Abstract
The asymmetric unit of the title compound, C18H18N2O2, consists of two independent molecules, each of which is located about a center of inversion. The molecules are not planar, showing dihedral angles of 55.84 (9) and 54.10 (8)° between the piperazinedione and the aromatic rings. The piperazine N atoms exhibit a planar configuration. The crystal packing is stabilized by intermolecular C—H⋯O hydrogen bonds.
Related literature
For background to the applications of piperazinedione and its derivatives, see: Acharya et al. (2001 ▶); Fischer (2003 ▶); Krchnak et al. (1996 ▶); Paradisi et al. (2002 ▶). For the syntheses and structures of piperazinediones, see: Wen et al. (2006 ▶); Zhang et al. (2007 ▶).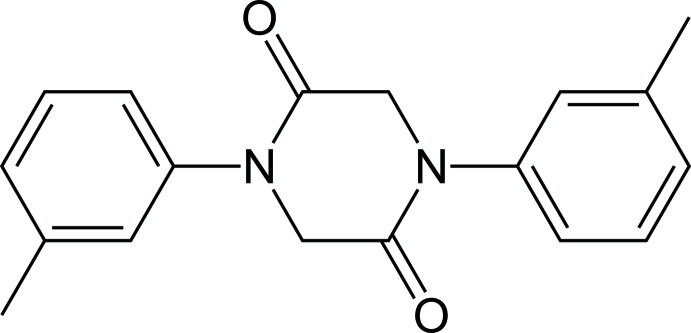
Experimental
Crystal data
C18H18N2O2
M r = 294.34
Monoclinic,

a = 12.6608 (15) Å
b = 6.1508 (7) Å
c = 19.223 (2) Å
β = 95.142 (2)°
V = 1490.9 (3) Å3
Z = 4
Mo Kα radiation
μ = 0.09 mm−1
T = 153 K
0.38 × 0.12 × 0.10 mm
Data collection
Bruker SMART CCD area-detector diffractometer
Absorption correction: multi-scan (SADABS; Sheldrick, 1996 ▶) T min = 0.968, T max = 0.991
7835 measured reflections
2836 independent reflections
2078 reflections with I > 2σ(I)
R int = 0.029
Refinement
R[F 2 > 2σ(F 2)] = 0.052
wR(F 2) = 0.137
S = 1.02
2836 reflections
199 parameters
H-atom parameters constrained
Δρmax = 0.19 e Å−3
Δρmin = −0.22 e Å−3
Data collection: SMART (Bruker, 2001 ▶); cell refinement: SAINT (Bruker, 2001 ▶); data reduction: SAINT; program(s) used to solve structure: SHELXTL (Sheldrick, 2008 ▶); program(s) used to refine structure: SHELXTL; molecular graphics: SHELXTL; software used to prepare material for publication: SHELXTL.
Supplementary Material
Crystal structure: contains datablock(s) I, global. DOI: 10.1107/S1600536812026372/zq2169sup1.cif
Structure factors: contains datablock(s) I. DOI: 10.1107/S1600536812026372/zq2169Isup2.hkl
Supplementary material file. DOI: 10.1107/S1600536812026372/zq2169Isup3.cml
Additional supplementary materials: crystallographic information; 3D view; checkCIF report
Table 1. Hydrogen-bond geometry (Å, °).
| D—H⋯A | D—H | H⋯A | D⋯A | D—H⋯A |
|---|---|---|---|---|
| C8—H8B⋯O1i | 0.97 | 2.53 | 3.494 (2) | 173 |
| C14—H14A⋯O2i | 0.93 | 2.45 | 3.325 (3) | 158 |
Symmetry code: (i)  .
.
Acknowledgments
The project was supported by the Promotive Research Fund for Excellent Young and Middle-Aged Scientists of Shandong Province (BS2011CL019).
supplementary crystallographic information
Comment
Piperazinediones form an important class of biologically active natural products (Fischer et al., 2003), and represent important precursors for the synthesis of peptides and non-natural amino acids (Paradisi et al., 2002). Recently, piperazinediones have gained importance in drug discovery (Krchnak et al., 1996), and opioid receptor agonists and antagonists (Acharya et al., 2001). Here, we report the crystal structure of the title compound.
