regulation. Antioxid Redox Signal. 2005;7(3–4):385–394. doi: 10.1089/ars.2005.7.385. [DOI] [PubMed] [Google Scholar]
- 56.Eggler AL, Gay KA, Mesecar AD. Molecular mechanisms of natural products in chemoprevention: induction of cytoprotective enzymes by Nrf2. Mol Nutr Food Res. 2008;52 (Suppl 1):S84–94. doi: 10.1002/mnfr.200700249. [DOI] [PubMed] [Google Scholar]
- 57.Mathers J, Fraser JA, McMahon M, Saunders RD, Hayes JD, McLellan LI. Antioxidant and cytoprotective responses to redox stress. Biochem Soc Symp. 2004;(71):157–176. doi: 10.1042/bss0710157. [DOI] [PubMed] [Google Scholar]
- 58.Zhang DD. Mechanistic studies of the Nrf2-Keap1 signaling pathway. Drug Metab Rev. 2006;38(4):769–789. doi: 10.1080/03602530600971974. [DOI] [PubMed] [Google Scholar]
- 59.Kundu JK, Surh YJ. Nrf2-Keap1 signaling as a potential target for chemoprevention of inflammation-associated carcinogenesis. Pharm Res. 2010;27(6):999–1013. doi: 10.1007/s11095-010-0096-8. [DOI] [PubMed] [Google Scholar]
- 60.Chen XL, Kunsch C. Induction of cytoprotective genes through Nrf2/antioxidant response element pathway: a new therapeutic approach for the treatment of inflammatory diseases. Curr Pharm Des. 2004;10(8):879–891. doi: 10.2174/1381612043452901. [DOI] [PubMed] [Google Scholar]
- 61.Niture SK, Kaspar JW, Shen J, Jaiswal AK. Nrf2 signaling and cell survival. Toxicol Appl Pharmacol. 2010;244(1):37–42. doi: 10.1016/j.taap.2009.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kaspar JW, Niture SK, Jaiswal AK. Nrf2:INrf2 (Keap1) signaling in oxidative stress. Free Radic Biol Med. 2009;47(9):1304–1309. doi: 10.1016/j.freeradbiomed.2009.07.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wakabayashi N, Dinkova-Kostova AT, Holtzclaw WD, Kang MI, Kobayashi A, Yamamoto M, Kensler TW, Talalay P. Protection against electrophile and oxidant stress by induction of the phase 2 response: fate of cysteines of the Keap1 sensor modified by inducers. Proc Natl Acad Sci U S A. 2004;101(7):2040–2045. doi: 10.1073/pnas.0307301101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kang MI, Kobayashi A, Wakabayashi N, Kim SG, Yamamoto M. Scaffolding of Keap1 to the actin cytoskeleton controls the function of Nrf2 as key regulator of cytoprotective phase 2 genes. Proc Natl Acad Sci U S A. 2004;101(7):2046–2051. doi: 10.1073/pnas.0308347100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kobayashi A, Kang MI, Okawa H, Ohtsuji M, Zenke Y, Chiba T, Igarashi K, Yamamoto M. Oxidative stress sensor Keap1 functions as an adaptor for Cul3-based E3 ligase to regulate proteasomal degradation of Nrf2. Mol Cell Biol. 2004;24(16):7130–7139. doi: 10.1128/MCB.24.16.7130-7139.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Zipper LM, Mulcahy RT. The Keap1 BTB/POZ dimerization function is required to sequester Nrf2 in cytoplasm. J Biol Chem. 2002;277(39):36544–36552. doi: 10.1074/jbc.M206530200. [DOI] [PubMed] [Google Scholar]
- 67.Dinkova-Kostova AT, Holtzclaw WD, Cole RN, Itoh K, Wakabayashi N, Katoh Y, Yamamoto M, Talalay P. Direct evidence that sulfhydryl groups of Keap1 are the sensors regulating induction of phase 2 enzymes that protect against carcinogens and oxidants. Proc Natl Acad Sci U S A. 2002;99(18):11908–11913. doi: 10.1073/pnas.172398899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Luo Y, Eggler AL, Liu D, Liu G, Mesecar AD, van Breemen RB. Sites of alkylation of human Keap1 by natural chemoprevention agents. J Am Soc Mass Spectrom. 2007;18(12):2226–2232. doi: 10.1016/j.jasms.2007.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.McMahon M, Thomas N, Itoh K, Yamamoto M, Hayes JD. Redox-regulated turnover of Nrf2 is determined by at least two separate protein domains, the redox-sensitive Neh2 degron and the redox-insensitive Neh6 degron. J Biol Chem. 2004;279(30):31556–31567. doi: 10.1074/jbc.M403061200. [DOI] [PubMed] [Google Scholar]
- 70.Zhang Y, Crouch DH, Yamamoto M, Hayes JD. Negative regulation of the Nrf1 transcription factor by its N-terminal domain is independent of Keap1: Nrf1, but not Nrf2, is targeted to the endoplasmic reticulum. Biochem J. 2006;399(3):373–385. doi: 10.1042/BJ20060725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Nioi P, Nguyen T, Sherratt PJ, Pickett CB. The carboxy-terminal Neh3 domain of Nrf2 is required for transcriptional activation. Mol Cell Biol. 2005;25(24):10895–10906. doi: 10.1128/MCB.25.24.10895-10906.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Jain AK, Bloom DA, Jaiswal AK. Nuclear import and export signals in control of Nrf2. J Biol Chem. 2005;280(32):29158–29168. doi: 10.1074/jbc.M502083200. [DOI] [PubMed] [Google Scholar]
- 73.Kobayashi M, Itoh K, Suzuki T, Osanai H, Nishikawa K, Katoh Y, Takagi Y, Yamamoto M. Identification of the interactive interface and phylogenic conservation of the Nrf2-Keap1 system. Genes Cells. 2002;7(8):807–820. doi: 10.1046/j.1365-2443.2002.00561.x. [DOI] [PubMed] [Google Scholar]
- 74.Katsuoka F, Motohashi H, Ishii T, Aburatani H, Engel JD, Yamamoto M. Genetic evidence that small maf proteins are essential for the activation of antioxidant response element-dependent genes. Mol Cell Biol. 2005;25(18):8044–8051. doi: 10.1128/MCB.25.18.8044-8051.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Li W, Kong AN. Molecular mechanisms of Nrf2-mediated antioxidant response. Mol Carcinog. 2009;48(2):91–104. doi: 10.1002/mc.20465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Zhang DD, Hannink M. Distinct cysteine residues in Keap1 are required for Keap1-dependent ubiquitination of Nrf2 and for stabilization of Nrf2 by chemopreventive agents and oxidative stress. Mol Cell Biol. 2003;23(22):8137–8151. doi: 10.1128/MCB.23.22.8137-8151.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Rachakonda G, Xiong Y, Sekhar KR, Stamer SL, Liebler DC, Freeman ML. Covalent modification at Cys151 dissociates the electrophile sensor Keap1 from the ubiquitin ligase CUL3. Chem Res Toxicol. 2008;21(3):705–710. doi: 10.1021/tx700302s. [DOI] [PubMed] [Google Scholar]
- 78.Nioi P, McMahon M, Itoh K, Yamamoto M, Hayes JD. Identification of a novel Nrf2-regulated antioxidant response element (ARE) in the mouse NAD(P)H:quinone oxidoreductase 1 gene: reassessment of the ARE consensus sequence. Biochem J. 2003;374(Pt 2):337–348. doi: 10.1042/BJ20030754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.MacLeod AK, McMahon M, Plummer SM, Higgins LG, Penning TM, Igarashi K, Hayes JD. Characterization of the cancer chemopreventive NRF2-dependent gene battery in human keratinocytes: demonstration that the KEAP1-NRF2 pathway, and not the BACH1-NRF2 pathway, controls cytoprotection against electrophiles as well as redox-cycling compounds. Carcinogenesis. 2009;30(9):1571–1580. doi: 10.1093/carcin/bgp176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Wasserman WW, Fahl WE. Functional antioxidant responsive elements. Proc Natl Acad Sci U S A. 1997;94(10):5361–5366. doi: 10.1073/pnas.94.10.5361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Sykiotis GP, Bohmann D. Stress-activated cap‘n’collar transcription factors in aging and human disease. Sci Signal. 2010;3(112):re3. doi: 10.1126/scisignal.3112re3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Dhakshinamoorthy S, Jain AK, Bloom DA, Jaiswal AK. Bach1 competes with Nrf2 leading to negative regulation of the antioxidant response element (ARE)-mediated NAD(P)H:quinone oxidoreductase 1 gene expression and induction in response to antioxidants. J Biol Chem. 2005;280(17):16891–16900. doi: 10.1074/jbc.M500166200. [DOI] [PubMed] [Google Scholar]
- 83.Rushmore TH, Morton MR, Pickett CB. The antioxidant responsive element. Activation by oxidative stress and identification of the DNA consensus sequence required for functional activity. J Biol Chem. 1991;266(18):11632–11639. [PubMed] [Google Scholar]
- 84.Zhang DD. The Nrf2-Keap1-ARE signaling pathway: The regulation and dual function of Nrf2 in cancer. Antioxid Redox Signal. 2010;13(11):1623–1626. doi: 10.1089/ars.2010.3301. [DOI] [PubMed] [Google Scholar]
- 85.Zhang DD, Lo SC, Sun Z, Habib GM, Lieberman MW, Hannink M. Ubiquitination of Keap1, a BTB-Kelch substrate adaptor protein for Cul3, targets Keap1 for degradation by a proteasome-independent pathway. J Biol Chem. 2005;280(34):30091–30099. doi: 10.1074/jbc.M501279200. [DOI] [PubMed] [Google Scholar]
