Summary
TLR, expressed on the surface of mast cells, respond to a variety of bacterial and viral components to induce and enhance high affinity IgE receptor (FcεRI)-mediated cytokine production. Recent reports have indicated that specific TLR-dependent responses in macrophages and dendritic cells are regulated by the ITAM-containing molecule, DAP12. When phosphorylated, DAP12 recruits Syk; a critical molecule for mast cell activation. We therefore examined whether DAP12 similarly regulates TLR-mediated responses in mast cells. DAP12 was confirmed to be expressed in both human and mouse mast cells and, upon phosphorylation, to recruit Syk. However, although TLR agonists induced cytokine production, and synergistically enhanced FcεRI-mediated cytokine production, surprisingly, they failed to increase DAP12 phosphorylation in mouse bone marrow-derived mast cells (BMMC). Furthermore, normal TLR-mediated responses were observed in DAP12−/− BMMC. However, DAP12 phosphorylation and subsequent Syk recruitment was observed in BMMC following Con A-induced aggregation of mannose-glycosylated receptors, and these responses, together with Con A-induced degranulation, were substantially reduced in the DAP12−/− BMMC. These data demonstrate that TLR have differential requirements for DAP12 for their function in different cell types and that the inability of TLR to influence mast cell degranulation may be linked to their inability to utilize DAP12 to recruit Syk.
Keywords: TLR, DAP12, Mast Cells, FcεRI, KIT, signal transduction
Introduction
Mast cells are widely recognized for their central role in initiating allergic reactions through the release of a wide variety of inflammatory mediators following receptor-mediated activation. Such activation primarily occurs as a consequence of aggregation of the FcεRI following the binding of multivalent antigen to IgE bound to these receptors [1]. Mast cells, however, also play a role in innate immunity by releasing multiple cytokines through the recognition of specific molecular patterns on bacteria and viruses by TLR expressed on the mast cell surface [2]. Signaling events initiated upon TLR activation, at least through TLR2 and TLR4, have been shown to interact with those initiated following FcεRI aggregation to markedly enhance cytokine production [3]. Such synergistic activation has also been observed when other receptors expressed on mast cells are ligated concurrently with FcεRI aggregation [4]; the most notable example of which is the marked enhancement of FcεRI-mediated degranulation and cytokine production observed when FcεRI aggregation is induced in the presence of SCF-mediated ligation and activation of KIT. Unlike with FcεRI-mediated signaling, however, TLR-induced signaling events do not induce degranulation nor do they enhance the FcεRI-mediated degranulation response [3]
The ability of cell surface receptors to activate the intracellular signaling pathways that are necessary for mediator release relies upon the recruitment of molecular components of these pathways to the plasma membrane and subsequent formation of macromolecular receptor-signaling complexes [5]. These events are regulated by both inducible and constitutive protein-protein and protein-lipid interactions. Inducible protein-protein interactions required for FcεRI-dependent and KIT-enhanced mast cell activation are primarily regulated by tyrosine kinase-dependent phosphorylation of binding motifs contained within receptor subunits and associating transmembrane and cytosolic adaptor molecules [6]. Due to their potential to create various combinations of protein signaling complexes, transmembrane adaptor proteins play an important role in the integration of signals from various receptors [7]. In mast cells, at least two transmembrane adaptor proteins appear to function as molecular scaffolds for signaling proteins following FcεRI aggregation; LAT and LAT2 (NTAL/LAB) [8].
Recent studies have revealed that TLR-mediated responses in various cells of hematopoietic lineage are alternatively regulated by DAP12, a small (~12 kDa) transmembrane protein which possesses a single ITAM motif. As for LAT2, DAP12 is reported to both positively and negatively regulate such responses [9]. In a similar manner to the signaling of the FcεRIγ chains, DAP12, once phosphorylated by Src tyrosine kinase family members [10], has the capacity to recruit Syk; a critical tyrosine kinase for mast cell activation. This occurs through binding of the SH2 domains of Syk to the phosphorylated ITAM of DAP12 [11]. Collectively, these data suggest that DAP12 would be expected to contribute to the integration of the signaling events required for the synergistic enhancement of mast cell mediator release following TLR engagement in the presence of FcεRI aggregation. We thus investigated the role of DAP12 in these responses in mast cells derived from bone marrow of wild type and DAP12-deficient mice.
As will be shown, phosphorylation of DAP12 in mast cells leads to the recruitment of Syk. Thus, DAP12 should have capacity to transduce the signals required for receptor-mediated mast cell activation. However, unlike the documented involvement of DAP12 in TLR-mediated responses in other cells of hematopoietic lineage, the ability of TLR agonists to enhance FcεRI-mediated mast cell activation is not affected by the absence of DAP12. Nevertheless, DAP12 becomes tyrosine phosphorylated and subsequently recruits Syk following activation of receptors with mannose-containing motifs and this response is critical for optimal degranulation induced by these receptors. These data illustrate that involvement of DAP12 in TLR-mediated responses may be dictated by the cell types in which they are expressed and furthermore suggest an explanation for the inability of TLR to induce degranulation of mast cells.
Results
Expression of DAP12 in mouse and human mast cells
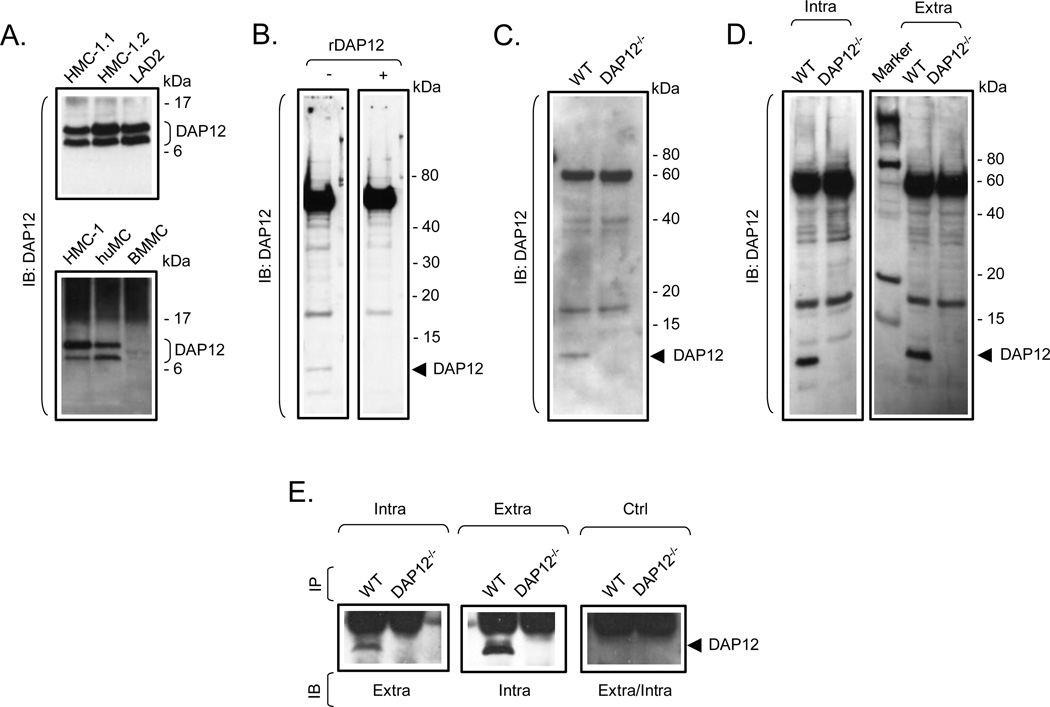
To explore the requirement for DAP12 in TLR-mediated mast cell activation, we first confirmed the expression of DAP12 in BMMC, and determined whether DAP12 was similarly expressed in human mast cells (huMC) and in human mast cell lines (HMC-1.1, HMC-1.2 and LAD2). As shown in Fig. 1A, a protein of the appropriate molecular weight ~12 kDa was strongly detected in mast cells of human origin. However, despite the expression of DAP12 mRNA in BMMC being confirmed by quantitative RT-PCR (data not shown), the commercially available antibodies only weakly detected DAP12 in mouse BMMC, although the antibodies are targeted towards DAP12 of both human and mouse origin. Regardless, where the antibodies detected the ~12 kDa protein, detection of the protein was abrogated when the antibodies were pre-treated with a diluted lysate of bacteria expressing GST-mouse DAP12 recombinant fusion protein (Fig. 1B) or when DAP12−/− BMMC were analyzed (Fig. 1C). In order to obtain antibodies with better performance for molecular and activation studies in mouse BMMC, we prepared DAP12-specific polyclonal antibodies by immunizing rabbits with keyhole limpet hemocyanin conjugated to peptides with sequences corresponding to a presumed extracellular or intracellular peptide fragment of mouse DAP12. As shown in Fig. 1D, these antibodies strongly recognized the ~12 kDa protein in wild type, (WT, DAP12+/+), but not in DAP12−/−, BMMC. In addition, mutual cross-immunoprecipitation studies conducted with these antibodies confirmed recognition of the same ~12 kDa protein in WT BMMC but not in DAP12−/− BMMC (Fig. 1E). Due to their better performance, these antibodies were used for the further studies.
Fig. 1. Expression of DAP12 in mast cells of human and mouse origin.
A. Cell lysates prepared from HMC-1.1, HMC-1.2, LAD2, huMC and BMMC were analyzed by immunoblotting (IB) with a DAP12-specific antibody (Santa Cruz). B. Cell lysates from BMMC were analyzed by immonoblotting with mouse DAP12-recognizing antibody (Santa Cruz) pre-incubated, or not, with diluted bacterial lysate containing GST-DAP12 recombinant fusion protein (rDAP12). C. Cell lysates from WT and DAP12−/− BMMC were analyzed by immonoblotting with mouse DAP12-recognizing antibody (Santa Cruz). D. Cell lysates from WT and DAP12−/− BMMC were analyzed by immunoblotting with the DAP12-specific antibodies generated against the intracellular (Intra) or extracellular (Extra) sequences of mouse DAP12. E. Cell lysates from WT and DAP12−/− BMMC were prepared and DAP12 was immunoprecipitated with newly generated DAP12-specific antibodies recognizing intracellular (Intra) or extracellular (Extra) sequence of DAP12. Immunoprecipitation from cell lysates with non-immunized rabbit sera was used as a negative control (Ctrl). The immunoprecipitates were cross-analyzed by immunoblotting with these DAP12 antibodies. In A–E, positions of the immune-detected proteins and/or molecular weight standards in kDa are indicated on the right. The blots are representative from at least three performed.
