Abstract
Visual processing studies have repeatedly shown impairment in patients with schizophrenia compared to healthy controls. Electroencephalography (EEG) and, specifically, visual evoked potential (VEP) studies have identified an early marker of this impairment in the form of a decrement in the P1 component of the VEP in patients and their clinically unaffected first-degree relatives. Much behavioral and neuroimaging research has implicated specific dysfunction of either the subcortical magnocellular pathway or the cortical visual dorsal stream in this impairment. In this study, EEG responses were obtained to the contrast modulation of checkerboard stimuli using the VESPA (Visual Evoked Spread Spectrum Analysis) method. This was done for a high contrast condition and, in order to bias the stimuli towards the magnocellular pathway, a low contrast condition. Standard VEPs were also obtained using high contrast pattern reversing checkerboards. Responses were measured using high-density electrical scalp recordings in 29 individuals meeting DSM-IV criteria for schizophrenia and in 18 control subjects. Replicating previous research, a large (Cohen’s d = 1.11) reduction in the P1 component of the VEP was seen in patients when compared with controls with no corresponding difference in the VESPA response to high contrast stimuli. In addition, the low-contrast VESPA displayed no difference between patients and controls. Furthermore, no differences were seen between patients and controls for the C1 components of either the VEP or the high-contrast VESPA. Based on the differing acquisition methods between VEP and VESPA, we discuss these results in terms of contrast gain control and the possibility of dysfunction at the cortical level with initial afferent activity into V1 along the magnocellular pathway being intact when processing is biased towards that pathway using low contrast stimuli.
Keywords: EEG, Visual Evoked Potential, VESPA, Schizophrenia, P1 component
1. Introduction
Deficits in visual processing have been widely reported in patients with schizophrenia (Butler et al., 2008). Electrophysiological research has provided measures of such dysfunction through the use of the electroencephalogram (EEG). In particular, studies involving the visual evoked potential (VEP; Foxe et al., 2001; Yeap et al., 2006, 2008a, 2008b) and the steady-state visual evoked potential (SSVEP; Butler et al., 2005; Kim et al., 2005; Krishnan et al., 2005) have consistently demonstrated that patients with schizophrenia exhibit relatively severe deficits in visual sensory processing. A number of such EEG studies, in addition to those using functional magnetic resonance imaging (fMRI) and behavioral measures, have suggested that these deficits may be the result of dysfunction in the dorsal visual stream (Foxe et al., 2001), with some, more specifically, attributing their results to subcortical processing abnormalities in the magnocellular visual pathway (Butler et al., 2007; Kéri et al., 2005; Martínez et al., 2008; Schechter et al., 2005).
An example of how visual processing in schizophrenia can be assessed using the VEP is seen in studies demonstrating a robust decrement in the amplitude of the occipital P1 component in patients (Butler et al., 2001, 2007; Doniger et al., 2002; Foxe et al., 2001, 2005; Haenschel et al., 2007; Schechter et al., 2005; Spencer et al., 2003). The P1 component is a positive deflection of the EEG wave that occurs around 100 ms post-stimulus. Scalp topographies and source analysis have suggested that these deficits may specifically reflect dysfunction of the dorsal visual stream (Foxe et al., 2001, 2005) which receives most of its input from the magnocellular visual pathway (Livingstone and Hubel, 1988).
Furthermore, a sizeable decrement in the P1 component in clinically unaffected first-degree relatives of schizophrenia patients (Yeap et al., 2006), and a possible genetic basis for the observed effects (Donohoe et al., 2008; O’Donoghue et al., 2012) suggest the potential use of P1 amplitude as an endophenotypic marker for schizophrenia (although it may be applicable more broadly to the psychotic disorders as we have recently shown similar P1 deficits in patients with bipolar disorder; Yeap et al., 2009). Despite the fact that the P1 deficit is typically of large effect size in group studies, the distributions of P1 amplitude within patients and controls usually display considerable overlap (Lalor et al., 2008; Yeap et al., 2006), which clearly restricts the utility of this measure as a diagnostic or risk-assessment tool. Thus, it would clearly be desirable to maximize the sensitivity of this measure through modification of the experimental setup as a step towards a diagnostic test facilitating early detection of schizophrenia in high-risk individuals.
One candidate method for increasing the sensitivity of the P1 amplitude measure between patients and controls was recently investigated (Lalor et al., 2008). This method, known as the Visual Evoked Spread Spectrum Analysis (VESPA), differs from the standard VEP in that it involves estimation of the impulse response of the visual system using continuous stimuli whose contrast is stochastically modulated across many levels typically between 0 and 100%. This is usually accomplished by assuming a linear relationship between the input contrast modulation signal and the output EEG. The resulting VESPA impulse response provides a measure of how changes in the input stimulus map to changes in the EEG a certain time later with the VESPA profile typically exhibiting a robust P1 peak. Because the VESPA exhibits a topographic scalp distribution that is distinct from that of the VEP (Lalor et al., 2006) – likely due to the differing cell subpopulations targeted by the method – it was hoped that this stimulus would result in a larger difference between P1 amplitudes in patients and controls. This did not turn out to be the case with no significant difference in P1 amplitude being observed in the VESPA responses for a group of patients and controls who displayed large and significant differences in VEP P1 amplitude (Lalor et al., 2008). A number of possible explanations for this were discussed. One suggestion was that the lack of any difference might have been due to the high contrast of the VESPA stimulus used in the study. Because magno cells effectively saturate at high contrasts (Kaplan and Shapley, 1986), the VESPA stimulus, which spent 98% of its time above 15% contrast, may have preferentially activated parvocellular pathways and, thus, may not have been sensitive to a magnocellularly-based deficit. However, the lack of a low-contrast stimulus condition precluded a definitive interpretation of these results.
