Abstract
Background
This study aimed to explore whether walking in nature may be beneficial for individuals with major depressive disorder (MDD). Healthy adults demonstrate significant cognitive gains after nature walks, but it was unclear whether those same benefits would be achieved in a depressed sample as walking alone in nature might induce rumination, thereby worsening memory and mood.
Methods
Twenty individuals diagnosed with MDD participated in this study. At baseline, mood and short term memory span were assessed using the PANAS and the backwards digit span (BDS) task, respectively. Participants were then asked to think about an unresolved negative autobiographical event to prime rumination, prior to taking a 50 minute walk in either a natural or urban setting. After the walk, mood and short-term memory span were reassessed. The following week, participants returned to the lab and repeated the entire procedure, but walked in the location not visited in the first session (i.e., a counterbalanced within-subjects design).
Results
Participants exhibited significant increases in memory span after the nature walk relative to the urban walk, p < .001, ηp2= .53 (a large effect-size). Participants also showed increases in mood, but the mood effects did not correlate with the memory effects, suggesting separable mechanisms and replicating previous work.
Limitations
Sample size and participants’ motivation.
Conclusions
These findings extend earlier work demonstrating the cognitive and affective benefits of interacting with nature to individuals with MDD. Therefore, interacting with nature may be useful clinically as a supplement to existing treatments for MDD.
Keywords: Major Depressive Disorder, memory, nature, intervention, mood, attention restoration
Major Depressive Disorder (MDD) is characterized by cognitive impairments such as compromised working memory (Lyubomirsky et al., 2003), and by affective impairments such as persistent negative mood (Nolen-Hoeksema et al., 2008). Prior research indicates that interacting with nature enhances cognitive functioning (Berman et al., 2008; Cimprich & Ronis, 2003; Kaplan & Berman, 2010; Taylor & Kuo, 2009) and specifically increases working-memory span and improves mood (Berman, et al., 2008).
Kaplan and colleagues (Kaplan, 1995; Kaplan & Berman, 2010) have proposed Attention Restoration Theory (ART) to explain how interacting with nature improves cognitive abilities. ART draws on research demonstrating that attention can be separated into two components: involuntary attention, in which attention is captured by salient stimuli, and voluntary or directed attention, in which attention is directed by cognitive-control processes. This distinction, first proposed by William James (James, 1892), has been validated by behavioral and neuroscience research (Buschman & Miller, 2007; Corbetta & Shulman, 2002; Fan et al., 2002). ART identifies directed attention as the cognitive mechanism that is restored by interacting with nature, and others have implicated a critical role for directed attention in many contexts (Diamond et al., 2007; Posner & Rothbart, 2007), including short-term memory performance (Jonides et al., 2008).
According to ART, interacting with environments that contain inherently fascinating stimuli (e.g., sunsets) modestly invoke involuntary attention, allowing directed-attention mechanisms a chance to replenish (Berman, et al., 2008; Kaplan, 1995; Kaplan & Berman, 2010). That is, the requirement for directed attention in such environments is minimized, and attention is captured in a bottom-up fashion by features of the environment itself. Thus, following an interaction with natural environments, individuals perform better on tasks that depend on directed-attention abilities. Unlike natural environments, urban environments contain bottom-up stimulation (e.g., car horns) that capture attention dramatically, requiring directed attention to overcome that stimulation (e.g., avoiding traffic, ignoring advertising, etc.), making urban environments less restorative.
Although interacting with natural environments has been found to be beneficial for healthy individuals, it’s not clear whether these benefits would generalize to individuals with MDD. On one hand, to the extent that interacting with natural environments (e.g., parks) replenish cognitive resources (Berman, et al., 2008; Kaplan & Berman, 2010), individuals with MDD may show the same or even greater cognitive gains than those demonstrated by healthy individuals. It has been hypothesized that individuals who are more attentionally fatigued may obtain greater benefits from interacting with nature (Kaplan & Berman, 2010), and fatigued participants have been found to gain greater benefits from other types of interventions (Masicampo & Baumeister, 2008). Given that individuals with depression are likely more mentally/attentionally fatigued than are nondepressed individuals due to their depressive symptoms (e.g. ruminations, psychomotor problems, etc.), it is possible that individuals with depression may show increased cognitive and affective gains from a nature interaction.
