Many multicellular organisms produce hard tissues such as bones, teeth, shells, skeletal units, and spicules (1). These hard tissues are biocomposites and incorporate both structural macromolecules (lipids, proteins, and polysaccharides) and minerals of, perhaps, 60 different kinds, including hydroxyapatite, calcium carbonate, and silica. A number of single-celled organisms (bacteria and algae) also produce inorganic materials either intracellularly or extracellularly (2). Examples include magnetotactic bacteria, which synthesize magnetite (3); chrysophytes (4), diatoms, and actinopoda (radiolarians; ref. 5), which synthesize siliceous materials; and S layer bacteria that have gypsum and calcium carbonate surface layers (6). Normally, hard tissues are mechanical devices (e.g., skeletal, cutting, grinding), or they serve a physical function (e.g., magnetic, optical, piezoelectric). Bioinorganics are also ion sources that are vital for physiological activities and, therefore, integral parts of the organisms (1, 2).
Recently, a different single-celled organism has been added to the list of inorganic particle producers. Klaus et al. (7) have found that single crystalline silver-based particles of well defined compositions and shapes are synthesized by Pseudomonas stutzeri AG259, a bacterial strain previously isolated from a silver mine (8). The study reports on the detailed structure and phase composition of the silver-containing particles with a flat morphology that form within the periplasmic space. Currently, neither the synthesis mechanism nor the physiological nature of the particles is known.
The presence of inorganic materials within organisms has broad implications in physical sciences, such as geology, mineralogy, physics, chemistry, and materials science (9, 10), as well as in biological fields, such as zoology, microbiology, morphology, physiology, evolution, and cellular biology (1, 2). The structures of biocomposites are highly controlled from the nanometer to the macroscopic levels, resulting in complex architectures that provide multifunctional properties. Therefore, there is much interest in inorganic material formation by organisms in these scientific fields (9–11). While biosciences are studying the implications of biomineralization in organismal physiology and its importance in species diversity and evolution, physical sciences focus on the mechanisms of formation and functional characteristics of inorganic materials. The synthesis mechanisms of inorganics by multicellular and single-celled organisms may be vastly different. Nonetheless, the presence of an inorganic compound in conjunction with a biological macromolecule within a tissue is intriguing in terms of the phase compatibility in these complex systems (9, 10). Furthermore, many aspects of composite materials biosynthesis are unusual from the traditional point of view of materials synthesis. These characteristics include the mineralogy of the inorganic; its phase composition, size, distribution, and morphology; its crystallography; its long-range orientational order of domains (texture); and its hierarchical organization.
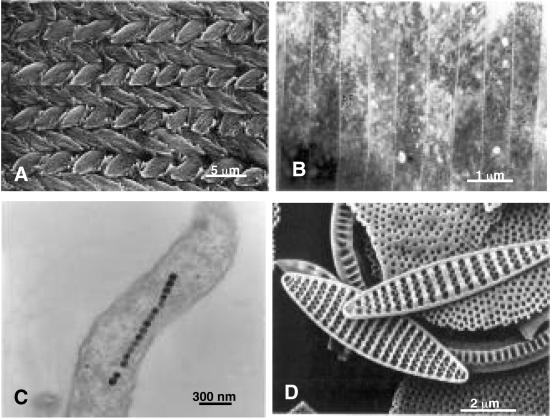
In multicellular organisms, bioinorganics are synthesized by a coordinated process involving cohort of similar cells, such as, in mammals, osteoblasts in bone (12) or dentinoblasts in dentin (13). Both of these hard tissues are extracellularly synthesized by these cells that control size, distribution, and morphology of the hydroxyapatite mineral particles (14). The resulting hard tissues are composites of particles within structural proteins (Fig. 1A). Dentin, enamel, and bone are known to be multifunctional, serving as load-bearing systems with piezoelectric properties. Similarly, in shell-forming molluscan species, mantel cells synthesize hard tissues that are differentiated into many different architectures. These include layered, columnar, and foliated structures of crystalline units that are allotropic forms of calcium carbonate (15). For example, in Haliotis rufescens, the gastropod commonly known as red abalone, columnar calcitic crystals constitute the prismatic section, whereas layered aragonitic platelets form the nacre (mother-of-pearl; Fig. 1B). Such a microarchitecture is a result of an evolutionary design for an ideal impact-resistant material providing armor to the mollusk (16, 17).