The title compound consists of two crystallographically independent C18H18N2O2 molecules in the asymmetric unit of the centrosymmetric space group P21/n (Fig. 1). Each molecule is located on a center of inversion. All bond lengths and angles of two independent molecules are comparable with those in the related compounds (Wen et al., 2006; Zhang et al., 2007). The molecules are not planar showing dihedral angles of 55.84 (9)° and 54.10 (8)° between the piperazinedione and the aromatic rings, which is different from the ortho-substituted isomer 1,4-bis(2-methylphenyl)piperazine-2,5-dione (Wen et al., 2006). The sum of the bond angles around N1 (360.00°) and N2 (359.98°) indicates the piperazine N atoms have also a planar configuration, different from the normal pyramidal configuration of the N atom. This difference is mainly due to the π-conjugation effects arising from the presence of the two C=O double bonds. In addition, the crystal packing exhibit intermolecular C—H···O hydrogen bonds (Table 1, Fig. 2).
Experimental
The title compound was prepared according to the literature method (Wen et al., 2006). Colourless single crystals of the title compound suitable for X-ray diffraction study were obtained by slow evaporation of an ethanol solution over a period of 20 d.
Refinement
All H atoms were positioned geometricallyand constrained to ride on their parent atoms, with C—H = 0.93 Å and Uiso(H) = 1.2Ueq(C) for aromatic H atoms, with C—H = 0.97 Å and Uiso(H) = 1.2Ueq(C) for methylene H atoms, and with C—H = 0.96 and Uiso(H) = 1.5Ueq(C) for methyl H atoms.
Figures
Fig. 1.
The molecular structure of the title compound, with atom labels and 50% probability displacement ellipsoids.
Fig. 2.
The packing diagram of the title compound, viewed down the c axis, showing the intermolecular hydrogen bonds (dashed lines).
Crystal data
| C18H18N2O2 | F(000) = 624 |
| Mr = 294.34 | Dx = 1.311 Mg m−3 |
| Monoclinic, P21/n | Mo Kα radiation, λ = 0.71073 Å |
| Hall symbol: -P 2yn | Cell parameters from 1825 reflections |
| a = 12.6608 (15) Å | θ = 3.2–23.9° |
| b = 6.1508 (7) Å | µ = 0.09 mm−1 |
| c = 19.223 (2) Å | T = 153 K |
| β = 95.142 (2)° | Block, colourless |
| V = 1490.9 (3) Å3 | 0.38 × 0.12 × 0.10 mm |
| Z = 4 |
Data collection
| Bruker SMART CCD area-detector diffractometer | 2836 independent reflections |
| Radiation source: fine-focus sealed tube | 2078 reflections with I > 2σ(I) |
| Graphite monochromator | Rint = 0.029 |
| phi and ω scans | θmax = 25.7°, θmin = 1.9° |
| Absorption correction: multi-scan (SADABS; Sheldrick, 1996) | h = −15→15 |
| Tmin = 0.968, Tmax = 0.991 | k = −7→7 |
| 7835 measured reflections | l = −23→12 |
Refinement
| Refinement on F2 | Primary atom site location: structure-invariant direct methods |
| Least-squares matrix: full | Secondary atom site location: difference Fourier map |