On the other hand, individuals with depression are characterized by high levels of rumination (Nolen-Hoeksema, et al., 2008). Rumination maintains and exacerbates negative mood, has been linked to impairments in short-term/working memory (Berman et al., 2011; Joormann & Gotlib, 2008; Landro et al., 2001), and may be particularly pronounced during time spent alone. Thus, asking a person with MDD to go for a solitary walk in a park may actually worsen, rather than improve, memory and mood by potentially taxing top-down/directed attention resources.
There are a variety of effective interventions for MDD, including psychotherapy (Robinson et al., 1990), medication (DeRubeis et al., 2005), and alternative treatments such as mindfulness meditation (Grossman et al., 2004). However, in a recent review, Kazdin and Blase (2011) called for more research to explore simple, portable and cost-effective interventions for mood and anxiety disorders. This study is a first attempt to discover if interacting with nature may be one such intervention possibility.
The current research
This study was designed to examine whether interacting with nature has beneficial effects on memory performance and affect in individuals diagnosed with MDD. Specifically, we examined whether interacting with nature could improve the typically impaired short-term memory/working memory performance in MDD (Berman, et al., 2011; Joormann et al., 2010; Landro, et al., 2001). We also examined whether mood would change differentially after a walk in nature vs. a walk in an urban environment, as well as the relation between mood and memory effects. Improvements in mood would be of particular interest given that MDD is characterized by low levels of positive affect (Watson & Naragon-Gainey, 2010).
A conservative task was employed to examine whether interacting with nature was beneficial for individuals with MDD by asking participants to reflect on an intense negative experience prior to going on their walks. In this way, we set the stage for an exposure to nature to maximize its impact on individuals with depression who were primed with negative thoughts and feelings.
Methods
Participants
Twenty individuals diagnosed with MDD (12 female, 8 male, mean age = 26) participated in this study. A diagnosis of MDD was made by clinicians who administered the Structured Clinical Interview (SCID) for DSM-IV (First & Gibbon, 1996). Participants were recruited from the University of Michigan and the greater Ann Arbor area through ads on Craigslist and Facebook, as well as fliers that were distributed around the University of Michigan campus and stores/shops in the greater Ann Arbor area. These ads asked participants if they were feeling sad, down or depressed and if they were interested in participating in research to e-mail our lab.
Participants were included if they met criteria for current MDD as determined by the SCID. All participants were run in the experimental sessions within two weeks of their SCID. The Beck Depression Inventory (BDI-II) (Beck et al., 1996) was also administered (M = 30.1, SD = 10.8). BDI scores of 20-28 indicate moderate depression, while scores of 29-65 indicate severe depression; thus our sample is in the moderate to severe range. Twelve participants had comorbid diagnoses (e.g., bulimia) and six were known to be on medication for depression. Participants gave informed consent as administered by the Institutional Review Board of the University of Michigan and were compensated $20/hour. Each session lasted 3 hours. One participant was removed for completing only the first session, leaving 19 participants with complete data.
Procedure
We first assessed participants’ mood with the Positive and Negative Affect Schedule (Watson et al., 1988), which yields separate scores for positive and negative affect. Then participants performed the backward digit span (BDS) task, in which digits were presented auditorily at a pace of 1 digit per second and were repeated aloud by the participant. Next, we primed participants to ruminate by instructing them to analyze their feelings surrounding an intense, unresolved negative autobiographical experience; a procedure used by others (Kross & Ayduk, 2008; Rusting & Nolen-Hoeksema, 1998). This was done to initiate rumination in participants to explore if nature walks remediate cognitive and affective difficulties in individuals with depression who were distressed. Finally, we reassessed participants’ mood.
Participants were then randomly assigned to take a 50- to 55-min walk in the Ann Arbor Arboretum (a park near campus) or in downtown Ann Arbor. The walks were predefined for participants and equated in total length (2.8 miles). Each participant was given a map displaying the path of each walk and wore a GPS watch to ensure compliance. The arboretum walk was tree-lined and secluded from traffic and people. The downtown walk was largely on traffic-heavy streets lined with university and office buildings. The walks were identical to those used in prior research, which has documented an effect of interacting with nature versus urban environments on cognitive functioning (Berman et al., 2008).