Figure 1.
(A) This intricately architectured mouse incisor tooth has enamel rods. (B) Layered composite structure of nacre (mother-of-pearl) from Nautilus pompelius shell. (C) Magnetite particles in Aquaspirillum magnetotacticum. (D) Siliceous skeletal structures in diatomaceous earth. Images in A and D were recorded by scanning electron microscopy, and B and C were recorded by transmission electron microscopy.
Small inorganic particles could be synthesized by many species of bacteria and algae, and these particles could be oxides, sulfides, carbonates, or phosphates (1, 2). These particles have highly intricate architectures and are ordered during assembly. In many cases, the particles have a well defined shape formed within a certain size range and have orientational (when they are crystalline) and geometrical (even when noncrystalline) symmetry. These structural features are species-specific, and all are thought to originate in the macromolecules that control particle synthesis. For example, in Aquaspirillum magnetotacticum, a magnetotactic bacterium, small magnetite particles form within cytoplasmic vesicular compartments (magnetosomes) in ordered geometries (3), and these particles are perfectly crystalline (Fig. 1C). The magnetic particles are magnetic-sensing devices that steer bacteria toward anaerobic sediments. Single-crystalline semiconducting particles, e.g., CdS, are synthesized in algae as a result of a toxification mechanism (18). Actinopoda and diatoms, single-celled organisms, synthesize amorphous siliceous units that are resting spores with highly intricate and symmetrical geometrical shapes (Fig. 1D). Similar structural skeletal units, spicules of SrSO4, are found in Acantharia (19). The cyst around a chrysophyte is a protective shell-like wall made of amorphous silica with elaborate geometries (4). Finally, in some cyanobacterial species, an outer cell surface proteinaceous membrane, an S layer, is a template for calcium-sulfate/carbonate synthesis. The thin layer of inorganic shell is a protective covering, consisting of highly organized two-dimensional ordered tiles (tessellations; ref. 6).
Based on their observations, Klaus et al. (7) note potential uses of bacteria for nanostructured thin film or particulate materials synthesis to support technological applications. These uses, in fact, are exciting prospects for all hard tissues in that the understanding of the principles of ultrafine particle (and hard tissue) synthesis could potentially be exploited in materials sciences. The use of biological principles in materials formation is an emerging field called biomimetics (9, 10). The fundamental premise in another emerging field, nanotechnology, is that the physical properties of materials are substantially determined by the length scales that characterize their structure and organization (20–22). For example, the mechanical properties of nanostructured composites, the electronic structure of low-dimensional semiconductors, the magnetic properties of superlattices, the properties of single-domained particles, and the solution properties of colloidal suspensions, all correlate directly to the nanometer-scale structures that characterize these systems (20). However, most traditional approaches to synthesis of nanoscale materials are energy inefficient, require stringent synthesis conditions (e.g., high temperature, pressure, pH), and often produce toxic byproducts. Furthermore, the quantities produced are small, and the resultant material is highly irreproducible because of the difficulty of control of agglomeration (23). Despite the premise of science and technology at the nanoscale, the control of structural properties and ordered assemblies of materials in two- and three-dimensions remains elusive (24, 25).
Materials produced by organisms, on the other hand, have properties that usually surpass those of analogous synthetically manufactured materials with similar phase compositions. Biological materials are assembled in aqueous environments under mild conditions by using biomacromolecules (26, 27). Organic macromolecules both collect and transport raw materials and consistently and uniformly self-assemble and coassemble subunits into short- and long-range ordered nuclei and substrates. The resulting structures are highly organized from molecular to nano, micro, and macro scales, often in a hierarchical manner, with intricate nanoarchitectures that ultimately make up a myriad of different tissues (28). They are simultaneously “smart,” dynamic, complex, self-healing, and multifunctional (29), characteristics difficult to achieve in purely synthetic systems. Therefore, biomimetics, the use of biological principles in materials synthesis and assembly, may be a path for realizing nanotechnology.