DAP12 tyrosine phosphorylation and association with Syk
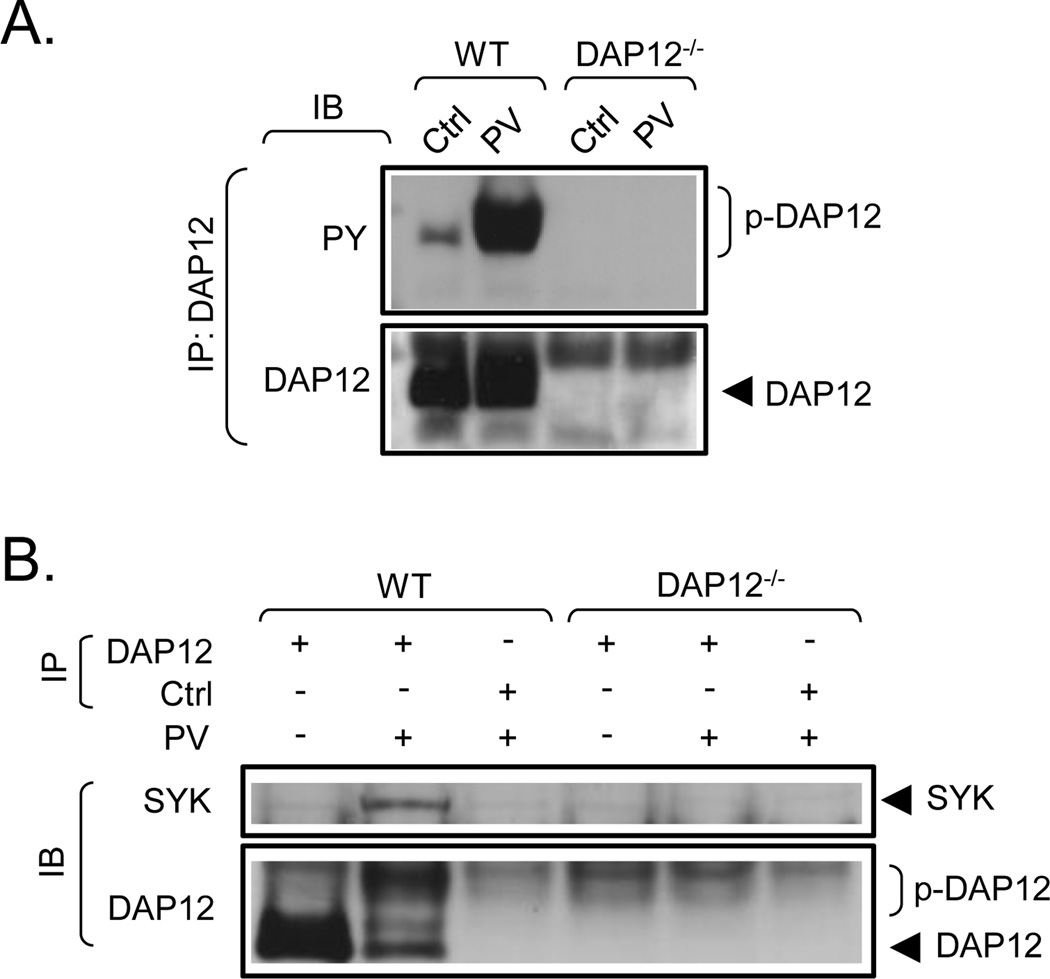
To determine whether DAP12 in mast cells could be phosphorylated and subsequently recruit Syk, we adopted an approach to ensure maximal possible phosphorylation. In resting cells, DAP12 is reported to be maintained in a dephosphorylated state by the action of the transmembrane protein tyrosine phosphatase CD45 [10]. We thus examined whether pervanadate, a non-specific phosphatase inhibitor [12], would enhance tyrosine phosphorylation of DAP12 and result in recruitment of Syk. In resting cells, there was slight, but still detectable, constitutive tyrosine phosphorylation of DAP12 (Fig. 2A). Treatment of WT mast cells with pervanadate induced a robust tyrosine phosphorylation of DAP12. As expected, the above responses were not observed in the BMMC derived from DAP12−/− mice. Under resting conditons, where DAP12 was minimally phosphorylated, Syk association with DAP12 was not detectable in WT BMMC (Fig. 2B). However, where tyrosine phosphorylation of DAP12 was observed in response to pervanadate treatment, Syk was found to co-immunoprecipitate with DAP12 in the WT BMMC (Fig. 2B). Again, as expected, this association was not observed in the DAP12−/− BMMC.
Fig. 2. Tyrosine phosphorylation of DAP12 in pervanadate-stimulated cells and its association with Syk.
A. Non-sensitized WT and DAP12−/− BMMC were activated with vehicle alone (Ctrl) or with pervanadate (PV, 0.2 mM sodium orthovanadate/0.2 mM hydrogen peroxide). After cell lysis, DAP12 was immunoprecipitated (IP) with the DAP12-specific antibody generated against the intracellular peptide fragment of mouse DAP12 as shown in Fig. 1E (this antibody was also used in further immunoblotting (IB) and IP experiments). The immunoprecipitates were analyzed by immunoblotting with a phosphotyrosine-specific antibody (PY) and the amount of the immunoprecipitated DAP12 was determined by immunoblotting with the DAP12-specific antibody used for IP (DAP12). B. DAP12 was immunoprecipitated from the vehicle (Ctrl) or pervanadate (PV)-stimulated cells as in A and samples were analyzed by immunoblotting with a Syk-specific antibody (Syk) and the same DAP12-specific antibody used for IP (DAP12). In both panels, the positions of Syk, DAP12 and its phosphorylated form (p-DAP12) are shown on the right. The blots are representative from at least three performed.
Characterization of DAP12−/− BMMC
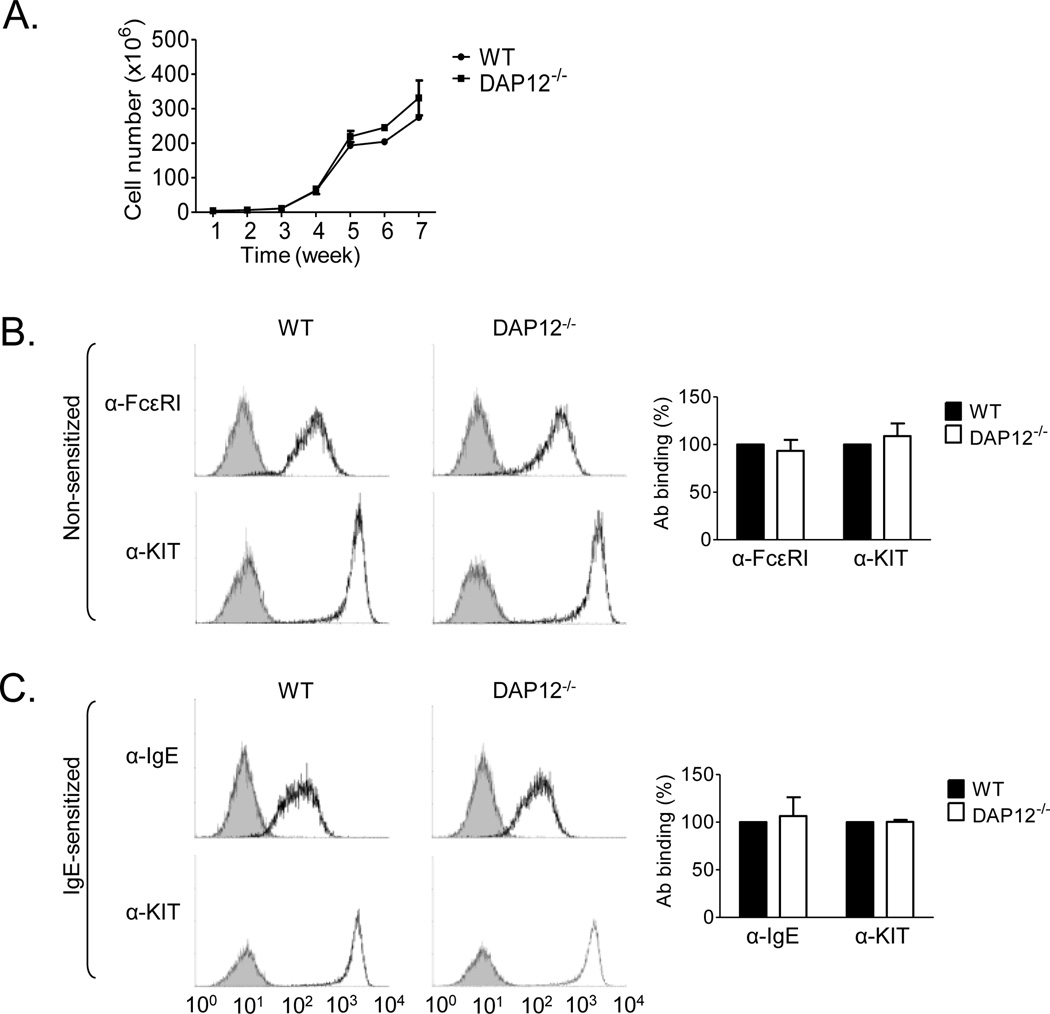
Having confirmed expression of DAP12 in both human and mouse mast cells, and its ability to recruit Syk following phosphorylation in WT mice, we investigated whether DAP12 regulated receptor-mediated mast cell mediator release. We first compared cell proliferation and surface expression of the two major mast cell receptors, FcεRI and KIT, in the WT and DAP12−/− cultures. As shown in Fig. 3A, the proliferative rate was comparable for WT and DAP12−/− BMMC. Similarly, 4–5 week old BMMC derived from WT and DAP12−/− mice also produced similar homogenous populations of cells with comparable expression of FcεRI and KIT. No differences in the expression of FcεRI and KIT in non-sensitized cells (Fig. 3B) or IgE binding and KIT expression in IgE-sensitized cells (Fig. 3C) were observed. In addition, there were also no gross differences in morphology between the 4–5 week old WT and DAP12−/− BMMC and no obvious differences in mast cell numbers or morphology within biopsies obtained from mouse ears and skin of WT and DAP12−/− mice (data not shown).
Fig. 3. Characterization of DAP12−/− BMMC.
A. BMMC from WT and DAP12−/− mice were cultured for times indicated and cell numbers were determined on a weekly basis. B–C. Flow cytometric analysis of FcεRI and KIT expression in 4-week old non-sensitized (B) or IgE-sensitized (C) WT or DAP12−/− BMMC using FcεRI-specific (α-FcεRI), IgE-specific (α-IgE) and KIT-specific (α-KIT) antibodies. The expression of the receptors in WT (black bars) and DAP12−/− (white bars) BMMC was normalized to that of WT BMMC. Data in A–C were evaluated from 3 independent isolates of BMMC (n=3) and are represented as means and S.D.