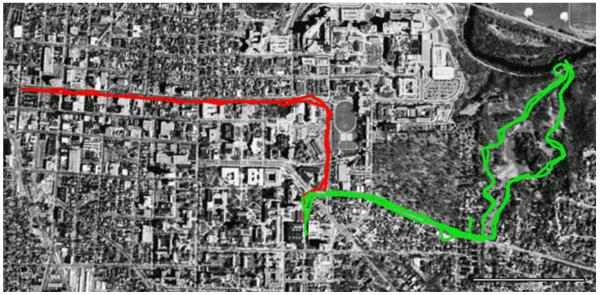
Upon their return, participants again completed the PANAS and BDS task. Participants’ walk GPS data were then analyzed and superimposed on a satellite image to ensure that they walked in the specified locations. Figure 1 shows a satellite image of the two walks from participant GPS data.
Figure 1.
Satellite images of the nature and urban walks obtained from participants’ GPS data. The nature walk is in green, and the urban walk in red. The nature walk shows data from two participants.
At the conclusion of the session participants were asked to respond on a scale of 0-2 (0 = no; 1 = sort-of; 2 = yes) if they thought about the memory that they generated. This scale indexed the extent to which participants perseverated during their walk about the negative autobiographical memory. While not a direct measure of rumination, responses to this question provided some indication of what participants were thinking about on their walks. One week later, participants returned to the lab and repeated the entire procedure, walking in the location that was not visited in the first session. The order of walking in nature versus an urban setting was counterbalanced across participants.
Analysis Parameters
A 2 (Time: pre-walk vs. post-walk) X 2 (Location: nature vs. urban) analysis of variance (ANOVA) was conducted separately on scores from the BDS task and the PANAS. Post-hoc t-tests were conducted to follow up significant interactions.
Results
Working Memory Capacity
The two-way ANOVA on BDS scores yielded no main effects of location or time (Fs < 3.39, ps > .08), but did yield a significant time X location interaction, F(1, 18) = 20.5, p < .001, ηp2 = .53, indicating that participants’ memory capacity increased more after the nature walk than after the urban walk. Indeed, the size of this effect was nearly 5 times larger than that found in our previous work (ηp2= .14) with a non-clinical sample (Berman, et al., 2008). This interaction was driven by reliable increases in BDS task performance after the nature walk, t(18) = 3.67, p < .005, and a trend toward decreases in BDS task performance after the urban walk, t(18) = −1.91, p = .07 (See Table 1). Moreover, although there were no differences in pre-nature and pre-urban BDS task performance, t(18) = 1.804, n.s. (i.e., no baseline differences in BDS performance), one participant did have a pre-nature BDS score that was nearly two standard deviations below the sample mean. Even after removing that participant, the same effects of greater increases in BDS task performance after the nature walk than after the urban walk were found, F(1, 17) = 17.88, p < .001, ηp2= .51 (see Table 1).
Table 1.
Means and standard deviations in parentheses for BDS and mood measures. The second set of BDS measures are when one participant was removed for having a low BDS score before the nature walk that was nearly two standard deviations below the sample mean.
| Measure | Walk Location |
Pre-Mood Induction |
Post-Mood Induction |
Post- Walk |
|---|---|---|---|---|
| BDS | Nature | 7.42 (3.00) | n/a | 8.63 (2.87) |
| Urban | 8.26 (2.51) | n/a | 7.84 (2.24) |
|
|
BDS (1 participant
removed) |
Nature | 7.72 (2.78) | n/a | 8.83 (2.81) |
| Urban | 8.33 (2.57) | n/a | 7.94 (2.26) |
|
| Positive Affect | Nature | 2.11 (0.82) | 1.48 (0.55) | 2.62 (1.03) |
| Urban | 1.92 (0.62) | 1.52 (0.44) | 2.26 (0.89) |
|
| Negative Affect | Nature | 2.04 (0.84) | 2.41 (0.96) | 1.53 (0.86) |
| Urban | 2.03 (0.88) | 2.58 (1.06) | 1.64 (0.92) |
Mood
As a manipulation check the mood induction was successful: positive affect (PA) was significantly reduced, and negative affect (NA) significantly increased after the participants reflected on their negative memories prior to their walks (ps < .05; see Table 1).
Positive affect
A 2 × 2 ANOVA yielded a significant effect of location (nature vs. urban), F(1,161) = 16.85, p < .001, but no significant effect of time (pre-walk vs. post-walk), F(1,16) = 2.04, n.s. Of most interest was the interaction – PA improved to a greater extent after the nature walk than the urban walk, as indicated by a significant interaction between location and time, F(1,16) = 6.62, p < .05, ηp2= .29. Follow-up tests showed that the main effect of location was driven by greater PA after the nature walk, t(16) = 2.30, p < .05, as no baseline differences in PA were found pre-nature vs. pre-urban, t(18) = .393, n.s. PA, however, did improve significantly after each walk: nature, t(16) = 4.31, p < .001; urban, t(18) = 3.67, p < .005 (see Table 1). Changes in PA did not correlate with changes in BDS performance after either walk (ps >.19), suggesting that the observed improvements in memory were not driven by mood, and that separate mechanisms may underlie the cognitive and affective effects of interacting with nature.
