Abstract
BACKGROUND
Methicillin-resistant Staphylococcus aureus (MRSA) can cause severe infection in patients who are undergoing vascular surgical operations. Testing all vascular surgery patients preoperatively for MRSA and attempting to decolonize those who have positive results may be a strategy to prevent MRSA infection. The economic value of such a strategy has not yet been determined.
METHODS
We developed a decision-analytic computer simulation model to determine the economic value of using such a strategy before all vascular surgical procedures from the societal and third-party payer perspectives at different MRSA prevalence and decolonization success rates.
RESULTS
The model showed preoperative MRSA testing to be cost-effective (incremental cost-effectiveness ratio, <$50,000 per quality-adjusted life year) when the MRSA prevalence is ≥0.01 and the decolonization success rate is ≥0.25. In fact, this strategy was dominant (ie, less costly and more effective) at the following thresholds: MRSA prevalence ≥0.01 and decolonization success rate ≥0.5, and MRSA prevalence ≥0.025 and decolonization success rate ≥0.25.
CONCLUSION
Testing and decolonizing patients for MRSA before vascular surgery may be a cost-effective strategy over a wide range of MRSA prevalence and decolonization success rates.
Methicillin-resistant Staphylococcus aureus (MRSA) is a common cause of postoperative infection.1 Patients who are undergoing vascular surgical procedures may be at particular risk for MRSA infection. MRSA can seed vascular graft material, and patients with vascular disease tend to be older and to have comorbidities such as diabetes and poor circulation, factors that may increase susceptibility to infection. A United Kingdom retrospective study found that from 1994 to 2000, the prevalence of patients with positive test results for MRSA in a vascular unit increased from 1% to 6%.2 In fact, studies indicate that MRSA has become the leading cause of infection among vascular surgery patients in the United States and the United Kingdom.1,3–5
Postoperative MRSA infection in vascular surgery patients can cause substantial morbidity and potential mortality.6 MRSA infection is associated with longer hospital stays, increased morbidity and mortality, and higher rates of amputation, revision amputation, and graft removal, compared with those of other infections.1,3,5,7–9 MRSA infection also correlates with lower limb-salvage rates, lower healing power, and delayed healing.3,4 Therefore, reducing the incidence of MRSA infection may be key to improving vascular surgery outcomes.10
Testing patients preoperatively for MRSA colonization and decolonizing patients with positive test results (ie, administering antibiotics or antiseptic medication to remove MRSA colonization) is a potential strategy to prevent postoperative MRSA infection. Patients with MRSA nasal colonization may be at greater risk for postoperative MRSA infection. One study found a 30.7% MRSA infection rate among vascular surgery patients with MRSA nasal colonization, compared with a 0.67% rate among vascular surgery patients without MRSA nasal colonization.11 This suggests that preoperative testing and decolonization may be important. However, studies have not established the cost-effectiveness of such a strategy. In addition, there is no consensus about what decolonization regimen should be used or how effective decolonization may be. Moreover, MRSA colonization prevalence may vary greatly from location to location.