Examination of DAP12 phosphorylation in TLR- and in TLR/FcεRI-stimulated mast cells
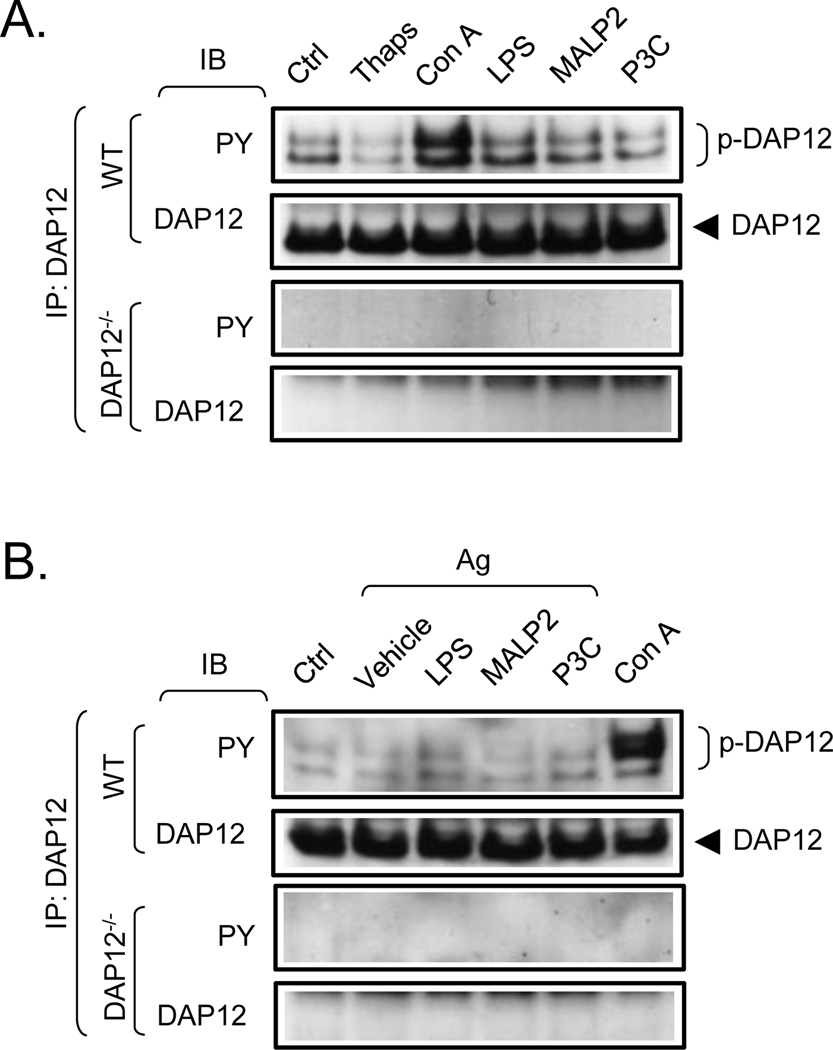
Multiple TLRs are expressed on mast cells [13] and it has been shown that TLR2 and TLR4 ligands interact synergistically with antigen to enhance cytokine production [3]. We thus investigated whether the ability of TLR to regulate cytokine production in mast cells was regulated by DAP12, as documented for TLR-mediated responses in other cells of hematopoietic origin [14–16], utilizing the WT and DAP12−/− BMMC. We initially examined whether challenge of BMMC with the TLR agonists, LPS (TLR4), macrophage-activating lipopeptide-2 (MALP2) (TLR2/6) and Pam3Cys-SKKKK (P3C) (TLR1/2), induces an increase in DAP12 phosphorylation. As a positive control, we included Con A (see later) in these studies. To determine if any observed DAP12 phosphorylation was regulated by an increase in cytosolic calcium levels, we also examined the ability of thapsigargin to induce DAP12 phosphorylation. In resting BMMC, we again detected constitutive phosphorylation of DAP12 (Fig. 4A). However, although Con A enhanced DAP12 phosphorylation, at both 10 µg/ml (Fig 4A) and 50 µg/ml (Fig 4B), thapsigargin and the three TLR agonists did not enhance DAP12 phosphorylation. Likewise, no discernable changes of DAP12 tyrosine phosphorylation were observed upon simultaneous engagement of TLR and FcεRI in IgE-sensitized mast cells despite the detection of a robust response to Con A (Fig. 4B). As expected, DAP12 phosphorylation was absent in the DAP12−/− BMMC. In addition to demonstrating that, in mast cells, TLR do not induce the phosphorylation of DAP12, these data also reveal that an increase in calcium levels is not sufficient to induce DAP12 phosphorylation (Fig 4A).
Fig. 4. Tyrosine phosphorylation of DAP12 in TLR-, thapsigargin-, and TLR/FcεRI- stimulated mast cells.
A. Non-sensitized WT and DAP12−/− BMMC were activated with vehicle alone (Ctrl), thapsigargin (Thaps, 2 µg/ml), Con A (10 µg/ml, positive control), LPS (200 ng/ml), MALP2 (100 ng/ml), or P3C (1 µg/ml). After cell lysis, DAP12 was immunoprecipitated (IP) from the lysates as in Fig. 2A and the immunoprecipitates were analyzed by immunoblotting (IB) with a phosphotyrosine-specific antibody (PY) and the DAP12-specific antibody used for IP (DAP12). B. IgE-sensitized WT and DAP12−/− BMMC were, or were not (Ctrl), simultaneously activated with IgE antigen (Ag, 20 ng/ml) in combination with vehicle alone (Vehicle), SCF (20 ng/ml) or LPS (200 ng/ml), MALP2 (100 ng/ml), or P3C (1 µg/ml). As a positive control, the IgE-stimulated cells were also stimulated with Con A (50 µg/ml).Tyrosine phosphorylation and DAP12 levels in these samples were subsequently analyzed as above in A. In A and B, positions of DAP12 and its tyrosine-phosphorylated forms (p-DAP12) are shown on the right. The blots are representative from at least three performed.
DAP12 is not required for TLR-and TLR/FcεRI-mediated signaling and cytokine production
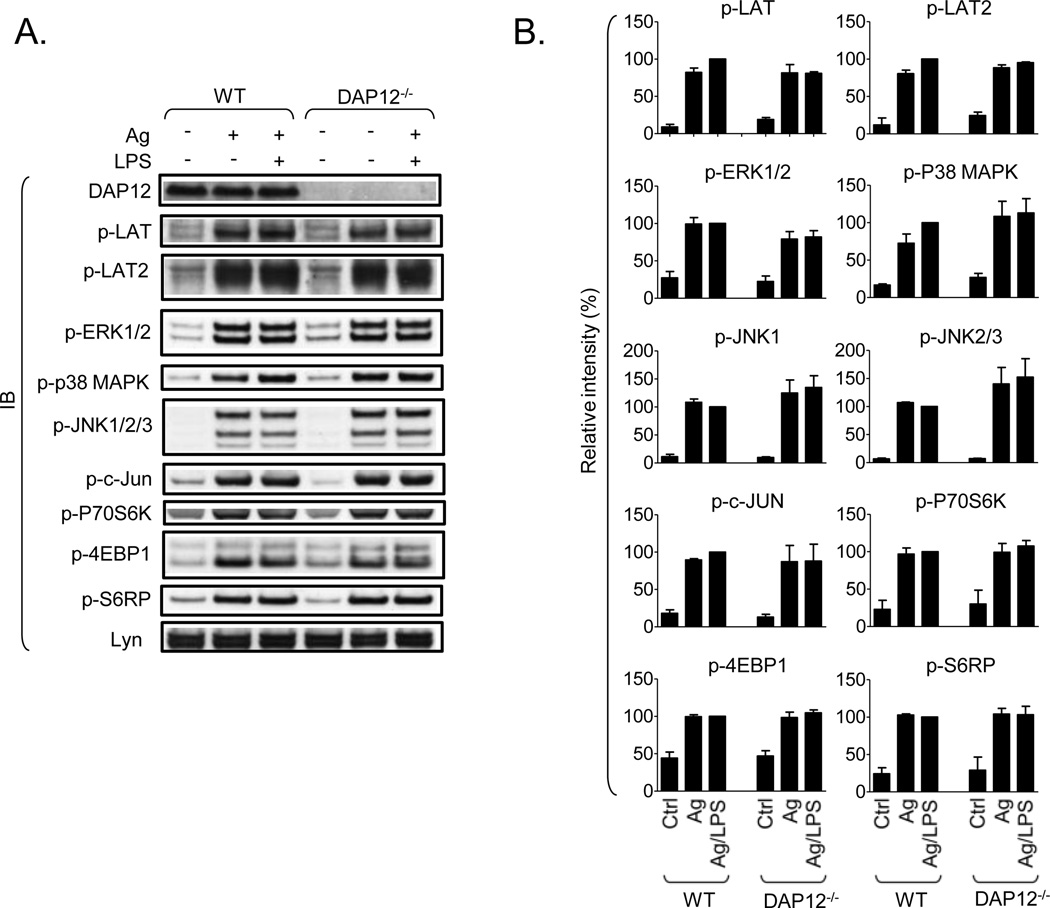
Although we could not detect an increase in DAP12 phosphorylation in cells challenged with the TLR agonists, the data obtained with pervanadate (Fig. 2) suggested that the constitutive DAP12 phosphorylation we observed in mast cells represents a balance between kinase and phosphatase activity. Thus, despite the lack of discernable Syk binding in the resting state (Fig. 2) the possibility existed that such association is below the sensitivity of the assay and that DAP12 is sufficiently phosphorylated in the resting state to contribute to TLR signaling. We therefore compared TLR/FcεRI-mediated signaling events and cytokine production in WT and DAP12−/− BMMC. As shown in Fig. 5A and B, FcεRI aggregation induced the phosphorylation of multiple critical signaling molecules including LAT, LAT2, ERK1/2, p38 MAPK, JNK, c-Jun, p70S6 kinase, 4E-binding protein 1 and S6 ribosomal protein required for degranulation and/or cytokine generation. However, we observed no enhanced phosphorylation of p38 MAPK, JNK or c-Jun when the cells were concurrently challenged with antigen and LPS (Fig. 5); which differs from the reported enhancement observed in MC/9 cell lines [3]. Regardless, there were also no differences in signaling events examined between the WT and DAP12−/− BMMC following challenge with FcεRI and/or LPS.
Fig. 5. DAP12 is not required for TLR/FcεRI-mediated signaling in mast cells.