In the present study, we seek to repeat the experiments of our previous study, but also to perform an additional low-contrast (0–10%) VESPA condition. Examining VESPA responses to both high and low contrast stimuli is directly in line with the most recent recommendations from the sixth meeting of the Cognitive Neuroscience Treatment Research to Improve Cognition in Schizophrenia (CNTRICS; Butler et al., 2012). These recommendations include the use of an experimental paradigm based on (steady-state) visual evoked potentials at both high and low contrasts to target possible differences in contrast gain control mechanisms between schizophrenia patients and controls (Butler et al., 2012). The underlying motivation for such a paradigm relates to the aforementioned evidence that visual deficits in schizophrenia may be underpinned by magnocellular-specific dysfunction and that the magnocellular and parvocellular pathways display markedly differing levels of contrast gain control. With respect to our VESPA paradigm, we hypothesized that, once again, we would find a reduced P1 in the VEP of patients with schizophrenia, that we would again find no difference between patients and controls in VESPA responses to stimuli modulated between 0 and 100% contrast, but that we would find differences between groups in the VESPA responses to low contrast (0–10%) stimuli due to previous reports of differing contrast gain between groups in this range.
2. Methods and Materials
2.1. Subjects
Written informed consent was obtained from 29 (7 female) patients with DSM-IV diagnosis of schizophrenia. The Ethics Committee of the Nathan Kline Institute approved the experimental procedures. Patients were aged 20 to 53 (mean±SD, 35.6±10.3 years) and had a mean illness duration of 11.16 years (SD±8.39). These patients had mean±SD scores on the Positive and Negative Syndrome Scale (PANSS) of 77.04 (±18.55) for the total score, 19.17 (±7.01) for the positive symptom subscale, and 18.79 (±6.23) for the negative symptom subscale. All of the patients were receiving antipsychotic medication at the time of testing with a mean chlorpromazine equivalent dose of 1258.27 mg/d (range, 300–3754 mg/d). The types of antipsychotics included atypicals, typicals or a combination of both.
Control subjects were recruited through local recruitment efforts in the institute’s catchment area. This group comprised 18 (6 female) paid volunteers aged 22 to 55 years (mean±SD, 38.6±10.2 years), all of whom reported to be free from neurological disease at the time of testing. While we did not expect any effect of age based on previous research (Yeap et al., 2008a), we noted that the mean age of patients and controls did not differ significantly (t45=0.94, P=0.34). All of the 18 controls, and 25 of the 29 patients were right-handed as assessed by the Edinburgh Handedness Inventory (Oldfield, 1971). None of the controls were receiving any psychotropic medication at the time of testing. Also, all controls were free of any psychiatric illness or symptoms by self-report using criteria from the Structured Clinical Interview for DSM-III-R–Non-Patient (SCID-NP), and all reported no history of alcohol or substance abuse.
2.2. Stimuli
For all experimental runs, the stimulus consisted of a checkerboard pattern with equal numbers of light and dark checks as in Figure 1. Each check subtended a visual angle of 0.65° both horizontally and vertically, while the checkerboard as a whole subtended visual angles of 5.25° vertically and horizontally. In all experimental runs the checkerboard was presented in the center of a monitor with a gray background.
Figure 1.
Stimuli: The VEP was elicited using pattern reversals of a 100% (right) contrast checkerboard. The standard VESPA was elicited using 68 contrast levels ranging from 0% (left) and 100% (right) contrast. The low-contrast VESPA was elicited using 8 contrast levels between 0% (left) and 10% (center) contrast.
VEPs were obtained by performing signal averaging time-locked to phase reversals of this checkerboard stimulus. These pattern reversals occurred periodically at a rate of 1.25 Hz. The checkerboard used to elicit the VEP had a contrast of 100% in terms of the range of the monitor.
For both types of VESPA stimulus condition the refresh rate of the monitor was set to 75 Hz and on every refresh the contrast of the checkerboard pattern was modulated by a stochastic signal with the mean luminance remaining constant. The stochastic signals used had their power distributed uniformly between 0 and 35 Hz.
2.3. Experimental procedure
Every subject underwent three VEP runs of 120 pattern reversals each as well as three runs of 96 s duration for two different VESPA conditions. In the first VESPA condition, hereafter known as the “standard” condition, the checkerboard contrast ranged from 0–100% in terms of the range of the monitor. The contrast range was restricted to 0–10% in the second VESPA condition known as the “low-contrast” condition. Subjects were instructed to maintain visual fixation on the center of the screen for the duration of each run. While abstaining from eye-blinks was not possible given the trial lengths, subjects were instructed to keep the number of eye-blinks to a minimum. A different stochastic signal was used for modulating the stimulus in each run, although each signal had the same temporal frequency distribution.
2.4. EEG acquisition and analysis
EEG data were recorded from 72 electrode positions referenced to the nasion, filtered over the range 0–134 Hz and digitized at a rate of 512 Hz using the BioSemi Active Two system. Subsequently, the EEG was digitally filtered with a high-pass filter with passband above 2 Hz and −60 dB response at 1 Hz and a low-pass filter with 0–35 Hz passband and −50 dB response at 45 Hz.
VEPs were calculated by averaging time-locked responses to the presentations of the display type described earlier. A time window of 540 ms starting 120 ms pre-stimulus was used. Any epochs where the EEG exceeded +/− 100 µV were rejected, resulting in a mean rejection rate of ~1%.
The VESPA is an estimate of the linear impulse response of the visual system (Lalor et al., 2006). It is based on the assumption that the EEG response to a stimulus, whose luminance or contrast is rapidly modulated by a stochastic signal, consists of a convolution of that signal with an unknown impulse response. Given the known stimulus signal and the measured EEG, this impulse response, i.e., the VESPA, can be estimated using the method of linear least squares. This explicit assumption of a linear relationship between stimulus contrast and EEG activity exactly implies that the VESPA should be exquisitely sensitive to any differences between patients and controls in terms of contrast gain control. More specifically, the VESPA should show marked differences between groups if those groups differ in the steepness of their contrast response functions at low contrasts as has been reported previously (Butler et al., 2007).
In the present study, as is typical, VESPAs were measured using a sliding window of 500 ms of data starting 100 ms pre-stimulus for both conditions. Both VEPs and VESPAs were baseline corrected by subtracting the mean of the interval −100 to 0ms from the average response for each subject.