We developed a computer simulation model to determine the economic value of the testing and decolonization strategy before vascular operations. The model simulated the decision of whether to perform preoperative MRSA testing on patients who were about to undergo vascular surgical procedures and to decolonize those with positive test results. Sensitivity analyses varied key model parameters and allowed us to delineate how the cost-effectiveness of such a strategy may vary by MRSA prevalence, decolonization success rate, and decolonization cost. The results of our model may help guide policy making and the design of future epidemiological and clinical studies.
METHODS
Model Structure
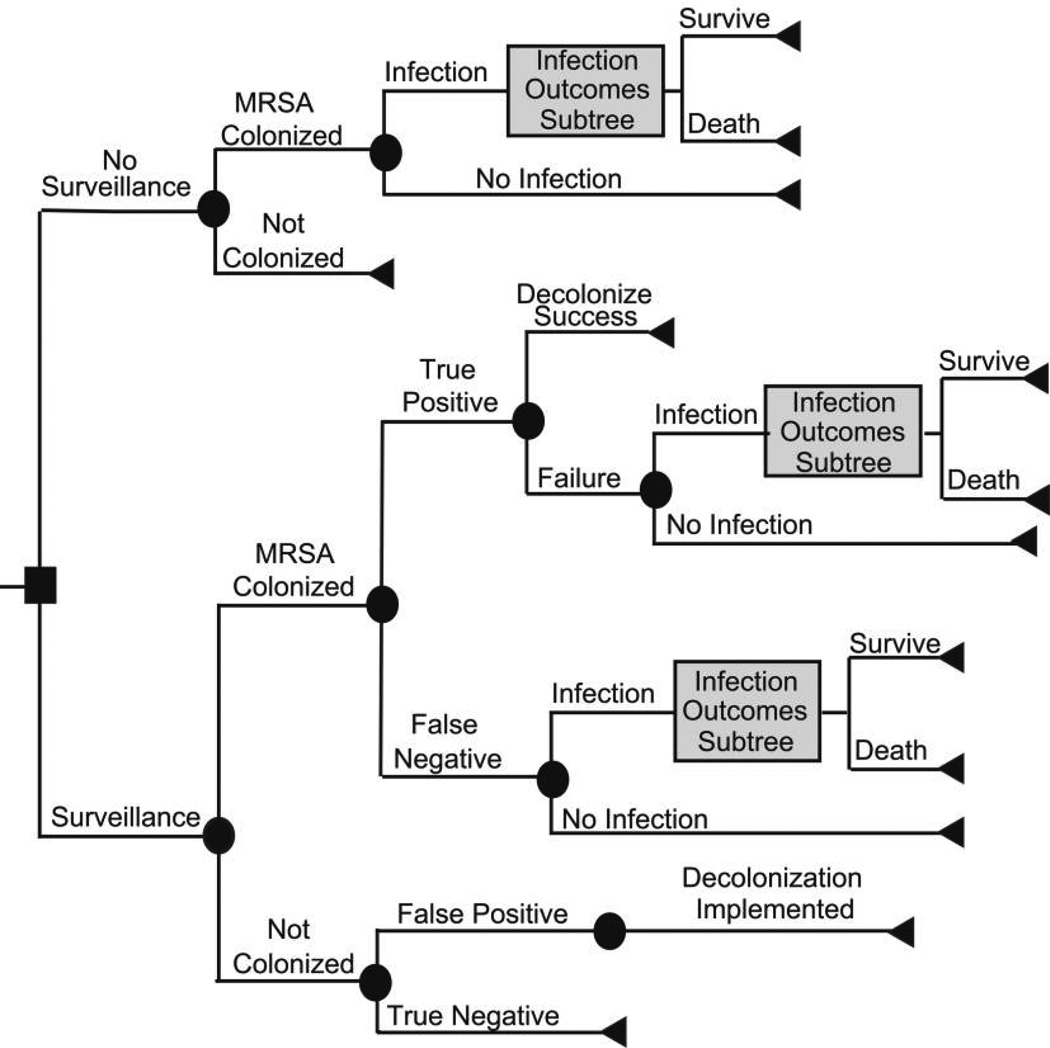
By means of the TreeAge Pro Suite 2008 (TreeAge Software), we constructed a cohort-based decision-analytic computer simulation model, evaluated through first- and second-order Monte Carlo techniques, that simulated the decision of whether to perform MRSA testing (culture of a single anterior nares sample) before all vascular surgeries; that is, the comparator was no MRSA testing before vascular surgery. Figure 1 shows the overall structure of the model. Each patient who enters the model is about to undergo a vascular surgery procedure and has a probability of being MRSA colonized that is based on local MRSA prevalence. The test result depends on whether the patient is MRSA colonized and on the specificity and sensitivity of the test. Patients who have positive test results (regardless of whether the patient is actually MRSA colonized) undergo a MRSA decolonization regimen. By contrast, patients who have negative test results (regardless of their true MRSA colonization status) do not receive MRSA decolonization. No decolonization is attempted for patients who are not tested. The decolonization regimen has a probability of success. Patients who are successfully decolonized cannot develop a MRSA infection. Patients not successfully decolonized have a probability of developing a MRSA infection.
FIGURE 1.
Structure of the surveillance decision model.
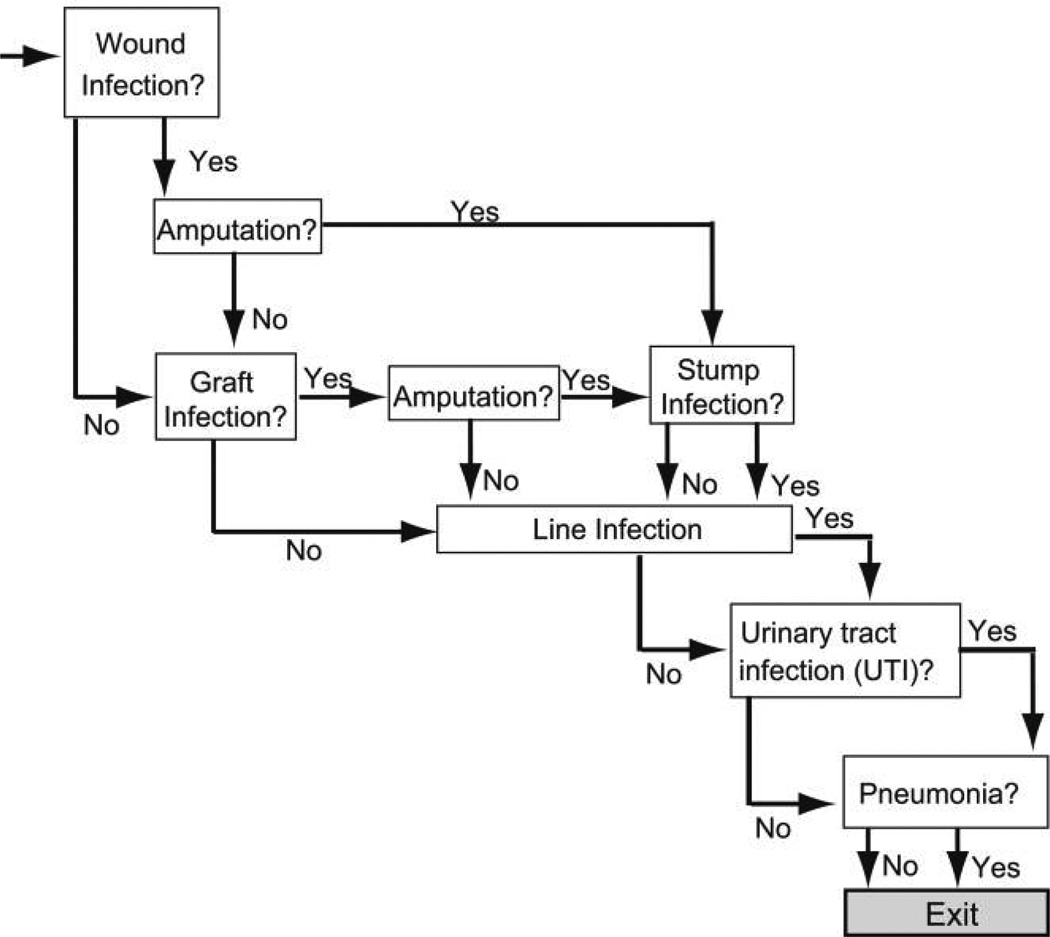
Patients who develop a MRSA infection then enter the MRSA infection outcome subtree (Figure 2). Each patient who enters this subtree has probabilities of developing any combination of the following syndromes: wound infection, graft infection, line infection, urinary tract infection, and pneumonia. Patients who develop either a wound infection or a graft infection have probabilities of requiring amputation. Those who undergo amputation have a probability of developing a stump infection. Every patient who enters the subtree has a chance of surviving or not surviving.