A. IgE-sensitized WT and DAP12−/− BMMC were, or were not, activated with IgE/antigen (Ag, 20 ng/ml) alone or together with LPS (200 ng/ml) for 30 min and total cell lysates were prepared. The samples were analyzed by immunoblotting with the DAP12-specific antibody used in Fig. 2A (DAP12), Lyn-specific antibody (Lyn), and with the following phospho-specific antibodies: p-LAT (Tyr191; also recognizes tyrosine-phosphorylated LAT2), p-ERK1/2 (Thr202/Tyr204), p-p38 MAPK (Thr180/Tyr182), p-JNK1 and p-JNK2/3 (Thr183/Tyr185), p-c-JUN (Ser63), p-p70S6 kinase (Thr389) (p-P70S6K), p-4E-binding protein (Thr37/46) (p-4EBP1), p-S6 ribosomal protein (Ser240/244) (p-S6RP). B. The phosphorylation of proteins in A in the non-activated (Ctrl), antigen-activated (Ag) and antigen/LPS-activated samples was normalized to the amount of Lyn and the maximal response obtained in WT samples. Values from non-activated samples are indicated as Ctrl. In B, data from 3 independent BMMC isolates (n=3) were evaluated and are represented as means and S.D.
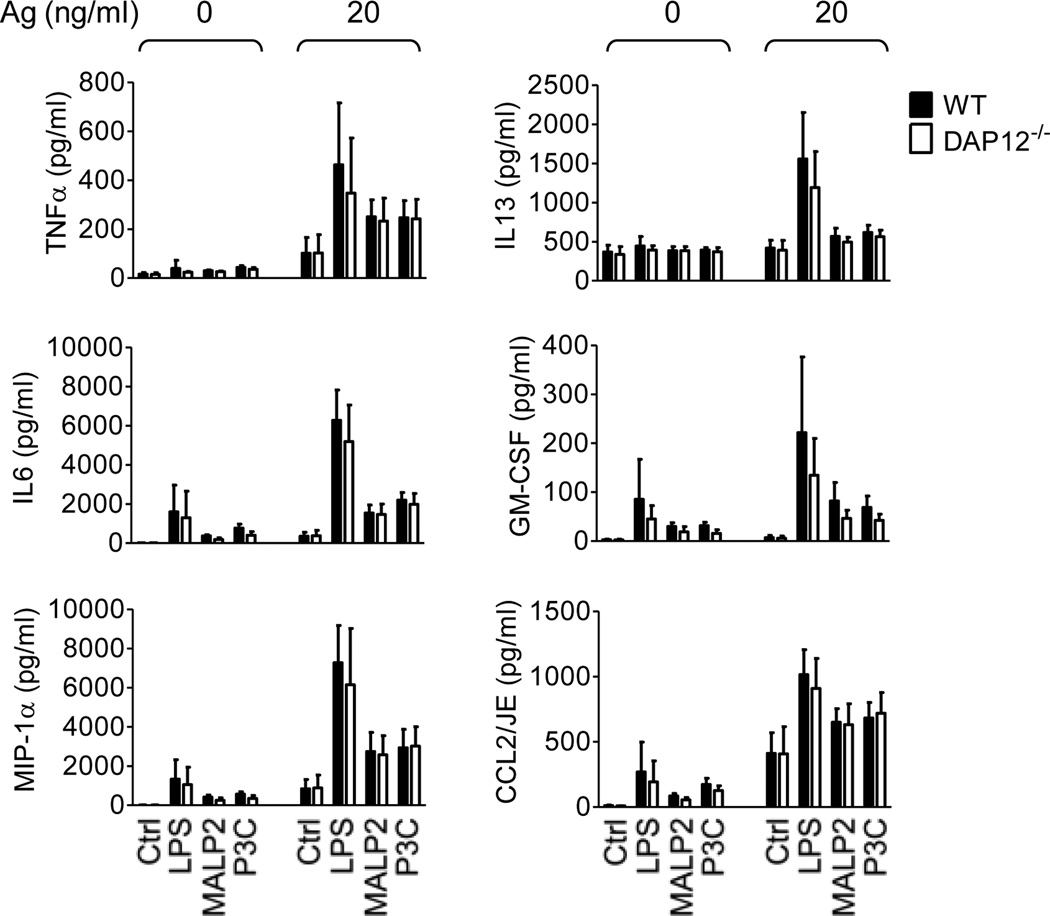
Next, we examined TLR/FcεRI-mediated cytokine production in WT and DAP12−/− BMMC. In agreement with the previous report [3], the TLR agonists markedly enhanced the ability of FcεRI to induce the production of multiple cytokines. Again, however, these responses were similar in both WT and DAP12−/− BMMC (Fig. 6). Taken together, these data demonstrate that, in contrast to what has been reported for macrophages and dendritic cells, DAP12 does not contribute to TLR-mediated responses in mast cells. It may be argued, however, that this difference may reflect reduced expression of DAP12 or TLRs in mast cells compared to the other cells types, or a lack of inducible phosphorylation of DAP12 by other receptors in mast cells. To investigate these possibilities, we compared the expression of DAP12 and TLR4 in WT and DAP12−/− mouse BMMC to WT and DAP12−/− macrophages. We then examined LPS/antigen-mediated cytokine production under conditions which induced DAP12 phosphorylation.
Fig. 6. DAP12 is not required for TLR/FcεRI-mediated cytokine production in mast cells.
IgE-sensitized WT (black bars) and DAP12−/− (white bars) BMMC were challenged with vehicle (Ctrl), LPS (200 ng/ml), MALP2 (100 ng/ml), or P3C (1 µg/ml) alone or together with IgE antigen (Ag, 20 ng/ml) for 6 h. The calculated concentrations (pg/ml) of cytokines released into the supernatants by 1.0×106/ml cell suspensions were determined. Data for LPS were evaluated from 5 independent BMMC isolates (n=5) performed in duplicates and data for MALP2 and P3C were evaluated from 2 independent BMMC isolates (n=2) performed in duplicates.
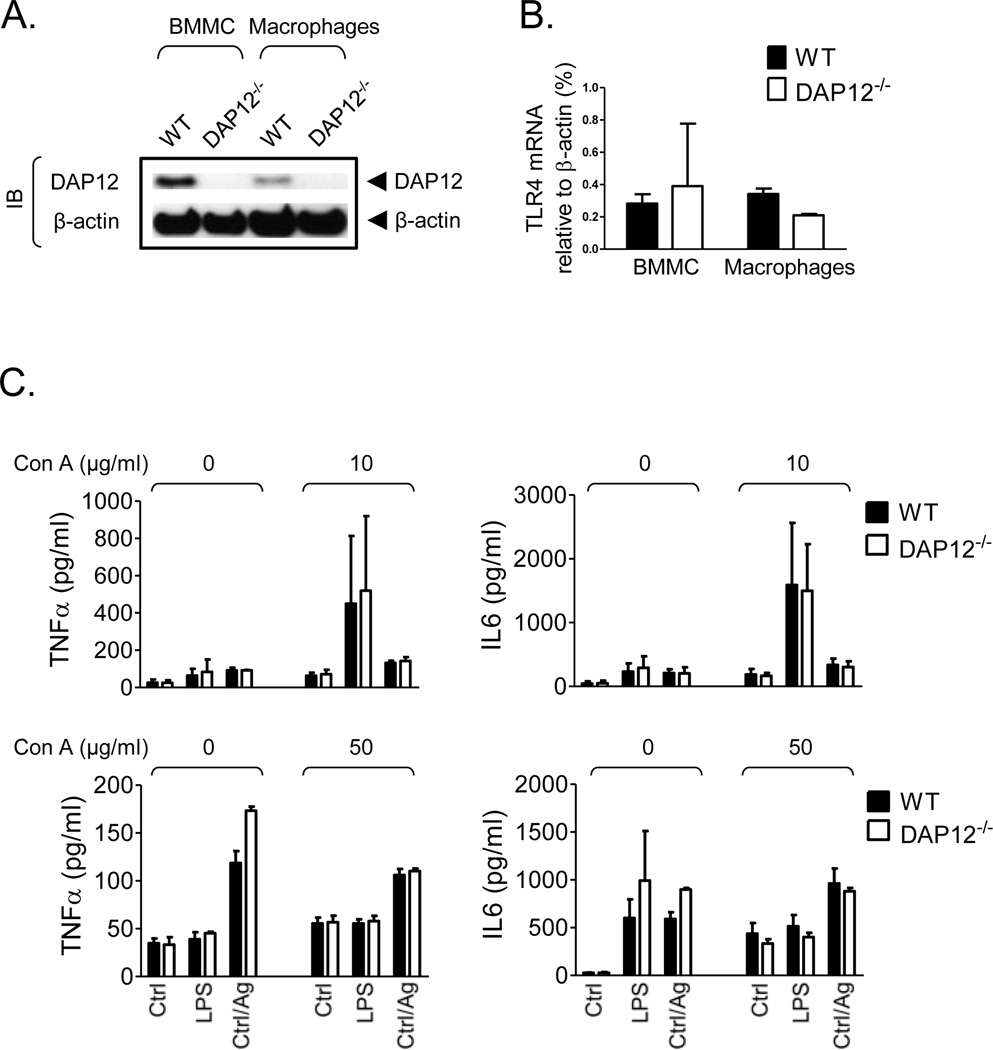
As shown in Fig. 7A, if anything, expression of DAP12 was higher in BMMC compared to macrophages. We found that the available antibodies did not convincingly recognize TLR4 in the mouse macrophages and mast cells, hence we examined TLR4 mRNA expression in both cell types by real-time PCR. Our data show that, as for DAP12, there was no evidence of reduced TLR4 expression in BMMC compared to macrophages (Fig. 7B). To determine whether a background of DAP12 phosphorylation would abrogate TLR-mediated responses, BMMC were challenged with LPS or antigen in the presence of Con A (10 and 50 µg/ml), which was demonstrated in Fig. 4A and 4B, respectively, to induce DAP12 phosphorylation. As shown in Fig. 7C, Con A failed to inhibit TLR- and FcεRI-mediated cytokine production whether alone or in combination. Although Con A (10 µg/ml) enhanced LPS-mediated cytokine production, this response was not dependent on DAP12 as it was not ablated in the DAP12−/− mast cells. These data, thus, further support our conclusion that DAP12 does not contribute to, or influence, TLR-mediated responses in mast cells.
Fig. 7. DAP12 and TLR4 expression in BMMC and peritoneal macrophages and TLR/FcεRI-mediated cytokine production in mast cells co-stimulated with Con A.