Given that the topography of the VEP P1 component differs from that of the VESPA (Lalor et al., 2006, 2008), we elected not to restrict our analysis to just one electrode. Rather we chose to assess the power of the P1 across the posterior scalp. We did this using a measure known as Global Field Power (GFP; Lehmann & Skrandies, 1980). The GFP represents the standard deviation of all electrode potentials at a given time and thus can be considered a reference-free quantification of response power over the scalp that is expressible in µV. In fact, because we were particularly interested in the posterior scalp, we adapted the GFP by calculating it just using the 29 electrodes posterior to the coronal central curve (i.e., posterior to Cz, C2, C3, etc.).
3. Results
In order to demonstrate our aforementioned contention that the VESPA should be sensitive to group difference in contrast gain at low frequencies, we first derived VESPA responses to simulated EEG based on two hypothetical contrast response curves (Figure 2a; curves loosely based on Figure 4 from Butler et al., 2007). Specifically, we generated a random Gaussian “contrast modulation” signal exactly like those used in our VESPA experiments. We used this signal to generate two simulated EEG traces by convolving the contrast modulation signal with a hypothetical VESPA response, where the response was scaled at each contrast level according to each of the two contrast response curves shown in Figure 2a. We then retrieved the impulse response function using our standard linear VESPA analysis. As can be seen in Figure 2b, the VESPA response based on the “patient” curve is significantly smaller than that based on the “control” curve.
Fig. 2.
(a): Hypothetical contrast response curves for controls and patients based on Figure 4 of Butler et al., 2007. (b): Simulated VESPA responses obtained using the assumption of a linear relationship between contrast and EEG when the simulated EEG was generated using the nonlinear hypothetical contrast response curves shown in (a).
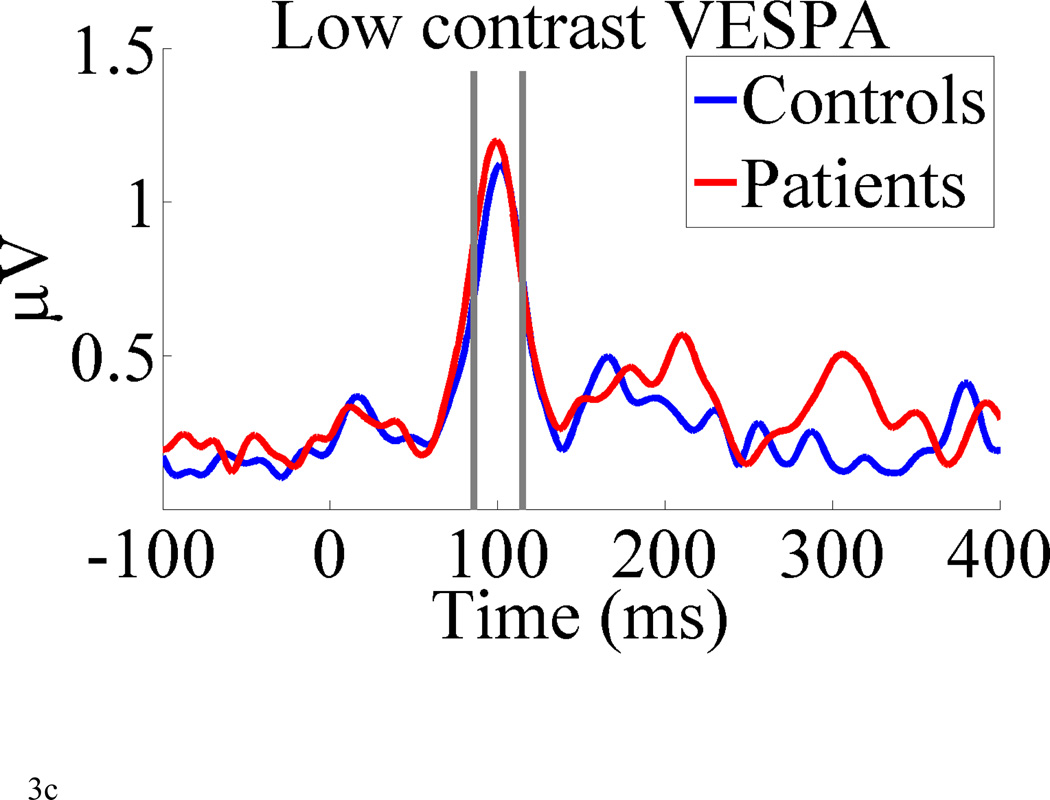
The observed data in the present study in no way resemble these simulations. Figure 3 shows the GFP (over the posterior 29 electrodes) of the grand average VEP, standard VESPA and low-contrast VESPA for both the control group and the patients. Defining the P1 component as the average amplitude in an interval around the major peak of the group average for each response (95–125 ms for the VEP and standard VESPA and 85–115 ms for the low-contrast VESPA; Figure 3), we first conducted a two-way 2×3 mixed ANOVA with factors of group (controls, patients) and response (VEP, VESPA, low-contrast VESPA). There was a significant main effect of response (F(2, 90) = 34.34, p < 0.0001) which simply reflects differences in response magnitudes between the three methods (see Figure 3). More importantly, an interaction was found between group and response (F(2, 90) = 9.19, p = 0.0002). As can be seen in Figure 3, this was driven by a much larger relative difference in P1 amplitudes between patients and controls for the VEP than for either of the VESPA responses. This difference also drove a main effect of group, F(1,45) = 10.72, p = 0.0013.
Figure 3.
Global field power of the pattern reverse VEP, the standard VESPA and the low-contrast VESPA for controls and patients for both methods averaged over 29 electrode locations posterior to the central midline. The vertical gray lines indicate the time intervals used to test for statistical differences in response peaks (see results).