- 86.Hong F, Sekhar KR, Freeman ML, Liebler DC. Specific patterns of electrophile adduction trigger Keap1 ubiquitination and Nrf2 activation. J Biol Chem. 2005;280(36):31768–31775. doi: 10.1074/jbc.M503346200. [DOI] [PubMed] [Google Scholar]
- 87.McMahon M, Thomas N, Itoh K, Yamamoto M, Hayes JD. Dimerization of substrate adaptors can facilitate cullin-mediated ubiquitylation of proteins by a “tethering” mechanism: a two-site interaction model for the Nrf2-Keap1 complex. J Biol Chem. 2006;281(34):24756–24768. doi: 10.1074/jbc.M601119200. [DOI] [PubMed] [Google Scholar]
- 88.Zhang Q, Pi J, Woods CG, Andersen ME. A systems biology perspective on Nrf2-mediated antioxidant response. Toxicol Appl Pharmacol. 2010;244(1):84–97. doi: 10.1016/j.taap.2009.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Tong KI, Kobayashi A, Katsuoka F, Yamamoto M. Two-site substrate recognition model for the Keap1-Nrf2 system: a hinge and latch mechanism. Biol Chem. 2006;387(10–11):1311–1320. doi: 10.1515/BC.2006.164. [DOI] [PubMed] [Google Scholar]
- 90.Tong KI, Padmanabhan B, Kobayashi A, Shang C, Hirotsu Y, Yokoyama S, Yamamoto M. Different electrostatic potentials define ETGE and DLG motifs as hinge and latch in oxidative stress response. Mol Cell Biol. 2007;27(21):7511–7521. doi: 10.1128/MCB.00753-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Tong KI, Katoh Y, Kusunoki H, Itoh K, Tanaka T, Yamamoto M. Keap1 recruits Neh2 through binding to ETGE and DLG motifs: characterization of the two-site molecular recognition model. Mol Cell Biol. 2006;26(8):2887–2900. doi: 10.1128/MCB.26.8.2887-2900.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Eggler AL, Small E, Hannink M, Mesecar AD. Cul3-mediated Nrf2 ubiquitination and antioxidant response element (ARE) activation are dependent on the partial molar volume at position 151 of Keap1. Biochem J. 2009;422(1):171–180. doi: 10.1042/BJ20090471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Sun Z, Zhang S, Chan JY, Zhang DD. Keap1 controls postinduction repression of the Nrf2-mediated antioxidant response by escorting nuclear export of Nrf2. Mol Cell Biol. 2007;27(18):6334–6349. doi: 10.1128/MCB.00630-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Kaspar JW, Jaiswal AK. An autoregulatory loop between Nrf2 and Cul3-Rbx1 controls their cellular abundance. J Biol Chem. 2010;285(28):21349–21358. doi: 10.1074/jbc.M110.121863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Lukosz M, Jakob S, Buchner N, Zschauer TC, Altschmied J, Haendeler J. Nuclear redox signaling. Antioxid Redox Signal. 2010;12(6):713–742. doi: 10.1089/ars.2009.2609. [DOI] [PubMed] [Google Scholar]
- 96.Stepkowski TM, Kruszewski MK. Molecular cross-talk between the NRF2/KEAP1 signaling pathway, autophagy, and apoptosis. Free Radic Biol Med. 2011;50(9):1186–1195. doi: 10.1016/j.freeradbiomed.2011.01.033. [DOI] [PubMed] [Google Scholar]
- 97.Wakabayashi N, Slocum SL, Skoko JJ, Shin S, Kensler TW. When NRF2 talks, who’s listening? Antioxid Redox Signal. 2010;13(11):1649–1663. doi: 10.1089/ars.2010.3216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Wakabayashi N, Shin S, Slocum SL, Agoston ES, Wakabayashi J, Kwak MK, Misra V, Biswal S, Yamamoto M, Kensler TW. Regulation of notch1 signaling by nrf2: implications for tissue regeneration. Sci Signal. 2010;3(130):ra52. doi: 10.1126/scisignal.2000762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Antelmann H, Helmann JD. Thiol-based redox switches and gene regulation. Antioxid Redox Signal. 2011;14(6):1049–1063. doi: 10.1089/ars.2010.3400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Kobayashi M, Li L, Iwamoto N, Nakajima-Takagi Y, Kaneko H, Nakayama Y, Eguchi M, Wada Y, Kumagai Y, Yamamoto M. The antioxidant defense system Keap1-Nrf2 comprises a multiple sensing mechanism for responding to a wide range of chemical compounds. Mol Cell Biol. 2009;29(2):493–502. doi: 10.1128/MCB.01080-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Yamamoto T, Suzuki T, Kobayashi A, Wakabayashi J, Maher J, Motohashi H, Yamamoto M. Physiological significance of reactive cysteine residues of Keap1 in determining Nrf2 activity. Mol Cell Biol. 2008;28(8):2758–2770. doi: 10.1128/MCB.01704-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.He X, Ma Q. NRF2 cysteine residues are critical for oxidant/electrophile-sensing, Kelch-like ECH-associated protein-1-dependent ubiquitination-proteasomal degradation, and transcription activation. Mol Pharmacol. 2009;76(6):1265–1278. doi: 10.1124/mol.109.058453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Lee JM, Li J, Johnson DA, Stein TD, Kraft AD, Calkins MJ, Jakel RJ, Johnson JA. Nrf2, a multi-organ protector? FASEB J. 2005;19(9):1061–1066. doi: 10.1096/fj.04-2591hyp. [DOI] [PubMed] [Google Scholar]
- 104.Lewis KN, Mele J, Hayes JD, Buffenstein R. Nrf2, a guardian of health span and gatekeeper of species longevity. Integr Comp Biol. 2010;50(5):829–843. doi: 10.1093/icb/icq034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Kwak MK, Kensler TW. Targeting NRF2 signaling for cancer chemoprevention. Toxicol Appl Pharmacol. 2010;244(1):66–76. doi: 10.1016/j.taap.2009.08.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Cornblatt BS, Ye L, Dinkova-Kostova AT, Erb M, Fahey JW, Singh NK, Chen MS, Stierer T, Garrett-Mayer E, Argani P, Davidson NE, Talalay P, Kensler TW, Visvanathan K. Preclinical and clinical evaluation of sulforaphane for chemoprevention in the breast. Carcinogenesis. 2007;28(7):1485–1490. doi: 10.1093/carcin/bgm049. [DOI] [PubMed] [Google Scholar]
- 107.Hatcher H, Planalp R, Cho J, Torti FM, Torti SV. Curcumin: from ancient medicine to current clinical trials. Cell Mol Life Sci. 2008;65(11):1631–1652. doi: 10.1007/s00018-008-7452-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Zhang Y, Munday R. Dithiolethiones for cancer chemoprevention: where do we stand? Mol Cancer Ther. 2008;7(11):3470–3479. doi: 10.1158/1535-7163.MCT-08-0625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Wang XJ, Sun Z, Villeneuve NF, Zhang S, Zhao F, Li Y, Chen W, Yi X, Zheng W, Wondrak GT, Wong PK, Zhang DD. Nrf2 enhances resistance of cancer cells to chemotherapeutic drugs, the dark side of Nrf2. Carcinogenesis. 2008;29(6):1235–1243. doi: 10.1093/carcin/bgn095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Gao L, Mann GE. Vascular NAD(P)H oxidase activation in diabetes: a double-edged sword in redox signalling. Cardiovasc Res. 2009;82(1):9–20. doi: 10.1093/cvr/cvp031. [DOI] [PubMed] [Google Scholar]
- 111.Hayes JD, McMahon M. The double-edged sword of Nrf2: subversion of redox homeostasis during the evolution of cancer. Mol Cell. 2006;21(6):732–734. doi: 10.1016/j.molcel.2006.03.004. [DOI] [PubMed] [Google Scholar]
- 112.Shibata T, Ohta T, Tong KI, Kokubu A, Odogawa R, Tsuta K, Asamura H, Yamamoto M, Hirohashi S. Cancer related mutations in NRF2 impair its recognition by Keap1-Cul3 E3 ligase and promote malignancy. Proc Natl Acad Sci U S A. 2008;105(36):13568–13573. doi: 10.1073/pnas.0806268105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Ohta T, Iijima K, Miyamoto M, Nakahara I, Tanaka H, Ohtsuji M, Suzuki T, Kobayashi A, Yokota J, Sakiyama T, Shibata T, Yamamoto M, Hirohashi S. Loss of Keap1 function activates Nrf2 and provides advantages for lung cancer cell growth. Cancer Res. 2008;68(5):1303–1309. doi: 10.1158/0008-5472.CAN-07-5003. [DOI] [PubMed] [Google Scholar]
- 114.Ren D, Villeneuve NF, Jiang T, Wu T, Lau A, Toppin HA, Zhang DD. Brusatol enhances the efficacy of chemotherapy by inhibiting the Nrf2-mediated defense mechanism. Proc Natl Acad Sci U S A. 2011;108(4):1433–1438. doi: 10.1073/pnas.1014275108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Tang X, Wang H, Fan L, Wu X, Xin A, Ren H, Wang XJ. Luteolin inhibits Nrf2 leading to negative regulation of the Nrf2/ARE pathway and sensitization of human lung carcinoma A549 cells to therapeutic drugs. Free Radic Biol Med. 2011;50(11):1599–1609. doi: 10.1016/j.freeradbiomed.2011.03.008. [DOI] [PubMed] [Google Scholar]
- 116.Suzuki M, Betsuyaku T, Ito Y, Nagai K, Nasuhara Y, Kaga K, Kondo S, Nishimura M. Down-regulated NF-E2-related factor 2 in pulmonary macrophages of aged smokers and patients with chronic obstructive pulmonary disease. Am J Respir Cell Mol Biol. 2008;39(6):673–682. doi: 10.1165/rcmb.2007-0424OC. [DOI] [PubMed] [Google Scholar]