| R[F2 > 2σ(F2)] = 0.052 | Hydrogen site location: inferred from neighbouring sites |
| wR(F2) = 0.137 | H-atom parameters constrained |
| S = 1.02 | w = 1/[σ2(Fo2) + (0.0664P)2 + 0.2932P] where P = (Fo2 + 2Fc2)/3 |
| 2836 reflections | (Δ/σ)max < 0.001 |
| 199 parameters | Δρmax = 0.19 e Å−3 |
| 0 restraints | Δρmin = −0.22 e Å−3 |
Special details
| Geometry. All e.s.d.'s (except the e.s.d. in the dihedral angle between two l.s. planes) are estimated using the full covariance matrix. The cell e.s.d.'s are taken into account individually in the estimation of e.s.d.'s in distances, angles and torsion angles; correlations between e.s.d.'s in cell parameters are only used when they are defined by crystal symmetry. An approximate (isotropic) treatment of cell e.s.d.'s is used for estimating e.s.d.'s involving l.s. planes. |
| Refinement. Refinement of F2 against ALL reflections. The weighted R-factor wR and goodness of fit S are based on F2, conventional R-factors R are based on F, with F set to zero for negative F2. The threshold expression of F2 > σ(F2) is used only for calculating R-factors(gt) etc. and is not relevant to the choice of reflections for refinement. R-factors based on F2 are statistically about twice as large as those based on F, and R- factors based on ALL data will be even larger. |
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2)
| x | y | z | Uiso*/Ueq | ||
| O2 | 0.14816 (10) | 0.1901 (2) | 0.50117 (7) | 0.0517 (4) | |
| N2 | 0.09833 (10) | 0.5171 (2) | 0.54147 (8) | 0.0372 (4) | |
| O1 | 0.53271 (11) | 1.1732 (2) | 0.59161 (8) | 0.0571 (4) | |
| N1 | 0.58560 (11) | 1.5000 (2) | 0.55280 (8) | 0.0423 (4) | |
| C15 | 0.19661 (13) | 0.5530 (3) | 0.58423 (9) | 0.0369 (4) | |
| C16 | 0.08291 (13) | 0.3358 (3) | 0.50265 (9) | 0.0383 (4) | |
| C14 | 0.25272 (14) | 0.7430 (3) | 0.57627 (10) | 0.0432 (5) | |
| H14A | 0.2275 | 0.8470 | 0.5438 | 0.052* | |
| C6 | 0.67677 (14) | 1.5123 (3) | 0.60368 (10) | 0.0434 (5) | |
| C7 | 0.52080 (14) | 1.3255 (3) | 0.55088 (11) | 0.0431 (5) | |
| C1 | 0.75220 (14) | 1.3485 (3) | 0.60645 (10) | 0.0442 (5) | |
| H1A | 0.7416 | 1.2280 | 0.5774 | 0.053* | |
| C10 | 0.23305 (13) | 0.4011 (3) | 0.63376 (9) | 0.0410 (5) | |
| H10A | 0.1942 | 0.2748 | 0.6391 | 0.049* | |
| C13 | 0.34654 (15) | 0.7763 (3) | 0.61707 (11) | 0.0499 (5) | |
| H13A | 0.3853 | 0.9026 | 0.6116 | 0.060* | |
| C8 | 0.56977 (15) | 1.6836 (3) | 0.50490 (11) | 0.0487 (5) | |
| H8A | 0.6345 | 1.7026 | 0.4821 | 0.058* | |
| H8B | 0.5608 | 1.8130 | 0.5326 | 0.058* | |
| C17 | 0.02008 (14) | 0.6913 (3) | 0.54148 (11) | 0.0480 (5) | |
| H17A | 0.0532 | 0.8236 | 0.5267 | 0.058* | |
| H17B | 0.0038 | 0.7134 | 0.5893 | 0.058* | |
| C12 | 0.38306 (15) | 0.6248 (3) | 0.66558 (11) | 0.0505 (5) | |
| H12A | 0.4468 | 0.6493 | 0.6924 | 0.061* | |