Unfortunately, the current understanding of the mechanisms of inorganic materials formation (biomineralization) in organisms and their regulation are far from complete. The macromolecules associated with hard tissues may act as nucleators, growth modifiers, anchoring units, compartments, or scaffolds in mineral formation (30). Their major role may be due to either templating (31–33) or enzymatic effects (34). A macromolecular template could provide stereochemistry and physisorption for the inorganic formation. On the other hand, an enzyme could regulate inorganic phase synthesis by controlling local chemistry. However, there has been only limited work completed in assessing the effects of these macromolecules in the regulation and control of biomineralization. Some elegantly performed research has studied functions of the proteins that were isolated from hard tissues and used them in a purified state in materials synthesis. Examples include amelogenins formation in human enamel (35) and lustrin in abalone nacre synthesis (36). In these cases, the effects of proteins in various mineral forms were investigated during in vitro biomineralization (37–39). These studies, however, are preliminary, because a large number of macromolecules are present in a hard tissue and may affect, independently or in concert, the assembly of hard tissue through control of the mineral synthesis. Further studies, therefore, are essential to elucidate the specific effects of macromolecules in simulated in vivo conditions that mimic natural, physiological synthesis (40).
In the study by Klaus et al. (7), it is not known whether the Ag-containing particles are a byproduct formed by some unknown, purely accidental mechanism, or perhaps, the organism uses the particles in a cellular activity with a parallel multifunctionality (similar to magnetite in bacteria; ref. 3). It is also plausible that this species of bacteria uses the silver ions as part of the electron-transport process in its reduction to elemental silver. It is interesting to note that the density of silver would result in an increased density of the cells in which it is formed, which would enhance their settling out in a water column. Because P. stutzeri is known to be a denitrifier, it can grown under anaerobic conditions by using nitrate in place of oxygen as an oxidant in respiration.
Silver particles in solution normally form equilibrium, cubooctahedral particle morphology based on their isomorphic crystal structure (41). This process is similar to Au formation, for example, via the reduction of its salts in an aqueous solution, a process known since Turkevich’s study (42). In P. stutzeri, the Ag-containing crystallites are located within the periplasmic space, and the flat shape could be a consequence of the confined space available for plate-like morphology. On the other hand, the shape could be a result of either an enzymatic reaction or templating of a macromolecule that might effect the growth kinetics. The isomorphic crystallite with flat, hexagonal, or triangular shape indicates that the crystallographic plane of the plate is the one with the highest atomic density. Plate morphology is preferred during crystal growth, because material is accumulated faster than during spherical growth. A flat crystallite with a high aspect ratio (edge size to thickness), e.g., 100/1, would have a surface-to-volume ratio two orders of magnitude larger than an equilibrium, symmetrical shape and, hence, could grow at a comparatively higher rate. This recent study (7), therefore, raises many interesting questions regarding inorganic formation processes and associated cellular mechanisms. It also opens up possibilities in physical sciences. Many strains of Pseudomonas are used in environmental cleanup; likewise, the present strain may be useful in bioremediation. In any case, the article by Klaus et al. (7) is another example revealing possibilities awaiting interesting research activities in biomimetics (43, 44).
The premise in biomimetics is that inorganic surface-specific proteins could be used as templating or enzymatic agents for controlled materials assembly either in vivo, through the genetic control of the organism, a long-term study, or in vitro, through genetic engineering techniques, a possible shorter-term study. There are several ways to obtain surface-specific proteins. The traditional approach, extraction from hard tissues, involves complex and time-consuming procedures including protein isolation, purification, amino acid analysis, and sequencing (45). Another way is to use existing proteins that are known to bind to inorganic surfaces. A number of proteins that bind to inorganic surfaces do so nonspecifically, and the primary mechanism is likely to be chemisorption (46). Therefore, their use is limited and mostly depends on solution chemistry. A more practical approach to obtain surface-specific proteins would be molecular design of recombinant proteins via genetic engineering techniques. Ideally, one would predict the surface topology of the desired inorganic crystal face and design the complementary molecule that could fit it tightly with high binding energy. Such designs can be accomplished by site-directed mutagenesis of existing proteins (47) or de novo selection of polypeptide motifs via phage (48) or cell surface (49) display libraries. In either case, it may ultimately be possible to construct a molecular “erector set” in which different types of proteins, each designed to perform a desired function, e.g., nucleation or growth modification, could assemble into intricate, hybrid structures composed of minerals and proteins. This type of construction would be a giant leap toward realizing genetically engineered technological materials.
