Abstract
44gSc was produced by 16 MeV proton irradiation of unenriched calcium metal with radionuclidic purity greater than 95%. The thick target yield at saturation for 44gSc was 213 MBq/μA, dwarfing the yields of contaminants 43Sc,44mSc, 47Sc and 48Sc for practical bombardment times of 1–2 h. Scandium was isolated from the dissolved calcium target by filtration, and reconstituted in small volumes of dilute HCl. Reactions with the chelate 1,4,7,10-tetraazacyclododecane-1,4,7,10-tetraacetic acid (DOTA) indicated a reactivity of 54±14 Gbq/μmol at end-of-bombardment.
Keywords: 44Sc, scandium-44, isotope production, PET, radiometal, thick target yield
1. Introduction
A 3.97 h1 half-life and 94% positron branch make 44gSc suitable for peptide radiolabeling for PET. Another scandium isotope, 43Sc, has matched characteristics with a 3.89 h half-life, and 88% positron branch. Both represent PET surrogates for the therapeutic isotope 47Sc, and possibly for 177Lu as shown by Majkowska-Pilip and Bilewicz (2011).
Recent developments in 44Ti/44gSc generator technology have intensified interest in 44gSc (Filosofov et al., 2010; Pruszyński et al., 2010, 2012). However, production of sufficient quantities of 44Ti (t1/2 =60 y) requires proton irradiation parameters exceeding the capabilities of most small medical cyclotrons. Therefore production is limited to the few willing facilities in the world (Zhernosekov et al., 2011; Alenitzky et al., 2005). In order to justify the procurement and custodianship of a long-lived generator, the need for 44gSc must be established through preclinical trials. To that end, direct production of 44gSc on small research cyclotrons is necessitated.
Abbas et al. (2011) investigated production via 44Ca(p,n)44gSc and showed that Ci levels of 44gSc are possible using an enriched target. Additionally, a thin target of enriched 44Ca produces the activity required for preclinical PET, and at current market prices such a target is not prohibitively expensive (currently 15 USD/mg metal mass, at 98% enrichment as carbonate; Isoflex, CA). However, to protect against the fluctuating prices of enriched isotopes, especially when anticipating the demand that may arise from successful tracers, it is worthwhile to develop alternative production routes.
43Sc, having lower energy concurrent gamma emissions and lower positron-branch endpoint energies than 44gSc, is produced by either the 43Ca(p,n) reaction or by alpha irradiation via the 40Ca(α,p) and 40Ca(α,n) channels. The method of alpha bombardment su3ers from a similar lack of available cyclotrons as 44Ti production. However with a 4-hour half-life and a production cross-section approaching 1 barn (Levkovskij, 1991), potential exists for regional distribution following mass production at a single alpha facility. The most suitable target for such irradiations is natural Ca metal, comprised at 97% by 40Ca.
To date, available literature does not address 44gSc and 43Sc production from a natural metal calcium target, but the properties are favorable. Calcium metal is malleable, has a melting point over 1100 K, is electrically conductive, and has a coe3cient of thermal conductivity comparable to that of aluminum (200 W/m K and 237 W/m K respectively). This is in contrast to CaO or CaCO3, insulating ceramics which dissipate neither heat nor charge in su3cient quantity to allow high-power targetry. The malleability of calcium allows it to be pressed into a water-cooled target body forming good thermal contact. Additionally, the intense reactivity of calcium with water lends itself towards fast target chemistry.