Negative affect
Results from the 2 × 2 ANOVA yielded no significant effect of location, F(1,16) = 2.75, n.s., but did yield a significant main effect of time, F(1,16) = 16.43, p < .001. Contrary to the results for PA, NA did not decrease more for the nature walk than for the urban walk, F(1,15) = .13, n.s., but decreases in NA were observed after both the nature walk t(16) = 4.34, p < .001 and the urban walk, t(18) = 3.72, p < .005. Changes in NA also did not correlate with changes in BDS performance after either walk (ps > .53).
Covariates
Walk order (nature first or urban first) was not a significant predictor for any mood or memory analysis when it was included as a between-subjects factor in the ANOVAs. Comorbid diagnosis was not a significant predictor for any memory analysis or any analysis of negative affect.
Thoughts during the walks
There was no difference in participants’ reports of thinking about their generated negative memory on the nature (M = 1.16; SD = .60) or the urban (M = 1.21; SD = .42) walk, t(18) = .37, p > .72, indicating that most participants thought about their negative autobiographical memory to some (and the same) degree on both walks. Finally, there were no significant correlations between thinking about the negative memory and changes in BDS task performance or mood scores for either walk (ps > .13).
Summary
Working-memory capacity and positive affect improved to a greater extent after the nature walk relative to the urban walk. Interestingly, these effects were not correlated, suggesting separable mechanisms. Lastly, participants’ thought about their negative autobiographical memories to an equal extent on both walks, therefore avoiding thinking about their negative memory was not a driving mechanism for the nature effects.
Discussion
This study examined whether interacting with nature has beneficial effects on cognitive and affective functioning in MDD. We found that individuals diagnosed with MDD exhibited cognitive and affective improvements after walking in a nature setting. These effects were observed even though participants were instructed prior to their walks to think about a painful negative experience, which has been shown to prime rumination (Kross & Ayduk, 2008), which in turn has been shown to disrupt working memory (Berman, et al., 2011).
These findings suggest that interacting with nature, even in the context of thinking about a painful memory, is beneficial for people suffering from MDD. Moreover, the effect sizes we observed for individuals with MDD in this study were nearly five times as large as the effect sizes observed in another study with healthy individuals (Berman, et al., 2008), suggesting that individuals with depression benefit even more from such interactions. Prior to this study it was not clear whether interacting with nature would harm or help those with MDD, especially given the negative mood induction prior to the walk. The fact that the nature walk was beneficial even while participants were thinking of a negative autobiographical memory suggests that the walk could be beneficial even in the midst of heightened ruminative processes. Importantly, the memory improvements we observed were not driven by changes in affect, replicating previous work (Berman, et al., 2008). Both positive and negative affect benefited after both walks, but only positive affect changed differentially for the nature walk compared to the urban walk. Increasing positive affect is important given that MDD is characterized by low levels of positive affect (Watson & Naragon-Gainey, 2010).
Some theories claim that increases in positive affect should lead to improvements in working-memory performance either by increasing dopamine levels (Ashby et al., 1999) or by broadening thought-action repertoires (Fredrickson, 2001). However, other researchers have found poorer cognitive-control in positive mood states (Oaksford et al., 1996), while still other investigators have found selective effects depending on task demands and stimuli (Gray, 2001; Phillips et al., 2002). For example, Phillips et al. (2002) suggest that induced positive moods improve performance on tasks that demand creativity and may impair performance on tasks that require more focused attention. It is possible that, had we administered a task that engaged more creative processes such as a verbal fluency task, we would have found a relation with our mood effects. While our data cannot rule out the possibility that affective and cognitive improvements are not related in all cases, the fact that memory and mood were unrelated in our study suggests that the cognitive benefits gained from interacting with nature are due to processes beyond simply increasing positive affect.
