Abstract
Hypothesis/Background
The subscapularis is an important mover and stabilizer of the glenohumeral joint and since the advent of shoulder arthroscopy, partial tears are found in 43% of rotator cuff patients. While partial tears to the upper band occur more commonly, little is known about the structure and mechanical behavior of the individual bands. Therefore, the objective of this study was to measure tensile mechanical properties, corresponding collagen fiber alignment, and histology in the upper and lower bands of the rat subscapularis tendon.
Materials and Methods
Thirty, adult Sprague-Dawley rats were euthanized and subscapularis tendons dissected out for mechanical, organization (n = 24), and histologic assessment (n = 6). Collagen organization was measured with a custom device during mechanical testing.
Results
Linear-region modulus at the insertion site was significantly lower in the upper band compared to the lower band while no differences were found at the midsubstance location. The upper band was found to be significantly less aligned and demonstrated a more rounded cell shape than the lower band at the insertion site.
Discussion
This study demonstrated that the two bands of the subscapularis tendon have differential mechanical, organizational, and histological properties. This suggests that a functional deficit exists to the upper band of the subscapularis and may be contributing to the prevalence of partial subscapularis tears.
Conclusions
Clinicians should be aware that the upper band of the subscapularis tendon may be at higher risk of developing tears due to the decreased mechanical properties and a more disorganized collagen fiber distribution.
Level of Evidence
Basic Science Study, Biomechanics, Animal Model.
Keywords: subscapularis tendon, rotator cuff, animal model, tendon injury
Introduction
The subscapularis is the largest of the four rotator cuff tendons and plays a considerable role in joint stability and function1. Due to its anterior positioning within the shoulder, provides dynamic restraint in the anterior and posterior directions1,20. The subscapularis has also been described as having two separate bands, which are distinct based on anatomical insertion, innervation, and muscle activity9,10,16,26. When examining the anatomical insertion sites, the upper band has a broader and more medial insertion compared to the lower band10. This has been thought to enhance biceps tendon stability3. The bands of the subscapularis can also be differentiated by the innervation of the subscapular nerve, which has been demonstrated to have 2 or 3 branches16. Previous research has shown the upper portion of the subscapularis is more active during arm elevation, indicating its potential for differential activation during functional movements26.
The anterior/posterior “force couple” has previously been described for the glenohumeral joint28. It states that the anterior subscapularis, the posterior infraspinatus, and the teres minor work in conjunction to provide dynamic joint stability28. Therefore, a subscapularis tendon tear can cause a disruption of the force couple and create joint instability. However, previous reports have demonstrated a very low incidence of subscapularis tears ranging from 2% to 10%11,14,18. Due to this information, the subscapularis tendon has received little attention both in terms of research and clinical practice. With the advent of shoulder arthroscopy, the incidence of subscapularis tendon tears has been on the rise. Reports now indicate a tear rate in upwards of 43%2, with most tears being upper band undersurface tears5,17. Prior to shoulder arthroscopy, surgeons were unable to visualize a partial undersurface tear during open rotator cuff repair and therefore, many subscapularis tears may have gone untreated decreasing functional outcomes6,23.
Although, clinicians are becoming more aware of upper band subscapularis tendon tears, the cause of these tears is still unknown. Due to the differences in anatomic insertion sites, innervations, and muscle activity between the upper and lower band of the subscapularis, the tendons may experience different loading environments that ultimately alter the mechanical strength and increase the likelihood of tears. However, well designed and controlled studies are often difficult with cadavers and the ability to perform prospective investigation is not possible. The rat shoulder model has been used previously and demonstrated to be valid for the study of rotator cuff disease27. Therefore, the objective of this study was to locally measure cross-sectional area (CSA), tensile mechanical properties, collagen fiber alignment during loading, and histology in the upper and lower bands of the rat subscapularis tendon, in order to identify structural differences that may result in the upper band being more susceptible to injury than the lower band. We hypothesize that the upper band will have an increased CSA, inferior mechanical properties, more disorganized collagen fiber distribution, and lower histology grades for cell shape and cellularity compared to the lower band.