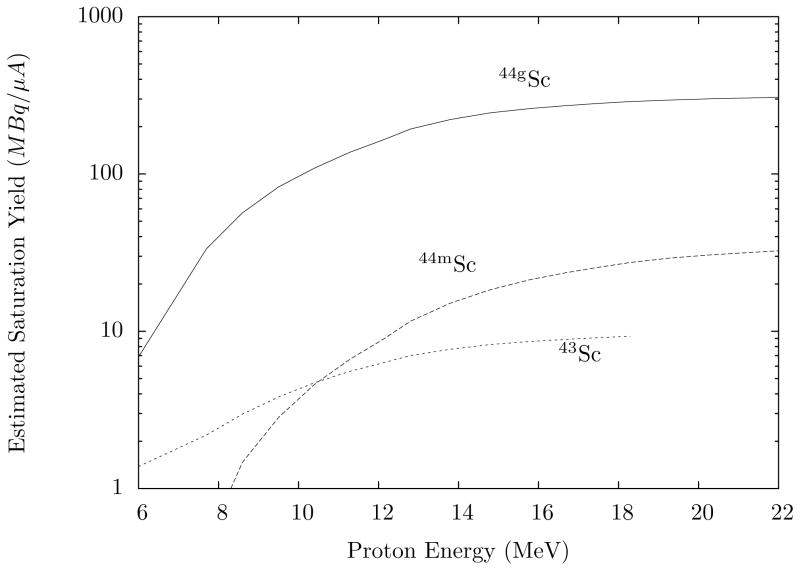
The development of Ca metal targetry and subsequent chemical separation begins with proton irradiations, producing 44gSc on a preclinical scale. Owing to a large reaction cross section, an unenriched calcium metal target provides ample 44gSc–in spite of the meager 2.09% natural abundance of 44Ca. Levkovskij (1991)2 determined the production cross-sections for 44mSc, 44gSc, and 43Sc. Coupling these values with the stopping power of calcium gives the expected end-of-saturation-bombardment (EOSB) yields shown in Fig. 1.
Figure 1.
Estimated thick target EOSB yields for production of 44gSc, 44mSc and 43Sc as a function of proton bombardment energy. This represents yields from an unenriched natural calcium metal target with the isotopic ratios listed in Table 2. The values curves were calculated using the cross-section data from Levkovskij (1991) and proton ranges from SRIM (Ziegler, 1985). Note: This graph shows saturation yields. When estimating a production yield, irradiation time must be considered.
The 44Ca(p,n)44gSc cross-section (peaking with 588 mb at 10.9 MeV) allows 264 MBq/μA 44gSc at EOSB for 16 MeV protons on a thick, unenriched metal target. The cross-sections for creation of other scandium radioisotopes are unpublished, and a measurement of their production rate concurrent with 44gSc is of utmost interest when assessing the potential for research use.
For eventual labeling of PET tracers, recovery of the 44gSc from calcium needs to remove bulk metal and give the final product in a small volume. A fortunate fact is that small amounts of residual calcium do not interfere with DOTA chelation (Abbas et al., 2011). Thus the chemical processing may be aimed at concentrating the activity rather than at rigorous purification. One such viable separation employs precipitation and filtration (Duval and Kurbatov, 1953). This approach takes advantage of the insolubility of Sc(OH)3 either as a precipitate or coprecipitate, and is similar to a technique developed for purifying yttrium from strontium (Avila-Rodriguez et al., 2008).
Majkowska-Pilip and Bilewicz (2011) showed that DOTA is a satisfactory candidate for bifunctional chelation of 44gSc to PET-tracer molecules. Pruszyński et al. (2012) report reaction conditions for labeling bifuncionalized DOTA, conjugated to octreotide (DOTATOC), with generator-produced 44Sc. They achieve near quantitative yields with a ratio of 44Sc to DOTATOC of roughly 9 GBq/μmol at 95 °C in pH 4 acetate bu3er for 30 min. When considering extracting 44gSc from a metallic calcium target, reactivity with DOTA in excess of 9 GBq/μmol is therefore a reasonable benchmark for evaluating the success of the chemical separation.
For this work, we measured the thick target yields of 44gSc at 16 MeV, along with contaminants 44mSc, 43Sc, 47Sc, and 48Sc. Additionally, we developed a filtration separation technique providing 44gSc in a small volume of dilute HCl. Finally, we determined the reactivity with DOTA by titration.
2. Materials and Methods
2.1. Cyclotron targetry and irradiations
To prepare the targets, several 100–150 mg dendritic chunks totalling 400–600 mg of natural 99.99% calcium metal (Sigma Aldrich, St. Louis MO) were pressed with a mechanical lever press into an aluminum target holder. The holder was a short 4.2 mm cylinder (3.8 cm diameter) with a 1.26 cm2 by 2.5 mm flat-bottom cavity in the center for holding the calcium. 1.7 mm of aluminum separated the calcium target from water-jet cooling applied to the backside of the holder. A piece of 12.5 μm aluminum foil placed over the irradiated face of the target protected the cyclotron from vaporized calcium.