| C11 | 0.32667 (14) | 0.4345 (3) | 0.67562 (10) | 0.0443 (5) | |
| C5 | 0.69087 (17) | 1.6911 (3) | 0.64691 (11) | 0.0563 (6) | |
| H5A | 0.6405 | 1.8015 | 0.6450 | 0.068* | |
| C9 | 0.92602 (17) | 1.1862 (4) | 0.65370 (13) | 0.0715 (7) | |
| H9A | 0.9037 | 1.0751 | 0.6205 | 0.107* | |
| H9B | 0.9353 | 1.1243 | 0.6997 | 0.107* | |
| H9C | 0.9920 | 1.2472 | 0.6420 | 0.107* | |
| C4 | 0.78057 (19) | 1.7042 (4) | 0.69301 (12) | 0.0656 (7) | |
| H4A | 0.7901 | 1.8230 | 0.7229 | 0.079* | |
| C2 | 0.84310 (15) | 1.3621 (4) | 0.65189 (10) | 0.0507 (5) | |
| C18 | 0.36529 (18) | 0.2729 (4) | 0.73046 (12) | 0.0676 (7) | |
| H18A | 0.3167 | 0.1528 | 0.7298 | 0.101* | |
| H18B | 0.3697 | 0.3412 | 0.7755 | 0.101* | |
| H18C | 0.4341 | 0.2210 | 0.7211 | 0.101* | |
| C3 | 0.85602 (18) | 1.5430 (4) | 0.69520 (12) | 0.0637 (6) | |
| H3A | 0.9166 | 1.5552 | 0.7261 | 0.076* |
Atomic displacement parameters (Å2)
| U11 | U22 | U33 | U12 | U13 | U23 | |
| O2 | 0.0451 (8) | 0.0387 (7) | 0.0687 (9) | 0.0118 (6) | −0.0098 (7) | −0.0062 (7) |
| N2 | 0.0339 (8) | 0.0303 (8) | 0.0456 (9) | 0.0019 (6) | −0.0069 (7) | 0.0003 (7) |
| O1 | 0.0545 (8) | 0.0430 (8) | 0.0724 (10) | −0.0054 (6) | −0.0018 (7) | 0.0148 (7) |
| N1 | 0.0392 (8) | 0.0338 (8) | 0.0535 (10) | −0.0026 (6) | 0.0027 (7) | 0.0021 (7) |
| C15 | 0.0342 (9) | 0.0362 (9) | 0.0392 (10) | 0.0025 (7) | −0.0029 (8) | −0.0016 (8) |
| C16 | 0.0383 (9) | 0.0311 (9) | 0.0445 (11) | 0.0019 (7) | −0.0019 (8) | 0.0034 (8) |
| C14 | 0.0472 (11) | 0.0374 (10) | 0.0439 (10) | −0.0030 (8) | −0.0015 (9) | 0.0029 (9) |
| C6 | 0.0432 (10) | 0.0423 (10) | 0.0455 (11) | −0.0072 (8) | 0.0087 (9) | 0.0010 (9) |
| C7 | 0.0400 (10) | 0.0313 (9) | 0.0592 (12) | 0.0005 (8) | 0.0102 (9) | 0.0007 (9) |
| C1 | 0.0455 (11) | 0.0457 (11) | 0.0413 (11) | −0.0040 (9) | 0.0031 (9) | −0.0003 (9) |
| C10 | 0.0378 (10) | 0.0401 (10) | 0.0441 (10) | −0.0011 (8) | −0.0018 (9) | 0.0047 (9) |
| C13 | 0.0447 (11) | 0.0495 (11) | 0.0544 (12) | −0.0134 (9) | −0.0009 (10) | −0.0011 (10) |
| C8 | 0.0428 (10) | 0.0357 (10) | 0.0672 (14) | −0.0047 (8) | 0.0027 (10) | 0.0067 (10) |
| C17 | 0.0431 (10) | 0.0355 (10) | 0.0621 (13) | 0.0075 (8) | −0.0134 (10) | −0.0099 (9) |
| C12 | 0.0364 (10) | 0.0625 (13) | 0.0506 (12) | −0.0052 (9) | −0.0078 (9) | −0.0038 (10) |
| C11 | 0.0387 (10) | 0.0530 (12) | 0.0401 (10) | 0.0060 (9) | −0.0025 (8) | 0.0015 (9) |
| C5 | 0.0569 (13) | 0.0472 (12) | 0.0662 (14) | −0.0086 (10) | 0.0129 (11) | −0.0103 (11) |
| C9 | 0.0516 (13) | 0.0829 (17) | 0.0775 (17) | 0.0030 (12) | −0.0078 (12) | 0.0164 (14) |
| C4 | 0.0691 (15) | 0.0647 (15) | 0.0635 (15) | −0.0252 (12) | 0.0093 (13) | −0.0206 (12) |
| C2 | 0.0451 (11) | 0.0616 (13) | 0.0454 (11) | −0.0088 (10) | 0.0037 (9) | 0.0078 (10) |
| C18 | 0.0633 (14) | 0.0744 (15) | 0.0606 (14) | 0.0066 (12) | −0.0191 (12) | 0.0146 (13) |