- 117.Marzec JM, Christie JD, Reddy SP, Jedlicka AE, Vuong H, Lanken PN, Aplenc R, Yamamoto T, Yamamoto M, Cho HY, Kleeberger SR. Functional polymorphisms in the transcription factor NRF2 in humans increase the risk of acute lung injury. FASEB J. 2007;21(9):2237–2246. doi: 10.1096/fj.06-7759com. [DOI] [PubMed] [Google Scholar]
- 118.Boutten A, Goven D, Artaud-Macari E, Boczkowski J, Bonay M. NRF2 targeting: a promising therapeutic strategy in chronic obstructive pulmonary disease. Trends Mol Med. 2011;17(7):363–371. doi: 10.1016/j.molmed.2011.02.006. [DOI] [PubMed] [Google Scholar]
- 119.Cho HY, Kleeberger SR. Nrf2 protects against airway disorders. Toxicol Appl Pharmacol. 2010;244(1):43–56. doi: 10.1016/j.taap.2009.07.024. [DOI] [PubMed] [Google Scholar]
- 120.Reddy SP. The antioxidant response element and oxidative stress modifiers in airway diseases. Curr Mol Med. 2008;8(5):376–383. doi: 10.2174/156652408785160925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.de Vries HE, Witte M, Hondius D, Rozemuller AJ, Drukarch B, Hoozemans J, van Horssen J. Nrf2-induced antioxidant protection: a promising target to counteract ROS-mediated damage in neurodegenerative disease? Free Radic Biol Med. 2008;45(10):1375–1383. doi: 10.1016/j.freeradbiomed.2008.09.001. [DOI] [PubMed] [Google Scholar]
- 122.Jazwa A, Rojo AI, Innamorato NG, Hesse M, Fernandez-Ruiz J, Cuadrado A. Pharmacological targeting of the transcription factor Nrf2 at the basal ganglia provides disease modifying therapy for experimental parkinsonism. Antioxid Redox Signal. 2011;14(12):2347–2360. doi: 10.1089/ars.2010.3731. [DOI] [PubMed] [Google Scholar]
- 123.Du Y, Wooten MC, Gearing M, Wooten MW. Age-associated oxidative damage to the p62 promoter: implications for Alzheimer disease. Free Radic Biol Med. 2009;46(4):492–501. doi: 10.1016/j.freeradbiomed.2008.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Tufekci KU, Civi Bayin E, Genc S, Genc K. The Nrf2/ARE Pathway: A Promising Target to Counteract Mitochondrial Dysfunction in Parkinson’s Disease. Parkinsons Dis. 2011;2011:314082. doi: 10.4061/2011/314082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Lee EB, Skovronsky DM, Abtahian F, Doms RW, Lee VM-Y. Secretion and Intracellular Generation of Truncated Aβ in β-Site Amyloid-β Precursor Protein-cleaving Enzyme Expressing Human Neurons. J Biol Chem. 2003;278(7):4458–4466. doi: 10.1074/jbc.M210105200. [DOI] [PubMed] [Google Scholar]
- 126.Ramsey CP, Glass CA, Montgomery MB, Lindl KA, Ritson GP, Chia LA, Hamilton RL, Chu CT, Jordan-Sciutto KL. Expression of Nrf2 in Neurodegenerative Diseases. J Neuropathol Exp Neurol. 2007;66(1):75–85. doi: 10.1097/nen.0b013e31802d6da9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Chen P-C, Vargas MR, Pani AK, Smeyne RJ, Johnson DA, Kan YW, Johnson JA. Nrf2-mediated neuroprotection in the MPTP mouse model of Parkinson’s disease: Critical role for the astrocyte. Proc Natl Acad Sci. 2009;106(8):2933–2938. doi: 10.1073/pnas.0813361106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Calabrese V, Cornelius C, Mancuso C, Pennisi G, Calafato S, Bellia F, Bates TE, Giuffrida Stella AM, Schapira T, Dinkova Kostova AT, Rizzarelli E. Cellular stress response: a novel target for chemoprevention and nutritional neuroprotection in aging, neurodegenerative disorders and longevity. Neurochem Res. 2008;33(12):2444–2471. doi: 10.1007/s11064-008-9775-9. [DOI] [PubMed] [Google Scholar]
- 129.van Muiswinkel FL, Kuiperij HB. The Nrf2-ARE Signalling pathway: promising drug target to combat oxidative stress in neurodegenerative disorders. Curr Drug Targets CNS Neurol Disord. 2005;4(3):267–281. doi: 10.2174/1568007054038238. [DOI] [PubMed] [Google Scholar]
- 130.Koenitzer JR, Freeman BA. Redox signaling in inflammation: interactions of endogenous electrophiles and mitochondria in cardiovascular disease. Ann N Y Acad Sci. 2010;1203:45–52. doi: 10.1111/j.1749-6632.2010.05559.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Ichikawa T, Li J, Meyer CJ, Janicki JS, Hannink M, Cui T. Dihydro-CDDO-trifluoroethyl amide (dh404), a novel Nrf2 activator, suppresses oxidative stress in cardiomyocytes. PLoS One. 2009;4(12):e8391. doi: 10.1371/journal.pone.0008391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Collins AR, Lyon CJ, Xia X, Liu JZ, Tangirala RK, Yin F, Boyadjian R, Bikineyeva A, Pratico D, Harrison DG, Hsueh WA. Age-accelerated atherosclerosis correlates with failure to upregulate antioxidant genes. Circ Res. 2009;104(6):e42–54. doi: 10.1161/CIRCRESAHA.108.188771. [DOI] [PubMed] [Google Scholar]
- 133.Sussan TE, Jun J, Thimmulappa R, Bedja D, Antero M, Gabrielson KL, Polotsky VY, Biswal S. Disruption of Nrf2, a key inducer of antioxidant defenses, attenuates ApoE-mediated atherosclerosis in mice. PLoS One. 2008;3(11):e3791. doi: 10.1371/journal.pone.0003791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Freigang S, Ampenberger F, Spohn G, Heer S, Shamshiev AT, Kisielow J, Hersberger M, Yamamoto M, Bachmann MF, Kopf M. Nrf2 is essential for cholesterol crystal-induced inflammasome activation and exacerbation of atherosclerosis. Eur J Immunol. 2011;41(7):2040–2051. doi: 10.1002/eji.201041316. [DOI] [PubMed] [Google Scholar]
- 135.Cheng X, Siow RC, Mann GE. Impaired redox signaling and antioxidant gene expression in endothelial cells in diabetes: a role for mitochondria and the nuclear factor-E2-related factor 2-Kelch-like ECH-associated protein 1 defense pathway. Antioxid Redox Signal. 2011;14(3):469–487. doi: 10.1089/ars.2010.3283. [DOI] [PubMed] [Google Scholar]
- 136.He X, Kan H, Cai L, Ma Q. Nrf2 is critical in defense against high glucose-induced oxidative damage in cardiomyocytes. J Mol Cell Cardiol. 2009;46(1):47–58. doi: 10.1016/j.yjmcc.2008.10.007. [DOI] [PubMed] [Google Scholar]
- 137.Wang X, Hai CX. ROS Acts as a Double-Edged Sword in the Pathogenesis of Type 2 Diabetes Mellitus: Is Nrf2a Potential Target for the Treatment? Mini Rev Med Chem. 2011 doi: 10.2174/138955711797247761. [DOI] [PubMed] [Google Scholar]
- 138.Jiang T, Huang Z, Lin Y, Zhang Z, Fang D, Zhang DD. The protective role of Nrf2 in streptozotocin-induced diabetic nephropathy. Diabetes. 2010;59(4):850–860. doi: 10.2337/db09-1342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Negi G, Kumar A, Joshi RP, Sharma SS. Oxidative stress and Nrf2 in the pathophysiology of diabetic neuropathy: old perspective with a new angle. Biochem Biophys Res Commun. 2011;408(1):1–5. doi: 10.1016/j.bbrc.2011.03.087. [DOI] [PubMed] [Google Scholar]
- 140.Theiss AL, Vijay-Kumar M, Obertone TS, Jones DP, Hansen JM, Gewirtz AT, Merlin D, Sitaraman SV. Prohibitin is a novel regulator of antioxidant response that attenuates colonic inflammation in mice. Gastroenterology. 2009;137(1):199–208. 208 e191–196. doi: 10.1053/j.gastro.2009.03.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Khor TO, Huang MT, Kwon KH, Chan JY, Reddy BS, Kong AN. Nrf2-deficient mice have an increased susceptibility to dextran sulfate sodium-induced colitis. Cancer Res. 2006;66(24):11580–11584. doi: 10.1158/0008-5472.CAN-06-3562. [DOI] [PubMed] [Google Scholar]
- 142.Aleksunes LM, Manautou JE. Emerging role of Nrf2 in protecting against hepatic and gastrointestinal disease. Toxicol Pathol. 2007;35(4):459–473. doi: 10.1080/01926230701311344. [DOI] [PubMed] [Google Scholar]
- 143.Nagai N, Thimmulappa RK, Cano M, Fujihara M, Izumi-Nagai K, Kong X, Sporn MB, Kensler TW, Biswal S, Handa JT. Nrf2 is a critical modulator of the innate immune response in a model of uveitis. Free Radic Biol Med. 2009;47(3):300–306. doi: 10.1016/j.freeradbiomed.2009.04.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Wruck CJ, Fragoulis A, Gurzynski A, Brandenburg LO, Kan YW, Chan K, Hassenpflug J, Freitag-Wolf S, Varoga D, Lippross S, Pufe T. Role of oxidative stress in rheumatoid arthritis: insights from the Nrf2-knockout mice. Ann Rheum Dis. 2011;70(5):844–850. doi: 10.1136/ard.2010.132720. [DOI] [PubMed] [Google Scholar]
- 145.Maicas N, Ferrandiz ML, Brines R, Ibanez L, Cuadrado A, Koenders MI, van den Berg WB, Alcaraz MJ. Deficiency of nrf2 accelerates the effector phase of arthritis and aggravates joint disease. Antioxid Redox Signal. 2011;15(4):889–901. doi: 10.1089/ars.2010.3835. [DOI] [PubMed] [Google Scholar]