Irradiations were performed on the UW-Madison PET-trace at 16 MeV. Typical irradiations lasted for 1 hour at currents ranging from 5–27 μA. In order to address the concern of target oxidation, a single 770 mg target was pressed into the aluminum holder and exposed to lab air for 3.5 days. The mass of the target was measured before and after pressing and after the waiting period.
2.2. Thick target yields
A 600 mg calcium target3, prepared as above, was irradiated for 330 s with an average current 6.1 μA at 16 MeV. The target was kept in the holder, and γ intensities were determined with a 60 cm3 high purity germanium (HPGe) detector (Canberra C1519) in 600 s and 1800 s segments for 3.5 days. The gamma lines used to determine yields are listed in Table 1. Peaks were selected from the spectra and their associated decay curves were fit with the appropriate half-lives to determine the activity at end-of-bombardment (EOB) and the activity distribution as a function of time. Gain shifts in the detector electronics were corrected by centroid fitting, and segments with poor resolution (indicating a gain shift during counting) were discarded.
Table 1.
Gamma emissions from the long-lived scandium radioisotopes used for determining the yields from proton irradiation of natCa.
| Nuclide | γ Energy (keV) | Branch (γ’s per decay) |
|---|---|---|
| 43Sc | 373 | 0.23 |
| 44gSc | 1157 | 1.0 |
| 44mSc | 271 | 0.87 |
| 46Sc | 889 | 1.0 |
| 47Sc | 159 | 0.68 |
| 48Sc | 1038 | 0.98 |
For all other irradiations, the activity at greater than 20 min post-EOB was measured with a Capintec CRC-15 Dual PET dose calibrator (setting 938) after dissolution and acidification of the target as described in Sec. 2.3.
2.3. Radiochemical separation
Irradiated calcium targets (400–600 mg) were removed from the target holder and dropped into 10 ml cold water in a 50 ml centrifuge tube resting in an ice bath. The calcium reacted on contact with the water creating a white sludge of CaO and Ca(OH)2. This was dissolved by addition of 2.5 ml concentrated trace-metals-grade HCl (Aristar Ultra, VWR, West Chester PA). For some of the dissolutions the calcium was dissolved with 2M HCl added 2–4 ml at a time instead of water. This had the effect of keeping the calcium in solution as hydrolysis commenced, reducing its propensity to bubble over. After dissolution, the pH was adjusted to 6.5–9.0 by addition of the appropriate volume of 1 M NH4OH (Optima, Fisher Scientific, Fair-lawn NJ). pH values were monitored during the procedure with an ion-sensitive field-effect transistor (ISFET) probe (I.Q. Scientific Instruments, Carlsbad CA). The solution was then pushed through a 0.22 μm Millex-GV 13 mm diameter syringe filter (Millipore Corporation, Billerica MA) where the majority of the activity was retained. To recover activity adhered to the tube walls, 2 ml 4M HCl was added to the dissolution vessel, vortexed, and the pH was readjusted to 6.5–9.0. This liquid was also pushed through the same filter. Then the filter was washed with 10 ml 0.1M ammonium hydroxide that had been adjusted to pH 8–9 with HCl.
Elution was performed slowly with 0.1 M HCl, at a rate of 150 μl/min at 70–90°C. In order to maximize the activity concentration, each 150 μl fraction was pulled back through the filter 2–3 times before it was finally expelled.
The entire process was also performed with a non-irradiated Ca target (462 mg), and an eluted sample was analyzed by ICP-MS at the State Hygiene Lab of Wisconsin for metal impurities.
2.4. Reactivity measurements
The pH of the first eluted fraction (0.1M HCl, 150 μl) was adjusted to 4.8 with 0.2 M ammonium acetate. This bu3ered solution was split into 9 fractions, each mixed with 100 μl of aqueous DOTA (Macrocyclics, CA) solutions of known concentrations ranging from 0.1 to 1000 nM, along with a no-DOTA control vial. The solutions were incubated at 80 °C for 30 min, and then spotted onto aluminum backed silica gel instant thin-layer liquid chromatography (ITLC) plates (EMD Chemicals, Gibbstown NJ). The plates were developed with water solvent for 15 min and dried. The activity distribution on the plates was assessed with a Packard Cyclone Phosphor-Plate imaging system.