| C3 | 0.0527 (13) | 0.0801 (17) | 0.0565 (13) | −0.0237 (12) | −0.0040 (11) | −0.0001 (13) |
Geometric parameters (Å, º)
| O2—C16 | 1.221 (2) | C8—C7ii | 1.499 (3) |
| N2—C16 | 1.346 (2) | C8—H8A | 0.9700 |
| N2—C15 | 1.445 (2) | C8—H8B | 0.9700 |
| N2—C17 | 1.459 (2) | C17—C16i | 1.500 (2) |
| O1—C7 | 1.221 (2) | C17—H17A | 0.9700 |
| N1—C7 | 1.349 (2) | C17—H17B | 0.9700 |
| N1—C6 | 1.446 (2) | C12—C11 | 1.393 (3) |
| N1—C8 | 1.460 (2) | C12—H12A | 0.9300 |
| C15—C10 | 1.383 (2) | C11—C18 | 1.499 (3) |
| C15—C14 | 1.383 (2) | C5—C4 | 1.379 (3) |
| C16—C17i | 1.500 (2) | C5—H5A | 0.9300 |
| C14—C13 | 1.379 (3) | C9—C2 | 1.505 (3) |
| C14—H14A | 0.9300 | C9—H9A | 0.9600 |
| C6—C5 | 1.380 (3) | C9—H9B | 0.9600 |
| C6—C1 | 1.386 (3) | C9—H9C | 0.9600 |
| C7—C8ii | 1.499 (3) | C4—C3 | 1.375 (3) |
| C1—C2 | 1.384 (3) | C4—H4A | 0.9300 |
| C1—H1A | 0.9300 | C2—C3 | 1.391 (3) |
| C10—C11 | 1.387 (2) | C18—H18A | 0.9600 |
| C10—H10A | 0.9300 | C18—H18B | 0.9600 |
| C13—C12 | 1.369 (3) | C18—H18C | 0.9600 |
| C13—H13A | 0.9300 | C3—H3A | 0.9300 |
| C16—N2—C15 | 121.08 (14) | N2—C17—C16i | 118.32 (15) |
| C16—N2—C17 | 122.92 (14) | N2—C17—H17A | 107.7 |
| C15—N2—C17 | 115.97 (14) | C16i—C17—H17A | 107.7 |
| C7—N1—C6 | 120.45 (16) | N2—C17—H17B | 107.7 |
| C7—N1—C8 | 123.32 (15) | C16i—C17—H17B | 107.7 |
| C6—N1—C8 | 116.22 (14) | H17A—C17—H17B | 107.1 |
| C10—C15—C14 | 120.26 (16) | C13—C12—C11 | 121.34 (17) |
| C10—C15—N2 | 120.36 (15) | C13—C12—H12A | 119.3 |
| C14—C15—N2 | 119.37 (15) | C11—C12—H12A | 119.3 |
| O2—C16—N2 | 123.79 (16) | C10—C11—C12 | 117.77 (17) |
| O2—C16—C17i | 117.46 (16) | C10—C11—C18 | 121.18 (18) |
| N2—C16—C17i | 118.75 (15) | C12—C11—C18 | 121.04 (17) |
| C13—C14—C15 | 119.16 (17) | C4—C5—C6 | 119.2 (2) |
| C13—C14—H14A | 120.4 | C4—C5—H5A | 120.4 |
| C15—C14—H14A | 120.4 | C6—C5—H5A | 120.4 |
| C5—C6—C1 | 120.31 (18) | C2—C9—H9A | 109.5 |
| C5—C6—N1 | 120.09 (18) | C2—C9—H9B | 109.5 |
| C1—C6—N1 | 119.54 (16) | H9A—C9—H9B | 109.5 |
| O1—C7—N1 | 123.53 (18) | C2—C9—H9C | 109.5 |
| O1—C7—C8ii | 118.20 (16) | H9A—C9—H9C | 109.5 |
| N1—C7—C8ii | 118.26 (17) | H9B—C9—H9C | 109.5 |
| C2—C1—C6 | 120.76 (18) | C3—C4—C5 | 120.5 (2) |
| C2—C1—H1A | 119.6 | C3—C4—H4A | 119.7 |
| C6—C1—H1A | 119.6 | C5—C4—H4A | 119.7 |
| C15—C10—C11 | 120.95 (17) | C1—C2—C3 | 118.2 (2) |
| C15—C10—H10A | 119.5 | C1—C2—C9 | 120.7 (2) |
| C11—C10—H10A | 119.5 | C3—C2—C9 | 121.12 (19) |
| C12—C13—C14 | 120.50 (18) | C11—C18—H18A | 109.5 |
| C12—C13—H13A | 119.8 | C11—C18—H18B | 109.5 |
| C14—C13—H13A | 119.8 | H18A—C18—H18B | 109.5 |
| N1—C8—C7ii | 118.38 (15) | C11—C18—H18C | 109.5 |
| N1—C8—H8A | 107.7 | H18A—C18—H18C | 109.5 |
| C7ii—C8—H8A | 107.7 | H18B—C18—H18C | 109.5 |
| N1—C8—H8B | 107.7 | C4—C3—C2 | 121.0 (2) |
| C7ii—C8—H8B | 107.7 | C4—C3—H3A | 119.5 |