Having demonstrated the salutary effects of nature, it is important to consider the potential mechanisms at play, which could help to refine the intervention more effectively. Although the present study does not allow us to examine this directly, according to ART, interacting with nature activates involuntary attention modestly, allowing replenishment of directed-attentional mechanisms (Berman, et al., 2008; Kaplan, 1995; Kaplan & Berman, 2010). Berman et al. (2008) showed this effect most directly in demonstrating that only cognitive tasks that had an executive component improved after a nature interaction. There are, of course, other potential mechanisms that could underlie the beneficial effects of nature. For example, the effects could be driven by stress reduction (Ulrich et al., 1991) or by other physiological changes. Future research is needed to examine the role that these processes play in mediating the observed effects.
Interestingly, there were no differences in what participants reported thinking about on the two walks. Therefore, it was not the case that participants thought about their negative experiences more on the urban walk than on the nature walk. There are at least two interpretations of this finding. First, the effects of nature on memory and positive affect may be independent of what participants think about during the walk. Alternatively, recent studies have demonstrated that people can reflect upon negative experiences either adaptively or maladaptively (Aldao & Nolen-Hoeksema, 2010; Kross & Ayduk, 2011). Thus, although participants reported thinking about their negative experience to the same degree on both walks, it is possible that they thought about it more adaptively when walking in nature versus an urban environment, which may in turn have given rise to the mood and memory effects we observed.
Limitations
In closing we should note that a limitation of our study is our relatively small sample size (19 participants). However, there are a few aspects of our design that mitigate concerns regarding sample size. First, our effect sizes were large. Second, our design was a within-subjects design, which helps to alleviate concerns regarding power and is a replication of previous work that used healthy participants (Berman, et al., 2008). Third, the sample size of this study matches that of other similar types of studies (Amir et al., 2009; Bismuth-Evenzal et al., 2012; Maalouf et al., 2011). Lastly, given our current effect size, we would need a sample of only 10 participants (half our current sample size) to have sufficient power to detect a significant interaction (i.e., power above .8), and our observed power to detect differences given or current sample size is .98, well above the .8 standard.
Despite the strengths of our design and the large effect sizes, it is difficult to rule out some alternative explanations. For example, we found no correlation between the mood effects and the cognitive effects. Although it is possible that this lack of a significant correlation is due to the small sample size in the present study, it is important to note that Berman et al. (2008) also reported no correlation between mood and cognitive effects with a sample size twice that of the current study. To rule out affective mechanisms, experiments that manipulate both mood and environmental setting are required. Future experiments should also include not only subjective measures of mood, but also physiological measures that may show relations to the cognitive effects even in the absence of relations to subjective mood measures. Finally, we did not have direct measures of adaptive versus maladaptive self-reflection during the walks. Thus, as noted earlier, we do not know whether nature influences the type of self-reflective process in which people engage.
Lastly, while all of our participants met criteria for depression as determined by the SCID, our participants were motivated enough to participate in a research study that involved mild physical activity, and not all participants with depression may have that same motivation. Therefore, an important challenge concerns how to motivate participants with depression to take nature walks given the motivational deficits that they suffer from. Although it is possible that the positive emotional and cognitive rewards may propel them to continue walking in nature in the future, additional work is needed both to motivate a broader range of participants to walk in nature and to develop methods to encourage participants to continue to walk in nature. We did not experience difficulties convincing participants in our study to walk in either location. These limitations notwithstanding, this study is an important first step in exploring the potential therapeutic benefit of interacting with nature for individuals with MDD.
Conclusion
Researchers have recently called for the development and exploration of brief, simple and portable interventions to treat mood disorders that can be widely disseminated at low-costs (Kazdin & Blase, 2011). The current research fits these aims well. Interacting with nature is, for the most part, widely accessible, simple and affordable. Yet we know virtually nothing about how this process affects mood and cognition in MDD. Although the current findings begin to address this issue, they also highlight important questions for future research. For example, how long-lasting are the effects of interacting with nature? Do individual differences (e.g., urban vs. rural dwellers) moderate their effects? How can we motivate participants with MDD to take these walks more often? Can interacting with nature provide an important supplement to existing empirically validated forms of treatment for MDD? Addressing these questions is important for refining knowledge concerning how interacting with nature influences depression.
These results are timely, as studies have indicated that urban living may adversely affect psychological functioning (Lederbogen et al., 2011) and increase psychopathology (Krabbendam & van Os, 2005; Pedersen & Mortensen, 2001; Peen et al., 2010; van Os et al., 2010). These results suggest that incorporating nearby nature into urban environments may counteract some of these adverse effects. Future research may examine whether nature interactions can supplement and enhance existing treatments for MDD and other psychopathologies to improve well-being.