A. Cell lysates from WT and DAP12−/− BMMC or peritoneal macrophages were analyzed by immunoblotting with DAP12- and β-actin-specific antibodies. B. Expression of TLR4 mRNA in WT and DAP12−/− BMMC or peritoneal macrophages were analyzed by quantitative real-time PCR and normalized to β-actin mRNA. C. IgE-sensitized WT (black bars) and DAP12−/− (white bars) BMMC were challenged with vehicle (Ctrl), LPS (200 ng/ml), or IgE/antigen (Ag, 20 ng/ml) in the absence or presence of Con A (10 µg/ml or 50 µg/ml) for 6 h. The calculated concentrations (pg/ml) of cytokines released into the supernatants by 1.0×106/ml cell suspensions were determined. In A, positions of DAP12 and β-actin are shown on the right. Representatives of at least three separate extractions are shown. In B, data from 2 independent BMMC isolates (n=2) and 2 independent macrophage extractions (n=2), and in C, data from 3 independent BMMC isolates (2 littermate pairs and 1 non-littermate WT, n=2(3)) were evaluated. Data are represented as means and S.D.
DAP12 is not required for FcεRI- and KIT-mediated responses
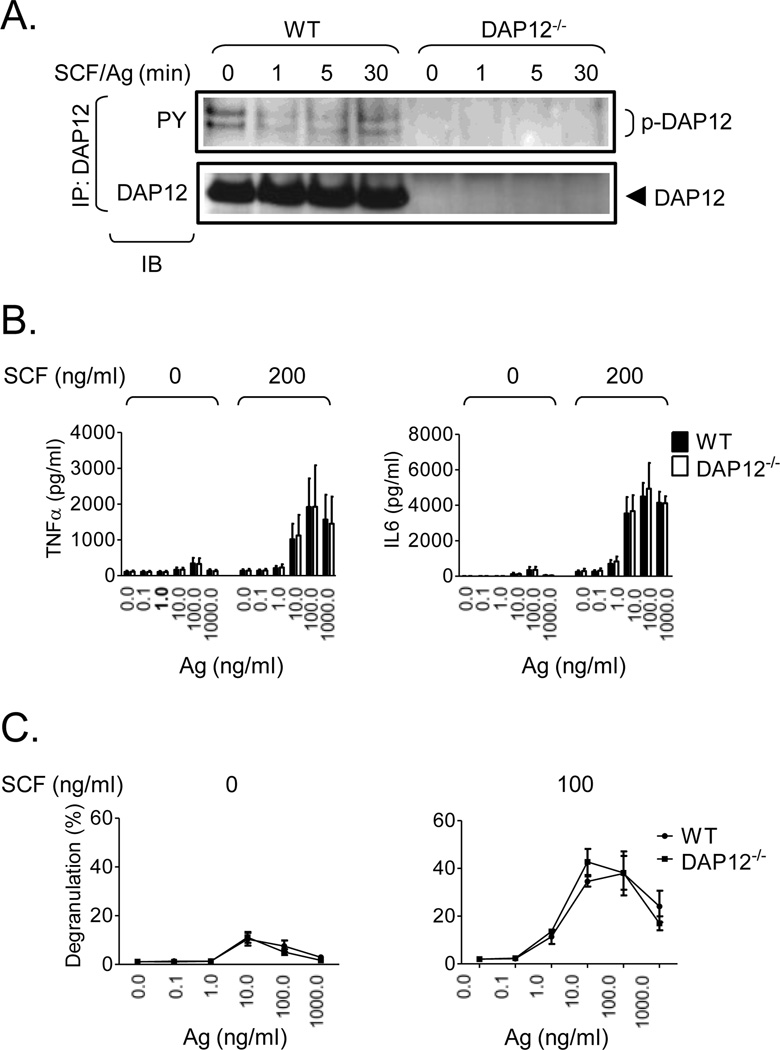
Antigen-mediated mast cell activation, a Syk-dependent process, can be dramatically enhanced by SCF. Thus, although DAP12 was not required for TLR-mediated responses and for FcεRI-mediated cytokine production in mast cells, the ability of phosphorylated DAP12 to recruit Syk suggested a potential role of DAP12 in antigen-mediated degranulation or the enhancement of this response by SCF. As with the TLR responses, however, we observed that SCF and antigen does not further enhance DAP12 phosphorylation (Fig. 8A). Furthermore, we also observed little difference in the phosphorylation of signaling molecules (data not shown), cytokine production (Fig. 8B) or degranulation (Fig. 8C) in the DAP12−/− BMMC compared to the WT cells. Thus, as for TLR/FcεRI integrated signaling events, KIT/FcεRI signaling does not require DAP12 to mediate critical signaling events.
Fig. 8. DAP12 phosphorylation and mediator release in KIT/FcεRI-stimulated WT and DAP12−/− BMMC cultures.
A. IgE-sensitized WT and DAP12−/− BMMC were simultaneously activated with mouse SCF and IgE antigen (Ag, 20 ng/ml) for the indicated time intervals. After cell lysis, DAP12 was immunoprecipitated (IP) from the lysates as in Fig. 2A and the immunoprecipitates were analyzed by immunoblotting (IB) with a phosphotyrosine-specific antibody (PY) and the DAP12-specific antibody used for IP (DAP12). The positions of DAP12 and its tyrosine-phoshorylated forms (p-DAP12) are shown on the right. B. IgE-sensitized WT (black bars) and DAP12−/− (white bars) BMMC were activated with different concentrations of IgE antigen (Ag) alone or together with mouse SCF (200 ng/ml). The calculated concentrations (pg/ml) of cytokines released into supernatants by 1.0×106/ml cell suspensions were determined. C. IgE-sensitized WT and DAP12−/− BMMC were simultaneously activated with indicated concentrations of SCF and IgE antigen (Ag) for 30 min and degranulation was determined. In A, representative experiments from at least three performed are shown. In B and C, data from 3 independent BMMC (n=3) isolates, in C performed in duplicates, were evaluated and represents means and S.D.
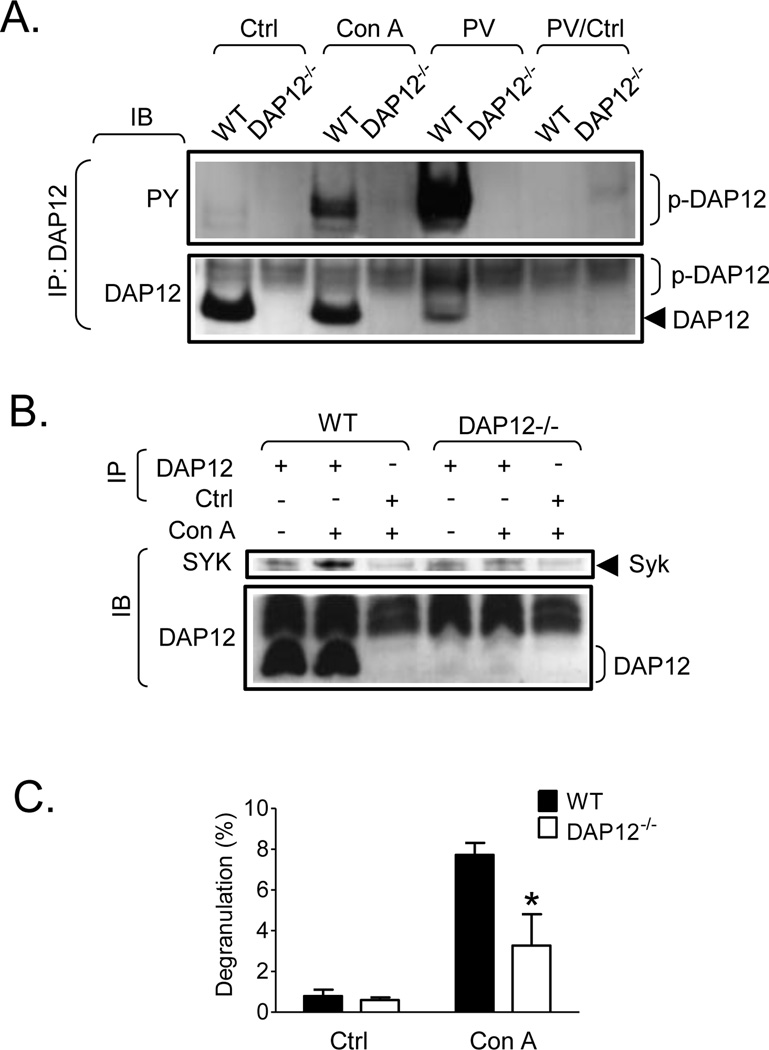
DAP12 is required for Con A-induced mast cell activation
To explore whether DAP12 might be required for signaling mediated by another receptor class expressed on mast cells, we again utilized Con A, which induces extensive aggregation of mannose-containing glycoconjugate receptors and is known to activate mast cells [17]. As can be seen in Fig. 9A, and as previously demonstrated in Fig. 4A and 4B, Con A induced DAP12 phosphorylation in WT BMMC. However, this response was lower than that observed with pervanadate treatment. Again, little DAP12 phosphorylation, and no Syk association (Fig. 9A and B), was observed under resting conditons. However, upon Con A challenge and DAP12 tyrosine phosphorylation, Syk was observed to co-immunoprecipitate with DAP12 in the WT BMMC (Fig. 9B). As expected, both DAP12 phosphorylation and Syk recruitment were not observed in the DAP12−/− BMMC. As shown in Fig. 9C, Con A induced degranulation in WT BMMC, but this was approximately half of that observed by antigen. Regardless, Con A-induced degranulation was markedly attenuated in DAP12−/− cells when compared to the WT responses. Taken together, these data demonstrate that DAP12 plays a role in mediator release induced by Con A but not TLR.
Fig. 9. Tyrosine phosphorylation of DAP12 in Con A-stimulated mast cells, its association with Syk, and its effect on Con A- induced mast cell degranulation.
A. Non-sensitized WT and DAP12−/− BMMC were activated with Con A (50 µg/ml), pervanadate (PV, 0.2 mM sodium orthovanadate/0.2 mM hydrogen peroxide, as a positive control) or vehicle alone (Ctrl). After cell lysis, DAP12 was immunoprecipitated (IP) from the lysates as in Fig. 2A and the immunoprecipitates were analyzed by immunoblotting (IB) with a phosphotyrosine-specific antibody (PY) and the DAP12-specific antibody used for IP (DAP12). As a negative control, immunoprecipitation from pervanadate activated cells with non-immunized rabbit sera was performed (PV/Ctrl). B. DAP12 was immunoprecipitated from resting or Con A-stimulated cells as in A. As a negative control, immunoprecipitation from Con A-stimulated cells with non-immunized rabbit sera was performed (Ctrl). The immunoprecipitated samples were analyzed by immunoblotting with a Syk-specific antibody (Syk) and the DAP12-specific antibody used for IP. C. Non-sensitized WT (black bars) and DAP12−/− BMMC were activated with vehicle alone (Ctrl) or Con A (10 µg/ml) for 30 min and degranulation was determined. In A and B, positions of Syk and DAP12 and its phosphorylated form (p-DAP12) are shown on the right. Representative experiments from at least three performed are shown. In C, data from 3 independent BMMC isolates (n=3) performed in duplicates were evaluated and represents means and S.D. Statistically significant differences between WT and DAP12−/− BMMC of the corresponding samples are indicated in DAP12−/− samples (*, p <0.05, unpaired two-tailed Student’s t test).