To examine the interaction between group and method further, post-hoc t-tests were carried out for each response separately. We used the aforementioned P1 magnitude, as the dependent measure. A significant difference was found between groups for the VEP (t = 3.6; p = 0.0007) whereas no differences were found between groups for the standard VESPA (t = 0.46, p = 0.65) or the low-contrast VESPA (t = −0.19, p = 0.85). A Cohen’s d effect size was calculated for the VEP P1 and found to be 1.11, which is considered a large effect size (i.e., > 0.8; Cohen, 1988). In order to assess the statistical validity of our null finding for the standard and low-contrast VESPA we conducted a post-hoc power analysis using the G*Power software package (Faul et al., 2007). Specifically we calculated the power of our independent two-sample t-test to detect a difference between patients (n = 29) and controls (n = 18) at the alpha = 0.05 level. Because we didn’t want to necessarily assume that, say, the low-contrast VESPA would be smaller for patients than for controls (as was the case for the VEP), we did this for a two-tailed t-test. Given the hypothesis underlying the study – that contrast gain at low contrasts is markedly different between patients and controls – we might have expected an effect size larger than that seen with the VEP (i.e., 1.11) for our low-contrast VESPA. Being more conservative than this, we conducted our power analysis at the minimum value considered to be large, i.e., d = 0.8. This gave us a power value of 0.74 which implies a probability of falsely accepting the null hypothesis of 0.26. This value, considered in light of the lack of an effect on the full contrast VESPA in the same cohort (as well as in our previous study) gives us confidence that our lack of a difference between patients and controls is meaningful.
Based on visual inspection of the GFP timecourse for the standard VESPA responses we also elected to conduct a t-test to assess differences between patients and controls in the interval 160–195ms. No significant difference was observed (t = 0.35, p = 0.72), in line with previous results (Lalor et al., 2008).
In addition, despite the fact that the study was aimed at assessing visual deficits in patients during the P1 timeframe, we also chose to investigate potential differences in C1 amplitude which have previously been reported to differ between groups (Butler et al., 2007). We did this by conducting a t-test between patients and controls on GFP amplitude during the interval 70–95 ms for both the VEP and the standard VESPA. No significant differences were observed (VEP: t = 0.81, p = 0.42; VESPA: t = 0.4, p = 0.69). It should be noted that our stimuli were not optimized for evoking large C1 components from each individual subject (e.g., see Kelly et al., 2008).
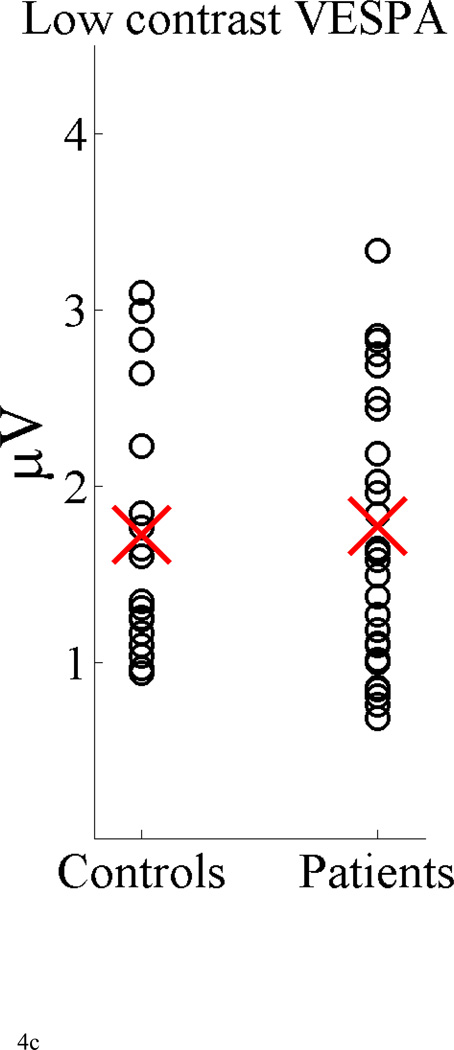
Figure 4 represents a scatter plot showing how the magnitude of the P1 component is distributed within the control and patients groups for all three methods. The mean values for each group and response are denoted in red with the overlap between distributions being apparent for all methods including the VEP. Another representation of this overlap for the VEP P1 can be seen in the receiver operating characteristic in Figure 5.
Figure 4.
Scatter plot, showing the distribution of the mean value of the P1 component in the interval 95–125 ms for the VEP and standard VESPA and in the interval 85–115ms for the low-contrast VESPA for all control subjects and patients. The P1 amplitude was based on the global field power of the most posterior 29 electrodes.
Figure 5.
Receiver operating characteristic plotting the rate of true positives versus false positives when classifying subjects as “controls” on the basis of their P1 amplitude.
4. Discussion
As in our previous study (Lalor et al., 2008), we have shown a difference in the sensitivities of the VEP and VESPA for detecting deficits in early visual processing in patients with schizophrenia. Specifically, a reduced P1 component was observed for schizophrenia patients when assessed using the transient VEP, while no difference in P1 component amplitude was seen when using the standard VESPA. In addition, when using a low-contrast VESPA stimulus to obtain responses biased toward the magnocellular pathway, we again see no difference in the amplitude of the response. Given that magnocellular pathway dysfunction has been suggested as a factor underpinning early visual deficits in schizophrenia (Butler et al., 2007; Kéri et al., 2005; Martínez et al., 2008), and that the contrast response curves of patients and controls have been shown to differ at low contrasts (Butler et al., 2007), we discuss here a number of possible reasons for the lack of a deficit in the VESPA in general and the low-contrast VESPA in particular.
Perhaps the most likely reason for the discrepancy in patient-control comparisons when using the VEP versus the VESPA is that the two responses are determined using different stimuli and different analysis methods. Unlike the VEP which is obtained by averaging responses to discrete stimuli, the VESPA (as used here) explicitly assumes a linear relationship between the stochastic modulation of contrast and the concurrently recorded EEG. Such a VESPA is likely to reflect largely the activity of visual cortical cells that modulate their activity in a relatively linear manner in response to contrast change across the range of contrasts that we employed. As discussed elsewhere (Murphy et al., in press), this is most likely to be true for cells in the early visual system.