Acknowledgements and Author Notes
This work was supported by NIMH grant MH60655 to JJ. We thank Alexa Erickson and Catherine Cherny for data collection; Phil Cheng and Hyang Sook Kim for diagnostic interviewing.
Role of the Funding Source This work was supported by NIMH grant MH60655 to John Jonides. The grant helped to pay for the post-doctoral fellow’s stipend (Marc G. Berman), research assistants’ hourly wages, participant payments and experimental equipment.
Footnotes
2 participants had missing mood data post-nature walk.
Conflicts of Interest The authors’ have no conflicts of interest to report.
Contributions Marc G. Berman headed study design, analysis, and manuscript composition
Ethan Kross helped to design the study and write the manuscript
Katherine M. Krpan helped to write the manuscript
Mary K. Askren helped to analyze the data and write the manuscript
Aleah Burson helped to design the study and write the manuscript
Patricia J. Deldin helped to design the study and write the manuscript
Stephen Kaplan helped to design the study and write the manuscript
Lindsey Sherdell helped to design the study and write the manuscript
Ian H. Gotlib helped to design the study and write the manuscript
John Jonides helped to design the study and write the manuscript
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aldao A, Nolen-Hoeksema S. Specificity of cognitive emotion regulation strategies: A transdiagnostic examination. Behaviour research and therapy. 2010;48(10):974–983. doi: 10.1016/j.brat.2010.06.002. [DOI] [PubMed] [Google Scholar]
- Amir N, Beard C, Burns M, Bomyea J. Attention Modification Program in Individuals With Generalized Anxiety Disorder. Journal of abnormal psychology. 2009;118(1):28–33. doi: 10.1037/a0012589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashby FG, Isen AM, Turken U. A neuropsychological theory of positive affect and its influence on cognition. Psychological review. 1999;106(3):529. doi: 10.1037/0033-295x.106.3.529. [DOI] [PubMed] [Google Scholar]
- Beck AT, Steer RA, Brown GK. Manual for the Beck Depression Inventory II (BDI-II) Psychological Corporation; San Antonio, TX: 1996. [Google Scholar]
- Berman MG, Jonides J, Kaplan S. The Cognitive Benefits of Interacting With Nature. Psychological Science. 2008;19(12):1207. doi: 10.1111/j.1467-9280.2008.02225.x. [DOI] [PubMed] [Google Scholar]
- Berman MG, Nee D, Casement M, Kim H, Deldin P, Kross E, Gonzalez R, Demiralp E, Gotlib I, Hamilton P, Joormann J, Waugh C, Jonides J. Neural and behavioral effects of interference resolution in depression and rumination. Cognitive, affective behavioral neuroscience. 2011 doi: 10.3758/s13415-010-0014-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bismuth-Evenzal Y, Gonopolsky Y, Gurwitz D, Iancu I, Weizman A, Rehavi M. Decreased serotonin content and reduced agonist-induced aggregation in platelets of patients chronically medicated with SSRI drugs. Journal of Affective Disorders. 2012;136(1-2):99–103. doi: 10.1016/j.jad.2011.08.013. [DOI] [PubMed] [Google Scholar]
- Buschman TJ, Miller EK. Top-down versus bottom-up control of attention in the prefrontal and posterior parietal cortices. Science. 2007;315(5820):1860. doi: 10.1126/science.1138071. [DOI] [PubMed] [Google Scholar]
- Cimprich B, Ronis DL. An environmental intervention to restore attention in women with newly diagnosed breast cancer. Cancer nursing. 2003;26(4):284. doi: 10.1097/00002820-200308000-00005. [DOI] [PubMed] [Google Scholar]
- Corbetta M, Shulman GL. Control of goal-directed and stimulus-driven attention in the brain. Nature Reviews Neuroscience. 2002;3(3):201. doi: 10.1038/nrn755. [DOI] [PubMed] [Google Scholar]
- DeRubeis RJ, Hollon SD, Amsterdam JD, Shelton RC, Young PR, Salomon RM, O’Reardon JP, Lovett ML, Gladis MM, Brown LL, Gallop R. Cognitive therapy vs medications in the treatment of moderate to severe depression. Archives of General Psychiatry. 2005;62(4):409–416. doi: 10.1001/archpsyc.62.4.409. [DOI] [PubMed] [Google Scholar]