3. Results
3.1. Cyclotron targetry and irradiations
Oxidation of the calcium target during handling was negligible. In all cases, no oxidation of the targets was observed during the short time that they were exposed to air before and after irradiation. The 770 mg target that was left out for 3.5 days gained only 40 mg mass, indicating minimal oxidation for the routine 5–20 minute target-prep procedure. All other targets gained no mass between pressing and irradiation.
The calcium targets were tested at successively higher beam currents between 5 μA and 27 μA. No adverse effects on the target occurred until 27 μA, where the aluminum foil over the target face oxidized and an insignificant layer of calcium was deposited, through vaporization, on the target body. All irradiations afterwards were limited to 25 μA.
3.2. Thick target yields
Table 2 lists the EOSB yields for the long-lived products of calcium irradiation. Reported uncertainties in the measurements consist of a 4% systematic uncertainty from the detector/source geometry and e3ciency calibration added in quadrature with counting statistics. Total yields from all thick target irradiations, as measured by the Capintec dose calibrator (setting 938), give an EOSB 44gSc yield of 215 ± 15 MBq/μA, consistent with the HPGe measurement.
Table 2.
Natural abundances of calcium isotopes, and their long-lived reaction products after proton bombardment at 16 MeV.
| Target | % | Product | t1/2 product | EOSB meas. (MBq/μA) | Yield @ 1 h (MBq/μA) |
|---|---|---|---|---|---|
| 40Ca | 96.94% | - | - | - | - |
| 42Ca | 0.647% | - | - | - | - |
| 43Ca | 0.135% | 43Sc | 3.89 h | 5.3 ± 0.4 | 0.85 |
| 44Ca | 2.09% | 44gSc | 3.97 h | 213. ± 9. | 34.1 |
| 44mSc | 58.6 h | 17.3 ± 0.6 | 0.21 | ||
| 46Ca | 0.004% | 46Sc | 83.8 d | unobs. | - |
| 48Ca | 0.187% | 48Sc | 43.7 h | 20.9 ± 0.9 | 0.33 |
| 47Sc | 80.4 h | 11.2 ± 0.4 | 0.09 |
3.3. Radiochemical separation
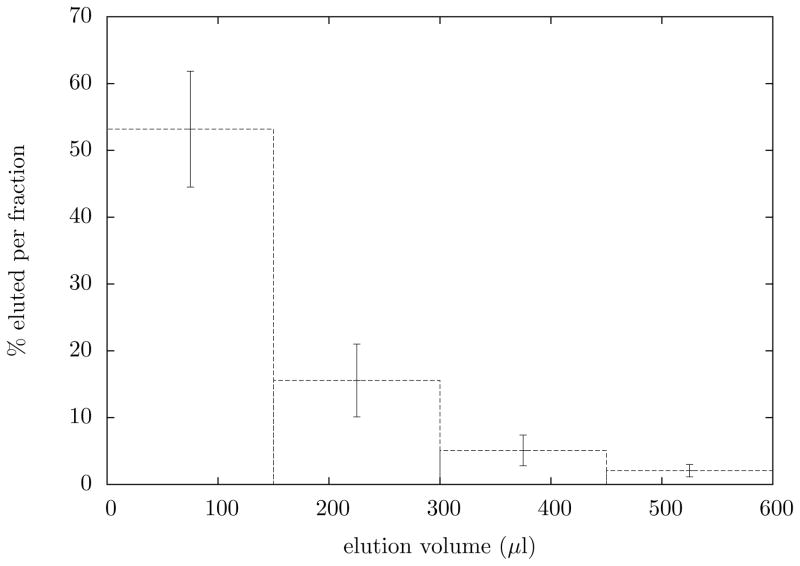
In the 11 separations performed, 73±14% of the activity was collected on the filter. Untrapped activity was split between breakthrough (11±9%) and retention on the walls of the dissolution vial (16 ± 13%). When eluted, 73 ± 12% of the activity retained on the filter was recovered in under 1 ml of 0.1 M HCl. The average elution profile is shown in Fig. 2. Clearly the highest activity concentration occurs in the first 150 μl fraction, representing 55% of the trapped activity, or 40% of the total produced scandium. The entire separation typically concluded less than one hour post-EOB.