Acknowledgments
This work was supported by the Air Force Office of Scientific Research and the Army Research Office.
Footnotes
See companion article on page 13611 in issue 24 of volume 96.
References
- 1.Lowenstam H A. Science. 1981;211:1126–1131. doi: 10.1126/science.7008198. [DOI] [PubMed] [Google Scholar]
- 2.Simkiss K, Wilbur K M. Biomineralization. New York: Academic; 1989. [Google Scholar]
- 3.Frankel R B, Blakemore R P, editors. Iron Biominerals. New York: Plenum; 1991. [Google Scholar]
- 4.Kristiansen J, Andersen R A, editors. Chrysophytes: Aspects and Problems. New York: Cambridge Univ. Press; 1986. [Google Scholar]
- 5.Margulis L, Schwartz K V. Five Kingdoms. New York: Freeman; 1998. [Google Scholar]
- 6.Schultze-Lam S, Harauz G, Beveridge T H. J Bacteriol. 1992;174:7971–7981. doi: 10.1128/jb.174.24.7971-7981.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Klaus T, Joerger R, Olsson E, Granqvist C G. Proc Natl Acad Sci USA. 1999;96:13611–13614. doi: 10.1073/pnas.96.24.13611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Haefeli C, Franklin C, Hardy K. J Bacteriol. 1984;158:389–404. doi: 10.1128/jb.158.1.389-392.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sarikaya M, Aksay I A, editors. Biomimetics: Design and Processing of Materials. New York: Am. Inst. Phys.; 1995. [Google Scholar]
- 10.Mann S, editor. Biomimetic Materials Chemistry. New York: VCH; 1996. [Google Scholar]
- 11.Rieke P C, Calvert P D, Alper M, editors. Materials Synthesis Using Biological Processes. Pittsburgh: Mater. Res. Soc.; 1990. [Google Scholar]
- 12.Glimcher J. In: Chemistry and Biology of Mineralized Tissues. Veis A, editor. Amsterdam: Elsevier; 1981. pp. 617–673. [Google Scholar]
- 13.Smith C E. Crit Rev Oral Biol Med. 1998;9:128–161. doi: 10.1177/10454411980090020101. [DOI] [PubMed] [Google Scholar]
- 14.Slavkin H, Price P. Chemistry and Biology of Mineralized Tissues. Amsterdam: Excerpta Medica; 1992. [Google Scholar]
- 15.Currey J D. J Mater Edu. 1987;9:118–296. [Google Scholar]
- 16.Jackson A P, Vincent J F V, Turner R F. Proc R Soc London Ser B. 1988;234:415–440. [Google Scholar]
- 17.Sarikaya M, Aksay I A. In: Structure, Cellular Synthesis, and Assembly of Biopolymers. Case S T, editor. Berlin: Springer; 1991. pp. 1–25. [Google Scholar]
- 18.Dameron C T, Reese R N, Mehra R K, Kortan A R, Carrol P J, Steigerwald M L, Brus L E, Winge D R. Nature (London) 1989;338:569–571. [Google Scholar]
- 19.Perry C C, Wilcock J R, Williams R J P. Experimentia. 1988;44:638–650. doi: 10.1007/BF01941024. [DOI] [PubMed] [Google Scholar]
- 20.Drexler K E. Nanotechnology. 1991;2:113–118. [Google Scholar]
- 21.Whitesides G M, Mathias J P, Seto C T. Science. 1991;254:1312–1319. doi: 10.1126/science.1962191. [DOI] [PubMed] [Google Scholar]
- 22.Knoll W, Angermeir L, Batz G, Fritz T, Fujisawa S, Furuno T, Guder H J, Hara M, Liley M, Niki K, et al. Syn Metals. 1993;6:5–11. [Google Scholar]
- 23.Komarneni K, Parker J C, Thomas G J, editors. Nanophase and Nanocomposite Materials. Pittsburgh: Mater. Res. Soc.; 1993. [Google Scholar]
- 24.Anders R P, Averbeck R S, Brown W L, Brus L E, Goddard W A, III, Kaldor A, Louie S G, Moskovitz M, Peercy P S, Riley S J, et al. J Mater Res. 1989;4:704–496. [Google Scholar]