Materials and Methods
Thirty male Sprague-Dawley rats (Charles River, 400–450 g) of similar age were used in this study, which was approved by the University of Pennsylvania Institutional Animal Care and Use Committee. The rats were sacrificed and subscapularis tendons were dissected out as a complete humerus tendon unit. The anterior shoulder anatomy of the rat was found to be very similar to the human with a prominent coracoid process, subscapularis tendon, and long head of the biceps tendon (Figure 1a and 1b). Samples designated for mechanical testing and alignment data were then fine dissected using a dissection microscope to remove all muscle and carefully separate the upper and lower bands based on natural delamination and insertion site location. Stain lines were placed on the tendons denoting the insertion site and tendon midsubstance for local measures of strain. CSA at each location was measured using a laser device13. The humerus was then mounted into a plastic pot and submerged in polymethylmethacrylate (PMMA) and allowed to cure prior to mechanical and alignment testing. After the PMMA had set, the humeral head was sanded down to prevent the bone from impeding light from passing through the tendon insertion site for polarized light analysis. Separate samples designated for histology were left intact with muscle and processed using standard paraffin procedures. Specifically, the samples were pinned to prevent tissue contracture during paraffin processing and the humeral head was maintained in neutral rotation.
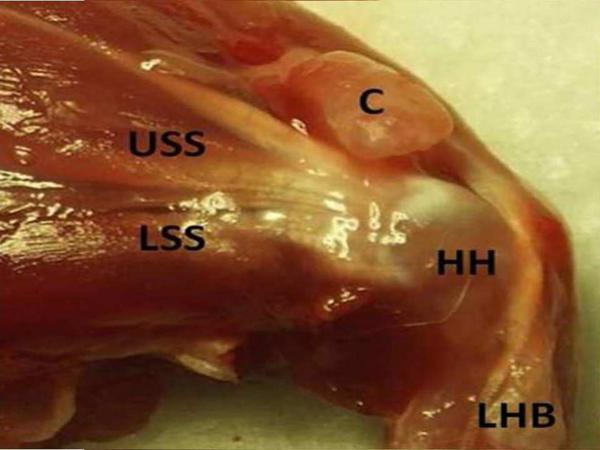
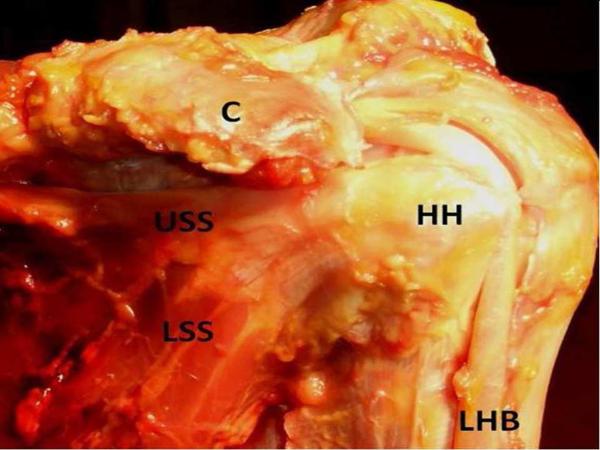
Figure 1.
These images demonstrate the comparative anatomy of the dissected anterior rat and human shoulder. A) The dissected anterior rat shoulder shows the humeral head (HH), the upper band of the subscapularis tendon (USS), the lower band of the subscapularis tendon (LSS), the coracoid process (C), and the long head of the biceps tendon (LHB). B) The dissected anterior human shoulder shows the humeral head (HH), the upper band of the subscapularis tendon (USS), the lower band of the subscapularis tendon (LSS), the coracoid process (C), and the long head of the biceps tendon (LHB). Specifically, the upper subscapularis of both the rat and the human passes under the coracoid process and inserts just medial to the long head of the biceps tendon.
The plastic pot was inserted into a specially designed testing fixture. The proximal end of the tendon was then held at the third stain line (7mm) in a screw clamp lined with fine grit sandpaper. The fixture and clamp where then mounted to a uniaxial testing system (Instron, Norwood, MA, USA) integrated with a polarized light setup, consisting of a backlight, 90°-offset rotating polarizer sheets on either side of the test sample, and a digital camera19. Prior to testing, the stepper motor encoder (Lin Engineering, Santa Clara, CA, USA) and polarizing sheets were synchronized in a position corresponding to 0° of angular rotation. The specimen was then immersed in a 37°C PBS bath, preloaded to 0.1N, preconditioned for 10 cycles from 0.1 to 0.5N at a rate of 1%/s, and held for 300s. Immediately following, a stress relaxation experiment was performed by elongating the specimen to a strain of 6% at a rate of 5%/s (0.575mm/s) followed by a 600s relaxation period. Specimens were then returned to the initial preload displacement and held for 60s, and ramp to failure was then applied at a rate of 0.3%/s. During the ramp to failure, sets of 14 images were acquired every 5 seconds as the polarizers rotated through a 125° range for measurement of fiber alignment. Using the applied stain lines, local tissue strain in each region of the tendon was measured optically with a custom program (MATLAB, Natick, MA, USA).