FIGURE 2.
Structure of the infection outcomes subtree.
Data Inputs
Table 1 shows the probability, cost, utility, and time input variables for our model and the distribution parameters and sources for each. We used β distributions for all probabilities and γ distributions for all cost variables. Hospitalization cost distributions came from the Healthcare Cost and Utilization Project National Inpatient Sample. All durations of antibiotics administration assumed a uniform distribution.
TABLE 1.
Data Inputs for Variables in Our Model
| Variable | Type of distribution |
Mea | Standard deviation |
Rangea |
|---|---|---|---|---|
| Cost, US$ | ||||
| Surveillanceb | γ | 9.52 | 1.62 | … |
| Decolonizationb | γ | 103.95 | 17.10 | … |
| Vancomycin, per day12 | γ | 9.01 | 5.00 | … |
| Procedures | ||||
| Graft replacement13 | γ | 1224.46 | 197.00 | … |
| Amputation13 | γ | 864.88 | 137.00 | … |
| Surgical revision of stump infection13 | γ | 625.03 | 93.00 | … |
| IV replacement14 | γ | 141.99 | 23.00 | … |
| Chest x-ray13 | γ | 42.36 | 4.5 | … |
| Hospitalization cost | ||||
| Wound infection13 | γ | 4,683 | 1,767 | … |
| Graft infection13 | γ | 12,958 | 365 | … |
| Amputation13 | γ | 11,922 | 4,061 | … |
| Infected stump13 | γ | 7,789 | 647 | … |
| Line infection14 | γ | 24,581 | 17,394 | … |
| Urinary tract infection13 | γ | 5,050 | 129 | … |
| Pneumonia13 | γ | 13,047 | 478 | … |
| Utilities, QALYs | ||||
| Wound infection15 | … | 0.642 | … | … |
| Graft infectionb | … | 0.53 | … | … |
| Amputation16 | … | 0.44 | … | … |
| Stump infection17 | … | 0.3 | … | … |
| Line infection18 | … | 0.642 | … | … |
| Urinary tract infection16 | … | 0.73 | … | … |
| Pneumonia16 | … | 0.58 | … | … |
| Probabilities | ||||
| Surveillance: true positive19 | β | 0.7985 | 0.0196 | … |
| Surveillance: false positive19 | β | 0.0415 | 0.0044 | … |
| Given MRSA colonization: | ||||
| MRSA infection11 | β | 0.2990 | 0.1233 | … |
| Given MRSA infection: | ||||
| Wound infection2,4–6,9,10,20 | β | 0.6071 | 0.1156 | … |
| Graft infection2–4 | β | 0.2133 | 0.4011 | … |
| Line infection5 | β | 0.0239 | 0.0015 | … |
| Urinary tract infection4,5 | β | 0.0833 | 0.0168 | … |
| Pneumonia2,5,6,9,10 | β | 0.1667 | 0.0738 | … |
| Given wound infection: | ||||
| Amputation5,7,9 | β | 0.4048 | 0.2495 | … |
| Given graft infection: | ||||
| Amputation1–3 | β | 0.6087 | 0.3059 | … |
| Given amputation: | ||||
| Stump infection8,20 | β | 0.5667 | 0.0471 | … |
| Mortality rate | ||||
| Wound infection4 | β | 0.5295 | 0.0050 | … |
| Graft infection1,2 | β | 0.3611 | 0.1964 | … |
| Line infectionb | β | 0.1005 | 0.0937 | … |
| Pneumonia2,9 | β | 0.1875 | 0.0884 | … |
| Urinary tract infectionb | β | 0.0498 | 0.0218 | … |
| Duration, days | ||||
| Antibiotic administration | ||||
| Wound infectionb | Uniform | … | … | 10–14 |
| Graft infectionb | Uniform | … | … | 28–42 |
| Amputationb | Uniform | … | … | 10–14 |
| Stump infectionb | Uniform | … | … | 10–14 |
| Line infectionb | Uniform | … | … | 14–28 |
| Urinary tract infectionb | Uniform | … | … | 10–14 |
| Pneumoniab | Uniform | … | … | 10–14 |
| Length of stayc | ||||
| Wound infection13 | … | 3.0 | … | … |
| Graft infection13 | … | 7.0 | … | … |
| Amputation13 | … | 8.0 | … | … |
| Stump infection13 | … | 5.0 | … | … |
| Line infection21 | … | 6.0 | … | … |
| Urinary tract infection13 | … | 4.0 | … | … |
| Pneumonia13 | … | 9.0 | … | … |
NOTE. QALY, quality-adjusted life year.