In fact, two recent studies from our group have shown that the VESPA is likely dominated by activity in very early retinotopic visual cortex. The first study showed that both the C1 and P1 components of the VESPA reverse in polarity for lower and upper-hemifield stimuli and are well accounted for by source models consisting of dipoles in calcarine cortex (Lalor et al., in review). The second study built on this finding by showing that the VEP C1 and VESPA C1 amplitudes are highly correlated across subjects and that the amplitudes of both the VEP C1 and VESPA C1 are highly correlated with the VESPA P1 amplitude across subjects (Murphy et al., in press). Using the smaller data set in the present study we conducted a similar analysis and found the same result: namely that the amplitude (as defined above) of the VEP C1 was significantly correlated with that of the VESPA C1 (Pearson’s r = 0.29; p = 0.045) and the VESPA P1 (Pearson’s r = 0.38; p = 0.009) across all subjects. Given that the VEP C1 is widely considered to reflect activity generated within early retinotopic visual cortex, especially V1 (Clark et al., 1995; Di Russo et al., 2001; 2005; Foxe and Simpson, 2002; Kelly et al., 2008, 2012), we infer a similarly early cortical origin for both VESPA components. Murphy et al. (in press) also showed no correlation between the amplitude of the VEP P1 and either the VESPA P1 or the VEP C1. This lack of correlation, in addition to the fact that the VEP P1 has a much more widespread scalp distribution than any of the other three components mentioned (Lalor et al., 2006, Murphy et al., in press), suggests that the underlying generators of the VEP P1 are more varied and widespread (see Murphy et al., in press, for further discussion).
This notion of more varied contributions to the VEP P1 is in line with the fact that multiple visual areas are active concurrently at relatively short latencies following the presentation of a discrete stimulus (Schroeder et al., 1998), which necessarily results in temporally and spatially overlapping cortical generators. Using VEP data in humans, it has been suggested that multiple visual areas are active within as little as 10–15 ms of the onset of the C1, with dorsolateral frontal cortex being active within just 30 ms (Foxe and Simpson, 2002). Evidence of multiple contributions to the P1 component in particular can be seen in papers utilizing source analysis to determine the neural generators of the VEP (Di Russo 2001, 2005), including those comparing the P1 generators in controls and patients with schizophrenia (Yeap et al., 2006). These studies posit the existence of a number of components that occur around the time frame of the P1. Among these are positive components whose activities are well accounted for by models comprising neural generators in dorsal extrastriate cortex of the middle occipital gyrus (Di Russo et al., 2001; onset VEP) and in area MT/V5 (Di Russo et al., 2005; pattern reversal VEP). This link between the VEP P1 and dorsal stream processing is particularly interesting with regard to our VESPA results, given previous reports of the specific involvement of dorsal stream processing in the reduced VEP P1 effect in schizophrenia (Doniger et al., 2002; Foxe et al., 2001; Sehatpour et al., 2010). In particular, it may be the case that the differences we see in the VEP P1 of schizophrenia patients are due to dysfunction of these extrastriate dorsal stream contributions or due to impairments in connectivity between V1 and the dorsal stream. This latter possibility cannot be overlooked given discussions in recent years that the pathophysiology of schizophrenia may be the result of a distributed impairment involving many cortical areas and their connectivity (Uhlhaas and Singer, 2010). The (contrast) VESPA, which appears to be dominated by activity in early retinotopic cortex, is probably not sensitive to activity in these dorsal stream areas, and thus would not be sensitive to any such dysfunction in patients. This applies equally to the low-contrast VESPA. The fact that neither the VEP C1 nor the VESPA C1 showed any difference between groups also supports the idea that the deficit may arise in extrastriate cortex given that both of these components have been suggested to arise in very early visual cortex.
Having suggested this, it is not immediately obvious how to reconcile our results with those studies that posit dysfunction of the subcortical magnocellular pathway (Schechter et al., 2005; Butler et al., 2007; Martínez et al., 2008). While we have shown no differences between patients and controls in early cortical responses to low contrast stimuli, a number of studies using such stimuli have shown reduced VEP P1 components in patients and have concluded that these effects originate subcortically (Butler et al., 2007; Schechter et al., 2005). While this notion has received a measure of support from structural studies using diffusion tensor imaging (DTI; Butler et al., 2006), it should be noted that these studies showed no significant difference in the C1 component to low contrast stimuli and, thus, our argument that the P1 reduction arises as a result of a dysfunctional extrastriate generator is not necessarily incompatible with these previous ERP results. Having said that, previous research has suggested that the C1 component may be dominated by parvocellular activity (see Foxe et al., 2008), so it remains a possibility that initial afferents to V1 through the subcortical magnocellular pathway are reflected in a reduced VEP P1. However, the lack of any effect on our low-contrast early cortical VESPA calls this into question. Finally, we note that reduced VEP C1 amplitudes have previously been reported in patients using high contrast stimuli (Schechter et al., 2005) which would be compatible with a subcortical deficit. The reason for the discrepancy between that result and our VEP C1 is not immediately apparent; although the authors of that study did point out that their C1 effect did not survive covariation for acuity.
The results of our study also run counter to what one would predict based on the previously published visual contrast response curves of patients and controls (Butler et al., 2005, 2007; see Figure 2). In one of those studies (Butler et al., 2005), the contrast response curves were fit on the basis of SSVEPs which likely contain contributions from extrastriate visual areas (Srinivasan et al, 2006; Di Russo et al., 2007; Lauritzen et al., 2010), and so differences in those curves do not necessarily indicate subcortical differences between groups. In the other study (Butler et al., 2007), separate sets of curves were fit using the amplitudes of several components. All of these curves showed differences between groups. However, as already mentioned, the P1 component (and the later N1) must contain extrastriate contributions. Also, the C1 component in this study occurred later than the P1, which implies that it was evoked after the initial volley into V1 and, thus, extrastriate contributions cannot be ruled out. As such, none of these sets of curves conclusively proves groups differences in contrast gain at the subcortical level and so neither of these reports is incompatible with our findings.