Discussion
In this study, we provide evidence which demonstrates that, unlike the recently described critical role for DAP12 in regulating signals for TLR in macrophages [15] as well as during TLR-induced systemic inflammatory responses [16], DAP12 does not regulate the ability of TLR1, TLR2, TLR4 and TLR6 agonists to potentiate FcεRI-mediated cytokine generation in mast cells. Furthermore, we show that DAP12 is not required for the ability of FcεRI or KIT to mediate mast cell activation. Nevertheless, we demonstrate that, in contrast to these receptors, mannose-containing plasma membrane glycoconjugates do require DAP12 to recruit the necessary signaling molecules for optimal downstream mast cell degranulation.
Although the signals elicited by the multiple classes of receptors that influence mast cell mediator release are diverse, a common theme for these processes is the necessity for recruitment of downstream signaling molecules to the receptor-signaling complexes via receptor subunits or adaptor molecules [6]. The documented role of DAP12 in TLR-mediated signaling in macrophages, in myeloid cells during systemic inflammatory responses, and the ability of phosphorylated DAP12 to recruit the critical mast cell signaling molecule Syk [11] initially led us to assume that DAP12 would contribute to the integration of the responses initiated by TLR2 and 4 and other receptors that result in synergistically enhanced mast cell cytokine production. This hypothesis was further strengthened by our confirmation that both human and mouse mast cells express DAP12 and our demonstration that phosphorylated DAP12 is able to recruit Syk (Fig. 1 and 2).
Despite recent reports having documented that DAP12 plays a regulatory role in TLR signaling, the results of these studies have been conflicting. While TLR-mediated inflammatory responses in DAP12-deficient mice were reported to be down-regulated, providing these mice with higher protection against septic shock induced by endotoxemia and cecal ligation and puncture [16], macrophages derived from DAP12-deficient mice have been shown, on the other hand, to be more sensitive to TLR agonists, leading to production of increased levels of pro-inflammatory cytokines [15]. In addition, a recent study demonstrated that DAP12-mediated negative regulation of specific TLR-mediated responses in bone marrow-derived dendritic cells is synergized by FcRIγ, an indispensable ITAM-containing subunit of FcεRI [14].
Certainly, our data on the synergistically enhanced production of cytokines in response to concurrent challenge of the cells with antigen and the TLR agonists LPS, MALP2 or P3C (Fig. 6) are in line with previous reports [3]. However, in contrast to the previous study, we were unable to detect enhancement of antigen-dependent phosphorylation of JNK, or indeed other MAP kinases (Fig. 5). The reasons for this disparity are not immediately apparent but may reflect the primary cultured BMMC used in the current study compared to the mouse mast cell line used in the previous study. Regardless, based on the lack of ability of the TLR agonists to enhance DAP12 phosphorylation (Fig. 4) and the lack of defective or enhanced signaling (Fig. 5), or altered cytokine production in the DAP12−/− BMMC (Fig. 6), we could conclude that, in mast cells, TLR signaling is independent of DAP12. In addition, the differences between the roles of DAP 12 and TLR signaling in macrophages and mast cells did not reflect defective expression of DAP12 or TLR4 in mast cells or lack of ability of other receptors to phosphorylate DAP12 (Fig. 7). Thus, these data imply that TLR have a differential requirement for DAP12 for the regulation of their actions depending on the cell type in which they are expressed. A number of adaptor molecules including MyD88 are documented to contribute to TLR signaling [18, 19]. Pathways, dependent on the MyD88, and pathways independent of this molecule are described for TLR signaling [20]. Certainly MyD88-dependent pathways do regulate TLR-mediated responses in mast cells [21, 22] and it may be that the combination of the utilization of MyD88 and other adaptor molecules in mast cells circumvents any potential requirement for DAP12 in the ability of TLR agonist to enhance FcεRI-mediated mast cell cytokine production. Of relevance may also be our observation that the ability of the MyD88-linked receptor for IL-33 to enhance FcεRI-mediated cytokine production is similarly unaltered by DAP12 deficiency in BMMC (S. Iwaki, M. A. Beaven, and A.M. Gilfillan, unpublished observations).
Both FcεRI and KIT require the initial activation of tyrosine kinases to promote downstream signaling events following receptor aggregation. In the case of the FcεRI, these events are regulated following activation of the Src family tyrosine kinases, Lyn and Fyn, whereas KIT possesses inherent tyrosine kinase activity [23]. Subsequent recruitment of Syk to the FcεRI signaling complex via interaction of its dual SH2 domains with phosphorylated FcεRIγ chain ITAMs and its subsequent activation is a critical step in antigen-mediated mast cell activation [24, 25]. The requirement of FcεRI for Syk for mediating degranulation and the inability of TLR to recruit Syk via DAP12 would provide an explanation for the documented inability of TLR to influence degranulation. However, despite the lack of influence of DAP12 on TLR-mediated responses in mast cells, the possibility existed that DAP12 may influence FcεRI-mediated mast cell activation or the potentiation of this response by KIT by either recruiting Syk to the receptor signaling complex or by competing for Syk binding. However, as for TLR signaling, the requirement of FcεRI and KIT to recruit necessary signaling molecules for cell activation was neither enhanced nor diminished in the DAP12−/− BMMC (Fig.8). Similarly, mast cell development from their bone marrow progenitors was not affected by a deficiency in DAP12 (Fig. 3). Thus, as with the TLR, the receptor subunits and associating transmembrane and cytosolic adaptor molecules negated a further requirement for DAP12 in recruiting critical signaling molecules for FcεRI- and KIT-mediated mast cell activation.
In contrast to the redundant role of DAP12 in mast cell responses mediated by TLR, FcεRI and KIT, our data do provide support for the conclusion that other receptors expressed on mast cells do require DAP12 for their function. This conclusion was supported by studies in which we demonstrated that Con A induced tyrosine phosphorylation of DAP12 and subsequent recruitment of Syk (Fig. 8), and that these responses, together with Con A-induced degranulation (Fig. 8) were reduced in DAP12−/− BMMC. Con A is recognized to aggregate mannose-glycosylated receptors in mast cells and thus induce degranulation [17]. The identity of the receptors eliciting these responses remains unknown, however, previous reports have documented that several of the glycosylated receptors expressed in a variety of cells of hematopoietic origin are associated with DAP12 [26–33]. Similarly, DAP12 has been shown to associate with the glycosylated surface receptors CD200R and leukocyte mono-IgE-like receptor 5/CLM-7 in mast cells. However, in mast cells, it is unclear whether ligation of these receptors induces DAP12 phosphorylation and promotes Syk recruitment. CD200R expression and thus the anaphylactic responses mediated by this receptor were reported to be absent in DAP12−/− mice [34] as were leukocyte mono-IgE-like receptor 5/CLM-7-mediated adhesion, degranulation and cytokine production. Thus it may be that these, or as yet unidentified glycosylated receptors, are responsible for the DAP12 phosphorylation and Syk recruitment observed in the BMMC triggered with Con A.
In summary, we have provided evidence that, unlike in macrophages, dendritic cells, or in vivo, TLR or indeed FcεRI or KIT circumvent the requirement for DAP12 to mediate their responses in mast cells. Regardless, certain glycosylated receptors require DAP12 to recruit Syk to induce downstream deganulation. These results illustrate that the role of DAP12 in TLR-mediated cellular responses may be dictated by the cell types in which these molecules are expressed and, furthermore, may provide an explanation as to why TLR fail to induce mast cell degranulation despite inducing cytokine production.
Materials and Methods
Animals and cells
C57BL/6J mice were obtained from The Jackson Laboratory, Bar Harbor, ME. DAP12-deficient mice were developed by Dr. Toshiyuki Takai, Tohoku University, Japan and backcrossed by Dr. Wayne Yokoyama, Washington University, St. Louis, MO, USA. The mice were originally generated on a 129s/v background then backcrossed onto C57BL/6J background. C57BL/6J and DAP12−/− were bred to obtain DAP12 heterozygote mice. The heterozygotes were then bred to obtain WT and DAP12−/− littermates which were subsequently used in the study. For genotyping, the following sets of primers were used: WT (5´-GATTAAGTCCCGTACAGGCC-3´, 5´-CTTCCGCTGTCCCTTGACC-3´), DAP12−/− (5´-CCCCTCTAGCTCTAGACTTC-3´, 5´-AGCCGATTGTCTGTTGTGCC-3´).
Mouse BMMC were developed from the bone marrow of littermates of the same sex as described [35]. BMMC of 4–6 weeks were used for experiments. HMC-1.1, HMC-1.2, LAD2 and primary human mast cells huMC cells were prepared as described [36]. All mouse studies were conducted under a protocol approved by the Institutional Animal Care and Use Committee at NIH and huMC were obtained under a protocol approved by the NIH IRB committee. Macrophages were obtained as described [37, 38]. Briefly, mouse bone marrow cells were plated onto tissue culture treated Petri dishes at 6 × 105 cells per ml in DMEM supplemented with 10% FBS, 5% Horse Serum, and 10 ng/ml M-CSF (Peprotech, Rocky Hill, NJ). On days 2 and 4, non-adherent cells were removed, and fresh M-CSF was added to the conditioned medium. On day 6, cells were harvested with ice cold PBS containing 1 mM EDTA. The macrophage phenotype of the cells was confirmed by flow cytometry using antibodies against F4/80 (Caltag, Carlsbad, CA), and Mac-1, GR-1, and MHC class II (BD Pharmingen, San Diego, CA).