Lower limit–upper limit for uniform distribution.
Source: expert consultation.
Length of stay is expressed as median, not mean.
To determine probabilities of different clinical outcomes, a PubMed search was conducted with the key words “vascular,” “vascular surgery,” “methicillin-resistant Staphylococcus aureus,” and “‘MRSA” and was limited to English-language articles published since 1999. We reviewed all abstracts from this search to determine their appropriateness for the model. We excluded case reports and case series, because denominators were needed to determine probabilities of different outcomes, and we included only studies that characterized the study populations (ie, clearly stated denominators) and that reported on the full set of clinical outcomes from that population (ie, properly defined the numerators). Model parameter distributions reflected the full distribution of values identified by the search.
Our model measured effectiveness in quality-adjusted life years (QALYs). Each clinical condition resulted in QALY decrements as listed in Table 1. The QALY decrement persisted during the expected duration of the clinical condition. To assess whether the surveillance strategy was cost-effective, the threshold used in our model was $50,000/QALY, which is a frequently cited criterion for cost-effectiveness analysis in the United States.22 According to nonparametric estimates by Shiroiwa et al23 in 2009, the US cost-effectiveness thresholds were similar to previously reported thresholds. The probability distributions of expected life expectancy came from the Human Mortality Database. Our model also assumed a 73-year-old median age of vascular surgery patients.11,24
Sensitivity Analyses
Sensitivity analyses examined the impact of varying the values of key variables in the model. We systematically varied the MRSA prevalence from 0.01 to 0.90 and the decolonization success rate from 0.25 to 1.00. To simulate the effect of using testing sites (eg, throat or axilla) in addition to the anterior nares, some scenarios looked at the effects of doubling the testing costs. Furthermore, for each simulation run, we conducted probabilistic sensitivity analyses that simultaneously varied all input parameters over the ranges listed in Table 1.
Opportunity Cost of Lost Bed-Days
An alternative approach reconducted our analyses by the Graves method to ascribe economic costs to hospital infections.25,26 The Graves method converts the increase in hospital length of stay (LOS) that is attributable to infection to economic cost by valuing the opportunity cost of lost bed-days. In other words, when a patient occupies a bed because of a MRSA infection, the hospital loses revenue because the bed could have been filled by another patient. We determined the additional LOS for each type of MRSA infection and the daily cost of a bed for a routine vascular surgery. Conducting a literature search of the effects of MRSA infection on LOS for vascular surgery patients helped verify our LOS estimates.
RESULTS
Overall Results
Each simulation run consisted of 1,000 trials of 1,000 patients. In other words, during each simulation run, 1,000,000 simulated patients traveled through the model. For each simulation run, we determined the incremental cost effectiveness ratio (ICER) of MRSA testing, defined as follows:
Table 2 shows how the ICER varies with MRSA prevalence and decolonization success rate. “Dominant” means testing dominates (ie, it is less costly and more effective than) no testing. For scenarios that use a culture of a single sample of the anterior nares, performing MRSA testing was the dominant strategy at MRSA prevalence ≥0.01 and decolonization success rate ≥0.5, and at MRSA prevalence ≥0.025 and decolonization success rate ≥0.25. Performing universal MRSA surveillance remained cost-effective (ie, ICER ≤$50,000/QALY) when MRSA prevalence was ≥0.01 and the decolonization success rate was ≥0.25.
TABLE 2.
Incremental Cost-Effectiveness Ratio of Performing Surveillance at Different MRSA Prevalence and Decolonization Success Rates
| MRSA prevalence | ||
|---|---|---|
| Decolonization success rate | 0.01 | 0.025 |
| Single-location surveillance | ||
| 0.25 | 1737 | D |
| 0.5 | D | D |
| 0.75 | D | D |
| 1 | D | D |
| 2-location surveillance | ||
| 0.25 | 7707 | 1789 |
| 0.5 | 2026 | D |
| 0.75 | 367 | D |
| 1 | D | D |
| Graves method | ||
| 0.25 | 2972 | D |
| 0.5 | D | D |
| 0.75 | D | D |
| 1 | D | D |
Note. D means surveillance is the dominant strategy. For MRSA prevalence (probability of colonization) of 0.1, 0.15, 0.2, 0.25, 0.3, 0.4, 0.5, 0.75, and 0.9, surveillance was the dominant strategy for all decolonization success rates (0.25, 0.5, 0.75, and 1), whether by single-location surveillance, 2-location surveillance, or the Graves method (opportunity cost of lost bed-days).