- 146.Wang XJ, Sun Z, Chen W, Li Y, Villeneuve NF, Zhang DD. Activation of Nrf2 by arsenite and monomethylarsonous acid is independent of Keap1-C151: enhanced Keap1-Cul3 interaction. Toxicol Appl Pharmacol. 2008;230(3):383–389. doi: 10.1016/j.taap.2008.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Dimmock JR, Elias DW, Beazely MA, Kandepu NM. Bioactivities of chalcones. Curr Med Chem. 1999;6(12):1125–1149. [PubMed] [Google Scholar]
- 148.Zhang L, Gavin T, Barber DS, Lopachin RM. Role of the Nrf2-ARE pathway in acrylamide neurotoxicity. Toxicol Lett. 2011;205(1):1–7. doi: 10.1016/j.toxlet.2011.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.McMahon M, Lamont DJ, Beattie KA, Hayes JD. Keap1 perceives stress via three sensors for the endogenous signaling molecules nitric oxide, zinc, and alkenals. Proc Natl Acad Sci U S A. 2010;107(44):18838–18843. doi: 10.1073/pnas.1007387107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Fourquet S, Guerois R, Biard D, Toledano MB. Activation of NRF2 by nitrosative agents and H2O2 involves KEAP1 disulfide formation. J Biol Chem. 2010;285(11):8463–8471. doi: 10.1074/jbc.M109.051714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Copple IM, Goldring CE, Kitteringham NR, Park BK. The keap1-nrf2 cellular defense pathway: mechanisms of regulation and role in protection against drug-induced toxicity. Handb Exp Pharmacol. 2010;(196):233–266. doi: 10.1007/978-3-642-00663-0_9. [DOI] [PubMed] [Google Scholar]
- 152.Eggler AL, Liu G, Pezzuto JM, van Breemen RB, Mesecar AD. Modifying specific cysteines of the electrophile-sensing human Keap1 protein is insufficient to disrupt binding to the Nrf2 domain Neh2. Proc Natl Acad Sci U S A. 2005;102(29):10070–10075. doi: 10.1073/pnas.0502402102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Dinkova-Kostova AT, Talalay P, Sharkey J, Zhang Y, Holtzclaw WD, Wang XJ, David E, Schiavoni KH, Finlayson S, Mierke DF, Honda T. An exceptionally potent inducer of cytoprotective enzymes: elucidation of the structural features that determine inducer potency and reactivity with Keap1. J Biol Chem. 2010;285(44):33747–33755. doi: 10.1074/jbc.M110.163485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Fahey JW, Dinkova-Kostova AT, Stephenson KK, Talalay P. The “Prochaska” microtiter plate bioassay for inducers of NQO1. Methods Enzymol. 2004;382:243–258. doi: 10.1016/S0076-6879(04)82014-7. [DOI] [PubMed] [Google Scholar]
- 155.Dinkova-Kostova AT, Fahey JW, Talalay P. Chemical structures of inducers of nicotinamide quinone oxidoreductase 1 (NQO1) Methods Enzymol. 2004;382:423–448. doi: 10.1016/S0076-6879(04)82023-8. [DOI] [PubMed] [Google Scholar]
- 156.Dinkova-Kostova AT, Massiah MA, Bozak RE, Hicks RJ, Talalay P. Potency of Michael reaction acceptors as inducersof enzymes that protect against carcinogenesis depends on their reactivity with sulfhydryl groups. Proc Natl Acad Sci U S A. 2001;98(6):3404–3409. doi: 10.1073/pnas.051632198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Nakamura Y, Miyoshi N. Electrophiles in foods: the current status of isothiocyanates and their chemical biology. Biosci Biotechnol Biochem. 2010;74(2):242–255. doi: 10.1271/bbb.90731. [DOI] [PubMed] [Google Scholar]
- 158.Calabrese EJ. Neuroscience and hormesis: overview and general findings. Crit Rev Toxicol. 2008;38(4):249–252. doi: 10.1080/10408440801981957. [DOI] [PubMed] [Google Scholar]
- 159.Ak T, Gülçin I. Antioxidant and radical scavenging properties of curcumin. Chem Biol Interact. 2008;174(1):27–37. doi: 10.1016/j.cbi.2008.05.003. [DOI] [PubMed] [Google Scholar]
- 160.Dinkova-Kostova AT, Talalay P. Relation of structure of curcumin analogs to their potencies as inducers of Phase 2 detoxification enzymes. Carcinogenesis. 1999;20(5):911–914. doi: 10.1093/carcin/20.5.911. [DOI] [PubMed] [Google Scholar]
- 161.Scapagnini G, Colombrita C, Amadio M, D’Agata V, Arcelli E, Sapienza M, Quattrone A, Calabrese V. Curcumin activates defensive genes and protects neurons against oxidative stress. Antioxid Redox Signal. 2006;8(3–4):395–403. doi: 10.1089/ars.2006.8.395. [DOI] [PubMed] [Google Scholar]
- 162.Balogun E, Hoque M, Gong P, Killeen E, Green CJ, Foresti R, Alam J, Motterlini R. Curcumin activates the haem oxygenase-1 gene via regulation of Nrf2 and the antioxidant-responsive element. Biochem J. 2003;371(Pt 3):887–895. doi: 10.1042/BJ20021619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.McNally SJ, Harrison EM, Ross JA, Garden OJ, Wigmore SJ. Curcumin induces heme oxygenase 1 through generation of reactive oxygen species, p38 activation and phosphatase inhibition. Int J Mol Med. 2007;19(1):165–172. [PubMed] [Google Scholar]
- 164.Thangapazham RL, Sharma A, Maheshwari RK. Multiple molecular targets in cancer chemoprevention by curcumin. AAPS J. 2006;8(3):E443–449. doi: 10.1208/aapsj080352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Garg R, Gupta S, Maru GB. Dietary curcumin modulates transcriptional regulators of phase I and phase II enzymes in benzo[a]pyrene-treated mice: mechanism of its anti-initiating action. Carcinogenesis. 2008;29(5):1022–1032. doi: 10.1093/carcin/bgn064. [DOI] [PubMed] [Google Scholar]
- 166.Goel A, Kunnumakkara AB, Aggarwal BB. Curcumin as “Curecumin”: from kitchen to clinic. Biochem Pharmacol. 2008;75(4):787–809. doi: 10.1016/j.bcp.2007.08.016. [DOI] [PubMed] [Google Scholar]
- 167.Dinkova-Kostova AT, Cory AH, Bozak RE, Hicks RJ, Cory JG. Bis(2-hydroxybenzylidene)acetone, a potent inducer of the phase 2 response, causes apoptosis in mouse leukemia cells through a p53-independent, caspase-mediated pathway. Cancer Lett. 2007;245(1–2):341–349. doi: 10.1016/j.canlet.2006.01.024. [DOI] [PubMed] [Google Scholar]
- 168.Michaluart P, Masferrer JL, Carothers AM, Subbaramaiah K, Zweifel BS, Koboldt C, Mestre JR, Grunberger D, Sacks PG, Tanabe T, Dannenberg AJ. Inhibitory effects of caffeic acid phenethyl ester on the activity and expression of cyclooxygenase-2 in human oral epithelial cells and in a rat model of inflammation. Cancer Res. 1999;59(10):2347–2352. [PubMed] [Google Scholar]
- 169.Kikuzaki H, Hisamoto M, Hirose K, Akiyama K, Taniguchi H. Antioxidant properties of ferulic acid and its related compounds. J Agric Food Chem. 2002;50(7):2161–2168. doi: 10.1021/jf011348w. [DOI] [PubMed] [Google Scholar]
- 170.Scapagnini G, Butterfield DA, Colombrita C, Sultana R, Pascale A, Calabrese V. Ethyl ferulate, a lipophilic polyphenol, induces HO-1 and protects rat neurons against oxidative stress. Antioxid Redox Signal. 2004;6(5):811–818. doi: 10.1089/ars.2004.6.811. [DOI] [PubMed] [Google Scholar]
- 171.Go ML, Wu X, Liu XL. Chalcones: an update on cytotoxic and chemoprotective properties. Curr Med Chem. 2005;12(4):481–499. doi: 10.2174/0929867053363153. [DOI] [PubMed] [Google Scholar]
- 172.Shibuya A, Onda K, Kawahara H, Uchiyama Y, Nakayama H, Omi T, Nagaoka M, Matsui H, Hirano T. Sofalcone, a gastric mucosa protective agent, increases vascular endothelial growth factor via the Nrf2-heme-oxygenase-1 dependent pathway in gastric epithelial cells. Biochem Biophys Res Commun. 2010;398(3):581–584. doi: 10.1016/j.bbrc.2010.06.124. [DOI] [PubMed] [Google Scholar]
- 173.Lee YM, Jeong GS, Lim HD, An RB, Kim YC, Kim EC. Isoliquiritigenin 2′-methyl ether induces growth inhibition and apoptosis in oral cancer cells via heme oxygenase-1. Toxicol In Vitro. 2010;24(3):776–782. doi: 10.1016/j.tiv.2009.12.024. [DOI] [PubMed] [Google Scholar]
- 174.Kumar V, Kumar S, Hassan M, Wu H, Thimmulappa RK, Kumar A, Sharma SK, Parmar VS, Biswal S, Malhotra SV. Novel chalcone derivatives as potent Nrf2 activators in mice and human lung epithelial cells. J Med Chem. 2011;54(12):4147–4159. doi: 10.1021/jm2002348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Smirnova NA, Haskew-Layton RE, Basso M, Hushpulian DM, Payappilly JB, Speer RE, Ahn YH, Rakhman I, Cole PA, Pinto JT, Ratan RR, Gazaryan IG. Development of neh2-luciferase reporter and its application for high throughput screening and real-time monitoring of nrf2 activators. Chem Biol. 2011;18(6):752–765. doi: 10.1016/j.chembiol.2011.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Prince M, Li Y, Childers A, Itoh K, Yamamoto M, Kleiner HE. Comparison of citrus coumarins on carcinogen-detoxifying enzymes in Nrf2 knockout mice. Toxicol Lett. 2009;185(3):180–186. doi: 10.1016/j.toxlet.2008.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Pokharel YR, Jeong JE, Oh SJ, Kim SK, Woo ER, Kang KW. Screening of potential chemopreventive compounds from Poncirus trifoliata Raf. Pharmazie. 2006;61(9):796–798. [PubMed] [Google Scholar]