Antibodies and reagents
The following phosphoprotein-specific antibodies were used: S6 ribosomal protein (Ser240/244), ERK1/2 (Thr202/Tyr204), p38 MAPK (Thr180/Tyr182), JNK1 and JNK2/3 (Thr183/Tyr185), c-Jun (Ser63), p70S6 kinase (Thr389), 4E-BP1 (Thr37/46) (Cell Signaling, Beverly, MA), KIT (Tyr721) (Invitrogen, Carlsbad, CA), LAT (Tyr191; also recognizes tyrosine-phosphorylated LAT2 [39] (Millipore Corporation, MA). Horseradish peroxidase-conjugated antibodies were phosphotyrosine-specific antibody PY-20 (BD Biosciences, Pharmingen, San Jose, CA), rabbit IgG-specific antibody (Amersham Biosciences, Piscataway, NJ), and mouse IgG Fc-specific antibody (Sigma-Aldrich, St. Louis, MO). The protein-specific antibodies labeled with fluorescent dyes were: CD117 (PE), rat IgG1 (PE) and mouse IgE (FITC) (BD Biosciences, Pharmingen), FcεRI (FITC) and Armenian hamster IgG (FITC) (eBioscience, San Diego, CA). The native protein-specific antibodies were as follows: DNP-specific IgE, β-actin (clone AC-15) (Sigma-Aldrich), Lyn (44) (Santa Cruz Biotechnology, Santa Cruz, CA), Syk [40]. Antibodies that are specific for the extracellular and intracellular domains of DAP12 were prepared by immunizing rabbits with keyhole limpet hemocyanin-conjugated to peptides of the following sequence: SDTFPRCDCSS, or CEGTRKQHIAETE, respectively (New England Peptide, Gardner, MA). Reagents used were: cell culture media (Invitrogen), cell culture growth factors (Pepro Tech, Rocky Hill, NJ), aprotinin (MP Biomedicals, Solon, OH), P3C and MALP2 (EMC microcollections GmbH, Tuebingen, Germany), LPS, Con A, DNP human serum albumin conjugate, sodium orthovanadate, sodium deoxycholate and all other chemicals were from Sigma-Aldrich. Pervanadate was prepared by a 20 min reaction of 10 mM sodium orthovanadate with hydrogen peroxide in water at room temperature and immediately used in experiments at the indicated concentration.
DNA constructs and recombinant proteins
To prepare recombinant protein of full length mouse DAP12, mouse DAP12 cDNA was amplified from a cloned mouse DAP12 cDNA (MGC Full-length, clone ID 6814478, Invitrogen) using the following pair of primers: 5´-CGTGGAATTCGGGGCTCTGGAGCCCTCCTG-3´, 5´-CGACGCGGCCGCATGACCGGATCCGGGCATCA-3´. The amplified fragment was cloned without the initial ATG codon into EcoRI and NotI cloning sites of the pGEX-4T-1 expression vector (Novagen, GE Healthcare, Life sciences) and the cloned fragment was verified by sequencing (Macrogen, Rockville, MD). Recombinant GST-DAP12 fusion protein was expressed at room temperature overnight in transfected bacteria TOP10 (Invitrogen) cultured in the presence of 0.2 mM isopropyl-β-D-thiogalactopyranoside. Bacteria were harvested, frozen and thawed, and resuspended in lysis buffer (50 mM Tris, pH 7.5, 50 mM NaCl, 2 mM EDTA, 0.05 % (w/v) sodium deoxycholate, 1 mg/ml lysozyme, 10 U/ml DNase, 1 mM PMSF, 10 µg/ml aprotinin). After 30 min, the bacterial lysate was extensively sonicated on ice. The insoluble material was separated by centrifugation at 20,000 × g for 10 min at 4 °C. The supernatant with recombinant GST-DAP12 fusion protein was used in experiments.
RNA isolation and RT-PCR
RNA was purified using RNeasy Mini Kit in combination with QIAshredder and RNase-Free DNase Set (Qiagen, Valencia, CA). For reverse transcription QuantiTect Reverse Transcription Kit was used (Qiagen, Valencia, CA). Gene expression was analyzed by ABI PRISM 7500 SDS system (Applied Biosystems, Foster City, CA) using QuantiTect SYBR Green PCR Kit in combination with gene-specific QuantiTect Primer Assays (Qiagen, Valencia, CA). All reactions were performed in triplicates for 40 cycles and annealing temperature 60 °C.
Cell activation, degranulation, and cytokine release
BMMC were sensitized overnight with DNP-specific IgE (100 ng/ml) in IL-3-free RPMI medium. For immunoprecipitation, immunoblotting and degranulation studies, cells were harvested and washed with buffered saline solution [BSS; 10 mM HEPES (N-2-hydroxyethylpiperazine-N'-2-ethanesulfonic acid), pH 7.4, 137 mM NaCl, 2.7 mM KCl, 0.4 mM Na2HPO4, 1.3 mM MgCl2, 1.8 mM CaCl2, 5.6 mM glucose, 0.05 % (w/v) BSA]. The cells were activated, for the indicated periods, at 37 °C by adding equal amount of twice-concentrated activators in BSS. Degranulation was determined by detection of β-hexosaminidase release as described [41] and data were calculated as a percentage of the intracellular β-hexosaminidase released into supernatant. For cytokine studies, the cells were washed with IL-3-free RPMI medium and then activated at 37 °C by adding equal amount of twice-concentrated activators in IL-3-free RPMI medium. The cells were subsequently cultured at 37 °C in the presence of 5 % (v/v) CO2. The cells in suspension (400 µl containing 0.4 × 106 cells) were activated for 6 h in wells of a 48-well plate, then separated by centrifugation and the supernatant collected. The content of cytokines released into the supernatants was determined by Quantikine ELISA kits (R&D Systems, Minneapolis, MN) according to manufacturer´s instructions and calculated as a concentration of cytokine released by 1.0 × 106/ml cells into the supernatant.
Immunoprecipitation, immunoblotting and flow cytometry
For immunoprecipitation studies, cells (1 × 107) were briefly centrifuged at the end of the activation period and the pellet was lysed in ice-cold lysis buffer [20 mM Tris-HCl, pH 8.0, 100 mM NaCl, 2 mM EDTA, 0.5 % (v/v) Triton X-100, 0.5 % (v/v) deoxycholate, 0.05% (w/v) SDS, 2 mM Na3VO4, 10 mM glycerolphosphate, 1 mM PMSF, 10 µg/ml aprotinin]. After 30 min incubation on ice, the lysates were centrifuged at 20,000 × g for 5 min at 4 °C and the supernatants were further processed. To immunoprecipitate DAP12, 1 ml aliquots of the cell lysates were incubated with 20 µl of serum from immunized rabbits for 2 h at 4 °C followed by incubation with UltraLink-immobilized protein A (Thermo Fisher Scientific, Waltham, MA) for 2 h at 4 °C. Immunoprecipitated proteins were eluted by incubation in reducing sample buffer [1:1 mixture of 4× NuPage sample buffer (Invitrogen) and protease inhibitor cocktail [42], 20 % (v/v) 2-mercaptoethanol)] at 90 °C for 10 min. For analysis of the total cellular content, 2 × 106/100 µl of the activated cells were mixed with an equal volume of reducing sample buffer pre-warmed to 90 °C. The samples were immediately incubated at 90 °C for 2–3 min. The contents of the samples were resuspended extensively by passing 10 times rapidly through a 28 gauge needle and boiled at 90 °C for an additional 5 min. The insoluble material was separated by centrifugation at 20,000 × g for 5 min at room temperature.
The immunoprecipitated proteins and total cell lysates were electrophoretically separated on 4–12 % or 12 % NuPAGE Bis-Tris gels (Invitrogen) and transferred onto nitrocellulose membranes. The membranes were immunostained with primary and then with the corresponding HRP-labeled secondary antibodies. HRP-labeled antibodies on the membranes were visualized by enhanced chemiluminescence (PerkinElmer Life Sciences, Shelton, CT). The chemiluminescence was detected on X-ray films and by Molecular Imager ChemiDoc XRS+ System (Bio-Rad, Hercules, CA). The acquired images from the Molecular Imager ChemiDoc XRS+ System were analyzed by Quantity One software to quantify the chemiluminescence.
To determine antibody binding to intact cells, cells were washed with ice-cold phosphate buffered saline (135 mM NaCl, 1.7 mM Na2HPO4 and 5 mM KH2PO4, pH 7.4) supplemented with 2% (w/v) BSA and stained on ice with fluorescent dye-labeled antibody for 60 min. The cells were washed and analyzed by flow cytometry using FACSCalibur with CellQuest software (Becton Dickinson, San Jose, CA).
Statistical evaluation
Means and S.D. were calculated from the number of experiments as indicated by n values. Statistical significance of intergroup differences was calculated by unpaired two-tailed Student’s t test. The intergroup differences were considered significant when p<0.05.
Acknowledgements
This work was supported by the Division of Intramural Research of NIAID and NCI within the National Institutes of Health. We thank Dr.’s Toshiyuki Tokai and Wayne Yokoyama for providing DAP12 deficient animals.
Abbreviations
- FcεRI
high affinity IgE receptor
- BMMC
bone marrow-derived mast cells
- huMC
human mast cells
- WT
wild type
- MALP2
macrophage-activating lipopeptide-2
- P3C
Pam3Cys-SKKKK
Footnotes
Conflict of interest
The authors report no conflicts of interests.