Figure 2.
The average elution profile of 44gSc from the membrane filter. Elution is performed with hot 0.1 M HCl, roughly 150μl at a time.
ICPMS of the first eluted fraction in the non-irradiated separation indicated that the most significant metal impurities were calcium (2.3 mM), iron (43 μM), and aluminum (107 μM). Using this final calcium concentration along with the original mass of the non-irradiated target (462 mg) and the typical 40% Sc recovery in 150 μl, gives a separation factor of 1.4×104.
3.4. Reactivity measurements
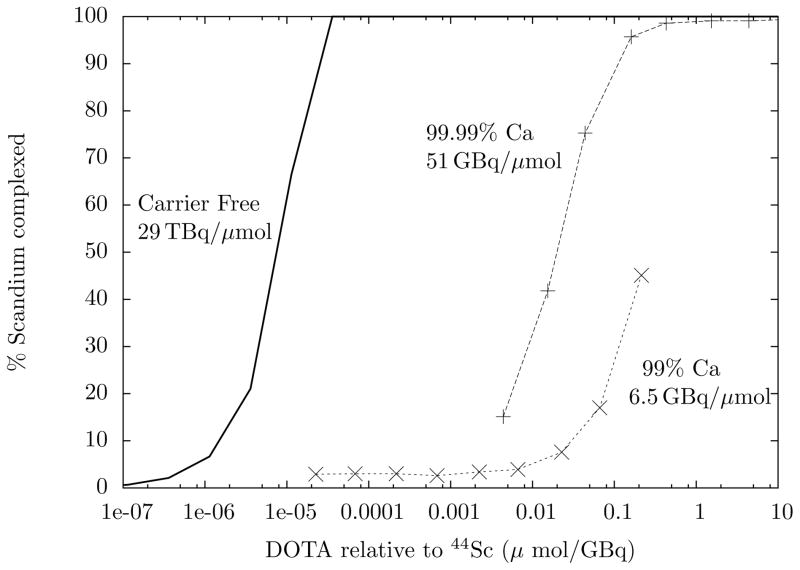
ITLC of the DOTA titration showed a peak at Rf = 0.8 – 0.9 consistent with the production of Sc-DOTA, i.e. the activity in the peak increased with increasing DOTA concentration and was not present in the control. A representative titration using 99.99% calcium is shown in Fig. 3. The y-values show the % of 4xSc activity in the Rf = 0.8 – 0.9 (Sc-DOTA) peak, along with the expected curve for carrier-free complexation. The reactivity is estimated using the assumption that 50% chelation yield occurs when the molar ratio of chelate to DOTA-binding metal impurities is 1:2. Here, the 99.99% curve implies an reactivity of 51 GBq/μmol. Overall, the reactivity was 54 ± 14 GBq/μmol (n=4) corrected to EOB. Fig. 3 also shows a representative titration when 99% pure calcium was used for production. An almost ten-fold increase in reactivity was observed when using 99.99% calcium over the less pure metal.
Figure 3.
Representative results of DOTA-Sc titrations with two di3erent grades of calcium target, along with the expected curve for carrier-free 44gSc. The reactivity of each measured sample is listed next to the curve, corrected to EOB. The carrier-free curve assumes log k = −26 and 75 μCi 44gSc in 200 μl bu3er.
The measured reactivity, divided by the activity concentration, gives a value for the concentration of non-radioactive, competitive, metal impurities in the product solution of 28±15 μM. This is consistent with the ICP-MS value for iron contamination of 43 μM.
4. Discussion
The 2% natural abundance of 44Ca is not prohibitive to production of 44gSc on a medical cyclotron. The targets presented here routinely yielded over 650 MBq for 1 hour irradiations at 20 μA, more than enough for preclinical PET imaging studies.