- 178.Song LL, Kosmeder JW, 2nd, Lee SK, Gerhauser C, Lantvit D, Moon RC, Moriarty RM, Pezzuto JM. Cancer chemopreventive activity mediated by 4′-bromoflavone, a potent inducer of phase II detoxification enzymes. Cancer Res. 1999;59(3):578–585. [PubMed] [Google Scholar]
- 179.Zhang W, Go ML. Functionalized 3-benzylidene-indolin-2-ones: inducers of NAD(P)H-quinone oxidoreductase 1 (NQO1) with antiproliferative activity. Bioorg Med Chem. 2009;17(5):2077–2090. doi: 10.1016/j.bmc.2008.12.052. [DOI] [PubMed] [Google Scholar]
- 180.Nakamura Y, Yoshida C, Murakami A, Ohigashi H, Osawa T, Uchida K. Zerumbone, a tropical ginger sesquiterpene, activates phase II drug metabolizing enzymes. FEBS Lett. 2004;572(1–3):245–250. doi: 10.1016/j.febslet.2004.07.042. [DOI] [PubMed] [Google Scholar]
- 181.Seo JY, Park J, Kim HJ, Lee IA, Lim JS, Lim SS, Choi SJ, Park JH, Kang HJ, Kim JS. Isoalantolactone from Inula helenium caused Nrf2-mediated induction of detoxifying enzymes. J Med Food. 2009;12(5):1038–1045. doi: 10.1089/jmf.2009.0072. [DOI] [PubMed] [Google Scholar]
- 182.Masutani H, Otsuki R, Yamaguchi Y, Takenaka M, Kanoh N, Takatera K, Kunimoto Y, Yodoi J. Fragrant unsaturated aldehydes elicit activation of the Keap1/Nrf2 system leading to the upregulation of thioredoxin expression and protection against oxidative stress. Antioxid Redox Signal. 2009;11(5):949–962. doi: 10.1089/ars.2008.2292. [DOI] [PubMed] [Google Scholar]
- 183.Yu A-L, Lu C-Y, Wang T-S, Tsai C-W, Liu K-L, Cheng Y-P, Chang HC, Lii C-K, Chen H-W. Induction of Heme Oxygenase 1 and Inhibition of Tumor Necrosis Factor α-Induced Intercellular Adhesion Molecule Expression by Andrographolide in EA.hy926 Cells. J Agric Food Chem. 2010;58(13):7641–7648. doi: 10.1021/jf101353c. [DOI] [PubMed] [Google Scholar]
- 184.Nishino H, Nishino A, Takayasu J, Hasegawa T, Iwashima A, Hirabayashi K, Iwata S, Shibata S. Inhibition of the tumor-promoting action of 12-O-tetradecanoylphorbol-13-acetate by some oleanane-type triterpenoid compounds. Cancer Res. 1988;48(18):5210–5215. [PubMed] [Google Scholar]
- 185.Dinkova-Kostova AT, Liby KT, Stephenson KK, Holtzclaw WD, Gao X, Suh N, Williams C, Risingsong R, Honda T, Gribble GW, Sporn MB, Talalay P. Extremely potent triterpenoid inducers of the phase 2 response: correlations of protection against oxidant and inflammatory stress. Proc Natl Acad Sci U S A. 2005;102(12):4584–4589. doi: 10.1073/pnas.0500815102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Sporn MB, Liby KT, Yore MM, Fu L, Lopchuk JM, Gribble GW. New synthetic triterpenoids: potent agents for prevention and treatment of tissue injury caused by inflammatory and oxidative stress. J Nat Prod. 2011;74(3):537–545. doi: 10.1021/np100826q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Liby K, Honda T, Williams CR, Risingsong R, Royce DB, Suh N, Dinkova-Kostova AT, Stephenson KK, Talalay P, Sundararajan C, Gribble GW, Sporn MB. Novel semisynthetic analogues of betulinic acid with diverse cytoprotective, antiproliferative, and proapoptotic activities. Mol Cancer Ther. 2007;6(7):2113–2119. doi: 10.1158/1535-7163.MCT-07-0180. [DOI] [PubMed] [Google Scholar]
- 188.Honda T, Dinkova-Kostova AT, David E, Padegimas EM, Sundararajan C, Visnick M, Bumeister R, Christian Wigley W. Synthesis and biological evaluation of 1-[2-cyano-3,12-dioxooleana-1,9(11)-dien-28-oyl]-4-ethynylimidazole. A novel and highly potent anti-inflammatory and cytoprotective agent. Bioorg Med Chem Lett. 2011;21(8):2188–2191. doi: 10.1016/j.bmcl.2011.03.018. [DOI] [PubMed] [Google Scholar]
- 189.Honda T, Yoshizawa H, Sundararajan C, David E, Lajoie MJ, Favaloro FG, Jr, Janosik T, Su X, Honda Y, Roebuck BD, Gribble GW. Tricyclic compounds containing nonenolizable cyano enones. A novel class of highly potent anti-inflammatory and cytoprotective agents. J Med Chem. 2011;54(6):1762–1778. doi: 10.1021/jm101445p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Haridas V, Hanausek M, Nishimura G, Soehnge H, Gaikwad A, Narog M, Spears E, Zoltaszek R, Walaszek Z, Gutterman JU. Triterpenoid electrophiles (avicins) activate the innate stress response by redox regulation of a gene battery. J Clin Invest. 2004;113(1):65–73. doi: 10.1172/JCI18699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Kansanen E, Kivela AM, Levonen AL. Regulation of Nrf2-dependent gene expression by 15-deoxy-Delta12,14-prostaglandin J2. Free Radic Biol Med. 2009;47(9):1310–1317. doi: 10.1016/j.freeradbiomed.2009.06.030. [DOI] [PubMed] [Google Scholar]
- 192.Kansanen E, Bonacci G, Schopfer FJ, Kuosmanen SM, Tong KI, Leinonen H, Woodcock SR, Yamamoto M, Carlberg C, Yla-Herttuala S, Freeman BA, Levonen AL. Electrophilic nitro-fatty acids activate NRF2 by a KEAP1 cysteine 151-independent mechanism. J Biol Chem. 2011;286(16):14019–14027. doi: 10.1074/jbc.M110.190710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Tsujita T, Li L, Nakajima H, Iwamoto N, Nakajima-Takagi Y, Ohashi K, Kawakami K, Kumagai Y, Freeman BA, Yamamoto M, Kobayashi M. Nitro-fatty acids and cyclopentenone prostaglandins share strategies to activate the Keap1-Nrf2 system: a study using green fluorescent protein transgenic zebrafish. Genes Cells. 2011;16(1):46–57. doi: 10.1111/j.1365-2443.2010.01466.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Wong DP, Wells G, Hagen T. Heteroaromatic 4-arylquinols are novel inducers of nuclear factor-erythroid 2-related factor 2 (Nrf2) Eur J Pharmacol. 2010;643(2–3):188–194. doi: 10.1016/j.ejphar.2010.06.040. [DOI] [PubMed] [Google Scholar]
- 195.Hur W, Sun Z, Jiang T, Mason DE, Peters EC, Zhang DD, Luesch H, Schultz PG, Gray NS. A small-molecule inducer of the antioxidant response element. Chem Biol. 2010;17(5):537–547. doi: 10.1016/j.chembiol.2010.03.013. [DOI] [PubMed] [Google Scholar]
- 196.Nakatani N. Phenolic antioxidants from herbs and spices. Biofactors. 2000;13(1–4):141–146. doi: 10.1002/biof.5520130123. [DOI] [PubMed] [Google Scholar]
- 197.Benson AM, Cha YN, Bueding E, Heine HS, Talalay P. Elevation of extrahepatic glutathione S-transferase and epoxide hydratase activities by 2(3)-tert-butyl-4-hydroxyanisole. Cancer Res. 1979;39(8):2971–2977. [PubMed] [Google Scholar]
- 198.Benson AM, Hunkeler MJ, Talalay P. Increase of NAD(P)H:quinone reductase by dietary antioxidants: possible role in protection against carcinogenesis and toxicity. Proc Natl Acad Sci U S A. 1980;77(9):5216–5220. doi: 10.1073/pnas.77.9.5216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Prochaska HJ, Bregman HS, De Long MJ, Talalay P. Specificity of induction of cancer protective enzymes by analogues of tert-butyl-4-hydroxyanisole (BHA) Biochem Pharmacol. 1985;34(21):3909–3914. doi: 10.1016/0006-2952(85)90443-5. [DOI] [PubMed] [Google Scholar]
- 200.Dinkova-Kostova AT, Wang XJ. Induction of the Keap1/Nrf2/ARE pathway by oxidizable diphenols. Chem Biol Interact. 2011;192(1–2):101–106. doi: 10.1016/j.cbi.2010.09.010. [DOI] [PubMed] [Google Scholar]
- 201.Prochaska HJ, De Long MJ, Talalay P. On the mechanisms of induction of cancer-protective enzymes: a unifying proposal. Proc Natl Acad Sci U S A. 1985;82(23):8232–8236. doi: 10.1073/pnas.82.23.8232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Wang XJ, Hayes JD, Higgins LG, Wolf CR, Dinkova-Kostova AT. Activation of the NRF2 signaling pathway by copper-mediated redox cycling of para- and ortho-hydroquinones. Chem Biol. 2010;17(1):75–85. doi: 10.1016/j.chembiol.2009.12.013. [DOI] [PubMed] [Google Scholar]
- 203.Hara H, Ohta M, Adachi T. Apomorphine protects against 6-hydroxydopamine-induced neuronal cell death through activation of the Nrf2-ARE pathway. J Neurosci Res. 2006;84(4):860–866. doi: 10.1002/jnr.20974. [DOI] [PubMed] [Google Scholar]
- 204.Shaw P, Mead R, Higginbottom A, Barber S. Therapeutics for neurological disorders. UK Patent Application. 2010 Priority Date 24.10.2008. Publ Date 05.05.2010.