- 25.Siegel R W. Phys Today. 1993;46:64–69. [Google Scholar]
- 26.Watabe N. Ultrastruct Res. 1965;12:351–370. doi: 10.1016/s0022-5320(65)80104-6. [DOI] [PubMed] [Google Scholar]
- 27.Crenshaw M A. In: Biological Mineralization and Demineralization. Nancollas G H, editor. Berlin: Springer; 1972. pp. 243–257. [Google Scholar]
- 28.Tirrel D A. Hierarchical Structures in Biology as a Guide for New Materials Technology. Washington, DC: Natl. Acad. Press; 1994. [Google Scholar]
- 29.Aksay I A, Baer E, Sarikaya M, Tirrell D A, editors. Hierarchically Structured Materials. Pittsburgh: Mater. Res. Soc.; 1992. [Google Scholar]
- 30.Addadi L, Weiner S. Angew Chem Int Ed Engl. 1992;31:153–169. [Google Scholar]
- 31.Mann S. Nature (London) 1988;33:119–123. [Google Scholar]
- 32.Knight C A, Cheng C C, DeVries A L. Biophys J. 1991;59:409–418. doi: 10.1016/S0006-3495(91)82234-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Addadi L, Weiner S. Proc Natl Acad Sci USA. 1985;82:4110–4114. doi: 10.1073/pnas.82.12.4110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Greenfield G, Wilson D C, Crenshaw M A. Am Zool. 1984;24:925–932. [Google Scholar]
- 35.Snead M L, Zeichner-David M, Chandra T, Robson K J, Woo S L, Slavkin H C. Proc Natl Acad Sci USA. 1983;80:7254–7258. doi: 10.1073/pnas.80.23.7254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Carioluo M A, Morse D E. J Comp Physiol B. 1987;157:717–729. [Google Scholar]
- 37.Falini G, Albeck S, Weiner S, Addadi L. Science. 1996;271:67–69. [Google Scholar]
- 38.Belcher A M, Wu X H, Christensen R J, Hansman P, Stucky G D, Morse D E. Nature (London) 1996;381:56–58. [Google Scholar]
- 39.Wierzbicki A, Sikes C S, Madura J D, Drake B. Calcif Tissue Int. 1994;54:133–141. doi: 10.1007/BF00296064. [DOI] [PubMed] [Google Scholar]
- 40.Alberts B, Bray D, Johnson A, Lewis J, Raff M, Roberts K, Walter P. Essential Cell Biology. New York: Garland; 1998. [Google Scholar]
- 41.Dana E S. Minerals. New York: Wiley; 1955. [Google Scholar]
- 42.Turkevich J, Stevenson P C, Hillier J. Trans Faraday Soc. 1951;11:55–75. [Google Scholar]
- 43.Heuer A A, Fink D J, Laraia V J, Arias J L, Calvert P D, Kendall K, Messing G L, Blackwell J, Rieke P C, Thompson D H, et al. Science. 1992;255:1098–1100. doi: 10.1126/science.1546311. [DOI] [PubMed] [Google Scholar]
- 44.Bunker B C, Rieke P C, Tarasevich B J, Campbell A A, Fryxell G E, Graff G L, Song L, Liu J, Virden J W, McVay G L. Science. 1994;265:1839–1841. doi: 10.1126/science.264.5155.48. [DOI] [PubMed] [Google Scholar]
- 45.Nakahara N, Bevelander G, Kakei M. Venus. 1982;41:34–46. [Google Scholar]
- 46.Geoghevan W D, Ackerman G A. J Histochem Cytochem. 1977;25:1187–2000. doi: 10.1177/25.11.21217. [DOI] [PubMed] [Google Scholar]
- 47.Scott J K, Smith G. Science. 1990;249:386–390. doi: 10.1126/science.1696028. [DOI] [PubMed] [Google Scholar]
- 48.Cwirla S E, Peters S E, Barret E A, Dower R W. Proc Natl Acad Sci USA. 1990;87:6378–6382. doi: 10.1073/pnas.87.16.6378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Brown S. Nat Biotechnol. 1997;15:269–272. doi: 10.1038/nbt0397-269. [DOI] [PubMed] [Google Scholar]