Stronger evidence for subcortical magnocellular dysfunction comes from utilizing stimuli with low spatial frequency. This results in an almost complete obliteration of the VEP in patients, including the earliest activity on the scalp (i.e., the C1; Butler et al., 2007), and is in line with the fact that the magnocellular pathway responds preferentially to low spatial frequency stimuli, whereas the parvocellular pathway is thought to dominate processing of high spatial frequencies (Merigan and Maunsell, 1993). While we have argued elsewhere that using low-contrast stimuli likely gives rise to responses dominated by activity from the magnocellular pathway (Lalor and Foxe, 2009, 2010), more work remains to be done to determine the reasons for the differing results obtained when using contrast manipulations versus spatial frequency manipulations (see Butler et al., 2007). The VESPA may have a role to play in examining these differences (Lalor et al., 2009).
Finally, although discussed before (Lalor et al., 2008), it is worth reiterating two other possible explanations for the different results obtained using the VEP and both the low and high contrast VESPAs. The first revolves around the linear assumption inherent to the VESPA. This assumption renders the VESPA insensitive to nonlinear processing in the magnocellular pathway, which is a feature of that pathway that has been highlighted in previous studies of visual differences between patients and controls (Kim et al., 2005). While this issue remains to be fully resolved, the simulations presented in the present paper clearly show that, even with the linear assumption, the VESPA should be sensitive to between group differences in the contrast response curves of early visual areas. The second explanation concerns the generative mechanisms of the transient VEP, and in particular, the possibility that phase-resetting of ongoing neural oscillations may contribute more to the VEP than it does to the continuous VESPA. As discussed elsewhere, we remain skeptical about this explanation (Murphy et al., in press).
In summary, we have once again shown a discrepancy between the VEP and VESPA when comparing responses from healthy controls to those of patients with schizophrenia. In addition we have seen that this discrepancy exists even when using a low-contrast VESPA stimulus biased towards the magnocellular pathway. We have posited a number of possible reasons for this, including higher visual cortex contributions to the VEP.
Acknowledgments
Primary support for this work was provided by an RO1 grant from the U.S. National Institute of Mental Health (MH74767 to M.I.K. & J.J.F.). Additional support was derived from MH85322 (to J.J.F.); the NIMH had no further role in study design; in the collection, analysis and interpretation of data; in the writing of the report; and in the decision to submit the paper for publication. Additional salary support to E.C.L. was provided by a Government of Ireland Postdoctoral Research Fellowship from the Irish Research Council for Science, Engineering and Technology.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Financial Disclosures
None of the authors have conflicts of interests to declare in relation to this study.
Contributors
Dr. Lalor designed the stimulus sequences, programmed all paradigms, analyzed all data and wrote the first draft of the manuscript. Dr. Foxe designed the experimental protocol and edited multiple drafts of the manuscript. Drs. De Sanctis and Krakowski collected all data. Dr. De Sanctis tabulated patient demographics and Dr. Krakowski performed the clinical ratings. All authors contributed to and have approved the final manuscript. The principle investigator, Dr. Foxe, takes responsibility for the integrity of the data and the accuracy of the data analysis, and attests that all authors had full access to all the data in the study.
References
- Butler PD, Schechter I, Zemon V, Schwartz SG, Greenstein VC, Gordon J, Schroeder CE, Javitt DC. Dysfunction of early-stage visual processing in schizophrenia. Am. J. Psychiatry. 2001;158(7):1126–1133. doi: 10.1176/appi.ajp.158.7.1126. [DOI] [PubMed] [Google Scholar]
- Butler PD, Zemon V, Schechter I, Saperstein AM, Hoptman MJ, Lim KO, Revheim N, Silipo G, Javitt DC. Early-stage visual processing and cortical amplification deficits in schizophrenia. Arch. Gen. Psychiatry. 2005;62(5):495–504. doi: 10.1001/archpsyc.62.5.495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler PD, Hoptman MJ, Nierenberg J, Foxe JJ, Javitt DC, Lim KO. Visual white matter integrity in schizophrenia. Am. J. Psychiatry. 2006;163(11):2011–2013. doi: 10.1176/appi.ajp.163.11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler PD, Martínez A, Foxe JJ, Kim D, Zemon V, Silipo G, Mahoney J, Shpaner M, Jalbrzikowski M, Javitt DC. Subcortical visual dysfunction in schizophrenia drives secondary cortical impairments. Brain. 2007;130(Pt 2):417–430. doi: 10.1093/brain/awl233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler PD, Silverstein SM, Dakin SC. Visual perception and its impairment in schizophrenia. Biol. Psychiatry. 2008;64(1):40–47. doi: 10.1016/j.biopsych.2008.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler PD, Chen Y, Ford JM, Geyer MA, Silverstein SM, Green MF. Perceptual measurement in schizophrenia: promising electrophysiology and neuroimaging paradigms from CNTRICS. Schizophr. Bull. 2012;38(1):81–91. doi: 10.1093/schbul/sbr106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark VP, Fan S, Hillyard SA. Identification of early visual evoked potential generators by retinotopic and topographic analyses. Hum. Brain Mapp. 1995;2:170–187. [Google Scholar]