- 205.Murakami A, Ashida H, Terao J. Multitargeted cancer prevention by quercetin. Cancer Lett. 2008;269(2):315–325. doi: 10.1016/j.canlet.2008.03.046. [DOI] [PubMed] [Google Scholar]
- 206.Tanigawa S, Fujii M, Hou DX. Action of Nrf2 and Keap1 in ARE-mediated NQO1 expression by quercetin. Free Radic Biol Med. 2007;42(11):1690–1703. doi: 10.1016/j.freeradbiomed.2007.02.017. [DOI] [PubMed] [Google Scholar]
- 207.Lee SE, Jeong SI, Yang H, Park CS, Jin YH, Park YS. Fisetin induces Nrf2-mediated HO-1 expression through PKC-delta and p38 in human umbilical vein endothelial cells. J Cell Biochem. 2011;112(9):2352–2360. doi: 10.1002/jcb.23158. [DOI] [PubMed] [Google Scholar]
- 208.Hernandez-Montes E, Pollard SE, Vauzour D, Jofre-Montseny L, Rota C, Rimbach G, Weinberg PD, Spencer JP. Activation of glutathione peroxidase via Nrf1 mediates genistein’s protection against oxidative endothelial cell injury. Biochem Biophys Res Commun. 2006;346(3):851–859. doi: 10.1016/j.bbrc.2006.05.197. [DOI] [PubMed] [Google Scholar]
- 209.Mann GE, Bonacasa B, Ishii T, Siow RC. Targeting the redox sensitive Nrf2-Keap1 defense pathway in cardiovascular disease: protection afforded by dietary isoflavones. Curr Opin Pharmacol. 2009;9(2):139–145. doi: 10.1016/j.coph.2008.12.012. [DOI] [PubMed] [Google Scholar]
- 210.Valenzano DR, Terzibasi E, Genade T, Cattaneo A, Domenici L, Cellerino A. Resveratrol prolongs lifespan and retards the onset of age-related markers in a short-lived vertebrate. Curr Biol. 2006;16(3):296–300. doi: 10.1016/j.cub.2005.12.038. [DOI] [PubMed] [Google Scholar]
- 211.Kode A, Rajendrasozhan S, Caito S, Yang SR, Megson IL, Rahman I. Resveratrol induces glutathione synthesis by activation of Nrf2 and protects against cigarette smoke-mediated oxidative stress in human lung epithelial cells. Am J Physiol Lung Cell Mol Physiol. 2008;294(3):L478–488. doi: 10.1152/ajplung.00361.2007. [DOI] [PubMed] [Google Scholar]
- 212.Ungvari Z, Bagi Z, Feher A, Recchia FA, Sonntag WE, Pearson K, de Cabo R, Csiszar A. Resveratrol confers endothelial protection via activation of the antioxidant transcription factor Nrf2. Am J Physiol Heart Circ Physiol. 2010;299(1):H18–24. doi: 10.1152/ajpheart.00260.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Butt MS, Sultan MT. Green tea: nature’s defense against malignancies. Crit Rev Food Sci Nutr. 2009;49(5):463–473. doi: 10.1080/10408390802145310. [DOI] [PubMed] [Google Scholar]
- 214.Sriram N, Kalayarasan S, Sudhandiran G. Epigallocatechin-3-gallate augments antioxidant activities and inhibits inflammation during bleomycin-induced experimental pulmonary fibrosis through Nrf2-Keap1 signaling. Pulm Pharmacol Ther. 2009;22(3):221–236. doi: 10.1016/j.pupt.2008.12.010. [DOI] [PubMed] [Google Scholar]
- 215.Shen G, Xu C, Hu R, Jain MR, Nair S, Lin W, Yang CS, Chan JY, Kong AN. Comparison of (−)-epigallocatechin-3-gallate elicited liver and small intestine gene expression profiles between C57BL/6J mice and C57BL/6J/Nrf2 (−/−) mice. Pharm Res. 2005;22(11):1805–1820. doi: 10.1007/s11095-005-7546-8. [DOI] [PubMed] [Google Scholar]
- 216.Martin D, Rojo AI, Salinas M, Diaz R, Gallardo G, Alam J, De Galarreta CM, Cuadrado A. Regulation of heme oxygenase-1 expression through the phosphatidylinositol 3-kinase/Akt pathway and the Nrf2 transcription factor in response to the antioxidant phytochemical carnosol. J Biol Chem. 2004;279(10):8919–8929. doi: 10.1074/jbc.M309660200. [DOI] [PubMed] [Google Scholar]
- 217.Satoh T, Kosaka K, Itoh K, Kobayashi A, Yamamoto M, Shimojo Y, Kitajima C, Cui J, Kamins J, Okamoto S, Izumi M, Shirasawa T, Lipton SA. Carnosic acid, a catechol-type electrophilic compound, protects neurons both in vitro and in vivo through activation of the Keap1/Nrf2 pathway via S-alkylation of targeted cysteines on Keap1. J Neurochem. 2008;104(4):1116–1131. doi: 10.1111/j.1471-4159.2007.05039.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Guzman-Beltran S, Espada S, Orozco-Ibarra M, Pedraza-Chaverri J, Cuadrado A. Nordihydroguaiaretic acid activates the antioxidant pathway Nrf2/HO-1 and protects cerebellar granule neurons against oxidative stress. Neurosci Lett. 2008;447(2–3):167–171. doi: 10.1016/j.neulet.2008.09.079. [DOI] [PubMed] [Google Scholar]
- 219.Miura T, Shinkai Y, Jiang HY, Iwamoto N, Sumi D, Taguchi K, Yamamoto M, Jinno H, Tanaka-Kagawa T, Cho AK, Kumagai Y. Initial response and cellular protection through the Keap1/Nrf2 system during the exposure of primary mouse hepatocytes to 1,2-naphthoquinone. Chem Res Toxicol. 2011;24(4):559–567. doi: 10.1021/tx100427p. [DOI] [PubMed] [Google Scholar]
- 220.Fahey JW, Zalcmann AT, Talalay P. The chemical diversity and distribution of glucosinolates and isothiocyanates among plants. Phytochemistry. 2001;56(1):5–51. doi: 10.1016/s0031-9422(00)00316-2. [DOI] [PubMed] [Google Scholar]
- 221.Halkier BA, Gershenzon J. Biology and biochemistry of glucosinolates. Annu Rev Plant Biol. 2006;57:303–333. doi: 10.1146/annurev.arplant.57.032905.105228. [DOI] [PubMed] [Google Scholar]
- 222.Cheung KL, Kong AN. Molecular targets of dietary phenethyl isothiocyanate and sulforaphane for cancer chemoprevention. AAPS J. 2010;12(1):87–97. doi: 10.1208/s12248-009-9162-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Hu C, Eggler AL, Mesecar AD, van Breemen RB. Modification of keap1 cysteine residues by sulforaphane. Chem Res Toxicol. 2011;24(4):515–521. doi: 10.1021/tx100389r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Ahn YH, Hwang Y, Liu H, Wang XJ, Zhang Y, Stephenson KK, Boronina TN, Cole RN, Dinkova-Kostova AT, Talalay P, Cole PA. Electrophilic tuning of the chemoprotective natural product sulforaphane. Proc Natl Acad Sci U S A. 2010;107(21):9590–9595. doi: 10.1073/pnas.1004104107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Hong F, Freeman ML, Liebler DC. Identification of sensor cysteines in human Keap1 modified by the cancer chemopreventive agent sulforaphane. Chem Res Toxicol. 2005;18(12):1917–1926. doi: 10.1021/tx0502138. [DOI] [PubMed] [Google Scholar]
- 226.Prawan A, Keum YS, Khor TO, Yu S, Nair S, Li W, Hu L, Kong AN. Structural influence of isothiocyanates on the antioxidant response element (ARE)-mediated heme oxygenase-1 (HO-1) expression. Pharm Res. 2008;25(4):836–844. doi: 10.1007/s11095-007-9370-9. [DOI] [PubMed] [Google Scholar]
- 227.Posner GH, Cho CG, Green JV, Zhang Y, Talalay P. Design and synthesis of bifunctional isothiocyanate analogs of sulforaphane: correlation between structure and potency as inducers of anticarcinogenic detoxication enzymes. J Med Chem. 1994;37(1):170–176. doi: 10.1021/jm00027a021. [DOI] [PubMed] [Google Scholar]