Local strain was measured optically and stress was calculated as force divided by initial area for both the insertion site and midsubstance separately. A bilinear curvefit was applied to the stress-strain data to quantify the moduli in the toe- and linear-regions. Stiffness was calculated from the optical load-displacement data. Fiber alignment was calculated from the image sets as previously described19. Circular variance (VAR), a measure of the distribution of collagen fiber alignment, was calculated for fiber distributions during zero displacement, at transition strain (intersection of the toe- and linear-regions), and at linear-region strain (last point in the linear region).
Sagittal sections (7 μm) were collected and stained with hematoxylin-eosin (H&E). Sections were then imaged at the articular side insertion site of each tendon using a microscope (Leica DM LB 100T, Buffalo Grove, IL, USA) at 200× magnification. This 108 location was chosen due to subscapularis tears occurring at the articular insertion site. H&E-stained sections were analyzed for changes in cell shape and cellularity. Histologic grading was performed by 3 blinded graders using previously described methods7
Paired t-tests were used to compare group (upper and lower band of the subscapularis) differences for CSA and mechanical properties. CSA and mechanical properties are presented as mean + SD. Since the alignment data was not normally distributed, non-parametric tests were used. Changes in fiber alignment were compared using a Friedman test for tendon band (upper vs. lower) and for the mechanical test region (toe- and linear-region). Significance was determined using Wilcoxon signed-rank post-hoc tests for VAR values. Nonparametric VAR data is presented as median + interquartile range. For histologic analyses, median grades were compared between groups for each tendon by use of a nonparametric Kruskal-Wallis test, and these data are presented as median + interquartile ranges. Significance was set at p <0.05. GraphPad Prism for Windows, Version 5.0 (GraphPad Software, La Jolla, CA, USA) was used for data analysis.
Results
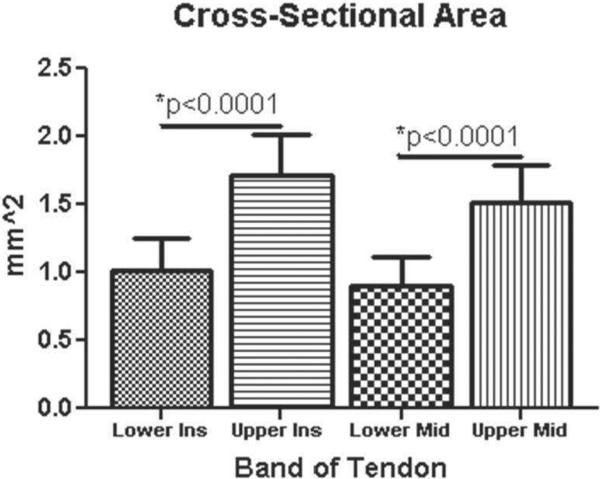