- Cohen J. Statistical Power Analysis for the Behavioral Sciences. second ed. New Jersey: Lawrence Erlbaum Associates; 1988. [Google Scholar]
- Di Russo F, Pitzalis S, Spitoni G, Aprile T, Patria F, Spinelli D, Hillyard SA. Identification of the neural sources of the pattern-reversal VEP. Neuroimage. 2005;24(3):874–886. doi: 10.1016/j.neuroimage.2004.09.029. [DOI] [PubMed] [Google Scholar]
- Di Russo F, Martínez A, Sereno MI, Pitzalis S, Hillyard SA. Cortical sources of the early components of the visual evoked potential. Hum. Brain Mapp. 2001;15(2):95–111. doi: 10.1002/hbm.10010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Russo F, Pitzalis S, Aprile T, Spitoni G, Patria F, Stella A, Spinelli D, Hillyard SA. Spatiotemporal analysis of the cortical sources of the steady-state visual evoked potential. Hum. Brain Mapp. 2007;28(4):323–334. doi: 10.1002/hbm.20276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doniger GM, Foxe JJ, Murray MM, Higgins BA, Javitt DC. Impaired visual object recognition and dorsal/ventral stream interaction in schizophrenia. Arch. Gen. Psychiatry. 2002;59(11):1011–1020. doi: 10.1001/archpsyc.59.11.1011. [DOI] [PubMed] [Google Scholar]
- Donohoe G, Morris DW, De Sanctis P, Magno E, Montesi JL, Garavan HP, Robertson IH, Javitt DC, Gill M, Corvin AP, Foxe JJ. Early Visual Processing Deficits in Dysbindin-Associated Schizophrenia. Biol. Psychiatry. 2008;63(5):484–489. doi: 10.1016/j.biopsych.2007.07.022. [DOI] [PubMed] [Google Scholar]
- Faul F, Erdfelder E, Lang AG, Buchner A. G*Power 3: A flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav. Res. Methods. 2007;39(2):175–191. doi: 10.3758/bf03193146. [DOI] [PubMed] [Google Scholar]
- Foxe JJ, Strugstad CE, Sehatpour P, Molholm S, Pasieka W, Schroeder CE, McCourt ME. Parvocellular and magnocellular contributions to the initial generators of the visual evoked potential: high-density electrical mapping of the “C1” component. Brain Topography. 2008;21:11–21. doi: 10.1007/s10548-008-0063-4. [DOI] [PubMed] [Google Scholar]
- Foxe JJ, Murray MM, Javitt DC. Filling-in in schizophrenia: a high density electrical mapping and source-analysis investigation of illusory contour processing. Cereb. Cortex. 2005;15(12):1914–1927. doi: 10.1093/cercor/bhi069. [DOI] [PubMed] [Google Scholar]
- Foxe JJ, Simpson GV. Flow of activation from V1 to frontal cortex in humans. A framework for defining "early" visual processing. Exp. Brain. Res. 2002;142(1):139–150. doi: 10.1007/s00221-001-0906-7. [DOI] [PubMed] [Google Scholar]
- Foxe JJ, Doniger GM, Javitt DC. Early visual processing deficits in schizophrenia: impaired P1 generation revealed by high-density electrical mapping. Neuroreport. 2001;12(17):3815–3820. doi: 10.1097/00001756-200112040-00043. [DOI] [PubMed] [Google Scholar]
- Haenschel C, Bittner RA, Haertling F, Rotarska-Jagiela A, Maurer K, Singer W, Linden DE. Contribution of impaired early-stage visual processing to working memory dysfunction in adolescents with schizophrenia: a study with eventrelated potentials and functional magnetic resonance imaging. Arch. Gen. Psychiatry. 2007;64(11):1229–1240. doi: 10.1001/archpsyc.64.11.1229. [DOI] [PubMed] [Google Scholar]
- Kaplan E, Shapley RM. The primate retina contains two types of ganglion cells, with high and low contrast sensitivity. Proc. Natl. Acad. Sci. USA. 1986;83:2755–2757. doi: 10.1073/pnas.83.8.2755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly SP, Gomez-Ramirez M, Foxe JJ. Spatial attention modulates initial afferent activity in human primary visual cortex. Cereb. Cortex. 2008;18:2629–2636. doi: 10.1093/cercor/bhn022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly SP, Schroeder CE, Lalor EC. What does polarity inversion of extrastriate activity tell us about striate contributions to the early VEP? A comment on Ales et al 2010. NeuroImage. 2012 doi: 10.1016/j.neuroimage.2012.03.081. [DOI] [PubMed] [Google Scholar]
- Kéri S, Kelemen O, Janka Z, Benedek G. Visual perceptual dysfunctions are possible endophenotypes of schizophrenia: Evidence from the psychophysical investigation of magnocellular and parvocellular pathways. Neuropsychology. 2005;19:649–656. doi: 10.1037/0894-4105.19.5.649. [DOI] [PubMed] [Google Scholar]
- Kim D, Zemon V, Saperstein A, Butler PD, Javitt DC. Dysfunction of early-stage visual processing in schizophrenia: harmonic analysis. Schizophr. Res. 2005;76(1):55–65. doi: 10.1016/j.schres.2004.10.011. [DOI] [PubMed] [Google Scholar]
- Krishnan GP, Vohs JL, Hetrick WP, Carroll CA, Shekhar A, Bockbrader MA, O'Donnell BF. Steady state visual evoked potential abnormalities in schizophrenia. Clin. Neurophysiol. 2005;116(3):614–624. doi: 10.1016/j.clinph.2004.09.016. [DOI] [PubMed] [Google Scholar]
- Lalor EC, Foxe JJ. Visual evoked spread spectrum analysis (VESPA) responses to stimuli biased towards magnocellular and parvocellular pathways. Vision Res. 2009;49(1):127–133. doi: 10.1016/j.visres.2008.09.032. [DOI] [PubMed] [Google Scholar]
- Lalor EC, Foxe JJ. Reply to Skottun and Skoyles: On interpreting responses to low contrast stimuli in terms of magnocellular activity - a few remarks. Vision Res. 2010;50(10):991–994. doi: 10.1016/j.visres.2009.04.030. [DOI] [PubMed] [Google Scholar]
- Lalor EC, Lucan JN, Foxe JJ. Estimation of the impulse response of the visual system using stochastic modulation of stimulus spatial frequency; Proceedings of the 4th International IEEE/EMBS Conference on Neural Engineering; 2009. [Google Scholar]
