Abstract
While there is solid evidence that cannabis use is heritable, attempts to identify genetic influences at the molecular level have yielded mixed results. Here, a large twin family sample (N=7452) was used to test for association between ten previously reported candidate genes and lifetime frequency of cannabis use using a gene-based association test. None of the candidate genes reached even nominal significance (p<.05). The lack of replication may point to our limited understanding of the neurobiology of cannabis involvement and also to potential publication bias and false-positive findings in previous studies.
Keywords: genes, cannabis, genetics, association
Twin and family studies have estimated the heritability of cannabis use phenotypes (lifetime use, frequency of use, and abuse/dependence) at between 40%-60% (Verweij, et al., 2010) and have also shown substantial overlap in the genetic factors influencing earlier (experimental/regular use) and later (abuse/dependence) stages of cannabis use, (Agrawal & Lynskey, 2006). Identification of the specific genes contributing to cannabis use variation could improve the knowledge regarding the biological processes that underlie cannabis use, and potentially substance use in general. Linkage and candidate gene association studies have identified a handful of potentially important genetic variants, but different studies have yielded inconsistent results (for an overview, see Agrawal & Lynskey, 2009). The present study tests for replication of previously identified candidate gene associations for cannabis use in a large population based sample of Australian twin families.
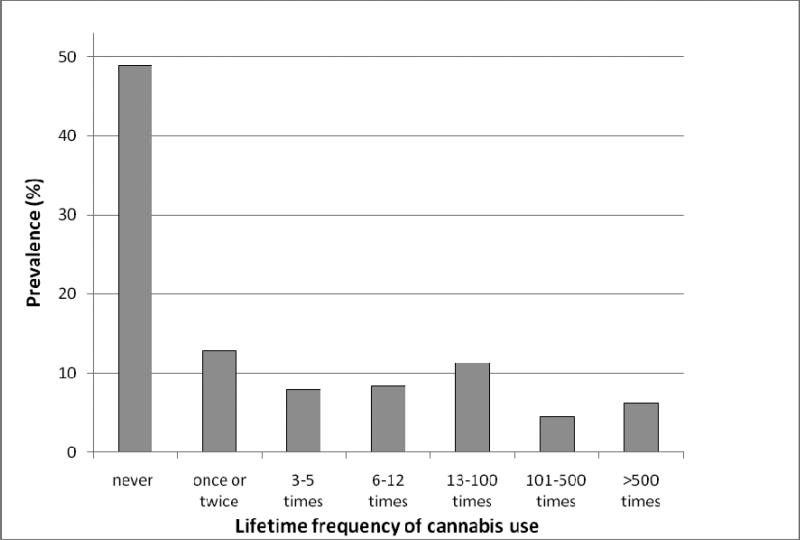
For this study we used data from 7452 Caucasian individuals (3334 males and 4118 females from 2595 independent families, mean age 43.2 ± 11.8 years) from the Australian Twin Registry for whom we have both genotypic and phenotypic data. Individuals participated in various studies between 1996 and 2004. The ATR is a population-based sample, but a subset of the sample was ascertained based on large sibship size, or having a relative with nicotine or alcohol dependence. The effective sample size (i.e. correcting for nonindependence of family members) was calculated to be 4312. Frequency of lifetime cannabis use used an open response format, but due to extreme skew was transformed by assigning values to seven bins (see Figure 1).
Figure 1.
Prevalence of the different response categories for lifetime frequency of cannabis use.
DNA samples were collected in accordance with standard protocols and were genotyped in different waves on different Illumina platforms (Illumina 317K, Illumina HumanCNV370-Quadv3, Illumina Human610-Quad). Imputation using MACH allowed combining data from different genotyping arrays and improved coverage of single nucleotide polymorphisms (SNPs) in the candidate genes (see Medland, et al., 2009).
We performed a genome-wide association (GWA) analysis where SNPs (N=2,380,486) across the entire genome were systematically tested for association with lifetime frequency of cannabis use. The analyses were performed in Merlin (Chen & Abecasis, 2007), which accounts for family relationships including MZ twins, and we included age and sex as covariates. Results of this GWA were then used to run a gene-based association test (VEGAS, Liu, et al., 2010). VEGAS summarises evidence for association on a per gene basis (N=17,591 autosomal genes) by considering the p-value of all SNPs within genes (including +/-50kb from the 5’ and 3’ UTR), while accounting for linkage disequilibrium (LD) and number of SNPs per gene. As such, the gene-based test identifies genes that show more signal of association than expected by chance given their length and LD between the SNPs. We tested for association between lifetime frequency of cannabis use with ten previously defined candidate genes for cannabis use disorders, as described by Agrawal and Lysnkey (2009). In their review they include genes that are posited to have specific influences on cannabis use because the genes relate to the action and metabolism of exogenous cannabinoids. They also describe non-specific candidate genes that potentially influence the biological basis of substance use in a more general way. For the latter cases, they included genes that encode the major neurotransmitter systems and have previously shown association with substance use. The selected candidate genes include genes that are thought to influence different stages of cannabis use. In total, we performed association analyses on ten candidate genes - based on a Bonferroni correction for multiple testing, we declared the significance level to be α=.005 (.05/10).