| H8A—C8—H8B | 107.1 | C2—C3—H3A | 119.5 |
| C16—N2—C15—C10 | −55.7 (2) | C14—C15—C10—C11 | −0.8 (3) |
| C17—N2—C15—C10 | 126.27 (19) | N2—C15—C10—C11 | −179.35 (17) |
| C16—N2—C15—C14 | 125.74 (19) | C15—C14—C13—C12 | −0.9 (3) |
| C17—N2—C15—C14 | −52.3 (2) | C7—N1—C8—C7ii | −2.2 (3) |
| C15—N2—C16—O2 | 1.0 (3) | C6—N1—C8—C7ii | 177.36 (16) |
| C17—N2—C16—O2 | 178.81 (19) | C16—N2—C17—C16i | 1.3 (3) |
| C15—N2—C16—C17i | −179.17 (17) | C15—N2—C17—C16i | 179.26 (16) |
| C17—N2—C16—C17i | −1.3 (3) | C14—C13—C12—C11 | −0.5 (3) |
| C10—C15—C14—C13 | 1.6 (3) | C15—C10—C11—C12 | −0.6 (3) |
| N2—C15—C14—C13 | −179.86 (17) | C15—C10—C11—C18 | 178.63 (19) |
| C7—N1—C6—C5 | −126.3 (2) | C13—C12—C11—C10 | 1.3 (3) |
| C8—N1—C6—C5 | 54.1 (2) | C13—C12—C11—C18 | −177.93 (19) |
| C7—N1—C6—C1 | 56.7 (2) | C1—C6—C5—C4 | −0.3 (3) |
| C8—N1—C6—C1 | −122.93 (19) | N1—C6—C5—C4 | −177.27 (19) |
| C6—N1—C7—O1 | 1.7 (3) | C6—C5—C4—C3 | 1.1 (3) |
| C8—N1—C7—O1 | −178.69 (18) | C6—C1—C2—C3 | 0.8 (3) |
| C6—N1—C7—C8ii | −177.35 (17) | C6—C1—C2—C9 | −178.7 (2) |
| C8—N1—C7—C8ii | 2.2 (3) | C5—C4—C3—C2 | −0.9 (4) |
| C5—C6—C1—C2 | −0.7 (3) | C1—C2—C3—C4 | 0.0 (3) |
| N1—C6—C1—C2 | 176.35 (17) | C9—C2—C3—C4 | 179.4 (2) |
Symmetry codes: (i) −x, −y+1, −z+1; (ii) −x+1, −y+3, −z+1.
Hydrogen-bond geometry (Å, º)
| D—H···A | D—H | H···A | D···A | D—H···A |
| C8—H8B···O1iii | 0.97 | 2.53 | 3.494 (2) | 173 |
| C14—H14A···O2iii | 0.93 | 2.45 | 3.325 (3) | 158 |
Symmetry code: (iii) x, y+1, z.
Footnotes
Supplementary data and figures for this paper are available from the IUCr electronic archives (Reference: ZQ2169).
References
- Acharya, A. N., Ostresh, J. M. & Houghten, R. A. (2001). J. Comb. Chem. 3, 612–623. [DOI] [PubMed]
- Bruker (2001). SMART and SAINT Bruker AXS Inc., Madison, Wisconsin, USA.
- Fischer, P. M. (2003). J. Pept. Sci. 9, 9–35. [DOI] [PubMed]
- Krchnak, V., Weichsel, A. S., Cabel, D., Flegelova, Z. & Lebl, M. (1996). Mol. Div. 1, 149–164. [DOI] [PubMed]
- Paradisi, F., Piccinelli, F., Porzi, G. & Sandri, S. (2002). Tetrahedron Asymmetry, 13, 497–502.
- Sheldrick, G. M. (1996). SADABS University of Göttingen, Germany.
- Sheldrick, G. M. (2008). Acta Cryst. A64, 112–122. [DOI] [PubMed]
- Wen, Y.-H., Zhang, S.-S., Yu, B.-H., Li, X.-M. & Liu, Q. (2006). Asian J. Chem. 18, 1032–1038.
- Zhang, S.-S., Wen, Y.-H., Tang, X.-F. & Li, X.-M. (2007). Chin. J. Chem. 25, 714–717.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Crystal structure: contains datablock(s) I, global. DOI: 10.1107/S1600536812026372/zq2169sup1.cif
Structure factors: contains datablock(s) I. DOI: 10.1107/S1600536812026372/zq2169Isup2.hkl
Supplementary material file. DOI: 10.1107/S1600536812026372/zq2169Isup3.cml
Additional supplementary materials: crystallographic information; 3D view; checkCIF report