- Diamond A, Barnett WS, Thomas J, Munro S. The early years - Preschool program improves cognitive control. Science. 2007;318:1387. doi: 10.1126/science.1151148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan J, McCandliss BD, Sommer T, Raz A, Posner MI. Testing the efficiency and independence of attentional networks. Journal of cognitive neuroscience. 2002;14(3):340–347. doi: 10.1162/089892902317361886. [DOI] [PubMed] [Google Scholar]
- First MB, Gibbon M. SCID-101 for DSM-IV Training Video for the Structured Clinical Interview for DSM-IV Axis I Disorders (SCID) Biometrics Research Department, New York State Psychiatric Institute; 1996. [Google Scholar]
- Fredrickson BL. The role of positive emotions in positive psychology - The broaden-and-build theory of positive emotions. American Psychologist. 2001;56(3):218. doi: 10.1037//0003-066x.56.3.218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray JR. Emotional modulation of cognitive control: Approach-withdrawal states double-dissociate spatial from verbal two-back task performance. Journal of Experimental Psychology-General. 2001;130(3):436–452. doi: 10.1037//0096-3445.130.3.436. [DOI] [PubMed] [Google Scholar]
- Grossman P, Niemann L, Schmidt S, Walach H. Mindfulness-based stress reduction and health benefits - A meta-analysis. Journal of Psychosomatic Research. 2004;57(1):35–43. doi: 10.1016/S0022-3999(03)00573-7. [DOI] [PubMed] [Google Scholar]
- James W, editor. Psychology: The Briefer Course. Holt; New York: 1892. [Google Scholar]
- Jonides J, Lewis RL, Nee DE, Lustig CA, Berman MG, Moore KS. The mind and brain of short-term memory. Annual Review of Psychology. 2008;59:193. doi: 10.1146/annurev.psych.59.103006.093615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joormann J, Gotlib IH. Updating the contents of working memory in depression: Interference from irrelevant negative material. Journal of abnormal psychology. 2008;117(1):182. doi: 10.1037/0021-843X.117.1.182. [DOI] [PubMed] [Google Scholar]
- Joormann J, Nee DE, Berman MG, Jonides J, Gotlib IH. Interference resolution in major depression. Cognitive, Affective, & Behavioral Neuroscience. 2010;10(1):21–33. doi: 10.3758/CABN.10.1.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan S. The Restorative Benefits of Nature - Toward an Integrative Framework. Journal of Environmental Psychology. 1995;15(3):169. [Google Scholar]
- Kaplan S, Berman MG. Directed Attention as a Common Resource for Executive Functioning and Self-Regulation. Perspectives on Psychological Science. 2010;5(1):43. doi: 10.1177/1745691609356784. [DOI] [PubMed] [Google Scholar]
- Kazdin AE, Blase SL. Rebooting Psychotherapy Research and Practice to Reduce the Burden of Mental Illness. Perspectives on Psychological Science. 2011;6(1):21–37. doi: 10.1177/1745691610393527. [DOI] [PubMed] [Google Scholar]
- Krabbendam L, van Os J. Schizophrenia and urbanicity: A major environmental influence - Conditional on genetic risk. Schizophrenia bulletin. 2005;31(4):795–799. doi: 10.1093/schbul/sbi060. [DOI] [PubMed] [Google Scholar]
- Kross E, Ayduk O. Facilitating adaptive emotional analysis: Distinguishing distanced-analysis of depressive experiences from immersed-analysis and distraction. Personality and Social Psychology Bulletin. 2008;34(7):924. doi: 10.1177/0146167208315938. [DOI] [PubMed] [Google Scholar]
- Kross E, Ayduk O. Making Meaning out of Negative Experiences by Self-Distancing. Current Directions in Psychological Science. 2011;20:187–191. [Google Scholar]
- Landro NI, Stiles TC, Sletvold H. Neuropsychological function in nonpsychotic unipolar major depression. Neuropsychiatry Neuropsychology and Behavioral Neurology. 2001;14(4):233–240. [PubMed] [Google Scholar]