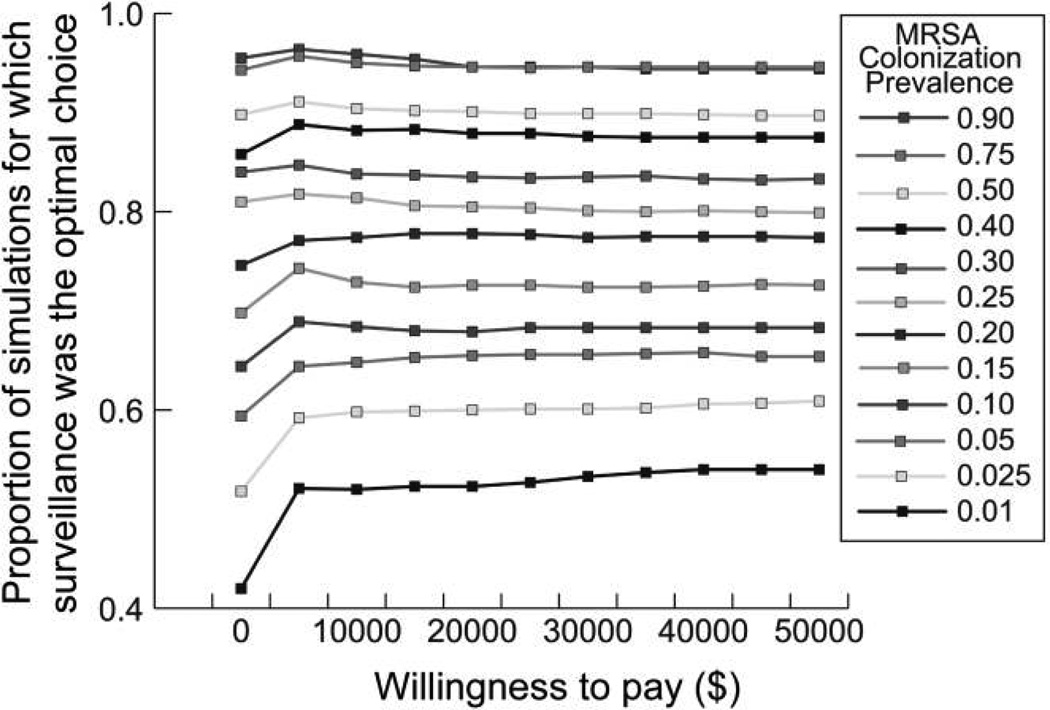
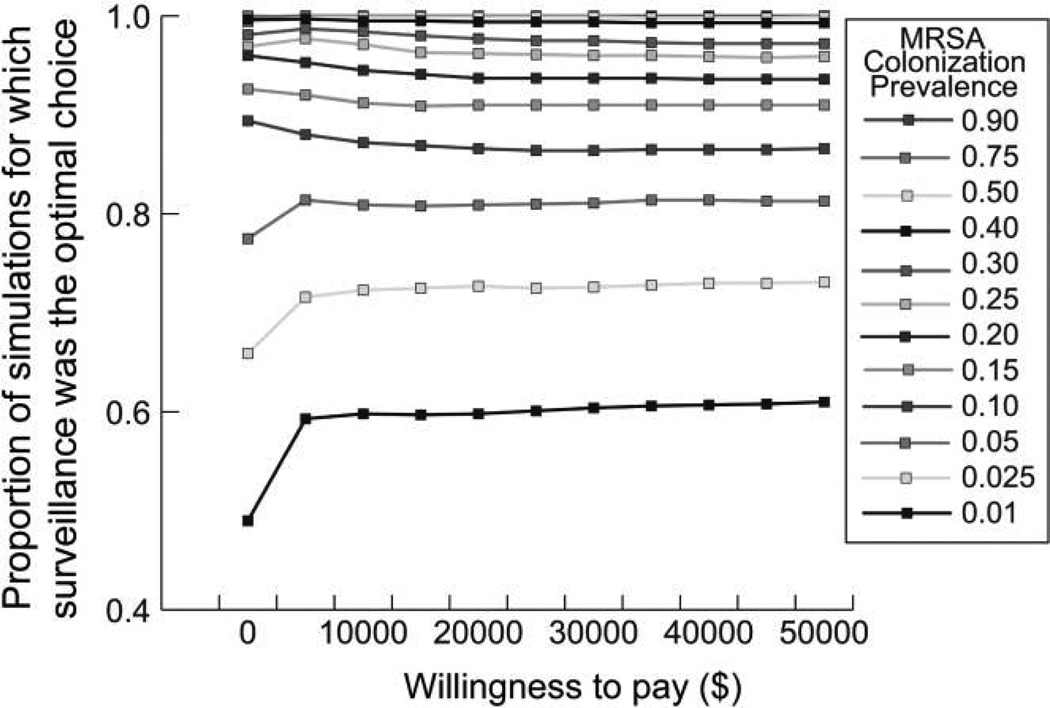
Figures 3 and 4 show the acceptability curves for different MRSA prevalence levels at decolonization success rates of 0.25 and 0.5, respectively. These curves display the proportion of patients in each 1,000,000-patient simulation for which testing was the more cost-effective choice (versus no testing) at different willingness-to-pay levels. The willingness-to-pay is the maximum amount a person would consent to pay, sacrifice, or exchange for a good (in our case, an additional QALY). There are no accepted criteria for willingness-to-pay thresholds: $50,000 per QALY gained is an often cited but controversial benchmark.23,27 However, in general, interventions that cost less than $20,000 per QALY gained are considered to have good evidence for adoption and those that cost more than $100,000 per QALY gained are considered to have poor evidence for adoption.28 For example, Figure 3 shows that when the decolonization success rate is 0.25 and MRSA prevalence is at least 0.025, testing is the better option (ie, less costly and more effective than no testing) for more than 50% of the simulated patients, even when the willingness to pay is barely greater than $0. Even when MRSA prevalence is 0.01, the decolonization success rate is 0.25, and the willingness to pay is $0, testing is the optimal choice in more than 40% of the simulated patients. MRSA testing becomes more cost-effective as MRSA prevalence and decolonization success rates increase.