The heritability of lifetime frequency of cannabis use was estimated in Merlin to be 45%, consistent with previous research (Verweij, et al., 2010). Table 1 shows the results of our gene-based test for association, including information regarding the number of tagged SNPs per gene, the ranking of the gene (out of 17,591 genes), the number of SNPs within the gene with a p-value below .01 or .05, and the percentage of the variance of the gene that is covered by our SNPs. Results indicate that none of the candidate genes reached even nominal significance (p<.05), and none of them was within the top 1000 genes.
Table 1.
Association results of the ten candidate genes with frequency of cannabis use.
| Gene | Chromosome | Start position gene* | End position gene* | Number of tagged SNPs | P-value | Rank | SNPs at α<.05/α<.01 | % of gene variance covered |
|---|---|---|---|---|---|---|---|---|
| CNR1 | 6 | 88906303 | 88911775 | 145 | .45 | 7814 | 10/3 | 96% |
| CNR2 | 1 | 24073046 | 24112404 | 15 | .06 | 1089 | 0/0 | 86% |
| FAAH | 1 | 46632525 | 46652107 | 90 | .96 | 16851 | 0/0 | 97% |
| MGLL | 3 | 128890598 | 129024741 | 170 | .25 | 4342 | 13/1 | 97% |
| TRPV1 | 17 | 3415489 | 3459454 | 116 | .31 | 5394 | 6/1 | 95% |
| GPR55 | 2 | 231480286 | 231498185 | 88 | .95 | 16591 | 0/0 | 91% |
| GABRA2 | 4 | 45946338 | 46086813 | 145 | .47 | 8179 | 0/0 | 98% |
| DRD2 | 11 | 112785526 | 112851211 | 197 | .89 | 15389 | 0/0 | 100% |
| OPRM1 | 6 | 154402135 | 154609693 | 402 | .37 | 6352 | 39/19 | 98% |
| COMT | 22 | 18309308 | 18336530 | 131 | .17 | 2956 | 17/8 | 98% |
CNR1/CNR2; cannabinoid receptors 1 and 2
FAAH; Fatty acid amide hydrolase
MGLL; Monoglyceride lipase
TRPV1; Transient receptor potential vanilloid 1
GPR55; Orphan cannabinoid receptor
GABRA2; Gamma-amino butyric acid
DRD2; Dopamine Receptor D2
OPRM1; Mu-opiod receptor 1
COMT; catecholamine-o-methyl transferase
note that the analyses included +/- 50kb from the gene border.
Because genes cover a lot of SNPs (up to 402 in our candidate genes) we also checked for association between specific SNPs (within our candidate genes) that have previously been found to be associated with cannabis use phenotypes (see Agrawal & Lynskey, 2009; Caspi, et al., 2005). Again, none of the candidate SNPs reached even nominal significance (see Table 2). Moreover, none of the other SNPs within the candidate genes reached Bonferroni corrected significance (α=.05).
Table 2.
Association results of the candidate SNPs as reported by Agrawal and Lynskey (2009) with frequency of cannabis use.
| SNP | Gene | p-value |
|---|---|---|
| rs2023239 | CNR1 | .39 |
| rs806379 | CNR1 | .88 |
| rs1535255 | CNR1 | Not tagged |
| rs806380 | CNR1 | .78 |
| rs6454674 | CNR1 | .39 |
| rs806368 | CNR1 | .50 |
| rs12720071 | CNR1 | .94 |
| rs806379 | CNR1 | .88 |
| rs2501432 | CNR2 | Not tagged |
| rs324420 | FAAH | .58 |
| rs279858 | GABRA2 | .27 |
| rs1799971 | OPRM1 | .12 |
| rs4680 | COMT | .31 |
CNR1/CNR2; cannabinoid receptors 1 and 2
FAAH; Fatty acid amide hydrolase
GABRA2; Gamma-amino butyric acid
OPRM1; Mu-opiod receptor 1
COMT; catecholamine-o-methyl transferase
Empirical power estimation using simulated data showed 97%, 51%, and 21% power to detect a candidate gene or SNP that accounts for 0.5%, 0.2%, or 0.1% of the variation at a Bonferroni-corrected alpha level, respectively. Even with this power, we could not replicate association for any of the previously identified candidate genes or SNPs. This suggests the biology underlying cannabis involvement is highly complex, and that our understanding of the biochemical and addictive processes governing cannabis use is nascent. These findings could also indicate that previous associations were false positives, and suggests that publication bias may misrepresent the actual association between the genetic variant and phenotype. This is not the first study demonstrating that candidate genes studies should be interpreted with caution - for example, Bosker et al. (2010) could not replicate most candidate genes previously associated with major depressive disorder.