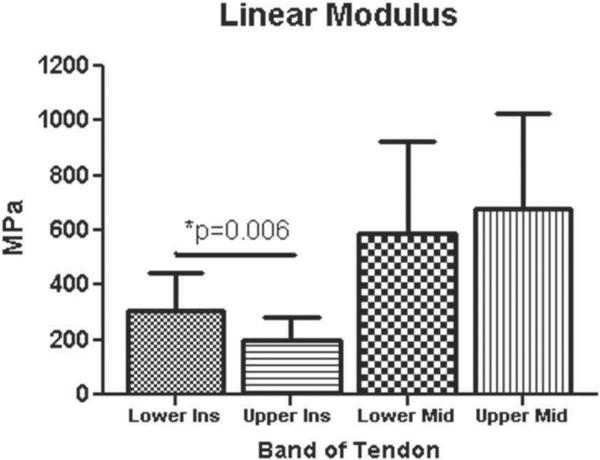
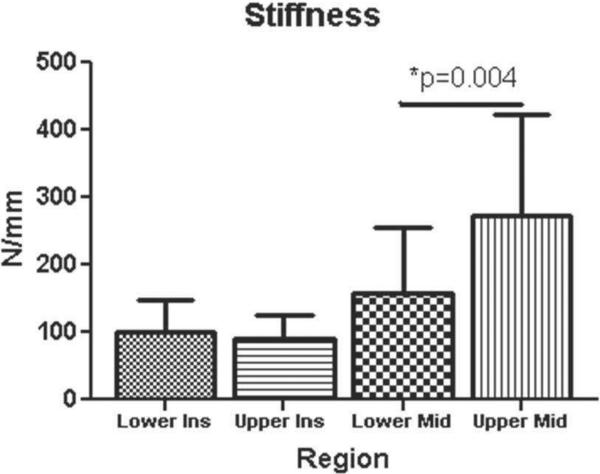
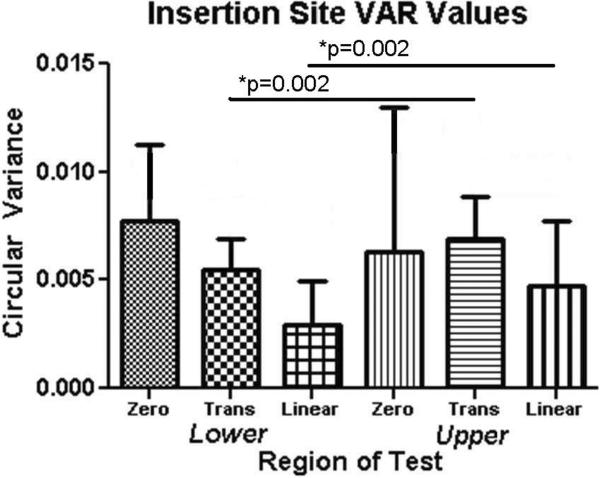
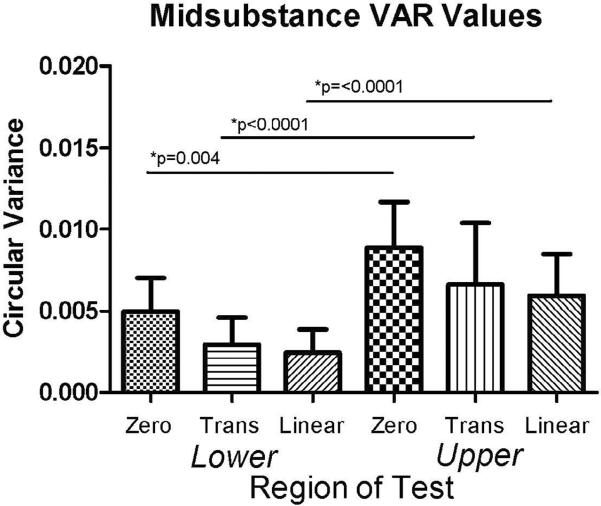
CSA was significantly larger for insertion site and midsubstance in the upper compared to the lower band (p = 0.0001) (Figure 2). Interestingly, modulus at the insertion site was significantly lower in the upper band compared to the lower band, while no differences were found at the midsubstance (p = 0.006) (Figure 3). Stiffness at the midsubstance was significantly higher for the upper band compared to the lower band (p = 0.004) (Figure 4). The upper band was found to be significantly less aligned (higher VAR) than the lower band at the toe- and linear-region of the insertion site (p = 0.002, p = 0.002) (Figure 5) and at all testing points in the midsubstance (p = 0.0004 at zero, <0.0001 at toe- and linear-regions) (Figure 6). The histology results demonstrated that the upper band had significantly more rounded cell shape compared to the lower band (p = 0.02) (Figure 7). There were no significant differences for cellularity between the upper and lower band (p = 0.38).
Figure 2.
The upper band had a significantly larger cross-sectional area (mm2) at both the insertion and the midsubstance compared to the lower band (data presented as mean + standard deviation).
Figure 3.
The upper band had a significantly lower linear modulus (MPa) at the insertion compared to the lower band (data presented as mean + standard deviation).
Figure 4.
The upper band had a significantly larger stiffness (N/mm) at the midsubstance compared to the lower band (data presented as mean + standard deviation).
Figure 5.
The upper band was significantly more disorganized as measured by circular variance (VAR) at the insertion site during both the toe and linear regions of the mechanical test compared to the lower band (data presented as median + interquartile range).
Figure 6.
The upper band was significantly more disorganized as measured by circular variance (VAR) at the midsubstance during all regions of the mechanical test compared to the lower band (data presented as median + interquartile range).