References
- 1.Kraft S, Kinet JP. New developments in FcεRI regulation, function and inhibition. Nat Rev Immunol. 2007;7:365–378. doi: 10.1038/nri2072. [DOI] [PubMed] [Google Scholar]
- 2.Marshall JS. Mast-cell responses to pathogens. Nat Rev Immunol. 2004;4:787–799. doi: 10.1038/nri1460. [DOI] [PubMed] [Google Scholar]
- 3.Qiao H, Andrade MV, Lisboa FA, Morgan K, Beaven MA. FcεR1 and toll-like receptors mediate synergistic signals to markedly augment production of inflammatory cytokines in murine mast cells. Blood. 2006;107:610–618. doi: 10.1182/blood-2005-06-2271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gilfillan AM, Tkaczyk C. Integrated signalling pathways for mast-cell activation. Nat Rev Immunol. 2006;6:218–230. doi: 10.1038/nri1782. [DOI] [PubMed] [Google Scholar]
- 5.Rivera J, Gilfillan AM. Molecular regulation of mast cell activation. J Allergy Clin Immunol. 2006;117:1214–1225. doi: 10.1016/j.jaci.2006.04.015. quiz 1226. [DOI] [PubMed] [Google Scholar]
- 6.Tkaczyk C, Jensen BM, Iwaki S, Gilfillan AM. Adaptive and innate immune reactions regulating mast cell activation: from receptor-mediated signaling to responses. Immunol Allergy Clin North Am. 2006;26:427–450. doi: 10.1016/j.iac.2006.05.011. [DOI] [PubMed] [Google Scholar]
- 7.Hořejší V, Zhang W, Schraven B. Transmembrane adaptor proteins: organizers of immunoreceptor signalling. Nat Rev Immunol. 2004;4:603–616. doi: 10.1038/nri1414. [DOI] [PubMed] [Google Scholar]
- 8.Iwaki S, Jensen BM, Gilfillan AM. NTAL/LAB/LAT2. Int J Biochem Cell Biol. 2007;39:868–873. doi: 10.1016/j.biocel.2006.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Turnbull IR, Colonna M. Activating and inhibitory functions of DAP12. Nat Rev Immunol. 2007;7:155–161. doi: 10.1038/nri2014. [DOI] [PubMed] [Google Scholar]
- 10.Mason LH, Willette-Brown J, Taylor LS, McVicar DW. Regulation of Ly49D/DAP12 signal transduction by Src-family kinases and CD45. J Immunol. 2006;176:6615–6623. doi: 10.4049/jimmunol.176.11.6615. [DOI] [PubMed] [Google Scholar]
- 11.McVicar DW, Taylor LS, Gosselin P, Willette-Brown J, Mikhael AI, Geahlen RL, Nakamura MC, Linnemeyer P, Seaman WE, Anderson SK, Ortaldo JR, Mason LH. DAP12-mediated signal transduction in natural killer cells. A dominant role for the Syk protein-tyrosine kinase. J Biol Chem. 1998;273:32934–32942. doi: 10.1074/jbc.273.49.32934. [DOI] [PubMed] [Google Scholar]
- 12.Huyer G, Liu S, Kelly J, Moffat J, Payette P, Kennedy B, Tsaprailis G, Gresser MJ, Ramachandran C. Mechanism of inhibition of protein-tyrosine phosphatases by vanadate and pervanadate. J Biol Chem. 1997;272:843–851. doi: 10.1074/jbc.272.2.843. [DOI] [PubMed] [Google Scholar]
- 13.Kulka M, Alexopoulou L, Flavell RA, Metcalfe DD. Activation of mast cells by double-stranded RNA: Evidence for activation through Toll-like receptor 3. J Allergy Clin Immunol. 2004;114:174–182. doi: 10.1016/j.jaci.2004.03.049. [DOI] [PubMed] [Google Scholar]
- 14.Chu CL, Yu YL, Shen KY, Lowell CA, Lanier LL, Hamerman JA. Increased TLR responses in dendritic cells lacking the ITAM-containing adapters DAP12 and FcRγ. Eur J Immunol. 2008;38:166–173. doi: 10.1002/eji.200737600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hamerman JA, Tchao NK, Lowell CA, Lanier LL. Enhanced Toll-like receptor responses in the absence of signaling adaptor DAP12. Nat Immunol. 2005;6:579–586. doi: 10.1038/ni1204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Turnbull IR, McDunn JE, Takai T, Townsend RR, Cobb JP, Colonna M. DAP12 (KARAP) amplifies inflammation and increases mortality from endotoxemia and septic peritonitis. J Exp Med. 2005;202:363–369. doi: 10.1084/jem.20050986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lawson D, Fewtrell C, Raff MC. Localized mast cell degranulation induced by concanavalin A-sepharose beads. Implications for the Ca2+ hypothesis of stimulus-secretion coupling. J Cell Biol. 1978;79:394–400. doi: 10.1083/jcb.79.2.394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ivashkiv LB. Cross-regulation of signaling by ITAM-associated receptors. Nat Immunol. 2009;10:340–347. doi: 10.1038/ni.1706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kenny EF, O'Neill LA. Signalling adaptors used by Toll-like receptors: An update. Cytokine. 2008;43:342–349. doi: 10.1016/j.cyto.2008.07.010. [DOI] [PubMed] [Google Scholar]
- 20.Oda K, Kitano H. A comprehensive map of the toll-like receptor signaling network. Mol Syst Biol. 2006;2 doi: 10.1038/msb4100057. 2006 0015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Furuta T, Imajo-Ohmi S, Fukuda H, Kano S, Miyake K, Watanabe N. Mast cell-mediated immune responses through IgE antibody and Toll-like receptor 4 by malarial peroxiredoxin. Eur J Immunol. 2008;38:1341–1350. doi: 10.1002/eji.200738059. [DOI] [PubMed] [Google Scholar]
- 22.Kikawada E, Bonventre JV, Arm JP. Group V secretory PLA2 regulates TLR2-dependent eicosanoid generation in mouse mast cells through amplification of ERK and cPLA2α activation. Blood. 2007;110:561–567. doi: 10.1182/blood-2006-10-052258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Linnekin D. Early signaling pathways activated by c-Kit in hematopoietic cells. Int J Biochem Cell Biol. 1999;31:1053–1074. doi: 10.1016/s1357-2725(99)00078-3. [DOI] [PubMed] [Google Scholar]
- 24.Costello PS, Turner M, Walters AE, Cunningham CN, Bauer PH, Downward J, Tybulewicz VL. Critical role for the tyrosine kinase Syk in signalling through the high affinity IgE receptor of mast cells. Oncogene. 1996;13:2595–2605. [PubMed] [Google Scholar]
- 25.Siraganian RP, Zhang J, Suzuki K, Sada K. Protein tyrosine kinase Syk in mast cell signaling. Mol Immunol. 2002;38:1229–1233. doi: 10.1016/s0161-5890(02)00068-8. [DOI] [PubMed] [Google Scholar]
- 26.Zarbock A, Abram CL, Hundt M, Altman A, Lowell CA, Ley K. PSGL-1 engagement by E-selectin signals through Src kinase Fgr and ITAM adapters DAP12 and FcRγ to induce slow leukocyte rolling. J Exp Med. 2008;205:2339–2347. doi: 10.1084/jem.20072660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Aoki N, Kimura Y, Kimura S, Nagato T, Azumi M, Kobayashi H, Sato K, Tateno M. Expression and functional role of MDL-1 (CLEC5A) in mouse myeloid lineage cells. J Leukoc Biol. 2009;85:508–517. doi: 10.1189/jlb.0508329. [DOI] [PubMed] [Google Scholar]
- 28.Gosselin P, Mason LH, Willette-Brown J, Ortaldo JR, McVicar DW, Anderson SK. Induction of DAP12 phosphorylation, calcium mobilization, and cytokine secretion by Ly49H. J Leukoc Biol. 1999;66:165–171. doi: 10.1002/jlb.66.1.165. [DOI] [PubMed] [Google Scholar]
- 29.Chen ST, Lin YL, Huang MT, Wu MF, Cheng SC, Lei HY, Lee CK, Chiou TW, Wong CH, Hsieh SL. CLEC5A is critical for dengue-virus-induced lethal disease. Nature. 2008;453:672–676. doi: 10.1038/nature07013. [DOI] [PubMed] [Google Scholar]
- 30.Smith KM, Wu J, Bakker ABH, Phillips JH, Lanier LL. Cutting Edge: Ly-49D and Ly-49H associate with mouse DAP12 and form activating receptors. J Immunol. 1998;161:7–10. [PubMed] [Google Scholar]
- 31.Dietrich J, Cella M, Seiffert M, Buhring HJ, Colonna M. Cutting Edge: signal-regulatory protein β1 is a DAP12-associated activating receptor expressed in myeloid cells. J Immunol. 2000;164:9–12. doi: 10.4049/jimmunol.164.1.9. [DOI] [PubMed] [Google Scholar]
- 32.Blasius AL, Cella M, Maldonado J, Takai T, Colonna M. Siglec-H is an IPC-specific receptor that modulates type I IFN secretion through DAP12. Blood. 2006;107:2474–2476. doi: 10.1182/blood-2005-09-3746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lanier LL, Corliss B, Wu J, Phillips JH. Association of DAP12 with activating CD94/NKG2C NK cell receptors. Immunity. 1998;8:693–701. doi: 10.1016/s1074-7613(00)80574-9. [DOI] [PubMed] [Google Scholar]
- 34.Kojima T, Obata K, Mukai K, Sato S, Takai T, Minegishi Y, Karasuyama H. Mast cells and basophils are selectively activated in vitro and in vivo through CD200R3 in an IgE-independent manner. J Immunol. 2007;179:7093–7100. doi: 10.4049/jimmunol.179.10.7093. [DOI] [PubMed] [Google Scholar]
- 35.Kuehn HS, Beaven MA, Ma HT, Kim MS, Metcalfe DD, Gilfillan AM. Synergistic activation of phospholipases Cγ and Cβ: a novel mechanism for PI3K-independent enhancement of FcεRI-induced mast cell mediator release. Cell Signal. 2008;20:625–636. doi: 10.1016/j.cellsig.2007.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kim MS, Kuehn HS, Metcalfe DD, Gilfillan AM. Activation and function of the mTORC1 pathway in mast cells. J Immunol. 2008;180:4586–4595. doi: 10.4049/jimmunol.180.7.4586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Turnbull IR, Gilfillan S, Cella M, Aoshi T, Miller M, Piccio L, Hernandez M, Colonna M. Cutting edge: TREM-2 attenuates macrophage activation. J Immunol. 2006;177:3520–3524. doi: 10.4049/jimmunol.177.6.3520. [DOI] [PubMed] [Google Scholar]
- 38.Whittaker GC, Orr SJ, Quigley L, Hughes L, Francischetti IMB, Zhang W, McVicar DW. The linker for activation of B cells (LAB)/non-T cell activation linker (NTAL) regulates triggering receptor expressed on myeloid cells (TREM)-2 signaling and macrophage inflammatory responses independently of the linker for activation of T cells. J Biol Chem. 285:2976–2985. doi: 10.1074/jbc.M109.038398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tkaczyk C, Horejsi V, Iwaki S, Draber P, Samelson LE, Satterthwaite AB, Nahm DH, Metcalfe DD, Gilfillan AM. NTAL phosphorylation is a pivotal link between the signaling cascades leading to human mast cell degranulation following Kit activation and FcεRI aggregation. Blood. 2004;104:207–214. doi: 10.1182/blood-2003-08-2769. [DOI] [PubMed] [Google Scholar]
- 40.Amoui M, Dráberová L, Tolar P, Dráber P. Direct interaction of Syk and Lyn protein tyrosine kinases in rat basophilic leukemia cells activated via type I Fcε receptors. Eur J Immunol. 1997;27:321–328. doi: 10.1002/eji.1830270146. [DOI] [PubMed] [Google Scholar]
- 41.Choi OH, Lee JH, Kassessinoff T, Cunha-Melo JR, Jones SVP, Beaven MA. Antigen and carbachol mobilize calcium by similar mechanisms in a transfected mast cell line (RBL-2H3 cells) that expresses ml muscarinic receptors. J Immunol. 1993;151:5586–5595. [PubMed] [Google Scholar]
- 42.Tkaczyk C, Metcalfe DD, Gilfillan AM. Determination of protein phosphorylation in FcεRI-activated human mast cells by immunoblot analysis requires protein extraction under denaturing conditions. J Immunol Methods. 2002;268:239–243. doi: 10.1016/s0022-1759(02)00210-7. [DOI] [PubMed] [Google Scholar]