The present study had several limitations. Firstly, the phenotype (lifetime frequency of use) is not the ideal replication phenotype for some of the genes, which were originally associated with late stages of cannabis use (abuse). However, some of the candidate genes are thought to be involved in earlier stages of cannabis use, and twin studies show the different stages of cannabis involvement are largely influenced by the same genetic factors (Agrawal & Lynskey, 2006), so we would still expect replication. Secondly, the non-normal distribution of our phenotype decreases statistical power, while also elevating the chance of false positives. However, the first problem would be heavily outweighed by the much larger sample than previous studies, and the latter is not an issue for our findings as there were no positive findings.
While our findings indicate that none of the ten candidate genes explain a substantial portion of the variance in frequency of lifetime cannabis use, this does not imply that lifetime cannabis use is not heritable. Other, unidentified common genetic variants may play a role in cannabis use, and the variants may be numerous with individually small effects. We therefore need to look systematically across the whole genome for these variants – that is, a large-scale GWA study is needed. Just such a study is now underway, incorporating multiple datasets from around the world.
Acknowledgements
We would like to thank the twins and their families registered at the ATR for their participation. We also thank Dixie Statham (sample collection); Lisa Bowdler, Steven Crooks (DNA processing); Scott Gordon (QC genotype data); David Smyth, Harry Beeby, and Daniel Park (IT support). Funding was provided by the Australian National Health and Medical Research Council (241944, 339462, 389927, 389875, 389891, 389892, 389938, 442915, 442981, 496739, 552485, 552498), the Australian Research Council (A7960034, A79906588, A79801419, DP0770096, DP0212016, DP0343921), the FP-5 GenomEUtwin Project QLG2-CT-2002-01254), and the U.S. National Institutes of Health (NIH grants AA07535, AA10248, AA13320, AA13321, AA13326, AA14041, DA12854, MH66206). A portion of the genotyping on which this study was based (Illumina 370K scans) was carried out at the Center for Inherited Disease Research, Baltimore (CIDR), through an access award to our late colleague Dr. Richard Todd (Psychiatry, Washington University School of Medicine, St Louis). Statistical analyses were carried out on the Genetic Cluster Computer, which is financially supported by the Netherlands Scientific Organization (NWO 480-05-003). K.J.H.V. is supported by an ANZ Trustees PhD scholarship in Medical Research. BPZ is supported by a UQ Postdoctoral Fellowship.
Footnotes
Author contribution: KJHV was responsible for the study concept and design of the study. NGM, ACH, PAFM, and GWM contributed to the data acquisition. KJHV performed the data analysis and interpretation of findings. SEM, and JZL assisted with data analysis. KJHV drafted the manuscript. BPZ, MTL, and AA, provided critical revision of the manuscript for important intellectual content. All authors critically reviewed content and approved final version for publication.
References
- Agrawal A, Lynskey MT. The genetic epidemiology of cannabis use, abuse and dependence. Addiction. 2006;101(6):801–812. doi: 10.1111/j.1360-0443.2006.01399.x. [DOI] [PubMed] [Google Scholar]
- Agrawal A, Lynskey MT. Candidate genes for cannabis use disorders: findings, challenges and directions. Addiction. 2009;104(4):518–532. doi: 10.1111/j.1360-0443.2009.02504.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosker FJ, Hartman CA, Nolte IM, Prins BP, Terpstra P, Posthuma D, et al. Poor replication of candidate genes for major depressive disorder using genome-wide association data. Molecular Psychiatry. 2010 doi: 10.1038/mp.2010.38. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- Caspi A, Moffitt TE, Cannon M, McClay J, Murray R, Harrington H, et al. Moderation of the effect of adolescent-onset cannabis use on adult psychosis by a functional polymorphism in the catechol-O-methyltransferase gene: Longitudinal evidence of a gene X environment interaction. Biological Psychiatry. 2005;57(10):1117–1127. doi: 10.1016/j.biopsych.2005.01.026. [DOI] [PubMed] [Google Scholar]
- Chen WM, Abecasis GR. Family-based association tests for genomewide association scans. American Journal of Human Genetics. 2007;81(5):913–926. doi: 10.1086/521580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu JZ, McRae AF, Nyholt DR, Medland SE, Wray NR, Brown KM, et al. A versatile gene-based test for genome-wide association studies. American Journal of Human Genetics. 2010;87(1):139–145. doi: 10.1016/j.ajhg.2010.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medland SE, Nyholt DR, Painter JN, McEvoy BP, McRae AF, Zhu G, et al. Common variants in the trichohyalin gene are associated with straight hair in Europeans. American Journal of Human Genetics. 2009;85(5):750–755. doi: 10.1016/j.ajhg.2009.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verweij KJH, Zietsch BP, Lynskey MT, Medland SE, Neale MC, Martin NG, et al. Genetic and environmental influences on cannabis use initiation and problematic use: a meta-analysis of twin studies. Addiction. 2010;105(3):417–430. doi: 10.1111/j.1360-0443.2009.02831.x. [DOI] [PMC free article] [PubMed] [Google Scholar]