FIGURE 3.
Acceptability curves for different MRSA prevalence rates at the decolonization success rate of 0.25.
FIGURE 4.
Acceptability curves for different MRSA prevalence rates at the decolonization success rate of 0.5.
The results displayed in Table 2 and Figures 3 and 4 are from scenarios in which the cost per patient of decolonization was $103.95 (standard deviation, $17.10). This implies that testing would remain the cost-effective choice for all decolonization costs less expensive than the cost distribution that we used. (We deliberately chose the higher end of decolonization cost estimates to conservatively estimate the value of MRSA testing.) Scenarios that looked at adding a testing site (eg, throat or axilla) to the anterior nares still found testing to be a dominant strategy in the conditions outlined in Table 2.
Opportunity Cost of Lost Bed-Days
Reconducting simulations by the Graves method yielded comparable results. Searching the literature for the effects of MRSA infection on hospital LOS in the vascular surgery population (with the key words “hospital-acquired infections,” “surgery,” and “length of stay”) uncovered only a handful of studies. A University of British Columbia retrospective study by Cowie et al6 determined that MRSA infection was associated with nearly 20 additional hospital days (24 days vs 5 days for the entire cohort). However, the small number of patients with MRSA infection (22 patients) did not allow full stratification for comorbidities. A prospective study by Taylor and Napolitano5 at the University of Maryland found a more conservative increase in mean hospital LOS for patients with MRSA infection: patients with MRSA infection had a mean LOS of 29.6 days (range, 2–174 days), which is nearly 7 days longer than the mean LOS of patients without MRSA infection (22.7 days) (P < .05). This was a larger study, and its prospective nature allowed for more extensive data collection. Attributing LOS differences in clinical studies to MRSA infection can be difficult. Other factors that coincide with MRSA infection, such as comorbidities that have been independently associated with an increase in LOS, may confound MRSA-attributable increases in LOS. Therefore, models may serve as complements to help separate these factors. In our model, MRSA infection resulted in a mean 5.9-day increase in LOS, which is more conservative than the numbers reported in the literature. Each lost bed-day corresponds to $2,079 in lost vascular surgery revenue. After we converted the costs by the Graves method, routine testing of vascular surgery patients remained a dominant strategy for the conditions outlined in Table 2.
DISCUSSION
Perioperative infection and postoperative infection are substantial problems. Infection can lead to substantial postoperative morbidity and mortality.1,3,5–9 MRSA is a leading cause of infection among vascular surgery patients and is associated with an increased risk of adverse clinical outcomes.1,3–5 Patients with vascular disease tend to be older and to have comorbidities that make them more susceptible to severe MRSA infection. Strategies to reduce the number of such infections could be extremely valuable but may be costly to implement. Therefore, there is a need for economic studies to determine the value of potential interventions, such as MRSA screening.
Our results suggest that testing patients for MRSA colonization before vascular surgery is cost-effective for a wide range of MRSA prevalence, decolonization cost, and decolonization success rates. In fact, our results indicate that this strategy quickly becomes economically dominant as MRSA prevalence and decolonization success rate increase. Economically dominant interventions not only are clinically effective but also are cost saving. This implies that covering the costs of MRSA testing could actually save third-party payers money.
There remains considerable debate about the effectiveness of various decolonization regimens. A number of different regimens exist. Long-term decolonization is harder to achieve than short-term decolonization, because many patients get recolonized when they return home or to other healthcare environments. For the purposes of avoiding perioperative or postoperative infection, decolonization needs to achieve only short-term success (enough time for the surgical wound to heal). Future studies may clarify the short-term success rates of different decolonization regimens. However, on the basis of our model, as long as decolonization is successful in 0.25 of attempts, implementing decolonization on patients with positive test results for MRSA would be cost-effective.
Although numbers may vary widely depending on a hospital’s location, local circumstances, and the decolonization regimen used, evidence suggests that decolonization success rates for the purpose of avoiding vascular surgery infection is likely at least 0.75 and that MRSA colonization prevalence is 1%–30.7% among surgical patients (ie, at least 1%).2,4,5,7,11 A systematic review of MRSA eradication trials showed the short-term (1 week) success rate of regimens that use topical mupirocin applied to the anterior nares to be .90.29 Under these conditions, routine MRSA testing would be a dominant strategy.
During the construction of the model, our intent was to remain conservative about the benefits of surveillance. We chose the higher end of decolonization costs, used less expensive potential procedures for each MRSA clinical condition, and did not include rare MRSA complications. Our model also did not consider how testing may prevent the spread of MRSA among patients. Identifying carriers may allow one to either decolonize or isolate a patient so that he or she will not transmit MRSA to other patients. Finally, we did not quantify the value of gaining information from surveillance (eg, MRSA colonization prevalence and infection incidence) that may help public health workers, hospital administrators, and researchers monitor MRSA spread and the effectiveness of interventions.
Rather than make decisions, computer models provide information to help individual surgeons, surgical unit administrators, hospital infection control personnel, and policy makers make decisions on the basis of their local circumstances. In the end, people, not computer models, make decisions. But models can help elucidate relationships and factors that are not readily apparent and can provide rough benchmarks. Decision makers can adapt model findings to the unique circumstances of their location and can use an appropriately tailored solution.