Discussion
Results support the first hypothesis that there would be an increased 134 CSA and a decreased linear modulus in the upper band of the subscapularis tendon compared to the lower band. The CSA was significantly larger at both the insertion site and the midsubstance of the upper band of the subscapularis tendon. This is in agreement with previous human cadaver studies that found the upper portion of the subscapularis to have a larger footprint or insertion site4,10. Similar to the human, the rat subscapularis tendon also demonstrated a 2/3 to 1/3 division between upper and lower bands. Typically, an uninjured tendon with a larger CSA is beneficial to support large loads through the glenohumeral joint without tendon damage. However, when examining the mechanical testing results, there was a decrease in modulus at the insertion site of the upper band compared to the lower band. Further, the midsubstance of the upper band was stiffer than the lower band. This finding in the rat is consistent with previous human cadaver data that sharply divided the SSC into four regions and found that the superior and mid-superior portions of the SSC demonstrated higher stiffness than the inferior portion15. Conversely, the moduli of the midsubstance locations were not different, further supporting the clinical finding that tears start and propagate near the insertion site of the tendon rather than at the midsubstance5,21. These results also suggest that the increases in upper band midsubstance stiffness are likely due to increases in cross sectional area and are not reflective of improved tissue quality. Adams et al2 has reported an increased incidence of partial subscapularis, which is isolated to the upper portion of the insertion site. These tears are commonly observed in the presence of a supraspinatus tear and have successful outcomes when repaired5. Although surgical repairs appear to be successful, the mechanism leading to upper band subscapularis tears is still unknown. Lo et al21 previously proposed the “roller-wringer” effect as the mechanism for upper band subscapularis tears. It suggests that when the arm is in a position of horizontal adduction and internal rotation the lesser tuberosity and the coracoid process act as the rollers in a roller-wringer and the upper band of the subscapularis gets compressed at the insertion site. If this occurs repetitively it could lead to tendon damage and eventually tearing. Previous research has demonstrated that compressive loading leads to decreased tensile mechanical properties7,25.
Results also support the second hypothesis that the upper band of the subscapularis would have a more disorganized collagen fiber distribution (higher VAR) compared to the lower band. The upper band was found to be significantly less organized (higher VAR) than the lower band in the toe- and linear-region of the insertion site and at all testing points in the midsubstance. These results are in agreement with the inferior mechanical properties at the insertion site of the upper band of the subscapularis tendon. In addition, the midsubstance results support the poor tissue quality of the larger upper band. It is likely that the decreased mechanical properties, in part, are due to a decrease in collagen fiber alignment in the loading direction. Previous research has demonstrated that when tendons are highly aligned they have increased strength in the direction of alignment19. Conversely, tendons or different regions of tendons that experience multidirectional loads are less aligned in the primary direction of loading19. This suggests that the upper band of the subscapularis may be experiencing multidirectional loads along its length. Due to the proximity of the upper band of the subscapularis to the coracoid process, it is likely that the tendon is being compressed during shoulder motion. Tendons that pass through bony arches have decreases in collagen organization7,25. This has been shown in the supraspinatus7 and is suggested to be one of the primary cause of rotator cuff tears.
Results are in partial agreement with the third hypothesis that the upper band of the subscapularis would have decreased histology grades for cell shape and cellularity. The upper band was found to have a more rounded cell shape compared to the lower band. However, there were no significant differences between the upper and lower bands of the subscapularis tendon for cellularity. These results are in agreement with our first two aims for tendon mechanics and collagen fiber alignment in the upper band of the subscapularis. Tendon cells are commonly spindle shaped and aligned in the direction of loading. This is mainly caused by the uniaxial mechanical load placed on the tendon during function19. If tendons experience compressive loads, the cells adapt from a spindle shaped to a more rounded cell shape. These results suggest that the upper band of the subscapularis tendon is experiencing repetitive compressive loads potentially from the coracoid process, which supports the “roller-wringer” hypothesis21. Similarly, previous studies have reported coracoid impingement in patients12,24,29. One study attempted to quantify the amount of impingement with the use of ultrasound to measure the coracohumeral interval (distance between the lesser tuberosity and the coracoid process) when patients were positioned in horizontal adduction and internal rotation29. They found a distance of 8mm in patients with diagnosed coracoid impingement and suggested that may be a good clinical indicator for diagnosis29. When repairing the subscapularis arthroscopically one study suggests that if a coracohumeral interval of less than 6mm exists then a coracoplasty should be performed to increase the interval to 8 or 10mm to improve healing and decrease the likelihood of re-injury by eliminating compressive loads to the tendon6,22. Future research should be performed to evaluate the role of the coracoid in subscapularis tendon injury.