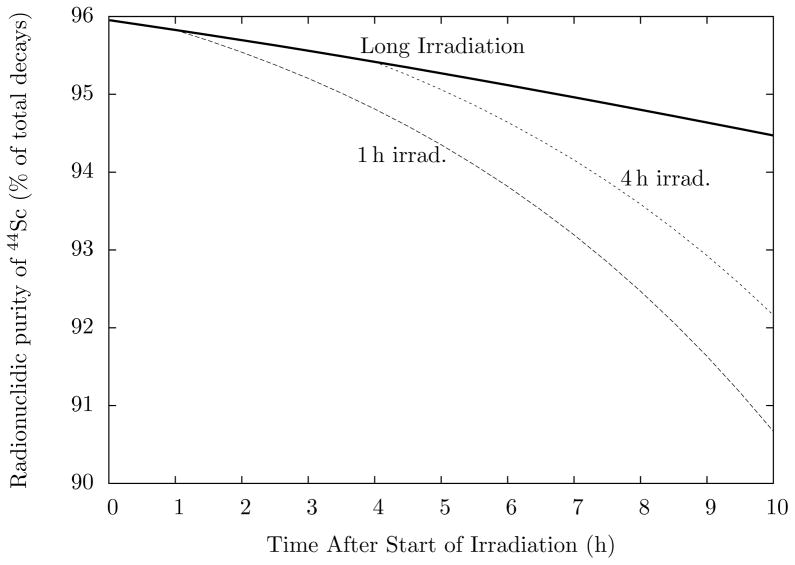
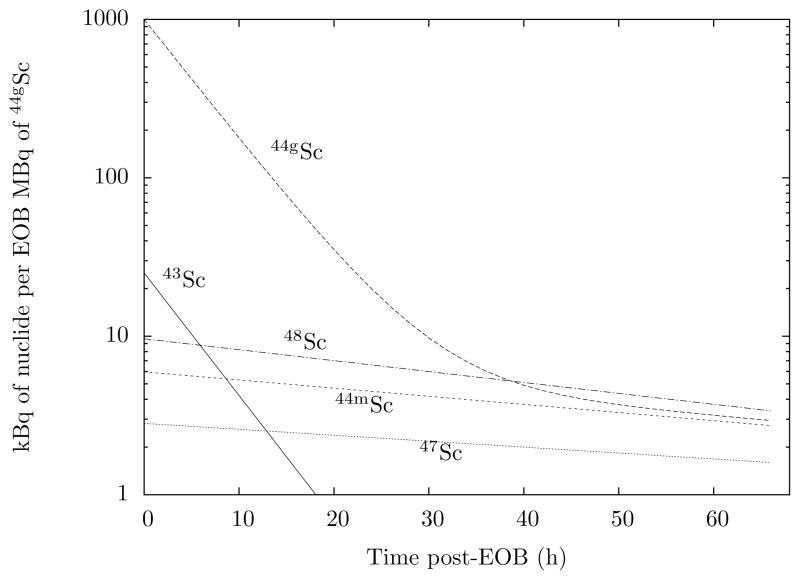
Fig. 4 shows the radionuclidic purity of 44gSc as a function of irradiation time. The purity exceeds 95% for irradiations up to 7 hours. Additionally, Fig. 5 shows the activity balance of Sc isotopes for a 1 hour irradiation. Notably, only two positron emitters are present, 44gSc and 43Sc. The comparable decay characteristics make 43Sc a non-interfering nuclide. Other impurities, 44mSc, 47Sc, and 48Sc, are limited by their longer half-lives and the lower isotopic abundances of their respective calcium feedstocks. Such impurities are tolerable for preclinical PET, especially considering that the combined activities of the three nuclides accounts for less than 3% of total decays at early timepoints. The contribution from these nuclides does not exceed 10% of the total activity until 10 hours post-EOB.
Figure 4.
Radionuclidic purity of 44gSc as a function of time after start of irradiation. The bold line represents the radionuclidic purity as a function of irradiation time, and the dashed lines show the purity of decaying samples after 1 hour and 4 hour irradiations.
Figure 5.
Activity balance of scandium radioisotopes as a function of time after a 1 hour irradiation of a thick target at 16 MeV.