Limitations
All computer simulation models are simplifications of real life and cannot completely represent every possible event and outcome that may result from MRSA colonization or infection. We selected the more common clinical outcomes for which data was available. The data inputs for our model came from different studies of varying quality. Our model did not fully reflect the sociodemographic and clinical heterogeneity of vascular surgery patients. Our model also focused on MRSA, which has disproportionately higher morbidity and mortality, compared with the morbidity and mortality of methicillin-susceptible S. aureus.
A potential limitation of the strategy that uses a culture of a single anterior nares sample is that surveillance for nasal carriage of MRSA is not 100% sensitive. Estimates of the sensitivity of swabbing the nares as a single site range 69%–91%.30 Adding sites such as the throat, perineum, and axilla may improve the rate of detection of MRSA carriage and therefore the value of MRSA screening (additional runs that factored in increased testing costs did not alter the dominance of the MRSA testing strategy).31
Conclusions
Our model suggests that preoperative testing of vascular surgery patients for MRSA is cost-effective over a wide range of MRSA prevalence, decolonization cost, and decolonization success rates. Testing may identify patients at risk for postoperative MRSA infection and prevent such infection. The potential savings from preventing these infections may outweigh the costs of testing and decolonization. Individual surgeons, surgical unit administrators, hospital infection control personnel, and policy makers can compare their local circumstances with the assumptions and outlined thresholds of our model to decide whether to implement preoperative MRSA testing. Future studies can help delineate MRSA prevalence in different vascular surgery populations and with different decolonization success rates.
ACKNOWLEDGMENTS
Financial support. National Institute of General Medical Sciences Models of Infectious Disease Agent Study (MIDAS) (U01-GM070708-05).
Footnotes
Potential conflicts of interest. All authors report no conflicts of interest relevant to this study.
REFERENCES
- 1.Naylor AR, Hayes PD, Darke S. A prospective audit of complex wound and graft infection in Great Britain and Ireland: the emergence of MRSA. Eur J Vasc Endovasc Surg. 2001;21:289–294. doi: 10.1053/ejvs.2001.1311. [DOI] [PubMed] [Google Scholar]
- 2.Nasim A, Thompson MM, Naylor AR, Bell PR, London NJ. The impact of MRSA on vascular surgery. Eur J Vasc Endovasc Surg. 2001;22:211–214. doi: 10.1053/ejvs.2001.1429. [DOI] [PubMed] [Google Scholar]
- 3.Chalmers RT, Wolfe JH, Cheshire NJ, et al. Improved management of infrainguinal bypass graft infection with methicillin-resistant Staphylococcus aureus. Br J Surg. 1999;86:1433–1436. doi: 10.1046/j.1365-2168.1999.01267.x. [DOI] [PubMed] [Google Scholar]
- 4.Grimble SA, Magee TR, Galland RB. Methicillin-resistant Staphylococcus aureus in patients undergoing major amputation. Eur J Vasc Endovasc Surg. 2001;22:215–218. doi: 10.1053/ejvs.2001.1436. [DOI] [PubMed] [Google Scholar]
- 5.Taylor MD, Napolitano LM. Methicillin-resistant Staphylococcus aureus infections in vascular surgery: increasing prevalence. Surg Infect (Larchmt) 2004;5:180–187. doi: 10.1089/sur.2004.5.180. [DOI] [PubMed] [Google Scholar]
- 6.Cowie SE, Ma I, Lee SK, Smith RM, Hsiang YN. Nosocomial MRSA infection in vascular surgery patients: impact on patient outcome. Vasc Endovascular Surg. 2005;39:327–334. doi: 10.1177/153857440503900404. [DOI] [PubMed] [Google Scholar]
- 7.Nather A, Bee CS, Huak CY, et al. Epidemiology of diabetic foot problems and predictive factors for limb loss. J Diabetes Complications. 2008;22:77–82. doi: 10.1016/j.jdiacomp.2007.04.004. [DOI] [PubMed] [Google Scholar]
- 8.Richards T, Pittathankel AA, Pursell R, Magee TR, Galland RB. MRSA in lower limb amputation and the role of antibiotic prophylaxis. J Cardiovasc Surg (Torino) 2005;46:37–41. [PubMed] [Google Scholar]
- 9.Murphy GJ, Pararajasingam R, Nasim A, Dennis MJ, Sayers RD. Methicillin-resistant Staphylococcus aureus infection in vascular surgical patients. Ann R Coll Surg Engl. 2001;83:158–163. [PMC free article] [PubMed] [Google Scholar]
- 10.Thompson M. An audit demonstrating a reduction in MRSA infection in a specialised vascular unit resulting from a change in infection control protocol. Eur J Vasc Endovasc Surg. 2006;31:609–615. doi: 10.1016/j.ejvs.2005.12.009. [DOI] [PubMed] [Google Scholar]
- 11.Morange-Saussier V, Giraudeau B, van der Mee N, Lermusiaux P, Quentin R. Nasal carriage of methicillin-resistant Staphylococcus aureus in vascular surgery. Ann Vasc Surg. 2006;20:767–772. doi: 10.1007/s10016-006-9088-x. [DOI] [PubMed] [Google Scholar]