There are some limitations to this study that should be acknowledged. First, a rat shoulder model was used to examine differences between the upper and lower band of the subscapularis tendon instead of a human model. The rat model has been previously evaluated and reported to be an appropriate model to study rotator cuff injury due to the similar anatomy and function as the human27. In addition, the rat model can be used to prospectively examine rotator cuff injury and healing8, which is very challenging in the human. Thus, having this normative data for the subscaularis in the rat model will be advantageous for future injury and healing studies. Unfortunately, we are not aware of any study that examined the cellular phenotype in both bands of the human subscapularis and therefore have difficultly comparing the histology results to human conditions. However, based on similar structure and function we are confident the histology results translate to the human condition. A second limitation is that the histology grading provides a semi-quantitative measure of cell morphology. However, group differences were observed for cell shape and suggest obvious differences were present between the upper and lower band of the subscapularis. Third, the stress relaxation test and subsequent return to preload displacement may cause crimping of collagen fibers, which may affect fiber alignment data in the zero displacement and transition strain regions. Unfortunately, there is no data or published research available to determine this. However, all experiments were performed identical and therefore we are confident in the consistency of the results.
Conclusion
Clinically, the data suggests that the upper band of the subscapularis tendon may be susceptible to injury due to the inferior mechanical properties, more disorganized collagen fiber distribution, and more rounded cell shape compared to the lower band. This information suggests that the upper band of the subscapularis is experiencing multidirectional loads that most likely involve compression forces. Future studies are needed to further define the role of the coracoid process in the development of altered tendon properties of the upper band of the subscapularis and its effect on tendon healing.
Figure 7a.
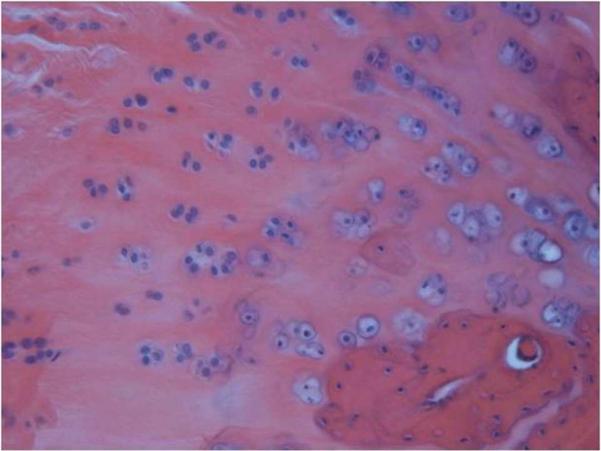
Microscope image of an H&E stained histology slide of the upper subscapularis tendons insertion site at 200× magnification. The upper band had a more rounded cell shape compared to the lower band.
Figure 7b.
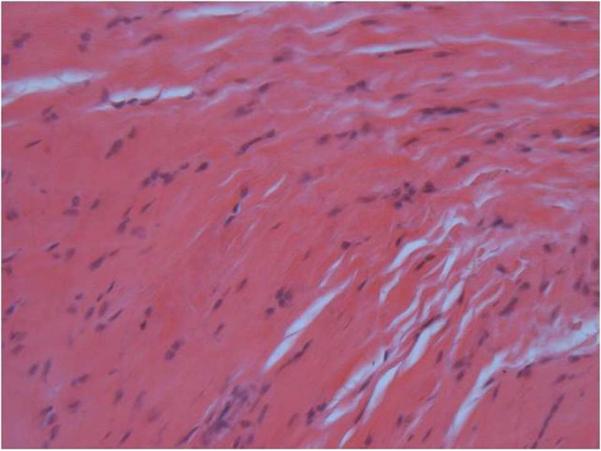
Microscope image of an H&E stained histology slide of the lower subscapularis tendons insertion site at 200× magnification. The lower band had a more spindle cell shape compared to the upper band.
Acknowledgements
“This study was supported in part by the Penn Center for Musculoskeletal Disorders, supported by award Number P30AR050950 from the NIAMS/NIH.”
This study was supported by the NIH 1R01AR051000-05.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
None of the authors have any financial biases associated with the present study.
None of the authors have a conflict of interest associated with the present study.