The separation of radioscandium by filtration is fast and e3cient, routinely trapping 70% of the produced activity. More than 70% of this can be eluted in a small volume of 0.1M HCl and immediatly used for radiolabeling. The reactivity of 44gSc for DOTA is over 1 Ci/μmol which is su3cient to label DOTA-bearing peptides. Target recycling is unnecessary, and the procedure requires minimal investment in equipment and time.
Unenriched calcium metal is a viable target material for cyclotron production of 44gSc.
The thick target yield for natCa(p,*)44gSc is 213MBq/μA (projected to saturation).
For short 16.4MeV proton irradiations of natural calcium, the radionuclidic purity of 44gSc exceeds 95%
Filtration separates radioscandium from near-neutral pH calcium matrices.
The reactivity of cyclotron-produced, filtration-separated, 44gSc with the bifunctional chelate DOTA is 54 GBq/μmol.
Acknowledgments
GWS and JWE gratefully acknowledge the support of the NIH Radiological Sciences Training Grant # 5T32CA9206-33. HFV is supported through the SciMed GRS Advanced Opportunity Fellowship.
We would like to thank Dr. Martin Shafer and everyone in the trace metals group at the State Hygiene Lab of Wisconsin for the ICP-MS study and analysis.
Footnotes
All nuclear physics data in this report come from Nuclear Data Sheets and were accessed via www.nndc.bnl.gov.
A multiplicative correction of 0.8 was applied to all proton reaction cross-section values taken from Levkovskij (1991). The correction is discussed by Takács et al. (2002), arising from an incorrect value for the monitor reaction cross-section. The shapes of the excitation functions are considered valid.
A thick target for 16 MeV protons in calcium is 400 mg/cm2 (Ziegler, 1985), or ≥ 504 mg for the geometry described.
The 43Sc produced from 44Ca(p,2n) is not distinguished from 43Ca(p,n) in this study.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Majkowska-Pilip A, Bilewicz A. J Inorg Biochem. 2011 doi: 10.1016/j.jinorgbio.2010.11.003. [DOI] [PubMed] [Google Scholar]
- Filosofov DV, Loktionova NS, Rösch F. Radiochim Acta. 2010;98:149. [Google Scholar]
- Pruszyński M, Loktionova N, Filosofov D, Rösch F. Appl Radiat Isotopes. 2010;68:1636. doi: 10.1016/j.apradiso.2010.04.003. [DOI] [PubMed] [Google Scholar]
- Pruszyński M, Majkowska-Pilip A, Loktionova N, Eppard E, Rösch F. Appl Radiat and Isotopes. 2012 doi: 10.1016/j.apradiso.2012.03.005. [DOI] [PubMed] [Google Scholar]
- Zhernosekov K, Bunka M, Hohn A, Schibli R, Türler A. J Labelled Compd Rad. 2011;54:S239. [Google Scholar]
- Alenitzky YG, Novgorodov AF, Skripnik AV, Filosofov DV, Kaplun VG, Suzikov AG, Eliseev IA, Rösch F. Tech Rep. Institute of Nuclear Chemistry, University of Mainz; 2005. 44Ti: Investigation of target preparation, irradiation and yields in the 45Sc(p,2n) process. [Google Scholar]
- Abbas K, Cydzik I, Simonell F, Krajewski S, Kasperek A, Bilewicz A. J Labelled Compd Rad. 2011;54:S53. [Google Scholar]
- Levkovskij VN. Activation cross section for nuclides with masses A = 40 – 100 by protons and alpha-particles with energies 10–50 MeV. 1991 accessed via EXFOR: entry A0510. [Google Scholar]
- Takács S, Tárkányi F, Sonck M, Hermanne A. Nucl Instrum Meth B. 2002;198:183. [Google Scholar]
- Ziegler JF. The Stopping and Ranges of Ions in Matter. Pergamon; 1985. [Google Scholar]
- Duval JE, Kurbatov MH. J Am Chem Soc. 1953;75:2246. http://pubs.acs.org/doi/pdf/10.1021/ja01105a065. [Google Scholar]
- Avila-Rodriguez MA, Nye JA, Nickles RJ. Appl Radiat Isotopes. 2008;66:9. doi: 10.1016/j.apradiso.2007.07.027. [DOI] [PubMed] [Google Scholar]