- 12.Bounthavong M, Hsu DI, Okamoto MP. Cost-effectiveness analysis of linezolid vs vancomycin in treating methicillin-resistant Staphylococcus aureus–complicated skin and soft-tissue infections using a decision analytic model. Int J Clin Pract. 2009;63:376–386. doi: 10.1111/j.1742-1241.2008.01958.x. [DOI] [PubMed] [Google Scholar]
- 13.Levit K (Thomson Reuters), Stranges E (Thomson Reuters), Ryan K (Thomson Reuters), Elixhauser A(AHRQ) Rockville, MD: Agency for Healthcare Research and Quality; 2008. [Accessed October 6, 1984]. HCUP Facts and Figures, 2006: Statistics on Hospital-Based Care in the United States. Available at: http://www.hcup-us.ahrq.gov/reports.jsp. [PubMed] [Google Scholar]
- 14.Shannon RP, Patel B, Cummins D, Shannon AH, Ganguli G, Lu Y. Economics of central line–associated bloodstream infections. Am J Med Qual. 2006;21(Suppl 6):7S–16S. doi: 10.1177/1062860606294631. [DOI] [PubMed] [Google Scholar]
- 15.Sackett DL, Torrance GW. The utility of different health states as perceived by the general public. J Chronic Dis. 1978;31:697–704. doi: 10.1016/0021-9681(78)90072-3. [DOI] [PubMed] [Google Scholar]
- 16.Gold MR, Franks P, McCoy KI, Fryback DG. Toward consistency in cost-utility analyses: using national measures to create condition-specific values. Med Care. 1998;36:778–792. doi: 10.1097/00005650-199806000-00002. [DOI] [PubMed] [Google Scholar]
- 17.Tengs TO, Wallace A. One thousand health-related quality-of-life estimates. Med Care. 2000;38:583–637. doi: 10.1097/00005650-200006000-00004. [DOI] [PubMed] [Google Scholar]
- 18.Selai C, Rosser R. Eliciting EuroQol descriptive data and utility scale values from inpatients: a feasibility study. Pharmacoeconomics. 1995;8:147–158. doi: 10.2165/00019053-199508020-00006. [DOI] [PubMed] [Google Scholar]
- 19.Reyes J, Hidalgo M, Díaz L, et al. Characterization of macrolide resistance in gram-positive cocci from Colombian hospitals: a countrywide surveillance. Int J Infect Dis. 2007;11:329–336. doi: 10.1016/j.ijid.2006.09.005. [DOI] [PubMed] [Google Scholar]
- 20.Cerveira JJ, Lal BK, Padberg FT, Jr, Pappas PJ, Hobson RW., 2nd Methicillin-resistant Staphylococcus aureus infection does not adversely affect clinical outcome of lower extremity amputations. Ann Vasc Surg. 2003;17:80–85. doi: 10.1007/s10016-001-0341-z. [DOI] [PubMed] [Google Scholar]
- 21.Zack J. Zeroing in on zero tolerance for central line–associated bacteremia. Am J Infect Control. 2008;36:S176.e1–S176.e 2. doi: 10.1016/j.ajic.2008.10.014. [DOI] [PubMed] [Google Scholar]
- 22.Bell C, Urbach D, Ray J, et al. Bias in published cost effectiveness studies: systematic review. BMJ. 2006;332:699–703. doi: 10.1136/bmj.38737.607558.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shiroiwa T, Sung YK, Fukuda T, Lang HC, Bae SC, Tsutani K. International survey on willingness-to-pay (WTP) for one additional QALY gained: what is the threshold of cost-effectiveness? Health Econ. 2009 doi: 10.1002/hec.1481. [epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 24.Wilmoth JR, Shkolnikov V. Human mortality database. [Accessed January 21, 2008]; Available at: http://www.humanmortality.de. [Google Scholar]
- 25.Graves N. Economics and preventing hospital-acquired infection. Emerg Infect Dis. 2004;10:561–566. doi: 10.3201/eid1004.020754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Graves N, Halton K, Lairson D. Economics and preventing hospital-acquired infection: broadening the perspective. Infect Control Hosp Epidemiol. 2007;28:178–184. doi: 10.1086/510787. [DOI] [PubMed] [Google Scholar]
- 27.Braithwaite RS, Meltzer DO, King JT, Jr, Leslie D, Roberts MS. What does the value of modern medicine say about the $50,000 per quality-adjusted life-year decision rule? Med Care. 2008;46:349–356. doi: 10.1097/MLR.0b013e31815c31a7. [DOI] [PubMed] [Google Scholar]
- 28.Laupacis A, Feeny D, Detsky AS, Tugwell PX. How attractive does a new technology have to be to warrant adoption and utilization? tentative guidelines for using clinical and economic evaluations. CMAJ. 1992;146:473–481. [PMC free article] [PubMed] [Google Scholar]
- 29.Ammerlaan HS, Kluytmans JA, Wertheim HF, Nouwen JL, Bonten MJ. Eradication of methicillin-resistant Staphylococcus aureus carriage: a systematic review. Clin Infect Dis. 2009;48:922–930. doi: 10.1086/597291. [DOI] [PubMed] [Google Scholar]
- 30.Lautenbach E, Nachamkin I, Hu B, et al. Surveillance cultures for detection of methicillin-resistant Staphylococcus aureus: diagnostic yield of anatomic sites and comparison of provider- and patient-collected samples. Infect Control Hosp Epidemiol. 2009;30:380–382. doi: 10.1086/596045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Marshall C, Spelman D. Re: is throat screening necessary to detect methicillin-resistant Staphylococcus aureus colonization in patients upon admission to an intensive care unit? J Clin Microbiol. 2007;45:3855. doi: 10.1128/JCM.01176-07. [DOI] [PMC free article] [PubMed] [Google Scholar]