IRB: University of Pennsylvania Institutional Animal Care and Use Committee approval was obtained prior to testing. Protocol number 801122
References
- 1.Abboud JA, Soslowsky LJ. Interplay of the Static and Dynamic Restraints in Glenohumeral Instability. Clinical Orthopaedics and Related Research. 2002;1:48–57. doi: 10.1097/00003086-200207000-00007. No doi. [DOI] [PubMed] [Google Scholar]
- 2.Adams CR, Schoolfield JD, Burkhart SS. Accuracy of preoperative magnetic resonance imaging in predicting a subscapularis tendon tear based on arthroscopy. Arthroscopy. 2010;26:1427–33. doi: 10.1016/j.arthro.2010.02.028. doi. 10.1016/j.arthro.2010.02.028. [DOI] [PubMed] [Google Scholar]
- 3.Arai R, Mochizuki T, Yamaguchi K, Sugaya H, Kobayashi M, Nakamura T, et al. Functional anatomy of the superior glenohumeral and coracohumeral ligaments and the subscapularis tendon in view of stabilization of the long head of the biceps tendon. J Shoulder Elbow Surg. 2010;19:58–64. doi: 10.1016/j.jse.2009.04.001. doi 10.1016/j.jse.2009.04.001. [DOI] [PubMed] [Google Scholar]
- 4.Arai R, Sugaya H, Mochizuki T, Nimura A, Moriishi J, Akita K. Subscapularis tendon tear: an anatomic and clinical investigation. Arthroscopy. 2008;24:997–1004. doi: 10.1016/j.arthro.2008.04.076. doi. 10.1016/j.arthro.2008.04.076. [DOI] [PubMed] [Google Scholar]
- 5.Bennett WF. Arthroscopic repair of anterosuperior (supraspinatus/subscapularis) rotator cuff tears: a prospective cohort with 2- to 4-year follow-up. Classification of biceps subluxation/instability. Arthroscopy. 2003;19:21–33. doi: 10.1053/jars.2003.50023. doi. 10.1053/jars.2003.50023. [DOI] [PubMed] [Google Scholar]
- 6.Burkhart SS, Brady PC. Arthroscopic subscapularis repair: surgical tips and pearls A to Z. Arthroscopy. 2006;22:1014–27. doi: 10.1016/j.arthro.2006.07.020. 10.1016/j.arthro.2006.07.020. [DOI] [PubMed] [Google Scholar]
- 7.Carpenter JE, Flanagan CL, Thomopoulos S, Yian EH, Soslowsky LJ. The effects of overuse combined with intrinsic or extrinsic alterations in an animal model of rotator cuff tendinosis. Am J Sports Med. 1998;26:801–7. doi: 10.1177/03635465980260061101. [DOI] [PubMed] [Google Scholar]
- 8.Carpenter JE, Thomopoulos S, Flanagan CL, DeBano CM, Soslowsky LJ. Rotator cuff defect healing: a biomechanical and histologic analysis in an animal model. J Shoulder Elbow Surg. 1998;7:599–605. doi: 10.1016/s1058-2746(98)90007-6. [DOI] [PubMed] [Google Scholar]
- 9.Chao S, Thomas S, Yucha D, Kelly JDt, Driban J, Swanik K. An electromyographic assessment of the “bear hug”: an examination for the evaluation of the subscapularis muscle. Arthroscopy. 2008;24:1265–70. doi: 10.1016/j.arthro.2008.01.022. 10.1016/j.arthro.2008.01.022. [DOI] [PubMed] [Google Scholar]
- 10.D'Addesi LL, Anbari A, Reish MW, Brahmabhatt S, Kelly JD. The subscapularis footprint: an anatomic study of the subscapularis tendon insertion. Arthroscopy. 2006;22:937–40. doi: 10.1016/j.arthro.2006.04.101. 10.1016/j.arthro.2006.04.101. [DOI] [PubMed] [Google Scholar]
- 11.Deutsch A, Altchek DW, Veltri DM, Potter HG, Warren RF. Traumatic tears of the subscapularis tendon. Clinical diagnosis, magnetic resonance imaging findings, and operative treatment. Am J Sports Med. 1997;25:13–22. doi: 10.1177/036354659702500104. [DOI] [PubMed] [Google Scholar]
- 12.Dines DM, Warren RF, Inglis AE, Pavlov H. The coracoid impingement syndrome. J Bone Joint Surg Br. 1990;72:314–6. doi: 10.1302/0301-620X.72B2.2312576. [DOI] [PubMed] [Google Scholar]
- 13.Favata M, Beredjiklian PK, Zgonis MH, Beason DP, Crombleholme TM, Jawad AF, et al. Regenerative properties of fetal sheep tendon are not adversely affected by transplantation into an adult environment. J Orthop Res. 2006;24:2124–32. doi: 10.1002/jor.20271. doi 10.1002/jor.20271. [DOI] [PubMed] [Google Scholar]