- Lederbogen F, Kirsch P, Haddad L, Streit F, Tost H, Schuch P, Wust S, Pruessner JC, Rietschel M, Deuschle M, Meyer-Lindenberg A. City living and urban upbringing affect neural social stress processing in humans. Nature. 2011;474(7352):498–501. doi: 10.1038/nature10190. [DOI] [PubMed] [Google Scholar]
- Lyubomirsky S, Kasri F, Zehm K. Dysphoric rumination impairs concentration on academic tasks. Cognitive Therapy and Research. 2003;27(3):309–330. [Google Scholar]
- Maalouf FT, Brent D, Clark L, Tavitian L, McHugh RM, Sahakian BJ, Phillips ML. Neurocognitive impairment in adolescent major depressive disorder: State vs. trait illness markers. Journal of Affective Disorders. 2011;133(3):625–632. doi: 10.1016/j.jad.2011.04.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masicampo EJ, Baumeister RF. Toward a physiology of dual-process reasoning and judgment - Lemonade, willpower, and expensive rule-based analysis. Psychological Science. 2008;19(3):255. doi: 10.1111/j.1467-9280.2008.02077.x. [DOI] [PubMed] [Google Scholar]
- Nolen-Hoeksema S, Wisco BE, Lyubomirsky S. Rethinking Rumination. Perspectives on Psychological Science. 2008;3(5):400. doi: 10.1111/j.1745-6924.2008.00088.x. [DOI] [PubMed] [Google Scholar]
- Oaksford M, Morris F, Grainger B, Williams JMG. Mood, reasoning, and central executive processes. Journal of Experimental Psychology-Learning Memory and Cognition. 1996;22(2):476. [Google Scholar]
- Pedersen CB, Mortensen PB. Evidence of a dose-response relationship between urbanicity during upbringing and schizophrenia risk. Archives of General Psychiatry. 2001;58(11):1039–1046. doi: 10.1001/archpsyc.58.11.1039. [DOI] [PubMed] [Google Scholar]
- Peen J, Schoevers RA, Beekman AT, Dekker J. The current status of urban-rural differences in psychiatric disorders. Acta Psychiatrica Scandinavica. 2010;121(2):84–93. doi: 10.1111/j.1600-0447.2009.01438.x. [DOI] [PubMed] [Google Scholar]
- Phillips LH, Bull R, Adams E, Fraser L. Positive mood and executive function: Evidence from Stroop and fluency tasks. Emotion (Washington D C) 2002;2(1):12–22. doi: 10.1037/1528-3542.2.1.12. [DOI] [PubMed] [Google Scholar]
- Posner MI, Rothbart MK. Research on attention networks as a model for the integration of psychological science. Annual Review of Psychology. 2007;58:1. doi: 10.1146/annurev.psych.58.110405.085516. [DOI] [PubMed] [Google Scholar]
- Robinson LA, Berman JS, Neimeyer RA. PSYCHOTHERAPY FOR THE TREATMENT OF DEPRESSION - A COMPREHENSIVE REVIEW OF CONTROLLED OUTCOME RESEARCH. Psychological bulletin. 1990;108(1):30–49. doi: 10.1037/0033-2909.108.1.30. [DOI] [PubMed] [Google Scholar]
- Rusting CL, Nolen-Hoeksema S. Regulating responses to anger: Effects of rumination and distraction on angry mood. Journal of personality and social psychology. 1998;74(3):790–803. doi: 10.1037//0022-3514.74.3.790. [DOI] [PubMed] [Google Scholar]
- Taylor AF, Kuo FE. Children With Attention Deficits Concentrate Better After Walk in the Park. Journal of Attention Disorders. 2009;12(5):402. doi: 10.1177/1087054708323000. [DOI] [PubMed] [Google Scholar]
- Ulrich RS, Simons RF, Losito BD, Fiorito E, Miles MA, Zelson M. Stress Recovery during Exposure to Natural and Urban Environments. Journal of Environmental Psychology. 1991;11(3):201. [Google Scholar]
- van Os J, Kenis G, Rutten BPF. The environment and schizophrenia. Nature. 2010;468(7321):203–212. doi: 10.1038/nature09563. [DOI] [PubMed] [Google Scholar]
- Watson D, Clark LA, Tellegen A. Development and Validation of Brief Measures of Positive and Negative Affect - the Panas Scales. Journal of personality and social psychology. 1988;54(6):1063. doi: 10.1037//0022-3514.54.6.1063. [DOI] [PubMed] [Google Scholar]
- Watson D, Naragon-Gainey K. On the specificity of positive emotional dysfunction in psychopathology: Evidence from the mood and anxiety disorders and schizophrenia/schizotypy. Clinical Psychology Review. 2010;30(7):839–848. doi: 10.1016/j.cpr.2009.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]