- 228.Jia Z, Zhu H, Trush MA, Misra HP, Li Y. Generation of superoxide from reaction of 3H-1,2-dithiole-3-thione with thiols: implications for dithiolethione chemoprotection. Mol Cell Biochem. 2008;307(1–2):185–191. doi: 10.1007/s11010-007-9598-z. [DOI] [PubMed] [Google Scholar]
- 229.Egner PA, Kensler TW, Prestera T, Talalay P, Libby AH, Joyner HH, Curphey TJ. Regulation of phase 2 enzyme induction by oltipraz and other dithiolethiones. Carcinogenesis. 1994;15(2):177–181. doi: 10.1093/carcin/15.2.177. [DOI] [PubMed] [Google Scholar]
- 230.Kwak MK, Wakabayashi N, Itoh K, Motohashi H, Yamamoto M, Kensler TW. Modulation of gene expression by cancer chemopreventive dithiolethiones through the Keap1-Nrf2 pathway. Identification of novel gene clusters for cell survival. J Biol Chem. 2003;278(10):8135–8145. doi: 10.1074/jbc.M211898200. [DOI] [PubMed] [Google Scholar]
- 231.Kensler TW, Groopman JD, Sutter TR, Curphey TJ, Roebuck BD. Development of cancer chemopreventive agents: oltipraz as a paradigm. Chem Res Toxicol. 1999;12(2):113–126. doi: 10.1021/tx980185b. [DOI] [PubMed] [Google Scholar]
- 232.Paonessa JD, Munday CM, Mhawech-Fauceglia P, Munday R, Zhang Y. 5,6-Dihydrocyclopenta[c][1,2]-dithiole-3(4H)-thione is a promising cancer chemopreventive agent in the urinary bladder. Chem Biol Interact. 2009;180(1):119–126. doi: 10.1016/j.cbi.2008.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Ariga T, Seki T. Antithrombotic and anticancer effects of garlic-derived sulfur compounds: a review. Biofactors. 2006;26(2):93–103. doi: 10.1002/biof.5520260201. [DOI] [PubMed] [Google Scholar]
- 234.Fisher CD, Augustine LM, Maher JM, Nelson DM, Slitt AL, Klaassen CD, Lehman-McKeeman LD, Cherrington NJ. Induction of drug-metabolizing enzymes by garlic and allyl sulfide compounds via activation of constitutive androstane receptor and nuclear factor E2-related factor 2. Drug Metab Dispos. 2007;35(6):995–1000. doi: 10.1124/dmd.106.014340. [DOI] [PubMed] [Google Scholar]
- 235.Wu CC, Sheen LY, Chen HW, Tsai SJ, Lii CK. Effects of organosulfur compounds from garlic oil on the antioxidation system in rat liver and red blood cells. Food Chem Toxicol. 2001;39(6):563–569. doi: 10.1016/s0278-6915(00)00171-x. [DOI] [PubMed] [Google Scholar]
- 236.Tchounwou PB, Centeno JA, Patlolla AK. Arsenic toxicity, mutagenesis, and carcinogenesis--a health risk assessment and management approach. Mol Cell Biochem. 2004;255(1–2):47–55. doi: 10.1023/b:mcbi.0000007260.32981.b9. [DOI] [PubMed] [Google Scholar]
- 237.Snow ET, Sykora P, Durham TR, Klein CB. Arsenic, mode of action at biologically plausible low doses: what are the implications for low dose cancer risk? Toxicol Appl Pharmacol. 2005;207(2 Suppl):557–564. doi: 10.1016/j.taap.2005.01.048. [DOI] [PubMed] [Google Scholar]
- 238.Elangovan S, Hsieh TC. Control of cellular redox status and upregulation of quinone reductase NQO1 via Nrf2 activation by alpha-lipoic acid in human leukemia HL-60 cells. Int J Oncol. 2008;33(4):833–838. [PubMed] [Google Scholar]
- 239.Shay KP, Moreau RF, Smith EJ, Smith AR, Hagen TM. Alpha-lipoic acid as a dietary supplement: molecular mechanisms and therapeutic potential. Biochim Biophys Acta. 2009;1790(10):1149–1160. doi: 10.1016/j.bbagen.2009.07.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.El-Sayed WM, Aboul-Fadl T, Lamb JG, Roberts JC, Franklin MR. Effect of selenium-containing compounds on hepatic chemoprotective enzymes in mice. Toxicology. 2006;220(2–3):179–188. doi: 10.1016/j.tox.2005.12.016. [DOI] [PubMed] [Google Scholar]
- 241.Emmert SW, Desai D, Amin S, Richie JP., Jr Enhanced Nrf2-dependent induction of glutathione in mouse embryonic fibroblasts by isoselenocyanate analog of sulforaphane. Bioorg Med Chem Lett. 2010;20(8):2675–2679. doi: 10.1016/j.bmcl.2010.01.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Schewe T. Molecular actions of ebselen--an antiinflammatory antioxidant. Gen Pharmacol. 1995;26(6):1153–1169. doi: 10.1016/0306-3623(95)00003-j. [DOI] [PubMed] [Google Scholar]
- 243.Sakurai T, Kanayama M, Shibata T, Itoh K, Kobayashi A, Yamamoto M, Uchida K. Ebselen, a seleno-organic antioxidant, as an electrophile. Chem Res Toxicol. 2006;19(9):1196–1204. doi: 10.1021/tx0601105. [DOI] [PubMed] [Google Scholar]
- 244.Terazawa R, Garud DR, Hamada N, Fujita Y, Itoh T, Nozawa Y, Nakane K, Deguchi T, Koketsu M, Ito M. Identification of organoselenium compounds that possess chemopreventive properties in human prostate cancer LNCaP cells. Bioorg Med Chem. 2010;18(19):7001–7008. doi: 10.1016/j.bmc.2010.08.019. [DOI] [PubMed] [Google Scholar]
- 245.Rao AV, Rao LG. Carotenoids and human health. Pharmacological Research. 2007;55(3):207–216. doi: 10.1016/j.phrs.2007.01.012. [DOI] [PubMed] [Google Scholar]
- 246.Linnewiel K, Ernst H, Caris-Veyrat C, Ben-Dor A, Kampf A, Salman H, Danilenko M, Levy J, Sharoni Y. Structure activity relationship of carotenoid derivatives in activation of the electrophile/antioxidant response element transcription system. Free Radic Biol Med. 2009;47(5):659–667. doi: 10.1016/j.freeradbiomed.2009.06.008. [DOI] [PubMed] [Google Scholar]
- 247.Valko M, Morris H, Cronin MT. Metals, toxicity and oxidative stress. Curr Med Chem. 2005;12(10):1161–1208. doi: 10.2174/0929867053764635. [DOI] [PubMed] [Google Scholar]
- 248.Joseph P. Mechanisms of cadmium carcinogenesis. Toxicol Appl Pharmacol. 2009;238(3):272–279. doi: 10.1016/j.taap.2009.01.011. [DOI] [PubMed] [Google Scholar]
- 249.He X, Chen MG, Ma Q. Activation of Nrf2 in defense against cadmium-induced oxidative stress. Chem Res Toxicol. 2008;21(7):1375–1383. doi: 10.1021/tx800019a. [DOI] [PubMed] [Google Scholar]
- 250.Kim NH, Oh MK, Park HJ, Kim IS. Auranofin, a gold(I)-containing antirheumatic compound, activates Keap1/Nrf2 signaling via Rac1/iNOS signal and mitogen-activated protein kinase activation. J Pharmacol Sci. 2010;113(3):246–254. doi: 10.1254/jphs.09330fp. [DOI] [PubMed] [Google Scholar]
- 251.Kelleher MO, McMahon M, Eggleston IM, Dixon MJ, Taguchi K, Yamamoto M, Hayes JD. 1-Cyano-2,3-epithiopropane is a novel plant-derived chemopreventive agent which induces cytoprotective genes that afford resistance against the genotoxic alpha,beta-unsaturated aldehyde acrolein. Carcinogenesis. 2009;30(10):1754–1762. doi: 10.1093/carcin/bgp182. [DOI] [PubMed] [Google Scholar]
- 252.Tarumoto T, Nagai T, Ohmine K, Miyoshi T, Nakamura M, Kondo T, Mitsugi K, Nakano S, Muroi K, Komatsu N, Ozawa K. Ascorbic acid restores sensitivity to imatinib via suppression of Nrf2-dependent gene expression in the imatinib-resistant cell line. Exp Hematol. 2004;32(4):375–381. doi: 10.1016/j.exphem.2004.01.007. [DOI] [PubMed] [Google Scholar]
- 253.Kweon M-H, Adhami VM, Lee J-S, Mukhtar H. Constitutive Overexpression of Nrf2-dependent Heme Oxygenase-1 in A549 Cells Contributes to Resistance to Apoptosis Induced by Epigallocatechin 3-Gallate. J Biol Chem. 2006;281(44):33761–33772. doi: 10.1074/jbc.M604748200. [DOI] [PubMed] [Google Scholar]