- 14.Flury MP, John M, Goldhahn J, Schwyzer HK, Simmen BR. Rupture of the subscapularis tendon (isolated or in combination with supraspinatus tear): when is a repair indicated? J Shoulder Elbow Surg. 2006;15:659–64. doi: 10.1016/j.jse.2005.07.013. doi 10.1016/j.jse.2005.07.013. [DOI] [PubMed] [Google Scholar]
- 15.Halder A, Zobitz ME, Schultz E, An KN. Structural properties of the subscapularis tendon. J Orthop Res. 2000;18:829–34. doi: 10.1002/jor.1100180522. [DOI] [PubMed] [Google Scholar]
- 16.Kato K. Innervation of the scapular muscles and its morphological significance in man. Anat Anz. 1989;168:155–68. [PubMed] [Google Scholar]
- 17.Koo SS, Burkhart SS. Subscapularis tendon tears: identifying mid to distal footprint disruptions. Arthroscopy. 2010;26:1130–4. doi: 10.1016/j.arthro.2010.06.017. doi 10.1016/j.arthro.2010.06.017. [DOI] [PubMed] [Google Scholar]
- 18.Kreuz PC, Remiger A, Erggelet C, Hinterwimmer S, Niemeyer P, Gachter A. Isolated and combined tears of the subscapularis tendon. Am J Sports Med. 2005;33:1831–7. doi: 10.1177/0363546505277118. doi. 10.1177/0363546505277118. [DOI] [PubMed] [Google Scholar]
- 19.Lake SP, Miller KS, Elliott DM, Soslowsky LJ. Effect of fiber distribution and realignment on the nonlinear and inhomogeneous mechanical properties of human supraspinatus tendon under longitudinal tensile loading. J Orthop Res. 2009;27:1596–602. doi: 10.1002/jor.20938. doi 10.1002/jor.20938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lippitt S, Matsen F. Mechanisms of glenohumeral joint stability. Clin Orthop Relat Res. 1993:20–8. [PubMed] [Google Scholar]
- 21.Lo IK, Burkhart SS. The etiology and assessment of subscapularis tendon tears: a case for subcoracoid impingement, the roller-wringer effect, and TUFF lesions of the subscapularis. Arthroscopy. 2003;19:1142–50. doi: 10.1016/j.arthro.2003.10.024. doi. 10.1016/j.arthro.2003.10.024. [DOI] [PubMed] [Google Scholar]
- 22.Lo IK, Burkhart SS. Arthroscopic coracoplasty through the rotator interval. Arthroscopy. 2003;19:667–71. doi: 10.1016/s0749-8063(03)00219-6. doi 10.1016/S0749-8063(03)00219-6. [DOI] [PubMed] [Google Scholar]
- 23.Lo IK, Burkhart SS. The comma sign: An arthroscopic guide to the torn subscapularis tendon. Arthroscopy. 2003;19:334–7. doi: 10.1053/jars.2003.50080. doi 10.1053/jars.2003.50080. [DOI] [PubMed] [Google Scholar]
- 24.Lo IK, Parten PM, Burkhart SS. Combined subcoracoid and subacromial impingement in association with anterosuperior rotator cuff tears: An arthroscopic approach. Arthroscopy. 2003;19:1068–78. doi: 10.1016/j.arthro.2003.10.016. doi 10.1016/j.arthro.2003.10.016. [DOI] [PubMed] [Google Scholar]
- 25.Malaviya P, Butler DL, Boivin GP, Smith FN, Barry FP, Murphy JM, et al. An in vivo model for load-modulated remodeling in the rabbit flexor tendon. J Orthop Res. 2000;18:116–25. doi: 10.1002/jor.1100180117. doi: 10.1002/jor.1100180117. [DOI] [PubMed] [Google Scholar]
- 26.Omi R, Sano H, Ohnuma M, Kishimoto KN, Watanuki S, Tashiro M, et al. Function of the shoulder muscles during arm elevation: an assessment using positron emission tomography. J Anat. 2010;216:643–9. doi: 10.1111/j.1469-7580.2010.01212.x. doi 10.1111/j.1469-7580.2010.01212.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Soslowsky LJ, Carpenter JE, DeBano CM, Banerji I, Moalli MR. Development and use of an animal model for investigations on rotator cuff disease. J Shoulder Elbow Surg. 1996;5:383–92. doi: 10.1016/s1058-2746(96)80070-x. [DOI] [PubMed] [Google Scholar]
- 28.Thompson WO, Debski RE, Boardman ND, 3rd, Taskiran E, Warner JJ, Fu FH, et al. A biomechanical analysis of rotator cuff deficiency in a cadaveric model. Am J Sports Med. 1996;24:286–92. doi: 10.1177/036354659602400307. [DOI] [PubMed] [Google Scholar]
- 29.Tracy MR, Trella TA, Nazarian LN, Tuohy CJ, Williams GR. Sonography of the coracohumeral interval: a potential technique for diagnosing coracoid impingement. J Ultrasound Med. 2010;29:337–41. doi: 10.7863/jum.2010.29.3.337. No doi. [DOI] [PubMed] [Google Scholar]