- Lalor EC, Pearlmutter BA, Reilly RB, McDarby G, Foxe JJ. The VESPA: a method for the rapid estimation of a visual evoked potential. Neuroimage. 2006;32(4):1549–1561. doi: 10.1016/j.neuroimage.2006.05.054. [DOI] [PubMed] [Google Scholar]
- Lalor EC, Yeap S, Reilly RB, Pearlmutter BA, Foxe JJ. Dissecting the cellular contributions to early visual sensory processing deficits in schizophrenia using the VESPA evoked response. Schizophr. Res. 2008;98(2008):256–264. doi: 10.1016/j.schres.2007.09.037. [DOI] [PubMed] [Google Scholar]
- Lalor EC, Kelly SP, Foxe JJ. Generation of the VESPA response to rapid contrast fluctuations is dominated by striate cortex: evidence from retinotopic mapping. doi: 10.1016/j.neuroscience.2012.05.067. Under Review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lauritzen TZ, Ales JM, Wade AR. The effects of visuospatial attention measured across visual cortex using source-imaged, steady-state EEG. J. Vis. 2010;10(14):39. doi: 10.1167/10.14.39. [DOI] [PubMed] [Google Scholar]
- Lehmann D, Skrandies W. Reference-free identification of components of checkerboard-evoked multichannel potential fields. Electroencephalogr. Clin. Neurophysiol. 1980;48:609–621. doi: 10.1016/0013-4694(80)90419-8. [DOI] [PubMed] [Google Scholar]
- Livingstone M, Hubel D. Segregation of form, color, movement, and depth: anatomy, physiology, and perception. Science. 1988;240(4853):740–749. doi: 10.1126/science.3283936. [DOI] [PubMed] [Google Scholar]
- Martínez A, Hillyard SA, Dias EC, Hagler DJ, Jr, Butler PD, Guilfoyle DN, Jalbrzikowski M, Silipo G, Javitt DC. Magnocellular Pathway Impairment in Schizophrenia: Evidence from Functional Magnetic Resonance Imaging. J. Neurosci. 2008;28(30):7492–7500. doi: 10.1523/JNEUROSCI.1852-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merigan WH, Maunsell JHR. How parallel are the primate visual pathways? Annu. Rev. Neurosci. 1993;16:369–402. doi: 10.1146/annurev.ne.16.030193.002101. [DOI] [PubMed] [Google Scholar]
- Murphy JW, Kelly SP, Foxe JJ, Lalor EC. Isolating early cortical generators of visual evoked activity: A systems identification approach. Exp. Brain Res. doi: 10.1007/s00221-012-3129-1. in press. [DOI] [PubMed] [Google Scholar]
- O'Donoghue T, Morris DW, Fahey C, Costa AD, Foxe JJ, Hoerold D, Tropea D, Gill M, Corvin A, Donohoe G. A NOS1 variant implicated in cognitive performance influences evoked neural responses during a high density EEG study of early visual perception. Hum. Brain Mapp. 2012;33(5):1202–1211. doi: 10.1002/hbm.21281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oldfield RC. The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia. 1971;9(1):97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- Schechter I, Butler PD, Zemon VM, Revheim N, Saperstein AM, Jalbrzikowski M, Pasternak R, Silipo G, Javitt DC. Impairments in generation of early-stage transient visual evoked potentials to magno- and parvocellular-selective stimuli in schizophrenia. Clin. Neurophysiol. 2005;116(9):2204–2215. doi: 10.1016/j.clinph.2005.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroeder CE, Mehta AD, Givre SJ. A spatiotemporal profile of visual system activation revealed by current source density analysis in the awake macaque. Cereb. Cortex. 1998;8(7):575–592. doi: 10.1093/cercor/8.7.575. [DOI] [PubMed] [Google Scholar]
- Sehatpour P, Dias EC, Butler PD, Revheim N, Guilfoyle DN, Foxe JJ, Javitt DC. Impaired visual object processing across an occipital-frontal-hippocampal brain network in schizophrenia: an integrated neuroimaging study. Arch. Gen. Psychiatry. 2010;67(8):772–782. doi: 10.1001/archgenpsychiatry.2010.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spencer KM, Nestor PG, Niznikiewicz MA, Salisbury DF, Shenton ME, McCarley RW. Abnormal neural synchrony in schizophrenia. J. Neurosci. 2003;23(19):7407–7411. doi: 10.1523/JNEUROSCI.23-19-07407.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srinivasan R, Bibi FA, Nunez PL. Steady-state visual evoked potentials: distributed local sources and wave-like dynamics are sensitive to flicker frequency. Brain Topogr. 2006;18(3):167–187. doi: 10.1007/s10548-006-0267-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uhlhaas PJ, Singer W. Abnormal neural oscillations and synchrony in schizophrenia. Nat. Rev. Neurosci. 2010;11(2):100–113. doi: 10.1038/nrn2774. [DOI] [PubMed] [Google Scholar]
- Yeap S, Kelly SP, Sehatpour P, Magno E, Javitt DC, Garavan H, Thakore JH, Foxe JJ. Early Visual Sensory Deficits as Endophenotypes for Schizophrenia: High-Density Electrical Mapping in Clinically Unaffected First-Degree Relatives. Arch. Gen. Psychiatry. 2006;63(11):1180–1188. doi: 10.1001/archpsyc.63.11.1180. [DOI] [PubMed] [Google Scholar]
- Yeap S, Kelly SP, Sehatpour P, Magno E, Garavan H, Thakore JH, Foxe JJ. Visual sensory processing deficits in Schizophrenia and their relationship to disease state. Eur Arch Psychiatry Clin Neurosci. 2008a;258(5):305–316. doi: 10.1007/s00406-008-0802-2. [DOI] [PubMed] [Google Scholar]
- Yeap S, Kelly SP, Thakore JH, Foxe JJ. Visual sensory processing deficits in first-episode patients with Schizophrenia. Schizophr Res. 2008b;102(1–3):340–343. doi: 10.1016/j.schres.2008.03.026. [DOI] [PubMed] [Google Scholar]
- Yeap S, Kelly SP, Reilly RB, Thakore JH, Foxe JJ. Visual sensory processing deficits in patients with bipolar disorder revealed through high-density electrical mapping. J. Psychiatry Neurosci. 2009;34(6):459–464. [PMC free article] [PubMed] [Google Scholar]