- 254.Wang XJ, Hayes JD, Henderson CJ, Wolf CR. Identification of retinoic acid as an inhibitor of transcription factor Nrf2 through activation of retinoic acid receptor alpha. Proc Natl Acad Sci U S A. 2007;104(49):19589–19594. doi: 10.1073/pnas.0709483104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Hitotsuyanagi Y, Kim I, Hasuda T, Yamauchi Y, Takeya K. A structure–activity relationship study of brusatol, an antitumor quassinoid. Tetrahedron. 2006;62:4262–4271. [Google Scholar]
- 256.Lin C-W, Wu M-J, Liu IYC, Su J-D, Yen J-H. Neurotrophic and Cytoprotective Action of Luteolin in PC12 Cells through ERK-Dependent Induction of Nrf2-Driven HO-1 Expression. J Agric Food Chem. 2010;58(7):4477–4486. doi: 10.1021/jf904061x. [DOI] [PubMed] [Google Scholar]
- 257.Cavin C, Delatour T, Marin-Kuan M, Holzhäuser D, Higgins L, Bezençon C, Guignard G, Junod S, Richoz-Payot J, Gremaud E, Hayes JD, Nestler S, Mantle P, Schilter B. Reduction in Antioxidant Defenses may Contribute to Ochratoxin A Toxicity and Carcinogenicity. Toxicol Sci. 2007;96(1):30–39. doi: 10.1093/toxsci/kfl169. [DOI] [PubMed] [Google Scholar]
- 258.Boesch-Saadatmandi C, Wagner AE, Graeser AC, Hundhausen C, Wolffram S, Rimbach G. Ochratoxin A impairs Nrf2-dependent gene expression in porcine kidney tubulus cells. J Anim Physiol Anim Nutr. 2009;93(5):547–554. doi: 10.1111/j.1439-0396.2008.00838.x. [DOI] [PubMed] [Google Scholar]
- 259.Kern JT, Hannink M, Hess JF. Disruption of the Keap1-containing ubiquitination complex as an antioxidant therapy. Curr TopMed Chem. 2007;7(10):972–978. doi: 10.2174/156802607780906825. [DOI] [PubMed] [Google Scholar]
- 260.Xu L, Wei Y, Reboul J, Vaglio P, Shin TH, Vidal M, Elledge SJ, Harper JW. BTB proteins are substrate-specific adaptors in an SCF-like modular ubiquitin ligase containing CUL-3. Nature. 2003;425(6955):316–321. doi: 10.1038/nature01985. [DOI] [PubMed] [Google Scholar]
- 261.Lo SC, Li X, Henzl MT, Beamer LJ, Hannink M. Structure of the Keap1:Nrf2 interface provides mechanistic insight into Nrf2 signaling. EMBO J. 2006;25(15):3605–3617. doi: 10.1038/sj.emboj.7601243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262.Li X, Zhang D, Hannink M, Beamer LJ. Crystal structure of the Kelch domain of human Keap1. J Biol Chem. 2004;279(52):54750–54758. doi: 10.1074/jbc.M410073200. [DOI] [PubMed] [Google Scholar]
- 263.Beamer LJ, Li X, Bottoms CA, Hannink M. Conserved solvent and side-chain interactions in the 1.35 Angstrom structure of the Kelch domain of Keap1. Acta Crystallogr D Biol Crystallogr. 2005;61(Pt 10):1335–1342. doi: 10.1107/S0907444905022626. [DOI] [PubMed] [Google Scholar]
- 264.Beamer LJ, Lo SC, Li XC, Henzl MT, Hannink M. Structure of the Keap1 : Nrf2 interface provides mechanistic insight into Nrf2 signaling. Embo J. 2006;25(15):3605–3617. doi: 10.1038/sj.emboj.7601243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.Padmanabhan B, Tong KI, Ohta T, Nakamura Y, Scharlock M, Ohtsuji M, Kang MI, Kobayashi A, Yokoyama S, Yamamoto M. Structural basis for defects of Keap1 activity provoked by its point mutations in lung cancer. Mol Cell. 2006;21(5):689–700. doi: 10.1016/j.molcel.2006.01.013. [DOI] [PubMed] [Google Scholar]
- 266.Chen Y, Inoyama D, Kong A-N, Beamer LJ, Hu L. Kinetic analysis of Keap1-Nrf2 interaction and determination of the minimal Nrf2 peptide sequence required for binding to Keap1 Kelch domain using Surface Plasmon Resonance. Chem Biol Drug Des. 2011;78(6):1014–1021. doi: 10.1111/j.1747-0285.2011.01240.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267.Zhao X, Sun G, Zhang J, Strong R, Dash PK, Kan YW, Grotta JC, Aronowski J. Transcription factor Nrf2 protects the brain from damage produced by intracerebral hemorrhage. Stroke. 2007;38(12):3280–3286. doi: 10.1161/STROKEAHA.107.486506. [DOI] [PubMed] [Google Scholar]
- 268.Ping Z, Liu W, Kang Z, Cai J, Wang Q, Cheng N, Wang S, Zhang JH, Sun X. Sulforaphane protects brains against hypoxic-ischemic injury through induction of Nrf2-dependent phase 2 enzyme. Brain Res. 2010;1343:178–185. doi: 10.1016/j.brainres.2010.04.036. [DOI] [PubMed] [Google Scholar]
- 269.Yang C, Zhang X, Fan H, Liu Y. Curcumin upregulates transcription factor Nrf2, HO-1 expression and protects rat brains against focal ischemia. Brain Res. 2009;1282:133–141. doi: 10.1016/j.brainres.2009.05.009. [DOI] [PubMed] [Google Scholar]
- 270.Zhao J, Moore AN, Redell JB, Dash PK. Enhancing expression of Nrf2-driven genes protects the blood brain barrier after brain injury. J Neurosci. 2007;27(38):10240–10248. doi: 10.1523/JNEUROSCI.1683-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271.Zhao J, Redell JB, Moore AN, Dash PK. A novel strategy to activate cytoprotective genes in the injured brain. Biochem Biophys Res Commun. 2011;407(3):501–506. doi: 10.1016/j.bbrc.2011.03.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272.Moehlenkamp JD, Johnson JA. Activation of antioxidant/electrophile-responsive elements in IMR-32 human neuroblastoma cells. Arch Biochem Biophys. 1999;363(1):98–106. doi: 10.1006/abbi.1998.1046. [DOI] [PubMed] [Google Scholar]
- 273.Wang Z, Gidwani V, Sun Z, Zhang DD, Wong PK. Development of a molecular assay for rapid screening of chemopreventive compounds targeting Nrf2. J AssocLab Autom. 2008;13:243–248. [Google Scholar]
- 274.Westerink WMA, Stevenson JCR, Horbach GJ, Schoonen WGEJ. The development of RAD51C, Cystatin A, p53 and Nrf2 luciferase-reporter assays in metabolically competent HepG2 cells for the assessment of mechanism-based genotoxicity and of oxidative stress in the early research phase of drug development. Mutat Res Gen Toxicol Environ Mutagens. 2010;696(1):21–40. doi: 10.1016/j.mrgentox.2009.12.007. [DOI] [PubMed] [Google Scholar]
- 275.Hannink M, Zhang DD, Lo SC, Cross JV, Templeton DJ. Keap1 is a redox-regulated substrate adaptor protein for a Cul3-dependent ubiquitin ligase complex. Mol Cell Biol. 2004;24(24):10941–10953. doi: 10.1128/MCB.24.24.10941-10953.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276.Gazaryan IG, Smirnova NA, Haskew-Layton RE, Basso M, Hushpulian DM, Payappilly JB, Speer RE, Ahn YH, Rakhman I, Cole PA, Pinto JT, Ratan RR. Development of Neh2-Luciferase Reporter and Its Application for High Throughput Screening and Real-Time Monitoring of Nrf2 Activators. Chem Biol. 2011;18(6):752–765. doi: 10.1016/j.chembiol.2011.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 277.Inoyama D, Chen Y, Huang X, Beamer L, Kong AN, Hu L. Optimization of fluorescently labled Nrf2 peptide probes and the development of a fluorescence polarization assay for the discovery of inhibitors Keap1-Nrf2 interaction. J Biomol Screen. 2011 doi: 10.1177/1087057111430124. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]